α-Catulin CTN-1 is required for BK channel subcellular localization in C. elegans body-wall muscle cells
Despite their importance, relatively little is known about the regulation of BK channel distribution and activity. Here, through a genetic screen in C. elegans, α-catulin is identified as a critical regulator of BK channel subcellular localization in muscle.
Keywords: BK channel, CTN-1, SLO-1, subcellular localization, α-catulin
Abstract
The BK channel, a voltage- and Ca2+-gated large-conductance potassium channel with many important functions, is often localized at specific subcellular domains. Although proper subcellular localization is likely a prerequisite for the channel to perform its physiological functions, little is known about the molecular basis of localization. Here, we show that CTN-1, a homologue of mammalian α-catulin, is required for subcellular localization of SLO-1, the Caenorhabditis elegans BK channel α-subunit, in body-wall muscle cells. CTN-1 was identified in a genetic screen for mutants that suppressed a lethargic phenotype caused by expressing a gain-of-function (gf) isoform of SLO-1. In body-wall muscle cells, CTN-1 coclusters with SLO-1 at regions of dense bodies, which are Z-disk analogs of mammalian skeletal muscle. In ctn-1 loss-of-function (lf) mutants, SLO-1 was mislocalized in body-wall muscle but its transcription and protein level were unchanged. Targeted rescue of ctn-1(lf) in muscle was sufficient to reinstate the lethargic phenotype in slo-1(gf);ctn-1(lf). These results suggest that CTN-1 plays an important role in BK channel function by mediating channel subcellular localization.
Introduction
The BK channel (also known as Slo1, KCa or MaxiK channel) is almost ubiquitously expressed and performs many important physiological functions. Malfunction of the channel may cause a variety of disorders, including epilepsy (Brenner et al, 2005; Du et al, 2005), hypertension (Brenner et al, 2000b), progressive hearing loss (Ruttiger et al, 2004), cerebellar ataxia (Sausbier et al, 2004), overactive bladder (Meredith et al, 2004), penile erectile dysfunction (Werner et al, 2005), impaired renal glomerular filtration and potassium excretion (Pluznick et al, 2003) and paroxysmal dyskinesia (Du et al, 2005). The central components of a BK channel are four α-subunits, which form the channel pore and contain voltage and calcium sensing domains (Adelman et al, 1992; Butler et al, 1993; Pallanck and Ganetzky, 1994). In addition, the α-subunits may interact with other proteins that modulate channel functional properties or expression, such as β-subunits and a MinK-related peptide in mammals (Knaus et al, 1994; Wallner et al, 1999; Xia et al, 1999, 2000; Uebele et al, 2000; Weiger et al, 2000; Brenner et al, 2000a; Levy et al, 2008), and Slob and dSLIP1 in Drosophila (Xia et al, 1998; Zhou et al, 1999).
BK channels are often localized/enriched at specific subcellular domains. For example, in neurons, BK channels are enriched at the presynaptic nerve terminal (Robitaille et al, 1993; Knaus et al, 1996; Zhou et al, 1999; Hu et al, 2001; Misonou et al, 2006), where they colocalize with voltage-gated Ca2+ channels (VGCCs) (Roberts et al, 1990; Robitaille et al, 1993; Issa and Hudspeth, 1994; Yazejian et al, 2000) to regulate neurotransmitter release (Robitaille et al, 1993; Hu et al, 2001; Wang et al, 2001; Raffaelli et al, 2004; Liu et al, 2007; Wang, 2008). In epithelial cells, BK channels are localized to the apical membrane, where they may regulate potassium secretion and cell volume (Segal and Reuss, 1990; Pacha et al, 1991; Takeuchi et al, 1992; Hirsch et al, 1993; James and Okada, 1994; Huang et al, 1999). In mouse inner hair cells of the cochlea, BK channels are localized to the apical membrane (Pyott et al, 2004) but their role in mammalian auditory function is unclear (Pyott et al, 2007). Proper subcellular localization of the channel is likely important to its physiological functions. However, this has not been experimentally demonstrated. The molecular basis of BK channel subcellular localization is also poorly understood. Although a recent study reported that ISLO-1, a protein with two putative membrane-spanning domains, contributes to SLO-1 subcellular localization in Caenorhabditis elegans (Kim et al, 2009), no homologues could be identified in mammals.
The C. elegans BK channel α-subunit SLO-1 is enriched at synaptic regions in the nervous system and clusters in the vicinity of dense bodies in body-wall muscle cells (Wang et al, 2001). In a genetic screen for suppressors of a lethargic phenotype caused by expressing a slo-1(gf) transgene, we identified CTN-1 as a protein indispensable for BK channel function in C. elegans body-wall muscle cells, presumably because of its function in channel subcellular localization. This finding may serve as a starting point for elucidating the molecular basis of BK channel subcellular localization in mammals.
Results
ctn-1 Mutants were isolated as suppressors of a lethargic phenotype caused by slo-1(gf)
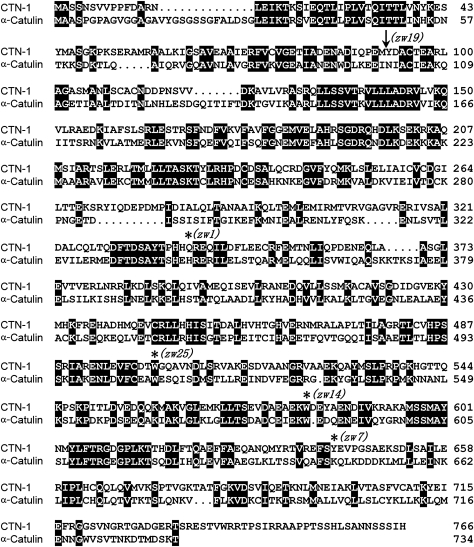
To identify novel molecules related to BK channel function in vivo, we screened for mutants that suppressed the lethargic phenotype of a worm strain expressing SLO-1(gf) under the control of slo-1 promoter (Pslo-1). SLO-1(gf) was created by mutating SLO-1 glutamate 350 to glutamine (E350Q). E350 is the same residue that was changed to lysine in a previously described slo-1(gf) mutant (Davies et al, 2003), and the equivalent of mouse Slo1 E321, which contributes to one of the two negative rings at the entrance to the intracellular vestibule of the channel (Brelidze et al, 2003). Worms expressing Pslo-1::SLO-1(E350Q) exhibited distorted locomotion waveform and greatly decreased locomotion speed (Supplementary Movies 1 and 2). From a screening of 24 000 haploid genomes, we isolated 25 mutants. Twelve of the isolated mutants belong to one gene, which was mapped to a 107-kb interval on chromosome I (2562–2669 kb) through single-nucleotide polymorphism (SNP)-based mapping (Davis et al, 2005). We then tested whether cosmids or PCR-amplified genomic DNA fragments of predicted genes within this interval could reinstate the lethargic phenotype of slo-1(gf) when they were expressed in one of the mutants that harboured the slo-1(gf) transgene. We found that two PCR-amplified overlapping genomic DNA fragments (total ∼15 kb) corresponding to the predicted ctn-1 gene (locus Y23H5A.5, http://www.wormbase.org) and 3 kb sequence upstream of its initiation site completely restored the lethargic phenotype of slo-1(gf) (not shown). The ctn-1 encodes a homologue of mammalian α-catulin (Janssens et al, 1999). Although several splice variants of ctn-1 have been identified (http://www.wormbase.org), we found that ctn-1d (Y23H5A.5d) is the predominant isoform based on reverse transcription PCR (RT–PCR). The predicted translational product of this isoform shares 41% identity with mammalian α-catulin (Janssens et al, 1999) in amino-acid sequence (Figure 1). Sequencing of five randomly picked ctn-1 mutants revealed molecular lesions of this gene in all of them (Figure 1). The ctn-1 (zw1), which is a putative null resulting from a premature stop codon, was used for all subsequent analyses.
