Growth habit determination by the balance of histone methylation activities in Arabidopsis
Plants have evolved different growth habits, such as annual, biennial, and perennial, as adaptations to specific climates and regions. This study reports on the function of histone methylation in the regulation of flowering time in Arabidopsis.
Keywords: FLC, growth habit, HDM, HMT
Abstract
In Arabidopsis, the rapid-flowering summer-annual versus the vernalization-requiring winter-annual growth habit is determined by natural variation in FRIGIDA (FRI) and FLOWERING LOCUS C (FLC). However, the biochemical basis of how FRI confers a winter-annual habit remains elusive. Here, we show that FRI elevates FLC expression by enhancement of histone methyltransferase (HMT) activity. EARLY FLOWERING IN SHORT DAYS (EFS), which is essential for FRI function, is demonstrated to be a novel dual substrate (histone H3 lysine 4 (H3K4) and H3K36)-specific HMT. FRI is recruited into FLC chromatin through EFS and in turn enhances EFS activity and engages additional HMTs. At FLC, the HMT activity of EFS is balanced by the H3K4/H3K36- and H3K4-specific histone demethylase (HDM) activities of autonomous-pathway components, RELATIVE OF EARLY FLOWERING 6 and FLOWERING LOCUS D, respectively. Loss of HDM activity in summer annuals results in dominant HMT activity, leading to conversion to a winter-annual habit in the absence of FRI. Thus, our study provides a model of how growth habit is determined through the balance of the H3K4/H3K36-specific HMT and HDM activities.
Introduction
Plants have evolved various growth habits, such as annual, biennial, and perennial, as evolutionary adaptations to specific climates and regions. Arabidopsis accessions can be either vernalization-requiring winter annuals or rapidly flowering summer annuals (Michaels et al, 2003). The winter-annual habit is conferred by the activation of the major floral repressor FLOWERING LOCUS C (FLC) by FRIGIDA (FRI; Koornneef et al, 1994; Lee et al, 1994; Johanson et al, 2000). Mutations in autonomous-pathway FLC repressors also result in winter-annual behaviour in the absence of functional FRI (Koornneef et al, 1991; Baurle and Dean, 2006). Although a number of genetic factors affecting Arabidopsis growth habits have been identified (Baurle and Dean, 2006), the biochemical function of the natural winter-annual determinant, FRI, and the molecular mechanism by which autonomous-pathway mutations delay flowering and convert a summer annual to a winter annual are not well understood.
EARLY FLOWERING IN SHORT DAYS (EFS or Su(var), E(z), and Trithorax (SET) DOMAIN GROUP 8) is essential for the winter-annual behaviour of lines with functional FRI (Kim et al, 2005; Zhao et al, 2005) and encodes a SET domain-containing protein grouped with Drosophila ASH1 (Springer et al, 2003), a controversial multi-catalytic histone methyltransferase (HMT; Beisel et al, 2002; Li et al, 2007; Tanaka et al, 2007). Although EFS is essential for FRI activity, its function in FLC regulation has been studied primarily in the absence of FRI. In fact, fri efs plants were reported to have reduced levels of histone H3 lysine 36 di- and tri-methylation (H3K36me2/me3) in the FLC-coding region (Zhao et al, 2005; Xu et al, 2008).
Histone methylation, which affects chromatin structure and gene transcription, is balanced by the activities of HMTs and histone demethylases (HDMs; Klose and Zhang, 2007). Here, we describe, at a molecular level, how the summer- versus winter-annual habit of Arabidopsis is determined by balanced activities of HMTs and HDMs acting at FLC. After being recruited by EFS, FRI allows for HMT activities to dominate at FLC. In the process, EFS and RELATIVE OF EARLY FLOWERING 6 (REF6; Noh et al, 2004) exert mutually antagonistic functions as novel dual substrate (H3K4 and H3K36)-specific HMT and HDM, respectively.
