Summary
Lineage based mechanisms are widely used to generate cell type diversity in both vertebrates and invertebrates. For the past few decades, the nematode C. elegans has served as a primary model system to study this process due to its fixed and well characterized cell lineage. Recent studies conducted at the level of single cells and individual cis-regulatory elements suggest a general model by which cellular diversity is generated in this organism. During its developmental history a cell passes through multiple transient regulatory states characterized by the expression of specific sets of transcription factors. The transition from one state to another is driven by a general binary decision mechanism acting at each successive division in a reiterative manner and ending up with the activation of the terminal differentiation program upon terminal division. A similar cell fate specification system seems to play a role in generating cellular diversity in the nervous system of more complex organisms such as Drosophila and vertebrates.
Keywords: transcription factors, cell fate, lineage, asymmetric division, Wnt, C. elegans
Introduction
Lineage-based mechanisms are widely used to generate cell fate diversity during animal development. For example the diversity of neuronal subtypes is produced in part via asymmetric divisions in vertebrates, Drosophila and C. elegans [1]. Work by J. E. Sulston in the 80s has established that the 558 cells that compose the C. elegans embryo at the end of embryogenesis are generated following an invariant lineage [2]. Since then C. elegans has become a primary model system to analyze the molecular mechanisms that regulate cell lineages.
The early cleavages of the C. elegans zygote generate 6 founder cells: AB (precursor of most of the nervous system and epidermis), MS (precursor of most of the mesoderm), E (precursor of the endoderm), C (generating epidermis, neurons and muscles), D (generating muscles) and P4 (precursor of the germline). Each cell of the embryo is then generated from these founders by a unique and stereotyped succession of asymmetric divisions oriented most of the time along the antero-posterior axis. For example, the left AIY interneuron is “ABplpapaaap”, indicating for each of the nine successive divisions of the AB blastomeres whether it derives from the anterior (a), posterior (p), right (r) or left (l) daughter. Therefore at the end of embryogenesis, each cell is defined by a unique “bar code” corresponding to its unique lineage history. Here we review recent studies conducted at the resolution of single cells and individual cis-regulatory elements that suggest a general model by which this lineage history is translated into specific cell fates. We also discuss parallels with the lineage-based mechanisms at play during the generation of the nervous system in Drosophila and vertebrates.
A succession of transient regulatory states
During the past few decades, the analysis of the expression pattern of several transcription factors has shown that a lot of them are expressed transiently in a restricted number of dividing cells during embryogenesis. For example during endoderm development the endoderm precursors express a different set of transcription factors following each division [3]. These local observations have been confirmed at a global genome wide level by microarray analysis at different time points during embryogenesis [4]. This study has shown that a lot of genes are expressed in a highly dynamic manner appearing and disappearing over a one cell cycle time scale. All together this suggests that during development a cell passes through successive transient regulatory states (defined by a specific combination of transcription factors), possibly one regulatory state per cell cycle. Interestingly the laboratory of R. H. Waterston has recently developed an automated method to determine the complete expression pattern of gene reporters during embryonic development with a cellular resolution [5]. Coupled with the recent project of the modENCODE Consortium to generate accurate reporters for most transcription factors in the C. elegans genome [6], this may lead to a comprehensive description of the expression pattern of transcription factors during embryogenesis at high temporal and spatial resolution in the near future. This will ultimately reveal the nature of the combinatorial code of transcription factors that defines each single cell.
A binary decision mechanism
How do cells shift from one regulatory state to another during C. elegans development? This transition seems tightly coupled to the process of asymmetric division. In C. elegans many asymmetric cell divisions seem to be regulated by a particular Wnt/β-catenin pathway called the Wnt/β-catenin asymmetry pathway. As the molecular details of this pathway have been described recently in two very good reviews [7,8], we only focus here on its transcriptional output and developmental effects. This pathway regulates both the nuclear export of the TCF transcription factor POP-1 and the degradation of the TCF coactivator, the β-catenin SYS-1. Following asymmetric division this pathway is differentially active between the two daughter cells. As a consequence there is a high SYS-1 to POP-1 ratio in the posterior daughter nucleus leading to the formation of a SYS-1/POP-1 complex which activates transcription. On the contrary there is a low SYS-1 to POP-1 ratio in the anterior daughter nucleus, POP-1 is mostly free of SYS-1 and represses transcription [9–11].