Figure 1.
The ctn-1 encodes a homologue of mammalian α-catulin. Shown is the alignment between predicted amino-acid sequences of CTN-1 and human α-catulin (41% identity). The molecular lesions of five ctn-1 alleles were determined. Four alleles have mutations leading to premature stop codon (marked with ‘*') and one allele (marked with an arrow) disrupts a splice donor site leading to a frame shift after amino-acid Y92 and then a stop codon (CFNGQPIMCM STOP).
ctn-1 was expressed in muscle cells and neurons
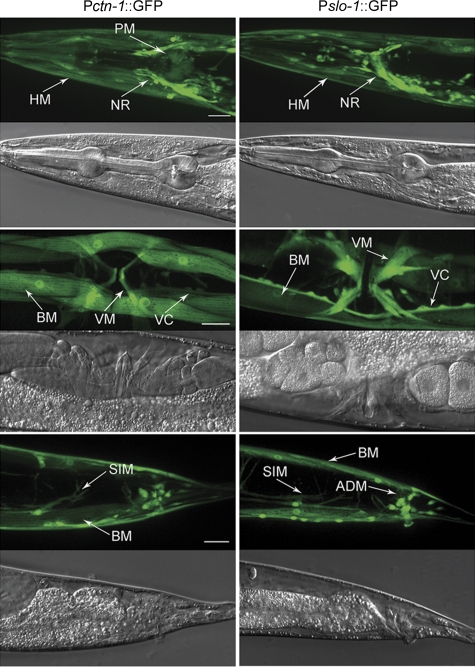
To understand how CTN-1 contributes to SLO-1 function in vivo, we first analysed the expression pattern of ctn-1 and compared it with that of slo-1. Two independent transgenic strains were created that expressed GFP under the control of the ctn-1 promoter (Pctn-1) and slo-1 promoter (Pslo-1), respectively. The expression pattern of ctn-1 largely overlapped with that of slo-1. Specifically, both ctn-1 and slo-1 were expressed in many neurons and several types of muscles, including body-wall muscle, vulval muscle and stomatointestinal muscle. However, slo-1 appeared to be expressed in more neurons in the head than ctn-1, whereas ctn-1 was expressed in pharyngeal muscle cells and some other unidentified cells that did not express slo-1 (Figure 2).
Figure 2.
The expression pattern of ctn-1 was similar to that of slo-1. The expression patterns were analysed by expressing GFP under the independent controls of slo-1 promoter (Pslo-1, 5.2 kb) and ctn-1 promoter (Pctn-1, 4.3 kb). Strong expression was observed in body-wall muscle (BM) (including head muscle, HM), vulval muscle (VM), stomatointestinal muscle (SIM), nerve ring (NR), and many neurons in the head (not labelled), ventral cord (VC) and tail (not labelled) with both transcriptional fusions. In addition, Pslo-1::GFP was expressed in more neurons in the head, and anal depressor muscle (ADM) in the tail, whereas Pctn-1::GFP was expressed in some pharyngeal muscles (PM) in the corpus and terminal bulb. Scale bar=20 μm. A full-colour version of this figure is available at The EMBO Journal Online.
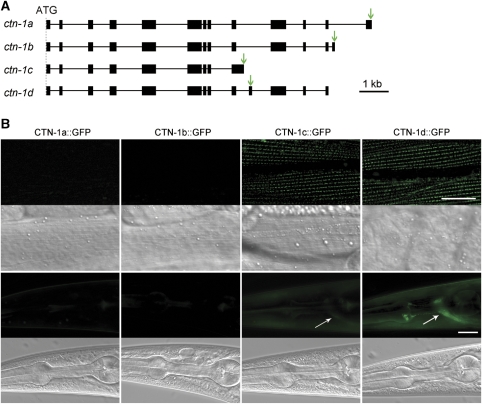
The identified ctn-1 splice variants (http://www.wormbase.org) include 9 to 13 exons. All of the splice variants share the first 8 exons but each of them has a unique exon afterward. To determine where these splice variants might be expressed, we created transgenic strains expressing ctn-1 genomic DNA with GFP-coding sequence inserted into each of the unique exons separately (Figure 3A). As GFP was fused to full-length CTN-1, GFP epifluorescence could reflect both the expression and subcellular localization patterns of the CTN-1 isoforms. We detected strong ctn-1d expression in both neurons and muscle cells, obvious ctn-1c expression in muscle cells but weak ctn-1c expression in neurons, and no ctn-1a or ctn-1b expression (Figure 3B). As CTN-1d::GFP appeared as puncta in neuronal processes but not in the soma, it is difficult to tell whether it is expressed in all or a subset of motoneurons in the ventral nerve cord. We then tested whether the expression of wild-type ctn-1c in a ctn-1;slo-1(gf) double mutant could reinstate the lethargic phenotype as ctn-1d did. However, such an effect of ctn-1c was not observed, suggesting CTN-1c is either unrelated or unimportant to SLO-1 function in vivo. Therefore, CTN-1d appeared to be the most important isoform with respect to SLO-1 function, and was used in subsequent experiments. CTN-1d is referred to as CTN-1 hereafter.
Figure 3.
Expression patterns of ctn-1 splicing variants. (A) Intron–exon organization of ctn-1 splicing variants. GFP was inserted into the unique exon of each splicing variant. The arrows indicate the locations of GFP insertion. (B) No expression was observed for CTN-1a and CTN-1b. Both CTN-1c and CTN-1d were strongly expressed in body-wall muscle cells. CTN-1d was also strongly expressed in neurons, as indicated by the bright fluorescence signal in the nerve ring (arrow), whereas CTN-1c appeared to be weakly expressed only in some neurons (arrow). A corresponding DIC image was shown below each fluorescent image. Scale bar=20 μm. A full-colour version of this figure is available at The EMBO Journal Online.
CTN-1 was required for SLO-1 function in body-wall muscle cells
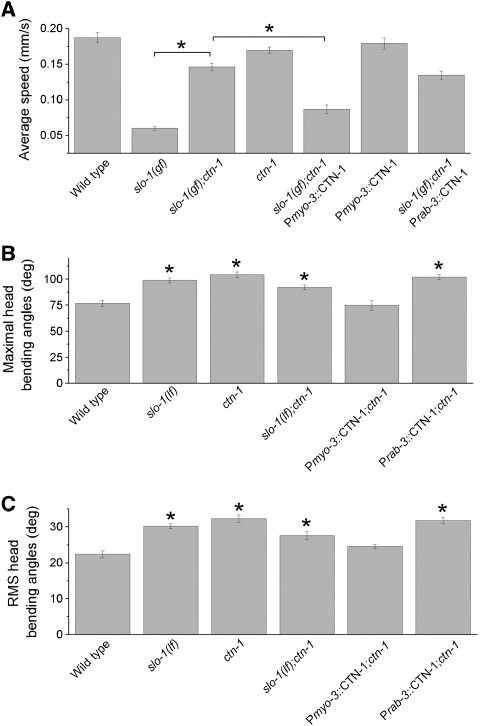
As the expression patterns of ctn-1 and slo-1 appeared to overlap in body-wall muscle cells and some neurons, the suppression of slo-1(gf) lethargic phenotype by ctn-1(lf) could be due to CTN-1 deficiency in muscle cells, neurons or both. To answer this question, we tested whether targeted expression of wild-type CTN-1 in body-wall muscle cells or neurons could reinstate the lethargic phenotype in the slo-1(gf);ctn-1(lf) double mutant. Quantitative analyses of locomotion speed showed that ctn-1(lf) partially but significantly suppressed the inhibitory effect of slo-1(gf) on locomotion, which could be reversed by expressing wild-type CTN-1 under the control of the muscle-specific myo-3 promoter (Pmyo-3) (Okkema et al, 1993) but not the pan-neuronal rab-3 promoter (Prab-3) (Nonet et al, 1997) (Figure 4A; Supplementary Movies 3, 4, 5, 6 and 7). These observations suggest that the suppression of slo-1(gf) phenotype by ctn-1(lf) resulted mainly from CTN-1 deficiency in muscle cells.