Results and Discussion
EFS is crucial for FRI recruitment into FLC chromatin and both EFS and FRI are required for elevated H3K4me/H3K36me
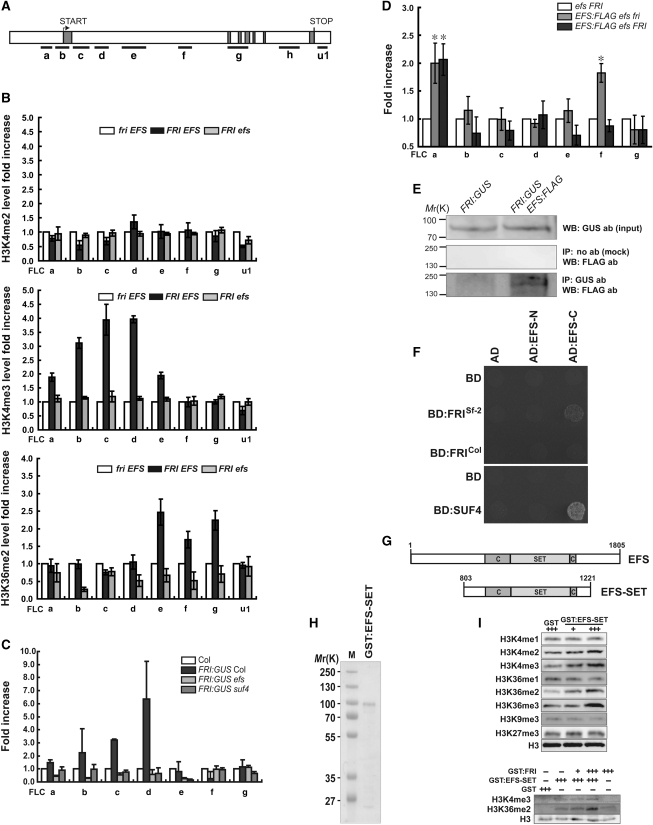
We evaluated the effect of EFS and FRI at the FLC locus by chromatin immunoprecipitation (ChIP) assays. Both FRI and EFS were required for the elevated levels of H3K4me3 and H3K36me2 (Figure 1A and B), markers related to transcriptional activation and elongation, respectively (Li et al, 2007). FRI- and EFS-mediated elevation of H3K4me3 was observed throughout the FLC regions evaluated, whereas H3K36me2 levels were elevated within the internal gene body. Partially functional FRI:β-glucuronidase (FRI:GUS; Supplementary Figure S1) and almost fully functional EFS:FLAG (Supplementary Figure S2) fusion proteins associated directly with the 5′ region and both the 5′ and internal regions of FLC, respectively (Figure 1C and D).
Figure 1.
FRI- and EFS-dependent H3K4 and H3K36 methylation of FLC chromatin. (A) FLC locus with exons (grey boxes) and introns (white boxes) showing regions tested for ChIP. (B) ChIP-quantitative real-time PCR (qPCR) analyses of FLC chromatin with indicated histone antibodies (abs). fri EFS is wild-type Columbia (Col). Error bars represent s.e. (B–D). (C, D) ChIP-qPCR to test the direct association of FRI:GUS (C) or EFS:FLAG (D) with FLC chromatin using GUS (C) or FLAG (D) ab. (*P<0.05 versus control in a Student's t-test). (E) Co-IP of FRI:GUS and EFS:FLAG. Western blot (WB) was performed with the indicated abs. (F) Interaction of EFS with FRI and SUF4 in yeast two-hybrid assays. The N-terminal (EFS-N) or C-terminal region (EFS-C) of EFS were fused to the GAL4 activation domain (AD). FRI from Sf-2 or Col and SUF4 were fused to the GAL4-binding domain (BD). (G) Domains in EFS and EFS-SET. (H) Coomassie-stained purified GST:EFS-SET. (I) HMT activity of GST:EFS-SET in assays using calf thymus histones (upper) or oligonucleosomes (lower) as substrates.
Surprisingly, the association of FRI:GUS with FLC chromatin was abolished in the absence of EFS or SUPPRESSOR OF FRIGIDA 4 (SUF4; Figure 1C), a FRI-interacting nuclear protein (Kim et al, 2006). However, the association of EFS:FLAG with the 5′ region of FLC was not significantly affected by FRI, whereas its interaction with the gene body was reduced by the presence of FRI (Figure 1D). Co-immunoprecipitation (Co-IP) of FRI:GUS and EFS:FLAG (Figure 1E) suggested that FRI and EFS might be present in the same complex, although the data did not exclude the possibility of interaction between the FLAG-tagged EFS and GUS instead of FRI. Consistent with a possible in vivo interaction, the C-terminal region of EFS interacted with a functional FRI (FRISf-2) and SUF4 but not with a non-functional FRI (FRICol) in yeast two-hybrid assays (Figure 1F). Together, these results demonstrate that EFS is crucial for the recruitment of FRI to FLC chromatin, as well as for the elevation of H3K4me3 and H3K36me2 levels at FLC.
FRI was reported to be required for the maintenance of FLC expression in late embryos, but dispensable for the initial reactivation in early embryos during reprogramming (Choi et al, 2009). Like FRI, EFS was also required for the maintenance, but not for the initial reactivation of FLC (Supplementary Figure S3). Therefore, FRI and EFS act at similar developmental stages.
EFS is a novel dual substrate (H3K4/H3K36)-specific HMT performing the functions of both SET1 and SET2 of yeast
In yeast and animals, H3K4 and H3K36 methylations are catalysed by SET1/Trithorax-class HMTs and SET2-class HMTs, respectively (Hampsey and Reinberg, 2003; Shilatifard, 2008). Arabidopsis Trithorax1 (ATX1) together with its homolog ATX2 and ATX-related 7 has a function in elevating H3K4me3 at FLC (Pien et al, 2008; Tamada et al, 2009). FRI was shown to be required for the recruitment of WDR5a into FLC chromatin, and WDR5a in turn interacts with ATX1 (Jiang et al, 2009). Hence, EFS-dependent elevation of H3K4me3 at FLC in lines with FRI might be catalysed either by ATX1/ATX2 recruited through WDR5a/FRI/EFS or by EFS directly.