The analysis of a mutant affecting this pathway suggests that it is involved in six successive asymmetric division rounds in the early embryo [12]. POP-1 and SYS-1 are also asymmetrically localized after many asymmetric divisions in the embryo [9,11,13]. All together this suggests that the Wnt/β-catenin asymmetry pathway may be a global binary cell fate decision mechanism regulating many asymmetric divisions during embryonic development.
Linking binary decisions and transient regulatory states
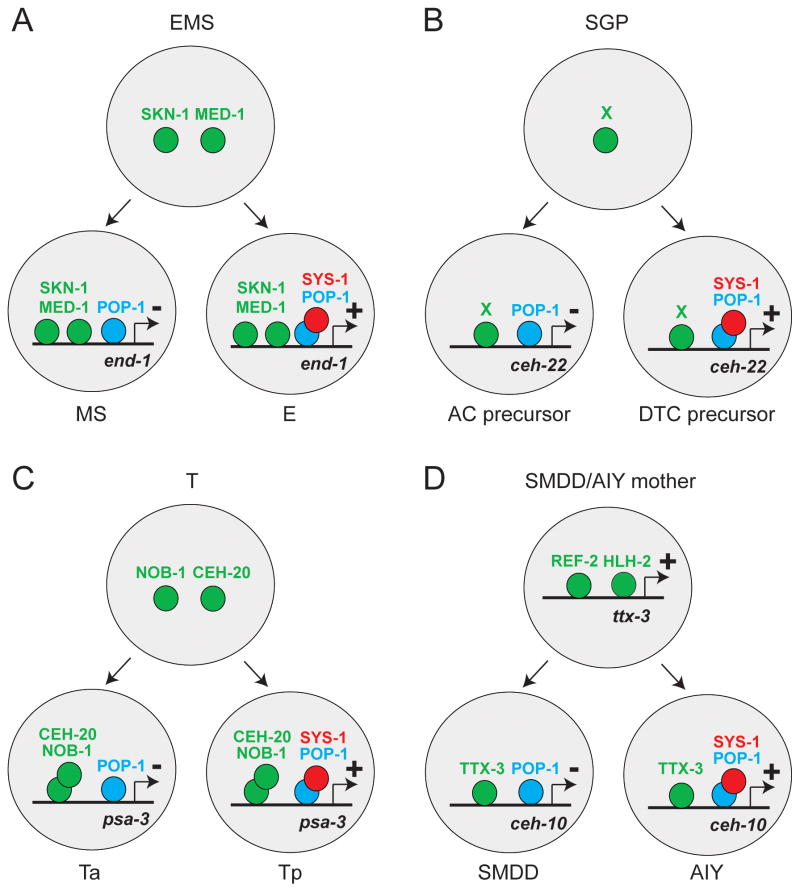
How does this binary decision system regulate the transition between regulatory states? Four recent studies conducted at single cells and individual cis-regulatory elements resolution level suggest a mechanism linking these two processes. In the first study, Maduro and colleagues analyze the regulation of the GATA transcription factor end-1 in the E blastomere (Figure 1A) [14]. In the early embryo the EMS blastomere divides asymmetrically to generate the MS blastomere (precursor of the mesoderm) and the E blastomere (precursor of the endoderm). The bZip transcription factor SKN-1 and the GATA transcription factor MED-1 are expressed in EMS, MS and E, and participate in the activation of end-1 expression. In the E blastomere the POP-1/SYS-1 activator complex cooperates with SKN-1 and MED-1 to activate end-1 expression while in the MS blastomere POP-1 alone represses end-1 activation by SKN-1 and MED-1. The promoter of end-1 contains binding sites for SKN-1, MED-1 and POP-1 suggesting that the lineage history (SKN-1 and MED-1) and the binary decision system (POP-1) are directly integrated at the level of the end-1 promoter.