Figure 4.
CTN-1 was required for SLO-1 function in body-wall muscle cells but not neurons. (A) The ctn-1(lf) mutant counteracted the inhibitory effect of slo-1(gf) on locomotion speed, and this effect could be reversed by expressing wild-type CTN-1 in body-wall muscle cells under the control of the myo-3 promoter (Pmyo-3) but not in neurons under the control of the pan-neuronal rab-3 promoter (Prab-3). (B, C) Quantification of the maximal head-bending angle (B) and root mean square (RMS) of head-bending angle (C). The ctn-1(lf) and slo-(lf) mutants showed increased head-bending angle, and the severity of this phenotype was nonadditive in the slo-1(lf);ctn-1(lf) double mutant. The abnormal bending phenotype of the ctn-1(lf) mutant could be rescued by expressing wild-type CTN-1 in muscle cells using Pmyo-3 but not in neurons using Prab-3. Data are shown as mean±s.e. The asterisk (*) indicates a statistically significant difference (P<0.01) for comparisons either between the specified groups (A), or between the wild type and a mutant strain (B, C). The number of worms in each group analysed was 20–30 for the data shown in (A) and 10 for the data shown in (B, C).
Both slo-1(lf) and ctn-1(lf) mutants appeared to be grossly distinct from the wild type in head movement behaviours. To further examine functional relationship between CTN-1 and SLO-1, we quantified the head-bending angle of the wild type and mutant animals using an automated tracking and analysis system. Consistent with a previous report (Kim et al, 2009), we observed a significant increase in head-bending angle in slo-1(lf). This phenotype was shared by ctn-1(lf), and that its severity was not additive in the ctn-1(lf);slo-1(lf) double mutant (Figure 4B and C), suggesting that CTN-1 and SLO-1 likely function together. The head-bending phenotype of ctn-1(lf) could be rescued by expressing wild-type CTN-1 in muscle cells but not in neurons (Figure 4B and C), suggesting that the mutant phenotype was mainly caused by CTN-1 dysfunction in muscle cells.
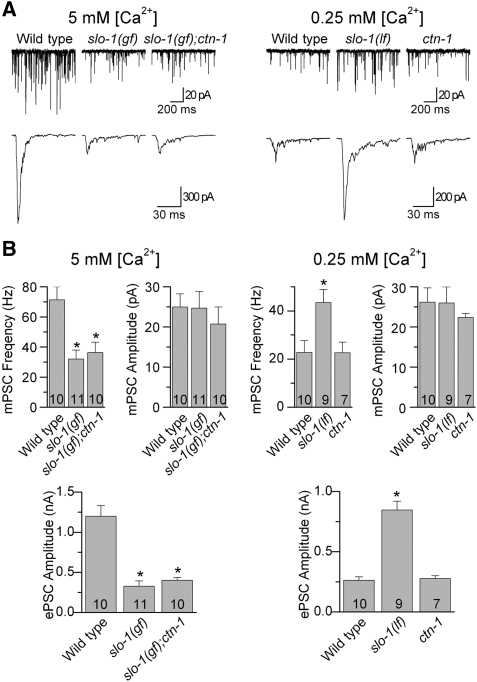
SLO-1 is an important negative regulator of neurotransmitter release at the C. elegans neuromuscular junction (NMJ) (Wang et al, 2001; Liu et al, 2007). The identification of ctn-1 expression in ventral cord motoneurons (Figure 2) raised the possibility that CTN-1 might be needed for SLO-1 function in regulating neurotransmitter release. To investigate this possibility, we analysed the effect of ctn-1(lf) on miniature and evoked postsynaptic currents (mPSCs and ePSCs) recorded from body-wall muscle cells at two different extracellular Ca2+ concentrations (5 and 0.25 mM). The higher [Ca2+] is more suitable for determining whether slo-1(gf) reduces ePSC amplitude and whether this effect may be reversed by ctn-1(lf), whereas the lower [Ca2+] is more suitable for testing whether ctn-1(lf) could increase ePSC amplitude as slo-1(lf) does (Liu et al, 2007).
ePSCs were evoked by photoactivation of motoneurons expressing channelrhodopsin-2 under the control of the unc-17 promoter (Liewald et al, 2008; Liu et al, 2009). At 5 mM [Ca2+]o, slo-1(gf) significantly decreased ePSC amplitude and mPSC frequency without affecting mPSC amplitude compared with the wild type, and these effects of slo-1(gf) were not suppressed by ctn-1(lf) (Figure 5). At 0.25 mM [Ca2+]o, slo-1(lf) significantly increased ePSC amplitude and mPSCs frequency without affecting mPSC amplitude, and these effects of slo-1(lf) were not shared by ctn-1(lf) (Figure 5). These observations suggest that CTN-1 is not required for the function of SLO-1 in regulating neurotransmitter release at the NMJ.
Figure 5.
The ctn-1(lf) mutant did not affect neurotransmitter release at the NMJs. (A) Representative traces of miniature postsynaptic currents (mPSCs) (top) and photo-evoked postsynaptic currents (ePSCs) (bottom) from the wild type, slo-1(gf), and slo-1(gf);ctn-1(lf), slo-1(lf), and ctn-1(lf) recorded at either 5 or 0.25 mM [Ca2+]o. (B) Comparisons of mPSC frequency, mPSC amplitude and ePSC amplitude between the different groups. Data are shown as mean±s.e. The asterisk (*) indicates P<0.01 compared with the wild type. The number of samples analysed is indicated inside each column. One-way ANOVA (with Bonferroni post hoc test) was used for statistical comparisons of the means.
CTN-1 was required for SLO-1 subcellular localization in body-wall muscle cells
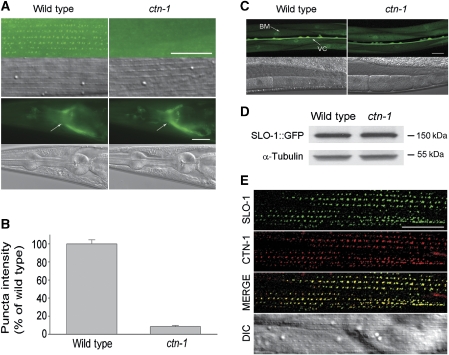
CTN-1 could contribute to SLO-1 function in body-wall muscle cells through several potential mechanisms. We first tested whether CTN-1 has a function in SLO-1 subcellular localization. We previously showed that SLO-1 is enriched at dense body areas in body-wall muscle cells and in the synapse-rich nerve ring in the nervous system, and that these subcellular localization patterns may be recapitulated by an SLO-1::GFP fusion protein (Wang et al, 2001). We created two transgenic strains expressing integrated Pslo-1::SLO-1::GFP and Pmyo-3::SLO-1::GFP for analysing SLO-1 subcellular localization in the nerve ring and body-wall muscle cells, respectively. These two transgenes were then separately crossed into ctn-1(lf) mutant. Although SLO-1::GFP appeared as puncta at locations matching dense bodies in body-wall muscle cells of the wild type, the fluorescent puncta were almost absent in ctn-1(lf) (Figure 6A and B). In contrast, SLO-1::GFP localization in the nerve ring appeared indistinguishable between the wild-type and ctn-1(lf) mutant (Figure 6A). We then asked whether ctn-1(lf) would affect the subcellular localization of two other proteins in body-wall muscle cells, including INX-11 and vinculin. INX-11 is an innexin that may form gap junctions or hemichannels, whereas vinculin is a membrane-cytoskeletal protein. Both proteins are expressed in body-wall muscle cells and localized to dense body regions (Francis and Waterston, 1985; Barstead and Waterston, 1989; Altun et al, 2009). We found that both GFP-tagged INX-11 and native vinculin were normally localized in the ctn-1(lf) mutant (Supplementary Figure S2). These observations suggest that CTN-1 may be specifically required for SLO-1 subcellular localization in body-wall muscle cells.