Therefore, it was of interest to examine the residue-specific HMT activity of EFS. Although full-length EFS could not be obtained, we could express the SET domain of EFS (EFS-SET), which contains the catalytic core elements (SET and cysteine-rich (C) pre-SET or post-SET domains; Figure 1G), as a GST fusion protein (Figure 1H). In assays using calf thymus histones as substrates followed by immunoblot analyses with various methylation-specific antibodies (abs), EFS-SET increased the levels of H3K4me2/me3 and H3K36me2/me3 but not of H3K4me1, H3K36me1, H3K9me3, and H3K27me3 (Figure 1I). When oligonucleosomes were used as substrates, the levels of H3K4me3 and H3K36me2 were also increased by EFS-SET, and the extent of H3K36me2 increased when FRI was added to the reaction (Figure 1I). Thus, EFS appears to possess intrinsic H3K4 and H3K36 di- and tri-methyltransferase activities that, at least in part, account for the FRI/EFS-mediated increased H3K4me3 and H3K36me2 levels within FLC chromatin (Figure 1B). The FRI-independent effects of EFS on H3K4 and H3K36 methylations at FLC are shown in Figure 3. In summary, EFS might have two functions in FLC activation: first, as a scaffold recruiting the FRI-containing transcriptional co-activator complex, and second, as a novel HMT with dual substrate (H3K4me1/me2 and H3K36me1/me2) specificity, thus performing both SET1 and SET2 functions.
REF6 is a novel dual substrate (H3K4/H3K36)-specific HDM repressing FLC chromatin together with FLD
Similar to the effects of functional FRI, the loss of autonomous-pathway members with non-functional fri also results in FLC activation and a winter-annual habit. REF6 (Noh et al, 2004) and FLOWERING LOCUS D (FLD; He et al, 2003) are two autonomous-pathway members predicted to have HDM activities based on the presence of the Jumonji C (JmjC) domain in REF6 and on the sequence similarity of FLD to human LSD1-class amine oxidases (AOs), although their biochemical functions have not yet been demonstrated. FCA and FPA, two other autonomous-pathway members, were also reported to be involved in chromatin silencing through an RNA-mediated pathway (Baurle et al, 2007), and FCA appears to require FLD for FLC repression (Liu et al, 2007).
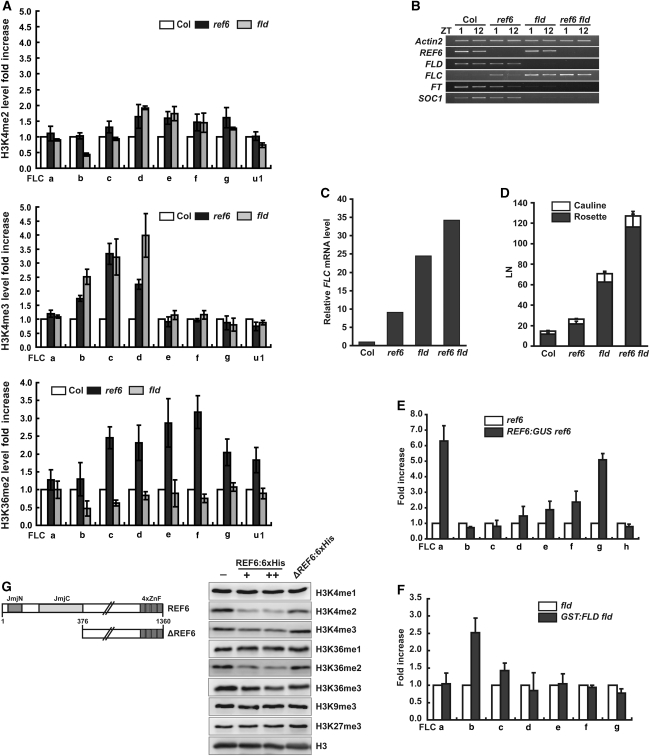
The JmjC domain of REF6 is most related to the JmjC domains of human JARID1 family members, which are H3K4me2/me3-specific demethylases (Christensen et al, 2007). AOs such as FLD are capable of demethylating H3K4me1/me2 (Shi et al, 2004). Consistent with the predicted functions of REF6 and FLD, H3K4me2/me3 levels in the 5′ region of FLC were increased in ref6 and fld mutants in comparison to wild type (Figure 2A). In addition, ref6 but not fld mutants also contained increased H3K36me2 levels in the internal gene body (Figure 2A). Although the lack of demethylase activity of AOs for tri-methylated lysines (Shi et al, 2004) makes it hard to explain the increased H3K4me3 levels in fld, these results suggest that REF6 and FLD might have additive functions in FLC repression as H3K4/H3K36- and H3K4-specific HDMs, respectively. The ref6 fld double mutants had higher FLC mRNA levels (Figure 2B and C) and showed a more delayed flowering (Figure 2D) than either of the single mutants, also supporting their independent repressive functions. Direct association of the functional REF6:GUS (Noh et al, 2004) with FLC chromatin was observed in the internal gene body as well as in the 5′ region, whereas the interaction of the functional GST:FLD (Supplementary Figure S4) only occurred in the 5′ region (Figure 2E and F). Thus, the two proteins bound to FLC chromatin in regions where histone methylation levels were also significantly increased in the corresponding mutants. The elevated level of FLC mRNA but not H3K36me2 at the FLC locus in fld mutants suggests that the deposition of H3K36me2 can be uncoupled from transcriptional activity.