Figure 1.
Integration of the Wnt/β-catenin asymmetric division machinery with the transcription factor cascade.
Asymmetric divisions of the EMS blastomere (A), the Somatic Gonadal Precursor (B), the T blast cell (C) and the SMDD/AIY mother (D).
In a second study, Lam and colleagues analyze the regulation of the Nkx transcription factor ceh-22 in the Distal Tip Cell (DTC) precursor (Figure 1B) [15]. In the larva, the Somatic Gonadal Precursor (SGP) divides asymmetrically to generate a DTC precursor and an Anchor Cell (AC) precursor. The expression of ceh-22 is reinforced in the DTC precursor versus the AC precursor by the POP-1/SYS-1 complex acting via POP-1 binding sites present in the ceh-22 promoter. However in this case transcription factors giving the specificity of response to the SGP lineage haven’t been identified yet (factor X in Figure 1B).
In the third case, Arata and colleagues study the regulation of the expression of the Meis transcription factor psa-3 in the Tp blast cell (Figure 1C) [16]. In the larva the T blast cell divides asymmetrically to generate the Ta cell (a precursor of epidermal cells) and the Tp cell (a precursor of neurons). The Hox transcription factor NOB-1 and the Pbx transcription factor CEH-20 are expressed in the T, Ta and Tp cells and contribute to the activation of psa-3 expression. In the Tp cell the POP-1/SYS-1 complex activates psa-3 expression while POP-1 alone represses this expression in the Ta cell. As in the case of end-1, the lineage specific information (NOB-1 and CEH-20) and the binary mechanism (POP-1) are directly integrated at the level of the psa-3 promoter via NOB-1 and POP-1 binding sites.
Finally the fourth study illustrates how lineage history and asymmetric division cue are integrated to activate the terminal differentiation program of specific neuronal cell types in the embryonic nervous system (Figure 1D) [17]. During neurulation the SMDD/AIY mother divides asymmetrically to generate the postmitotic motorneuron SMDD and the postmitotic interneuron AIY. The Zic transcription factor REF-2 and the bHLH transcription factor HLH-2 are transiently expressed in the SMDD/AIY mother where they directly activate the expression of the ttx-3 homeodomain transcription factor. Following division, TTX-3 is inherited in both SMDD and AIY, and directly activates the expression of the homeodomain transcription factor ceh-10 in AIY by cooperating with the POP-1/SYS-1 activator complex. In SMDD POP-1 alone represses ceh-10 expression. Finally TTX-3 and CEH-10 cooperate to directly activate a large battery of terminal differentiation genes responsible for AIY differentiation and to automaintain their expression throughout the life of the worm [17,18].
These four studies suggest a general model explaining how a binary decision system drives the transition between successive regulatory states (Figure 2). The mother cell is characterized by a regulatory state A corresponding to the expression of transcription factor A (SKN-1 and MED-1 for the EMS cell; NOB-1 and CEH-20 for the T cell; REF-2, HLH-2 and TTX-3 for the SMDD/AIY mother). Following cell division transcription factor A cooperates with the POP-1/SYS-1 activator complex in one of the two daughter cells to directly activate the expression of transcription factor B (END-1 in the E cell, CEH-22 in the DTC precursor; PSA-3 in the Tp cell and CEH-10 in the AIY neuron). In the other daughter cell transcription factor A may cooperate with POP-1 alone to activate the expression of a transcription factor C. This activation may involve a derepression mechanism by which POP-1 alone could repress the expression of a repressor (Rep) of factor C transcription. Such a repressor has not been identified so far and alternative scenarios such as a more direct role for POP-1 in activating factor C transcription are also possible. This model illustrates how the POP-1 binary decision system can generate from one regulatory state A in the mother cell, two new regulatory states B and C in the daughter cells. This general mechanism can be used in an reiterative manner (Figure 2) to generate a high diversity of cell fates in complex lineages. This model, by including mechanisms of integration by cis-regulatory elements, expands the “POP-1 coordinate system” model initially proposed by Lin and colleagues [9].
Figure 2.
Model for the generation of cell fate diversity by reiterative use of the Wnt/β-catenin asymmetry pathway.