Figure 6.
CTN-1 mediates SLO-1 subcellular localization in body-wall muscle cells. (A) SLO-1::GFP was mislocalized in body-wall muscle cells but not neurons of ctn-1(lf), as indicated by the absence of fluorescent puncta (upper panel) in muscle cells but apparently normal epifluorescence in the nerve ring (indicated with arrow) (lower panel). (B) Quantification of the intensity of SLO-1::GFP puncta in body-wall muscle cells. The intensity in ctn-1(lf) was normalized to that in the wild type. Data are shown as mean±s.e. Compared with the wild type, the intensity was significantly reduced in the ctn-1(lf) mutant (P<0.01, unpaired t-test). The numbers of cells analysed were 24 and 22 for the wild-type and ctn-1 mutant, respectively. (C) slo-1 Transcription was unaltered in ctn-1(lf). A slo-1 promoter and GFP transcriptional fusion was expressed in wild-type animals and ctn-1(lf). GFP epifluorescence in body-wall muscle cells, imaged under identical exposure conditions, was indistinguishable between the two groups. The body muscle (BM) and ventral cord (VC) are indicated (arrows). (D) Western blot shows that the level of total SLO-1::GFP protein was comparable between wild-type and ctn-1(lf) mutant worms. α-Tubulin was blotted to show equal loading of the protein samples. (E) SLO-1 and CTN-1 colocalized in dense body areas of body-wall muscle cells. GFP-tagged SLO-1 (green) and mStrawberry-tagged CTN-1 (red) were coexpressed in body-wall muscle cells under the control of the myo-3 promoter. The merged picture shows colocalization of the two fusion proteins. Scale bar=20 μm.
The disappearance of SLO-1::GFP puncta in body-wall muscle cells of ctn-1(lf) mutants could be due to decreased gene transcription or decreased protein synthesis/stability. To determine whether CTN-1 controls slo-1 transcription in muscle cells, we compared the expression of a Pslo-1::GFP transcriptional fusion between the wild-type and ctn-1(lf) mutant. GFP expression in body-wall muscle cells was similar between the two groups (Figure 6C). To determine whether CTN-1 has an effect on SLO-1 protein level, we compared the total SLO-1::GFP protein level between the wild-type and ctn-1(lf) mutant by western blot using a GFP antibody but found no difference (Figure 6D). These observations suggest that the apparent SLO-1 mislocalization observed in ctn-1(lf) mutant did not result from a deficiency in slo-1 transcription or SLO-1 protein synthesis/stability. We also asked whether CTN-1 regulates SLO-1 surface protein level by performing biotinylation assays with transfected HEK293 cells, and found that both the total and surface SLO-1 protein levels were comparable between cells transfected with SLO-1 alone and SLO-1 plus CTN-1 (Supplementary Figure S3), suggesting that CTN-1 probably does not regulate SLO-1 trafficking to the plasma membrane. However, given that HEK293 cells are different from C. elegans body-wall muscle cells in many ways, we cannot exclude a function of CTN-1 in SLO-1 membrane trafficking in vivo solely based on this observation.
CTN-1 physically interacted with SLO-1 both in vivo and in vitro
To determine whether CTN-1 mediates SLO-1 subcellular localization through a local effect, we analysed subcellular localization pattern of a CTN-1::EGFP fusion protein in body-wall muscle cells. The fusion protein was expected to recapitulate the subcellular localization pattern of wild-type CTN-1 because it reinstated the lethargic phenotype when expressed in the slo-1(gf);ctn-1(lf) double mutant (not shown). We found that CTN-1::EGFP was enriched at dense body regions (Supplementary Figure S4A), which was independent of SLO-1 (Supplementary Figure S4B). Furthermore, we found that CTN-1::mStrawberry, which was also fully functional, colocalized with SLO-1::GFP at dense body regions (Figure 6E). These observations suggest that CTN-1 likely mediates SLO-1 subcellular localization through a local effect.
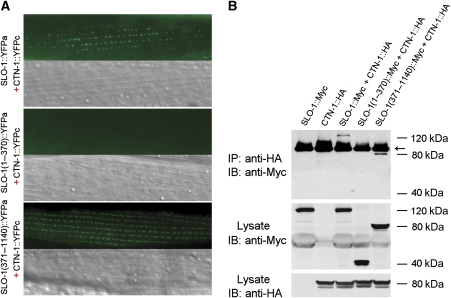
The colocalization data shown in Figure 6E did not have enough resolution to suggest whether CTN-1 physically interacts with SLO-1. To address this question, we performed bimolecular fluorescence complementation (BiFC) assays (Chen et al, 2007; Shyu et al, 2008), which tells not only whether but also where two proteins interact in vivo. In this assay, the nonfluorescent amino- and carboxyl-terminal portions of yellow fluorescent protein (YFPa and YFPc) are fused separately to a pair of proteins of interest. The fluorophore of YFP may be reconstituted if these two proteins are physically very close (Shyu et al, 2008). We inserted YFPa into the linker region between the two RCK domains of SLO-1 and fused YFPc to the carboxyl terminus of CTN-1, and coexpressed these two fusion proteins in body-wall muscle cells under the control of Pmyo-3. Fluorescent puncta were observed in body-wall muscle cells coexpressing SLO-1::YFPa and CTN-1::YFPc (Figure 7A), suggesting that SLO-1 and CTN-1 are physically very close in the muscle cells. SLO-1 may be divided into two major structural components, including the amino-terminal portion (1–352 aa) that contains seven membrane-spanning domains and the channel pore domain, and the cytoplasmic carboxyl terminal portion (353–1140) that contains two RCK domains (Wang et al, 2001; Jiang et al, 2002; Salkoff et al, 2006; Yusifov et al, 2008; Yuan et al, 2010). To determine which part of SLO-1 is important for the interaction with CTN-1, we tested whether the amino- or carboxyl-terminal portion of SLO-1 is required for BiFC with CTN-1. In these experiments, YFPa was either fused to the carboxyl terminus of SLO-1(1–370) or inserted into the linker region between the two RCK domains of SLO-1(371–1140). We found that SLO-1(371–1140) but not SLO-1(1–370) allowed BiFC with CTN-1 (Figure 7A). These observations suggest that CTN-1 is physically very close to SLO-1, and likely interacts with the carboxyl terminal portion of SLO-1 to mediate SLO-1 subcellular localization.
Figure 7.