Figure 2.
Repression of FLC chromatin by REF6 and FLD. (A) ChIP–qPCR analyses of FLC chromatin for regions described in Figure 1A. Error bars represent s.e. (B, C) Expression of flowering genes at zeitgeber (ZT) 1 and 12 in ref6, fld, and ref6 fld as studied by reverse transcription (RT)–PCR (B) or RT–qPCR at ZT2 (C). FT: FLOWERING LOCUS T. SOC1: SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1. The values are the means of three technical replicates (C). (D) Flowering time of ref6, fld, and ref6 fld in LD as scored by the leaf number (LN). Error bars represent s.d. (E, F) ChIP–qPCR was used to test the direct association of REF6:GUS (E) or GST:FLD (F) with FLC chromatin using GUS (E) or GST (F) ab. Error bars represent s.e. (G) Domains in REF6 and ΔREF6 (left), and HDM activity of REF6:6xHis (right).
As no JmjC proteins have been demonstrated to demethylate both H3K4me and H3K36me, we tested the intrinsic HDM activity of REF6 by using 6xhistidine-tagged full-length REF6 (REF6:6xHis; Figure 2G). REF6:6xHis, but not ΔREF6:6xHis, which lacks the JmjN and JmjC domains of REF6, strongly reduced the levels of H3K4me2 and H3K36me2 in the substrate calf thymus histones and also moderately reduced the levels of H3K4me3 and H3K36me3 (Figure 2G). H3K4me1, H3K36me1, H3K9me3, and H3K27me3 levels were not affected by REF6:6xHis in the assays. Together with the increased levels of H3K4me2/me3 and H3K36me2 at FLC in ref6 mutants (Figure 2A) and the direct binding of REF6:GUS with FLC chromatin (Figure 2E), these results indicate that REF6 is an H3K4me2/me3- and H3K36me2/me3-specific HDM.
The HMT activity of EFS is antagonized by the HDM activities of REF6/FLD in FLC regulation
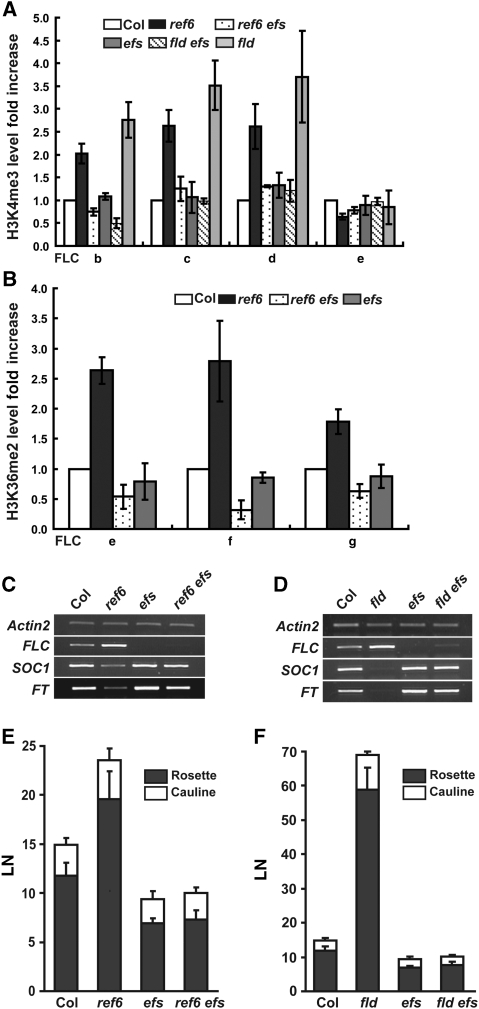
The results shown in Figures 1 and 2 suggest potential antagonism between EFS and REF6 for H3K4me and H3K36me and between EFS and FLD for H3K4me at FLC. To test these possibilities, we first measured H3K4me3 levels at FLC using ref6 efs and fld efs double mutants. The increased H3K4me3 caused by the ref6 or fld mutation was suppressed in the double mutants to the levels found in the efs single mutant (Figure 3A). Similarly, the ref6 mutation-induced increase of H3K36me2 was also suppressed by efs (Figure 3B). Changes of the histone markers were correlated with the steady-state mRNA levels of FLC (Figure 3C and D) and flowering time (Figure 3E and F). The efs mutation efficiently suppressed ref6- or fld-induced increases of FLC mRNA expression and delayed flowering. The minor residual expression of FLC in fld efs (Figure 3D) implies an EFS-independent portion of FLD activity. However, the results in Figure 3 indicate that the FRI-independent H3K4/H3K36 methyltransferase activity of EFS is largely responsible for the de-repression of FLC in ref6 and fld and that EFS has antagonistic biochemical functions to REF6 or FLD in H3K4me/H3K36me or H3K4me, respectively, at the FLC locus.