Note that the activation of target genes by POP-1 via the repression of a repressor (REP) is hypothetical as no such repressor as been identified so far.
This model by its simplicity seems conceptually attractive but it may display some limitations. Even though the study of some Wnt/β-catenin asymmetry pathway mutants and the observation of POP-1 and SYS-1 asymmetries suggest that this pathway may be broadly used during C. elegans embryonic development [9,11–13,17], more analysis on the single cell and cis-regulatory level are required to understand the full extent to which this principle applies. Already it is clear that other mechanisms of binary cell fate decisions also play a role during C. elegans embryonic development such as the PAR system in the AB versus P1 decision [19], Notch signaling during the ABa versus ABp decision [20] or the asymmetric distribution of the unrelated protein HAM-1 during terminal asymmetric divisions generating cell death [21]. While these different binary cell fate decision mechanisms may appear conceptually relatively similar their molecular details are intrinsically very different. What promotes the selection of one binary mechanism over another in a specific context is an intriguing question.
A similar regulatory logic in other organisms ?
Passing through a succession of regulatory states seems a general property of cells during development as well illustrated for example in studies of early development of sea urchin [22] and ascidians [23,24] or nervous system development in Drosophila [25,26] or vertebrates [27–29].
The transitions between regulatory states are regulated by diverse types of mechanisms and among them lineage based binary specification is used by several other animals in addition to C. elegans. For example, in the annelid Platynereis, during most embryonic cell divisions β-catenin accumulates to high levels in one of the daughter cell and to low levels in the other daughter [30]. This β-catenin asymmetry is required in a reiterative manner to generate different fates in the daughter cells. This is remarkably similar to the asymmetry of the β-catenin SYS-1 and its role in reiterative binary cell fate decisions in C. elegans. In vertebrates the Wnt/β-catenin pathway has been implicated in the choice of neuronal progenitors between proliferation and differentiation. It has been suggested that β-catenin could play a role in the asymmetric division of ventricular zone progenitors [31–34]. Even though further experiments are clearly required to confirm this hypothesis, these results raise the possibility that a binary cell fate decision mechanism based on the Wnt/β-catenin pathway may play a role in several distantly related animal phyla.
Another lineage based binary decision mechanism, this one involving the Notch pathway, plays an important role in the generation of cell fate diversity in the nervous system of Drosophila and vertebrates. For example in the Drosophila midline the MP3 precursor cell divides asymmetrically to generate the H-cell neuron and its sister neuron called H-cell sib (Figure 3A) [35]. MP3 and its daughters express the transcription factors Nubbin and Pdm2. Following cell division the level of Notch signaling is different between the two daughters leading to the expression of the transcription factors SoxNeuro and Tailup in the H-cell, and of the transcription factors Forkhead, Lim1 and Single Minded in H-cell sib. In vertebrates one well characterized example is the generation of the V2a and V2b spinal interneurons (Figure 3B) [36–38]. A common progenitor p2 which expresses the transcription factors FoxN4, Gata2, Mash1 and Lhx3 divides asymmetrically to generate V2a and V2b. After division a differential level of Notch activity leads to the expression of the transcription factor Chx10 in V2a, and of the transcription factors Scl and Gata3 in V2b. In these examples the Notch pathway seems to play a role similar to the one of the Wnt/β-catenin asymmetry pathway in the C. elegans embryo, driving cells through successive regulatory states. However, the detailed mechanism by which the Notch asymmetry signal is integrated into the transcription factor cascade remains to be established. It would be interesting to determine whether the lineage specific factors and the binary decision system are directly integrated at the level of the cis-regulatory regions of daughter cell specific transcription factors as observed in C. elegans. The use of recent techniques such as chromatin immunoprecipitation may help answer this question.
Figure 3.
Regulation of terminal asymmetric divisions of neuronal precursors by Notch signaling.
(A) Division of the MP3 precursor in the Drosophila midline. (B) Division of the p2 precursor in the vertebrate spinal cord. In red: genes activated by Notch signaling; in blue: genes repressed by Notch signaling.