CTN-1 physically interacts with SLO-1. (A) CTN-1 and SLO-1 were physically very close. The nonfluorescent amino- and carboxyl-terminal portions of YFP (YFPa and YFPc) were fused to SLO-1 (or its variants) and CTN-1, respectively. Bimolecular fluorescence complementation (BiFC) assays were performed by coexpressing CTN-1::YFPc with each of the SLO-1 fusions (full-length SLO-1::YFPa, SLO-1(1–370)::YFPa and SLO-1(371–1140)::YFPa) in body-wall muscle cells under the control of the myo-3 promoter. Fluorescent puncta were observed in dense body areas when CTN-1::YFPc was coexpressed with either full-length SLO-1::YFPa (upper panel) or SLO-1(371–1140)::YFPa (lower panel) but not with SLO-1(1–370)::YFPa (middle panel). The negative result with SLO-1(1–370)::YFPa was not due to poor expression of the fusion protein because SLO-1(1–370)::YFPa was able to reconstitute YFP epifluorescence when coexpressed with another protein (not shown). Scale bar=20 μm. (B) CTN-1 and SLO-1 coimmunoprecipitated from transfected HEK293 cells. CTN-1 coimmunoprecipitated with either full-length SLO-1 or SLO-1(371–1140) but not with SLO-1(1–370). CTN-1 was tagged with HA, whereas SLO-1 and its variants were tagged with Myc. Immunoprecipitation (IP) and immunoblot (IB) were performed with Myc and HA antibodies. All specific bands are indicated by their relative molecular sizes. The antibody heavy chain dimmer is indicated with an arrow. A full-colour version of this figure is available at The EMBO Journal Online.
To obtain independent evidence showing the interaction between CTN-1 and SLO-1, we performed coimmunoprecipitation experiments with HA-tagged CTN-1, and Myc-tagged full-length SLO-1 or SLO-1 amino- and carboxyl-terminal portions. We found that either full-length SLO-1 or SLO-1(371–1140) coimmunoprecipitated with CTN-1 whereas SLO-1(1–370) did not (Figure 7B). These observations reinforced the notion that the cytoplasmic carboxyl terminal portion of SLO-1 is important to the interaction with CTN-1.
CTN-1 did not modulate SLO-1 current properties
The physical closeness between CTN-1 and SLO-1, as revealed by the biochemical assays, suggested that CTN-1 might be able to directly modulate SLO-1 functional properties. We examined this possibility by coexpressing CTN-1 with SLO-1 in Xenopus oocytes, and analysing SLO-1 currents recorded from inside–out patches at three different cytoplasmic Ca2+ concentrations (10 μM, 100 μM and 1 mM). CTN-1 did not show a significant effect on SLO-1 conductance–voltage relationship (Supplementary Figure S5). SLO-1 current amplitude and kinetics also appeared to be unaltered by CTN-1 (not quantified). These observations indirectly suggest that proper subcellular localization of SLO-1 is critical to its function in body-wall muscle cells.
CTN-1 was normally localized in dys-1 mutant
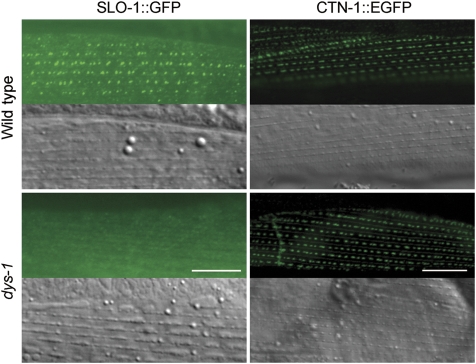
A recent study suggested that ISLO-1 contributes to SLO-1 subcellular localization in body-wall muscle cells by interacting with the dystrophin-associated protein complex (DAPC). SLO-1 is mislocalized in mutants of either islo-1 or dys-1 (dystrophin) (Kim et al, 2009). We were able to reproduce the reported SLO-1 mislocalization in the dys-1(cx18) mutant (Figure 8). To determine whether CTN-1 depends on DAPC for localization, we compared CTN-1::EGFP expression and subcellular localization in body-wall muscle cells between the wild-type and dys-1(cx18) mutant but found no difference (Figure 8), suggesting that dystrophin is not required for CTN-1 subcellular localization in body-wall muscle cells. To address whether mutation of CTN-1 would have an effect on the assembly of the DAPC, we cloned the cDNA of α-dystrobrevin gene (dyb-1), which encodes a major component of the DAPC (Blake et al, 2002), and expressed mStrawberry-tagged DYB-1 (DYB-1::mStrawberry) in body-wall muscle cells of the wild-type and ctn-1 mutant. We found that DYB-1::mStrawberry was localized in dense body areas in both the wild-type and ctn-1 mutant (Supplementary Figure S6). These results suggest that CTN-1 and DAPC might be required for SLO-1 localization independently.
Figure 8.
SLO-1 but not CTN-1 was mislocalized in dys-1 mutant. SLO-1::GFP and CTN-1::EGFP fusions were expressed separately in body-wall muscle cells of the wild type and dys-1(cx18) under the control of the myo-3 promoter. The mutation of dys-1 mislocalized SLO-1::GFP but not CTN-1::EGFP. Scale bar=10 μm. A full-colour version of this figure is available at The EMBO Journal Online.
Discussion
This study showed that CTN-1-mediated SLO-1 subcellular localization was important to SLO-1 function in vivo. This conclusion is supported by the disruption of SLO-1 localization in body-wall muscle cells of ctn-1(lf) and the suppression of slo-1(gf)-induced lethargy by ctn-1(lf). Although there may be other proteins implicated in BK channel subcellular localization, the fact that 12 independent alleles of ctn-1 were isolated in the genetic screen suggests that CTN-1 is an important player in SLO-1 function.
What might be the physiological significance of localizing SLO-1 to dense body regions by CTN-1? The function of body-wall muscle cells is to contract and relax in response to changes in [Ca2+]i. EGL-19, an L-type (CaV1.1) VGCC (Bargmann, 1998), is also localized to dense body regions (Kim et al, 2009) and is the predominant carrier of inward currents in C. elegans body-wall muscle cells (Jospin et al, 2002). It is well established that Ca2+ entry through VGCCs creates local high Ca2+ concentration domains known as Ca2+ microdomains at the inner openings of the channels, where [Ca2+] could be as high as over 100 μM (Adler et al, 1991; Llinas et al, 1992; Yazejian et al, 2000). The high [Ca2+] may serve as a strong activator of colocalized SLO-1. Indeed, the activity of BK channels has been used as a measure of Ca2+ concentrations at the presynaptic terminal resulting from the opening of VGCCs (Yazejian et al, 2000). Thus, localization of SLO-1 to the vicinity of EGL-19 by CTN-1 potentially allows SLO-1 to be activated by Ca2+ entering through EGL-19, and SLO-1 could in turn downregulate the activity of EGL-19 through its effect on the membrane potential.
The mammalian CTN-1 homologue α-catulin is almost ubiquitously expressed (Janssens et al, 1999; Park et al, 2002). However, there are only a few published studies on α-catulin (Janssens et al, 1999; Demacio and Ray, 2001; Park et al, 2002; Merdek et al, 2004; Wiesner et al, 2008). As α-catulin shares sequence homology with α-catenin, which binds a variety of cell adhesion or cytoskeletal proteins, such as α-catenin, β-catenin, zona occludence protein 1 (ZO-1) and α-actinin (Knudsen et al, 1995; Nieset et al, 1997; Muller et al, 2005; Nelson, 2008), CTN-1 may also bind such proteins, thus contributing to BK channel subcellular localization by serving as a cytoplasmic linker between the channel and cytoskeletons. Recently, ISLO-1 was suggested to mediate SLO-1 subcellular localization in C. elegans body-wall muscle by interacting with the DAPC (Kim et al, 2009), which raises the possibility that CTN-1 might also interact with the DAPC to localize SLO-1. However, mutation of dystrophin did not disrupt CTN-1 subcellular localization in body-wall muscle cells (Figure 8), and ctn-1(lf) did not affect the assembly of dystrophin complex (Supplementary Figure S6), suggesting that SLO-1 subcellular localization in body-wall muscle cells likely involves two independent mechanisms, and disrupting one of them is sufficient to mislocalize SLO-1.