Figure 3.
HMT activity of EFS is antagonized by HDM activities of REF6/FLD in FLC regulation. (A) ChIP-qPCR analyses of FLC chromatin with H3K4me3 ab. Regions tested are as described in Figure 1A and error bars represent s.e. (A, B). (B) ChIP-qPCR analyses of FLC chromatin with H3K36me2 ab. (C, D) Expression of flowering genes in ref6 efs (C) and fld efs (D) as analysed by RT–PCR. (E, F) Flowering time of ref6 efs (E) and fld efs (F) in LD. Error bars represent s.d.
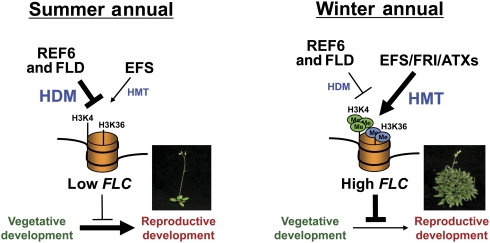
The summer- versus winter-annual habit of Arabidopsis is determined by the balance of H3K4/H3K36-specific HMT and HDM activities
On the basis of our results, we propose a model for the summer- versus winter-annual habit in Arabidopsis through balanced histone methylation activities (Figure 4). In non-functional fri-containing summer annuals, the HDM activities of REF6 and FLD are dominant to the HMT activity of EFS because of lack of FRI, resulting in low H3K4me/H3K36me and FLC mRNA levels. However, in mutants of the HDMs or certain other autonomous-pathway members, the HMT activity of EFS overrides the decreased HDM activities, and as a result, the H3K4me/H3K36me and FLC mRNA levels are increased, leading to winter-annual behaviour. In FRI-containing winter-annual types, EFS recruits FRI as a scaffold, and in turn, FRI enhances the HMT activity of EFS and recruits additional H3K4-specific HMTs (ATXs). Thus, the bifurcate enhancement of HMT activities driven by the EFS/FRI complex causes a winter-annual habit.
Figure 4.
H3K4me- and H3K36me-mediated determination of summer- or winter-annual habit in Arabidopsis. See text for details.
The mechanism of the FRI enhancement of EFS activity remains unknown. FRI and its relatives form a small, plant-specific gene family (Michaels et al, 2004). In the crucifer lineage, FRI appears to have been recruited for FLC activation (Johanson et al, 2000). It will be of future interest to explore whether other FRI family members act in higher plants as plant-specific co-activators for H3K4 and H3K36 methyltransferases, or other histone-modifying complexes, at a range of target loci.
Materials and methods
Plant materials, growth conditions, and flowering time analyses
All Arabidopsis mutants used in this study are in the Columbia (Col) background and were described previously: FRI (Lee et al, 1994), ref6-3 (Noh et al, 2004), fld-3 (He et al, 2003), efs-3 (Kim et al, 2005), and suf4 (Kim et al, 2006). Plants were grown and their flowering times were measured as described (Han et al, 2007). At least 15 plants for each genotype were scored for the flowering time analyses, and data are shown with means±s.d. Photoperiods used in the study were 16 h light and 8 h dark for LD and 8 h light and 16 h dark for SD.
FRI:GUS, EFS:FLAG, REF6:GUS, and 35S∷GST:FLD
The FRI:GUS (Choi et al, 2009) and REF6:GUS (Noh et al, 2004) constructs were described previously. The EFS fragment including the native promoter region was amplified by PCR from genomic DNA and cloned into pEarleyGate302. The FLD cDNA was cloned into pGEX4T and the GST-fused FLD fragment was PCR amplified and cloned behind the CaMV35S promoter in Cameleon-YC2.1, resulting in the binary 35S∷GST:FLD construct. Binary plasmids were introduced into Agrobacterium tumefaciens, and the resulting strains were used to transform Arabidopsis plants using the floral dip method (Clough and Bent, 1998). Sequences of primers used for constructions are available on request.