Finally, in the Drosophila and vertebrate nervous system, cell fate diversity can also be generated via stem cell-like asymmetric cell divisions [39]. In this process a stem cell progresses through different temporal states by expressing different transcription factors in successive divisions to generate different postmitotic neurons over time. While the mechanisms that drive the transition between temporal states seem complex and are still under investigation, a yet to be identified binary system could be part of it.
Conclusion
Studies of C. elegans embryonic development at single cells and individual cis-regulatory elements resolution level suggest that cell fate diversity is generated via a general Wnt/β-catenin binary decision mechanism which drives cells through a succession of transient regulatory states. While the molecular mechanisms integrating this pathway to the cascade of transcription factors is emerging several key questions remain unanswered such as how gene expression is activated in the daughter cell where β-catenin is absent. The Wnt/β-catenin pathway is also involved in asymmetric divisions in other organisms like Platynereis or vertebrates and it will be interesting to characterize the similarities and differences between these systems in the future. Finally in both C. elegans and other organisms different types of lineage based binary decision mechanisms exist in addition to the Wnt/β-catenin pathway, and it would be interesting to determine the specific constraints that influence the choice of one mechanism over another.
Acknowledgments
We thank Francois Guillemot and Weimin Zhong for helpful comments on the manuscript. Our work is funded by the National Institutes of Health (R01NS039996-05; R01NS050266-03), the Howard Hughes Medical Institute and postdoctoral fellowships by the EMBO and HFSPO to V.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sawa H. Specification of neurons through asymmetric cell divisions. Curr Opin Neurobiol. 2010;20:44–49. doi: 10.1016/j.conb.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 3.Maduro MF, Rothman JH. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol. 2002;246:68–85. doi: 10.1006/dbio.2002.0655. [DOI] [PubMed] [Google Scholar]
- 4.Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. doi: 10.1242/dev.00302. [DOI] [PubMed] [Google Scholar]
- *5.Murray JI, Bao Z, Boyle TJ, Boeck ME, Mericle BL, Nicholas TJ, Zhao Z, Sandel MJ, Waterston RH. Automated analysis of embryonic gene expression with cellular resolution in C. elegans. Nat Methods. 2008;5:703–709. doi: 10.1038/nmeth.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes an automated method to determine the expression pattern of fluorescent reporters during embryonic development with single cell resolution
- 6.Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM, et al. Unlocking the secrets of the genome. Nature. 2009;459:927–930. doi: 10.1038/459927a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizumoto K, Sawa H. Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol. 2007;17:465–473. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Phillips BT, Kimble J. A new look at TCF and beta-catenin through the lens of a divergent C. elegans Wnt pathway. Dev Cell. 2009;17:27–34. doi: 10.1016/j.devcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin R, Hill RJ, Priess JR. POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–239. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- 10.Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–772. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BT, Kidd AR, 3rd, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:3231–3236. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaletta T, Schnabel H, Schnabel R. Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature. 1997;390:294–298. doi: 10.1038/36869. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Shetty P, Robertson SM, Lin R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1. Development. 2007;134:2685–2695. doi: 10.1242/dev.008268. [DOI] [PubMed] [Google Scholar]
- 14.Maduro MF, Kasmir JJ, Zhu J, Rothman JH. The Wnt effector POP-1 and the PAL-1/Caudal homeoprotein collaborate with SKN-1 to activate C. elegans endoderm development. Dev Biol. 2005;285:510–523. doi: 10.1016/j.ydbio.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Lam N, Chesney MA, Kimble J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr Biol. 2006;16:287–295. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arata Y, Kouike H, Zhang Y, Herman MA, Okano H, Sawa H. Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev Cell. 2006;11:105–115. doi: 10.1016/j.devcel.2006.04.020. [DOI] [PubMed] [Google Scholar]