Although ctn-1 appeared to be also expressed in many neurons, we were unable to detect a significant effect of CTN-1 in the nervous system through analyses of locomotion behaviours and postsynaptic currents at the NMJ. These observations, however, do not exclude the possibility that CTN-1 has a function in the nervous system but was not detected in this study because our analyses were biased toward detecting defects in muscle cells, motoneurons or other neurons important to locomotion. Thus, the function of CTN-1 in the nervous system remains to be further investigated.
An elucidation of the molecular basis of BK channel subcellular localization could be a key to understanding how the channel performs its various physiological functions. Given that the primary sequences of both SLO-1 and CTN-1 share high level of homology with their mammalian counterparts, it would be interesting to know whether α-catulin has a similar function in localizing mammalian BK channels, and whether an analogous mechanism is used to localize the channel in other types of cells.
Materials and methods
Growth and culture of C. elegans
C. elegans hermaphrodites were grown on agar plates with a layer of OP50 Escherichia coli at room temperature (21–22°C) or inside an environmental chamber (21°C).
Mutant screening
An integrated transgenic strain expressing Pslo-1::SLO-1(E350Q) was used for mutant screen. Synchronized L4-stage slo-1(gf) worms were treated with the chemical mutagen ethyl methanesulfonate (50 mM) for 4 h at room temperature. The F2 progeny were screened for animals that moved better than the original slo-1(gf) animals. Isolated mutants were grouped through complementation tests.
Behavioural assay
Locomotion velocity was determined using a technique described previously (Liu et al, 2006), whereas the head-bending angle was quantified using a newly developed worm tracking and analysing system. Specifically, a single adult hermaphrodite was transferred to an agar plate with a thin layer of OP50 E. coli. After allowing ∼30 s for recovery from the transfer, snapshots of the worm were taken at 15 frames per second for 30 s using a VGA FireWire camera (XCD-V60, Sony, Tokyo, Japan) mounted on a stereomicroscope (SMZ800, Nikon, Tokyo, Japan). The worm was constantly kept in the centre of the view field with a motorized microscope stage (OptiScanTM ES111, Prior Scientific, Inc., Rockland, MA). Both the camera and the motorized stage were controlled by a custom program running in MATLAB (The MathWorks, Inc., Natick, MA).
Cloning of ctn-1
ctn-1(zw1) was used for SNP-based genetic mapping (Davis et al, 2005). After mapping the mutation to a small interval, the candidate gene was identified by testing whether cosmids and PCR-amplified genomic DNA fragments covering this interval could reinstate the lethargic phenotype in ctn-1(lf);slo-1(gf). Full-length cDNA of the candidate gene was obtained through RT–PCR. Molecular lesions of ctn-1 were identified by sequencing the cDNA prepared from five randomly chosen mutant alleles.
Analysis of expression pattern and subcellular localization
The expression pattern of ctn-1 was determined by expressing GFP under the control of 4.3 kb ctn-1 promoter (Pctn-1::GFP, wp761), whereas that of slo-1 by expressing EGFP under the control of 5.2-kb slo-1 promoter (Pslo-1::EGFP, wp758). The plasmids were separately injected into the lin-15(n765) strain using a lin-15 rescue plasmid as a transformation marker. Cells expressing the fluorescent protein were visualized and photographed with a Zeiss Axiovert 200 M fluorescence microscope (× 40 objective) with an apotome device (Zeiss) for optical sectioning.
To determine the expression patterns of different splicing variants of ctn-1, we cloned ctn-1 genomic DNA including 4.3 kb upstream of the initiation site and 0.6 kb downstream of the last exon, and inserted GFP into a unique axon of each splicing variant. As the genomic DNA of ctn-1 was too long (∼16 kb) to be cloned into one plasmid, it was cloned into two plasmids as two separate fragments with 0.6 kb overlap. Homologous recombination in vivo would result in a full-length CTN-1::GFP translational fusion. After linearization, the plasmids were coinjected with a lin-15 rescue plasmid into the lin-15(n765) strain. Transformed worms were identified as lacking a multivulval phenotype. Recombination of the two ctn-1 gene fragments in transgenic worms was verified by PCR using a GFP primer and a primer specific to the plasmid that did not contain GFP-coding sequence. Epifluorescence of the fusion protein in transgenic animals was visualized and photographed with a Nikon TE2000-U inverted microscope (× 60 objective) and a monochrome-cooled CCD camera (F-View II, Olympus).
Subcellular localization of CTN-1 was determined by fusing EGFP to its carboxyl terminus and expressing the fusion protein in body-wall muscle cells under the control of Pmyo-3 (Okkema et al, 1993) (Pmyo-3::CTN-1::EGFP, wp771). To determine whether SLO-1 protein expression or subcellular localization was altered in ctn-1(lf) mutant, a transgenic strain expressing Pmyo-3::SLO-1::GFP (wp746) or Pslo-1::SLO-1::GFP (wp5) was integrated through γ-irradiation, backcrossed with wild-type worms three times, and crossed into ctn-1(lf). To determine whether CTN-1 and SLO-1 are colocalized in muscle cells, CTN-1 was fused with mStrawberry (Pmyo-3::CTN-1::mStrawberry, wp804) and coexpressed with Pmyo-3::SLO-1::GFP. Epifluorescence of the fusion proteins in transgenic animals was visualized and photographed with a Nikon TE2000-U inverted microscope (× 60 objective) and the F-view II digital camera.
BiFC assay
The DNA sequences encoding YFP amino and carboxyl terminals (YFPa and YFPc) were amplified by PCR from pCE-BiFC-VN173 and pCE-BiFC-VC155 vectors (Hiatt et al, 2008), respectively, to make the following plasmids: Pmyo-3::SLO-1::YFPa (wp805), Pmyo-3::SLO-1(1–370)::YFPa (wp913), Pmyo-3::SLO-1(371–1140)::YFPa (wp914) and Pmyo-3::CTN-1::YFPc (wp772). The plasmid encoding SLO-1::YFPa was first injected into lin-15(n765) to establish independent transgenic lines, with a lin-15 plasmid coinjected to serve as a transformation marker. A representative transgenic line thus obtained was then injected with the plasmids encoding CTN-1::YFPc and the transformation marker Pmyo-2::DsRED2 (wp568). Epifluorescence of transgenic worms was visualized and photographed as described above.
Coimmunorecipitation and western blot
HA-tagged CTN-1 and Myc-tagged SLO-1 were cloned into the pIRES2-mCherry and pIRES2-EGFP vectors (Clontech), respectively, to generate the following plasmids: CTN-1::HA-IRES2-mCherry (wp847), SLO-1::Myc-IRES2-EGFP (wp857), SLO-1(1–370)::Myc-IRES2-EGFP (wp932) and SLO-1(371–1140)::Myc-IRES2-EGFP (wp933). HEK293 cells were cultured in DMEM with 10% FBS, and transiently transfected with Lipofectamine 2000 (Invitrogen). Cells were harvested 48 hours after transfection, and lysed in 1% CHAPS/150 mM NaCl/1 mM CaCl2/62.5 mM Tris, pH 6.8 plus protease inhibitor (Roche). The supernatants of cell lysates were incubated with a Myc antibody (Santa Cruz Biotechnology) for 3 h at 4°C, and immunoprecipitated with protein A/G PLUS agarose (Santa Cruz Biotechnology) for 2 h at 4°C. Immune complexes were separated on 4–12% SDS–PAGE gels and probed with a HA antibody (NeoMarkers).