ChIP assay
Fourteen-day-old seedlings grown in LD were used for the ChIP experiments as previously described (Han et al, 2007). ChIP DNA was analysed by qPCR as described in the RT–PCR and qPCR section using FLC genomic primers FLC a to h (Liu et al, 2007) and U1 (Sung and Amasino, 2004). ‘Input' is 10% of the nuclear extract used and ‘mock' refers to the control lacking ab. Abs used are described in the ‘In vitro HDM assay' section. For the ChIP assays using FRI:GUS and EFS:FLAG, GUS ab (Invitrogen A5790) and FLAG ab (Sigma A8592) were used, respectively. Each ChIP assay was repeated at least three times with independent samples. The values are the means of these biological replicates and error bars represent s.e.
Histochemical GUS imaging
The FLC:GUS transgene in FRI EFS was introduced into fri EFS and FRI efs by crossing. The GUS activity in the gametophytes and seeds was analysed as described previously (Choi et al, 2009).
Co-IP
For the Co-IP assay, we generated the double transgenic EFS:FLAG- and FRI:GUS-containing homozygous plants by crossing. Cross-linked nuclear proteins were extracted from LD-grown 14-day-old seedlings according to the ChIP protocol. After dilution of the nuclear proteins with immunoprecipitation buffer (50 mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2.5 mM DTT, 1 mM PMSF), FRI:GUS was immunoprecipitated with GUS ab (Invitrogen A5790). The FRI:GUS-containing immunocomplex was resolved by SDS–PAGE and detected by FLAG ab (Sigma A8592).
GST:EFS-SET and GST:FRI protein expression
The cDNA sequences encoding the pre-SET, SET, and post-SET domains (amino acids 803–1221) of EFS or functional FRI were amplified by RT–PCR and subcloned into the SalI and either BamHI or EcoRI sites of the pGEX-4T vector (GE Healthcare). Sequences of primers used for construction are available on request. Recombinant GST, GST:EFS-SET, and GST:FRI fusion proteins were expressed in Escherichia coli strain BL21 and resuspended in 20 ml of extraction buffer (50 mM Tris pH 7.5, 150 mM NaCl, 10 mM EDTA, 1 mM DTT, 0.4% Triton X-100, 100 μM PMSF, 1 mg/ml lysozyme), followed by sonication. Cleared soluble proteins obtained after centrifugation were purified using glutathione-Sepharose beads (GE Healthcare) according to the manufacturer's instructions.
In vitro HMT assay
Following a previously published protocol (Rea et al, 2000) with modifications, the in vitro HMT assay was carried out with 5 μg of calf thymus histones (Sigma) or 1 μg of oligonucleosomes (12-nucleosome array reconstituted with Xenopus histones on the G4E4 fragment) as substrates and 0.2 μM of S-adenosyl-L-methionine (Sigma) as the methyl donor in 100 μl of methyltransferase buffer (50 mM Tris pH 8.0, 20 mM KCl, 10 mM MgCl2, 10 mM β-mercaptoethanol, 250 mM sucrose). We routinely used 8 μg (+) or 24 μg (+++) of purified GST fusion proteins for the assays. After 13 h incubation at 30°C, reactions were stopped by boiling in SDS loading buffer, the proteins were separated by 15% SDS–PAGE and analysed by western blot with methylation-specific abs, which are described in the ‘In vitro HDM assay' section.
RT–PCR and qPCR
Total RNA was extracted from 14-day-old seedlings grown in LD using the TRI Reagent (Sigma) according to the manufacturer's instructions. cDNA was synthesized from total RNA using the SuperScript First-Strand Synthesis System (Invitrogen) with an oligo(dT) primer. RT–PCR was performed using gene-specific primers as previously described (Song et al, 2009).
qPCR was performed with the Applied Biosystems 7300 Real-time PCR System using iQ™ SYBR Green Supermix (Bio-Rad). Absolute quantification was performed by generating standard curves using serial dilutions of Actin2 and FLC sequence-containing clones. The relative mRNA levels represent the fold change over the control. The values are the means of three technical replicates and error bars represent s.d. To compare the relative amounts of the amplified products for ChIP experiments, genomic fragments of the FLC locus were amplified by qPCR and calculated according to the 2ΔΔCT method (Livak and Schmittgen, 2001), similar to the controls. Actin2 with primers JP1565 and JP1596 (Johnson et al, 2002) was used as an internal control and for normalization between samples. Control levels were set to 1 after normalization and others were expressed as relative values to the control levels.
REF6:6xHis protein expression
For REF6 expression in yeast, a PCR-amplified full-length REF6 cDNA fragment was cloned first into the pENTR/SD/D-TOPO entry vector, and then transferred into the pYES-DEST52 destination vector using the Gateway cloning technology (Invitrogen). The resulting plasmid was transformed into yeast strain BY4741 (MATa, ura3Δ0, leu2Δ0, his3Δ1, met15Δ0; Open Biosystems). The ΔREF6 mutant clone was created by amplifying a REF6 cDNA fragment lacking the coding regions for the JmjN and JmjC domains and by cloning into the same entry and destination vectors with the full-length REF6. Sequences of primers used for constructions are available on request.