- **17.Bertrand V, Hobert O. Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans. Dev Cell. 2009;16:563–575. doi: 10.1016/j.devcel.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a mechanism by which the lineage history and the general asymmetric division machinery are integrated to trigger the terminal differentiation program of postmitotic neurons in C. elegans
- 18.Wenick AS, Hobert O. Genomic cis-Regulatory Architecture and trans-Acting Regulators of a Single Interneuron-Specific Gene Battery in C. elegans. Dev Cell. 2004;6:757–770. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Nance J. PAR proteins and the establishment of cell polarity during C. elegans development. Bioessays. 2005;27:126–135. doi: 10.1002/bies.20175. [DOI] [PubMed] [Google Scholar]
- 20.Priess JR. Notch signaling in the C. elegans embryo. Community CeR: WormBook. WormBook. 2005 doi: 10.1895/wormbook.1.4.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 21.Guenther C, Garriga G. Asymmetric distribution of the C. elegans HAM-1 protein in neuroblasts enables daughter cells to adopt distinct fates. Development. 1996;122:3509–3518. doi: 10.1242/dev.122.11.3509. [DOI] [PubMed] [Google Scholar]
- 22.Davidson EH, McClay DR, Hood L. Regulatory gene networks and the properties of the developmental process. Proc Natl Acad Sci U S A. 2003;100:1475–1480. doi: 10.1073/pnas.0437746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai KS, Hino K, Yagi K, Satoh N, Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131:4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- 24.Imai KS, Levine M, Satoh N, Satou Y. Regulatory blueprint for a chordate embryo. Science. 2006;312:1183–1187. doi: 10.1126/science.1123404. [DOI] [PubMed] [Google Scholar]
- 25.Zhong W. Diversifying neural cells through order of birth and asymmetry of division. Neuron. 2003;37:11–14. doi: 10.1016/s0896-6273(02)01178-9. [DOI] [PubMed] [Google Scholar]
- **26.Baumgardt M, Karlsson D, Terriente J, Diaz-Benjumea FJ, Thor S. Neuronal subtype specification within a lineage by opposing temporal feed-forward loops. Cell. 2009;139:969–982. doi: 10.1016/j.cell.2009.10.032. [DOI] [PubMed] [Google Scholar]; By using a combination of lineaging experiments and mutant analysis the authors describe a very precise transcription factor cascade regulating the generation of different neurons in a complex neuronal lineage of Drosophila
- 27.Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 28.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 29.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider SQ, Bowerman B. beta-Catenin asymmetries after all animal/vegetal- oriented cell divisions in Platynereis dumerilii embryos mediate binary cell-fate specification. Dev Cell. 2007;13:73–86. doi: 10.1016/j.devcel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- 33.Stocker AM, Chenn A. Focal reduction of alphaE-catenin causes premature differentiation and reduction of beta-catenin signaling during cortical development. Dev Biol. 2009;328:66–77. doi: 10.1016/j.ydbio.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand V, Hobert O. Wnt asymmetry and the terminal division of neuronal progenitors. Cell Cycle. 2009;8:1973–1974. doi: 10.4161/cc.8.13.9024. [DOI] [PubMed] [Google Scholar]
- *35.Wheeler SR, Stagg SB, Crews ST. Multiple Notch signaling events control Drosophila CNS midline neurogenesis, gliogenesis and neuronal identity. Development. 2008;135:3071–3079. doi: 10.1242/dev.022343. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the role of Notch signaling in regulating terminal asymmetric divisions in the midline of Drosophila
- *36.Kimura Y, Satou C, Higashijima S. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135:3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]; By using lineaging techniques in zebrafish, the authors establish that the V2a and V2b neurons are generated by a terminal asymmetric division regulated by Notch signaling
- 37.Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–827. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Barrio MG, Taveira-Marques R, Muroyama Y, Yuk DI, Li S, Wines-Samuelson M, Shen J, Smith HK, Xiang M, Rowitch D, et al. A regulatory network involving Foxn4, Mash1 and delta-like 4/Notch1 generates V2a and V2b spinal interneurons from a common progenitor pool. Development. 2007;134:3427–3436. doi: 10.1242/dev.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob J, Maurange C, Gould AP. Temporal control of neuronal diversity: common regulatory principles in insects and vertebrates? Development. 2008;135:3481–3489. doi: 10.1242/dev.016931. [DOI] [PubMed] [Google Scholar]