To examine SLO-1::GFP protein level expressed in worms, worms of mixed stages were homogenized in a lysis buffer (2% SDS/100 mM NaCl/10% glycerol/50 mM Tris, pH 6.8). Soluble protein extracts were separated on 4–12% SDS–PAGE gels and probed with GFP (Molecular Probes) and α-tubulin (Santa Cruz Biotechnology) antibodies. Anti-mouse IgG HRP (Santa Cruz Biotechnology) was used as the secondary antibody for detection by enhanced chemiluminescence (Pierce).
Surface biotinylation
Biotinylation assays were performed in transiently transfected HEK293 cells using the Cell Surface Protein Isolation Kit (Pierce). Surface proteins were biotinylated 48 hours after the transfection, precipitated with neutrAvidin-agarose beads, and eluted with SDS sample buffer (1% SDS/50 mM DTT/10% glycerol/62.5 mM Tris, pH 6.8). Total lysate or biotinylated proteins were separated by 4–12% SDS–PAGE, and the blots were detected as described above.
Recording of postsynaptic currents
An integrated Punc-17::ChR-2::mCherry transgene in the genetic background of wild-type worms (Liu et al, 2009) was crossed into slo-1(md1745), ctn-1(zw1), slo-1(gf) and slo-1(gf);ctn-1(zw1) strains. Both the Punc-17::ChR-2::mCherry and Pslo-1::SLO-1(gf) transgenes appeared to be integrated into the X chromosome at close proximity because we were unable to identify a worm homozygous for both transgenes from several hundreds of cross progeny. Therefore, worms heterozygous for both transgenes were used for experiments involving comparisons with slo-1(gf) mutant (Figure 5), whereas worms homozygous for Punc-17::ChR-2::mCherry were used for experiments that did not involve comparisons with slo-1(gf) (Figure 5). Both mPSCs and ePSCs were recorded from body-wall muscle cells using the voltage-clamp technique with the membrane potential held at −60 mV, as described previously (Liu et al, 2005, 2007). ePSCs were evoked by applying a pulse (3 ms) of blue light using a 470±20 nm excitation filter (59222, Chroma Technology Corp.), and a light source equipped with a shutter (Lambda XL with SmartShutter, Sutter Instrument). The recording pipette solution contained (in mM) 120 KCl, 20 KOH, 5 Tris, 0.25 CaCl2, 4 MgCl2, 36 sucrose, 5 EGTA and 4 Na2ATP (pH 7.2). Two external solutions with different [Ca2+] (5 and 0.25 mM) were used. The external solution with the higher [Ca2+] contained (in mM) 140 NaCl, 5 KCl, 5 CaCl2, 5 MgCl2, 11 dextrose and 5 HEPES (pH 7.2). This solution was modified by reducing CaCl2 to 0.25 mM and increasing NaCl to 145 mM to make the external solution with the lower [Ca2+].
Xenopus oocyte expression
Capped cRNAs were synthesized using the mMessage mMachine Kit (Ambion). Approximately 50 nl cRNA (1 ng/nl) was injected into each oocyte using a Drummond Nanoject II injector (Drummond Scientific). Inside–out patches were obtained from the oocyte 2–3 days after cRNA injection. Macroscopic currents induced by voltage steps (−80 to +160 mV in 20 mV increments, 50 ms duration) were amplified with a Multiclamp 700B amplifier (Molecular Devices), and acquired with the Clampex software (version 10.2, Molecular Devices). Data were sampled at 10 kHz after filtering at 2 kHz.
Compositions of the pipette solution included (in mM) 140 K+ gluconate, 1 Mg2+ gluconate and 5 HEPES (pH 7.2). Three cytoplasmic solutions (pH 7.2) that differed in free [Ca2+] were used to perfuse the cytoplasmic side of the patches. All cytoplasmic solutions contained 140 mM K+ gluconate and 10 mM HEPES. Besides, other components were added to adjust free [Ca2+] (1 mM Ca2+ gluconate for 1 mM [Ca2+], 0.1 mM Ca2+ gluconate for 100 μM [Ca2+] and 3.39 mM Ca2+ gluconate plus 5 mM HEDTA for 10 μM free [Ca2+]). Free [Ca2+] was calculated using online software (http://maxchelator.stanford.edu).
Data analysis
Electrophysiological data. Amplitude and frequency of mPSCs were analysed using MiniAnalysis (Synaptosoft, Inc., Decatur, GA). A detection threshold of 10 pA was used in initial automatic analysis, followed by visual inspections to include missed smaller events (5 pA or larger) and to exclude false events resulting from baseline fluctuations. Amplitudes of ePSCs were measured with the Clampfit software (Molecular Devices). The average of the two largest ePSCs from each experiment was used for statistical analysis. Peak macroscopic currents from isolated oocyte patches were determined and used to plot the conductance–voltage relationship.
Behavioural data. The longest series of successive worm images showing continuous forward locomotion (10–20 s) was chosen from the 30-s recording of each worm. To find head-bending angles, a custom MATLAB program was used to detect the shape of the worm, place 13 equally spaced markers along the midline, and distinguish the head and tail (Supplementary Figure S1A). The angle supplementary to the angle formed by two straight lines connecting the marker points 1 and 2, and the marker points 2 and 3 (Supplementary Figure S1B) was plotted over successive frames to produce a sinusoidal head-bending trace (Supplementary Figure S1D). From the head-bending trace, the maximal-bending angle was found as the full amplitude of bending between the ventral and dorsal sides (Supplementary Figure S1C). Although the maximal-bending angle metric provides an intuitive description of the data, it does not account for smaller and/or irregular oscillations. To mathematically quantify the magnitude of the head bending as a whole, the root mean square of the trace was also calculated.