REF6:6xHis and ΔREF6:6xHis proteins were prepared as follows: overnight cultured yeast cells grown in a synthetic SD medium with essential amino acids (Clontech) and 2% glucose were inoculated into 3 l of CSM-URA 2% (w/v) Raffinose and further grown at 30°C to an optical density at 600 nm (OD600) of 0.8. For protein induction, 2% (w/v) peptone, 1% (w/v) yeast extract, and 2% (w/v) galactose were added and further incubated at 30°C to an OD600 of 4–5. Yeast cells were collected by centrifugation and the pellets were suspended in 150 ml of lysis buffer (50 mM Tris pH 7.9, 400 mM NaCl, 1 mM PMSF). Acid-washed glass beads (1 g/10 ml buffer) were added, and the mixture was transferred into a bead beater (Biospec). The suspension was subjected to bursts for 30 s followed by 90 s cooling periods on ice for 2 h. The lysate was clarified by centrifugation and subjected to further purification steps according to the manufacturer's instructions for His-tagged proteins (Qiagen). Purified recombinant REF6 proteins were dialyzed against Hepes buffer (40 mM Hepes pH 7.9, 50 mM KCl, 10% glycerol, 1 mM DTT, 0.2 mM PMSF) overnight at 4°C. On the next day, the dialyzed proteins were concentrated approximately five-fold by ultrafiltration in Microcon centrifugal filters with a 30-kDa molecular cutoff (Millipore) and stored at −20°C until further use.
In vitro HDM assay
In vitro HDM assays were performed as previously described (Whetstine et al, 2006). Briefly, 10 μg (+) or 20 μg (++) of purified REF6:6xHis or 20 μg of purified ΔREF6:6xHis was incubated with 40 μg of calf thymus histones type II-A (Sigma) in the demethylation reaction buffer (20 mM Tris–HCl pH 7.3, 150 mM NaCl, 50 μM (NH4)2Fe(SO4)2 6(H2O), 1 mM α-ketoglutarate, 2 mM ascorbic acid) for 5 h at 37°C. Histone modifications were detected by western blot with the following abs: H3K4me1 (Upstate 07-436), H3K4me2 (Upstate 07-030), H3K4me3 (Abcam ab8580), H3K36me1 (Abcam ab9048), H3K36me2 (Upstate 07-369), H3K36me3 (Abcam ab9050), H3K9me3 (Upstate 07-442), H3K27me3 (Upstate 07-449), and H3 (Abcam Ab1791-100).
Yeast two-hybrid assay
Vectors and yeast strains were obtained from Clontech (Matchmaker GAL4 Two-Hybrid System 3). Assays were carried out according to the manufacturer's instructions. The cDNA sequences encoding FRISf-2, FRICol, EFS-N (amino acids 1–1218) and EFS-C (amino acids 1210–1805) were amplified by RT–PCR. FRISf−2 and EFS-N were subcloned into BamHI and EcoRI sites of pGBKT7 and pGADT7, respectively. EFS-C was subcloned into BamHI sites of pGADT7. FRICol was subcloned into SmaI sites of pGBKT7. Sequences of primers used for construction are available on request. The SUF4 construct was previously described (Kim et al, 2006). Plasmids were co-transformed into yeast AH109 strain by lithium acetate method, and transformants were incubated on the synthetic dropout media lacking Trp and Leu for 4 days at 30°C. Then, cells were transferred to the synthetic dropout media lacking Trp, Leu, His, and adenine and incubated at 30°C until colonies appeared.