Data graphing and statistical analyses. Graphing and statistical analyses were performed with Origin (version 8.0, OriginLab). One-way ANOVA (followed by Bonferroni's post hoc test) was used for statistical comparisons. P<0.01 is considered to be statistically significant. All values are expressed as mean±s.e. n is the number of patches or muscle cells analysed.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (1R01MH085927 and 5R01GM083049, ZWW) and National Science Foundation (0619427, ZWW). We thank Eric Jorgensen for providing the Punc-17::ChR-2::mCherry strain, Chang-Deng Hu for worm BiFC vectors, R de Martin for an α-catulin plasmid and Audrey Fraser at the Sanger Institute for providing cosmids. The MH24 antibody developed by Robert H Waterson was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Science, Iowa City, IA 52242.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA (1992) Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9: 209–216 [DOI] [PubMed] [Google Scholar]
- Adler EM, Augustine GJ, Duffy SN, Charlton MP (1991) Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci 11: 1496–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun ZF, Chen B, Wang ZW, Hall DH (2009) High resolution map of Caenorhabditis elegans gap junction proteins. Dev Dyn 238: 1936–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282: 2028–2033 [DOI] [PubMed] [Google Scholar]
- Barstead RJ, Waterston RH (1989) The basal component of the nematode dense-body is vinculin. J Biol Chem 264: 10177–10185 [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329 [DOI] [PubMed] [Google Scholar]
- Brelidze TI, Niu X, Magleby KL (2003) A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc Natl Acad Sci USA 100: 9017–9022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW (2005) BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759 [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW (2000a) Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461 [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW (2000b) Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876 [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L (1993) mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261: 221–224 [DOI] [PubMed] [Google Scholar]
- Chen B, Liu Q, Ge Q, Xie J, Wang ZW (2007) UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr Biol 17: 1334–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL (2003) A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 115: 655–666 [DOI] [PubMed] [Google Scholar]
- Davis MW, Hammarlund M, Harrach T, Hullett P, Olsen S, Jorgensen EM (2005) Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demacio PC, Ray PN (2001) Alpha-catulin maps to the familial dysautonomia region on 9q31. Genome 44: 990–994 [DOI] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK (2005) Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37: 733–738 [DOI] [PubMed] [Google Scholar]
- Francis GR, Waterston RH (1985) Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J Cell Biol 101: 1532–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt SM, Shyu YJ, Duren HM, Hu CD (2008) Bimolecular fluorescence complementation (BiFC) analysis of protein interactions in Caenorhabditis elegans. Methods 45: 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J, Leipziger J, Frobe U, Schlatter E (1993) Regulation and possible physiological role of the Ca2+-dependent K+ channel of cortical collecting ducts of the rat. Pflugers Arch 422: 492–498 [DOI] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF (2001) Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci 21: 9585–9597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chung YW, Wong PY (1999) Potassium channel activity recorded from the apical membrane of freshly isolated epithelial cells in rat caudal epididymis. Biol Reprod 60: 1509–1514 [DOI] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ (1994) Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA 91: 7578–7582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AF, Okada Y (1994) Maxi K+ channels from the apical membranes of rabbit oviduct epithelial cells. J Membr Biol 137: 109–118 [DOI] [PubMed] [Google Scholar]
- Janssens B, Staes K, van Roy F (1999) Human alpha-catulin, a novel alpha-catenin-like molecule with conserved genomic structure, but deviating alternative splicing. Biochim Biophys Acta 1447: 341–347 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (2002) Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417: 515–522 [DOI] [PubMed] [Google Scholar]
- Jospin M, Jacquemond V, Mariol MC, Segalat L, Allard B (2002) The L-type voltage-dependent Ca2+ channel EGL-19 controls body wall muscle function in Caenorhabditis elegans. J Cell Biol 159: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Pierce-Shimomura JT, Oh HJ, Johnson BE, Goodman MB, McIntire SL (2009) The dystrophin complex controls bk channel localization and muscle activity in Caenorhabditis elegans. PLoS Genet 5: e1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R (1994) Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem 269: 17274–17278 [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G (1996) Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci 16: 955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ (1995) Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol 130: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DI, Wanderling S, Biemesderfer D, Goldstein SA (2008) MiRP3 acts as an accessory subunit with the BK potassium channel. Am J Physiol Renal Physiol 295: F380–F387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A (2008) Optogenetic analysis of synaptic function. Nat Methods 5: 895–902 [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen B, Gaier E, Joshi J, Wang ZW (2006) Low conductance gap junctions mediate specific electrical coupling in body-wall muscle cells of Caenorhabditis elegans. J Biol Chem 281: 7881–7889 [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen B, Ge Q, Wang ZW (2007) Presynaptic Ca2+/calmodulin-dependent protein kinase II modulates neurotransmitter release by activating BK channels at Caenorhabditis elegans neuromuscular junction. J Neurosci 27: 10404–10413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen B, Yankova M, Morest DK, Maryon E, Hand AR, Nonet ML, Wang ZW (2005) Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J Neurosci 25: 6745–6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Hollopeter G, Jorgensen EM (2009) Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc Natl Acad Sci USA 106: 10823–10828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Silver RB (1992) Microdomains of high calcium concentration in a presynaptic terminal. Science 256: 677–679 [DOI] [PubMed] [Google Scholar]
- Merdek KD, Nguyen NT, Toksoz D (2004) Distinct activities of the alpha-catenin family, alpha-catulin and alpha-catenin, on beta-catenin-mediated signaling. Mol Cell Biol 24: 2410–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW (2004) Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752 [DOI] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS (2006) Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol 496: 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G (2005) The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem 280: 3747–3756 [DOI] [PubMed] [Google Scholar]
- Nelson WJ (2008) Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 36 (Part 2): 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ (1997) Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci 110 (Part 8): 1013–1022 [DOI] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ (1997) Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci 17: 8061–8073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A (1993) Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135: 385–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha J, Frindt G, Sackin H, Palmer LG (1991) Apical maxi K channels in intercalated cells of CCT. Am J Physiol 261(4 Part 2): F696–F705 [DOI] [PubMed] [Google Scholar]
- Pallanck L, Ganetzky B (1994) Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet 3: 1239–1243 [DOI] [PubMed] [Google Scholar]
- Park B, Nguyen NT, Dutt P, Merdek KD, Bashar M, Sterpetti P, Tosolini A, Testa JR, Toksoz D (2002) Association of Lbc Rho guanine nucleotide exchange factor with alpha-catenin-related protein, alpha-catulin/CTNNAL1, supports serum response factor activation. J Biol Chem 277: 45361–45370 [DOI] [PubMed] [Google Scholar]
- Pluznick JL, Wei P, Carmines PK, Sansom SC (2003) Renal fluid and electrolyte handling in BKCa-beta1-/- mice. Am J Physiol Renal Physiol 284: F1274–F1279 [DOI] [PubMed] [Google Scholar]
- Pyott SJ, Glowatzki E, Trimmer JS, Aldrich RW (2004) Extrasynaptic localization of inactivating calcium-activated potassium channels in mouse inner hair cells. J Neurosci 24: 9469–9474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, Meredith AL, Fodor AA, Vazquez AE, Yamoah EN, Aldrich RW (2007) Cochlear function in mice lacking the BK channel alpha, beta1, or beta4 subunits. J Biol Chem 282: 3312–3324 [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E (2004) BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol 557(Part 1): 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ (1990) Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci 10: 3664–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP (1993) Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron 11: 645–655 [DOI] [PubMed] [Google Scholar]
- Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M (2004) Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA 101: 12922–12927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A (2006) High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931 [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P (2004) Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA 101: 9474–9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal Y, Reuss L (1990) Maxi K+ channels and their relationship to the apical membrane conductance in Necturus gallbladder epithelium. J Gen Physiol 95: 791–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu YJ, Hiatt SM, Duren HM, Ellis RE, Kerppola TK, Hu CD (2008) Visualization of protein interactions in living Caenorhabditis elegans using bimolecular fluorescence complementation analysis. Nat Protoc 3: 588–596 [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Marcus DC, Wangemann P (1992) Maxi K+ channel in apical membrane of vestibular dark cells. Am J Physiol 262 (6 Part 1): C1430–C1436 [DOI] [PubMed] [Google Scholar]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R (2000) Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem 275: 23211–23218 [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L (1999) Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci USA 96: 4137–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW (2008) Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol Neurobiol 38: 153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW, Saifee O, Nonet ML, Salkoff L (2001) SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32: 867–881 [DOI] [PubMed] [Google Scholar]
- Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, Ge P, Wang C, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, DiStefano PS, Curtis R (2000) A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J Neurosci 20: 3563–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT (2005) Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J Physiol 567 (Part 2): 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner C, Winsauer G, Resch U, Hoeth M, Schmid JA, van Hengel J, van Roy F, Binder BR, de Martin R (2008) Alpha-catulin, a Rho signalling component, can regulate NF-kappaB through binding to IKK-beta, and confers resistance to apoptosis. Oncogene 27: 2159–2169 [DOI] [PubMed] [Google Scholar]
- Xia X, Hirschberg B, Smolik S, Forte M, Adelman JP (1998) dSLo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci 18: 2360–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ (1999) Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci 19: 5255–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Zeng XH, Duan KL, Lingle CJ (2000) Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J Neurosci 20: 4890–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazejian B, Sun XP, Grinnell AD (2000) Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels. Nat Neurosci 3: 566–571 [DOI] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y, Mackinnon R (2010) Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science 329: 182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusifov T, Savalli N, Gandhi CS, Ottolia M, Olcese R (2008) The RCK2 domain of the human BKCa channel is a calcium sensor. Proc Natl Acad Sci USA 105: 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Schopperle WM, Murrey H, Jaramillo A, Dagan D, Griffith LC, Levitan IB (1999) A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron 22: 809–818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.