Supplementary Material
Acknowledgments
We are grateful to J-Y Hong for assistance with mutant analysis. Oligonucleosomes were gift from J-J Song. This work was supported by the Global Research Laboratory Program and the BK21 Program of the Ministry of Education, Science, and Technology (MEST) and by the Bio-Green21 Program of the Rural Development Administration. BN was supported by a grant from the MEST to the Environmental Biotechnology National Core Research Center (R15-2003-012-01001-0) and by a grant from the Korea Research Foundation (KRF-2008-314-C00359). YH was supported by the BK21 Program. Work in RMA's laboratory is also supported by grants from the NSF and the NIH. BN and YSN designed research; JHK, IM, YT, and YH performed research; YC, RMA, BN, and YSN contributed new reagents/analytic tools; JHK, BN, and YSN analysed data; and JHK, RMA, BN, and YSN wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Baurle I, Dean C (2006) The timing of developmental transitions in plants. Cell 125: 655–664 [DOI] [PubMed] [Google Scholar]
- Baurle I, Smith L, Baulcombe DC, Dean C (2007) Widespread role for the flowering-time regulators FCA and FPA in RNA-mediated chromatin silencing. Science 318: 109–112 [DOI] [PubMed] [Google Scholar]
- Beisel C, Imhof A, Greene J, Kremmer E, Sauer F (2002) Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419: 857–862 [DOI] [PubMed] [Google Scholar]
- Choi J, Hyun Y, Kang MJ, Yun HI, Yun JY, Lister C, Dean C, Amasino RM, Noh B, Noh YS, Choi Y (2009) Resetting and regulation of FLOWERING LOCUS C expression during Arabidopsis reproductive development. Plant J 57: 918–931 [DOI] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K (2007) RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell 128: 1063–1076 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Hampsey M, Reinberg D (2003) Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113: 429–432 [DOI] [PubMed] [Google Scholar]
- Han SK, Song JD, Noh YS, Noh B (2007) Role of plant CBP/p300-like genes in the regulation of flowering time. Plant J 49: 103–114 [DOI] [PubMed] [Google Scholar]
- He Y, Michaels SD, Amasino RM (2003) Regulation of flowering time by histone acetylation in Arabidopsis. Science 302: 1751–1754 [DOI] [PubMed] [Google Scholar]
- Jiang D, Gu X, He Y (2009) Establishment of the winter-annual growth habit via FRIGIDA-mediated histone methylation at flowering locus c in Arabidopsis. Plant Cell 21: 1733–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347 [DOI] [PubMed] [Google Scholar]
- Johnson LM, Cao XF, Jacobsen SE (2002) Interplay between two epigenetic marks: DNA methylation and histone H3 lysine 9 methylation. Curr Biol 16: 1360–1367 [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K, Park C, Hwang HJ, Lee I (2006) SUPPRESSOR OF FRIGIDA4, encoding a C2H2-type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis FLOWERING LOCUS C. Plant Cell 18: 2985–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, He YH, Jacob Y, Noh YS, Michaels S, Amasino R (2005) Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell 17: 3301–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Zhang Y (2007) Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol 8: 307–318 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229: 57–66 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Vrles HB, Hanhart C, Soppe W, Peeters T (1994) The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta wild-type. Plant J 6: 911–919 [Google Scholar]
- Lee I, Michaels SD, Amasino RM (1994) The late-flowering phenotype of FRIGIDA and mutations in LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J 6: 903–909 [Google Scholar]
- Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128: 707–719 [DOI] [PubMed] [Google Scholar]
- Liu FQ, Quesada V, Crevillen P, Baurle I, Swiezewski S, Dean C (2007) The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to down regulate FLC. Mol Cell 28: 398–407 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Bezerra IC, Amasino RM (2004) FRIGIDA-related genes are required for the winter-annual habit in Arabidopsis. Proc Natl Acad Sci USA 101: 3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, He Y, Scortecci KC, Amasino RM (2003) Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc Natl Acad Sci USA 100: 10102–10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Lee SH, Kim HJ, Yi G, Shin EA, Lee M, Jung KJ, Doyle MR, Amasino RM, Noh YS (2004) Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell 16: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien S, Fleury D, Mylne JS, Crevillen P, Inze D, Avramova Z, Dean C, Grossniklaus U (2008) Arabidopsis trithorax1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell 20: 580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599 [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119: 941–953 [DOI] [PubMed] [Google Scholar]
- Shilatifard A (2008) Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol 20: 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HR, Song JD, Cho JN, Amasino RM, Noh B, Noh YS (2009) The RNA-binding protein ELF9 directly reduces SUPPRESSOR OF OVEREXPRESSION OF CO 1 transcript levels in Arabidopsis, possibly via nonsense-mediated mRNA decay. Plant Cell 21: 1195–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer NM, Napoli CA, Selinger DA, Pandey R, Cone KC, Chandler VL, Kaeppler HF, Kaeppler SM (2003) Comparative analysis of set domain proteins in maize and Arabidopsis reveals multiple duplications preceding the divergence of monocots and dicots. Plant Physiol 132: 907–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Amasino RM (2004) Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427: 159–164 [DOI] [PubMed] [Google Scholar]
- Tamada Y, Yun JY, Woo SC, Amasino RM (2009) ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell 21: 3257–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Katagiri Z, Kawahashi K, Kioussis D, Kitajima S (2007) Trithorax-group protein ASH1 methylates histone H3 lysine 36. Gene 397: 161–168 [DOI] [PubMed] [Google Scholar]
- Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y (2006) Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125: 467–481 [DOI] [PubMed] [Google Scholar]
- Xu L, Zhao Z, Dong A, Soubigou-Taconnat L, Renou JP, Steinmetz A, Shen WH (2008) Di- and tri- but not mono-methylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol Cell Biol 28: 1348–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Yu Y, Meyer D, Wu CJ, Shen WH (2005) Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3K36. Nat Cell Biol 7: 1256–1260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.