Abstract
A better understanding of factors that affect cognition could lead to improved health and greater independence for older adults. We examined the association of four modifiable factors (leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension) with changes in two aspects of fluid intelligence (verbal memory and inductive reasoning). Data for 626 adults collected over 14 years (three time points) were analyzed by multi-level modeling. A component of self-directed work, higher work control, was associated with better verbal memory (p < .05) and inductive reasoning (p < .01). There were no significant interactions among these factors. The findings suggest that a strong sense of control at work may be protective for fluid intelligence in adults.
Keywords: Aging, Cognition, Memory, Reasoning, Multilevel Modeling
Intact cognition is critical for the maintenance of activities of daily living and independence in older adults who are at increased risk for cognitive impairment and dementia (Hebert, Scherr, Bienjas, Bennett, & Evans, 2003; Hogan, 2005; Snowdon & Nun, 2003). Cognition is often categorized into crystallized and fluid intelligence. Crystallized intelligence refers to accumulated knowledge of the world; fluid intelligence refers to the ability to flexibly and adaptively identify complex relationships and draw inferences based on crystallized intelligence (Craik & Bialystok, 2006; Craik & Salthouse, 2008). Crystallized intelligence increases remarkably during childhood, increases at a slower rate throughout adulthood, and remains stable into advanced old age. Fluid intelligence peaks during the late teens and early twenties, declines gradually thereafter, and is more susceptible to internal and external influences (Craik & Bialystok). Although aspects of fluid intelligence such as verbal memory and inductive reasoning typically show reliable population-level decline as individuals reach their 60s (Finkel, Reynolds, McArdle, Gatz, & Pedersen, 2003), there is considerable inter-individual variability in the rate and timing of the decline (Christensen et al., 1999). Thus, understanding modifiable factors that affect individual differences in verbal memory and inductive reasoning might help adults maintain fluid intelligence well into old age. This knowledge could be applied to inform future interventions aimed at reducing the occurrence and impact of cognitive decline, and possibly dementia.
The purpose of this study was to examine the association of four modifiable factors -- leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension -- with changes in verbal memory and inductive reasoning in a sample of adults followed for 14 years. Data were drawn from the Seattle Longitudinal Study (Schaie, 2005) and analyzed with multilevel modeling (MLM).
Review of the Literature
A synthesis of the entire array of risk and protective factors for fluid intelligence across adulthood is beyond the scope of this article. Thus, we focused our literature review on the four modifiable factors examined in our analyses: leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension.
Leisure-time physical activity is a potential protective factor for cognition and is gaining attention because of its possible relationship to physical fitness. Leisure-time physical activity includes all muscular activities and might or might not include regular exercise training that is aimed at improving physical fitness. There is growing evidence that high levels of physical fitness are related to improved function of the pre-frontal lobe, where inductive reasoning is thought to be located, and of the hippocampal region, where verbal memory is thought to be located (Lambert, Fernandez, & Frick, 2005). Physical activity of low intensity was associated with greater cognitive decline 10 years later in 295 older men compared to physical activity of high intensity (van Gelder et al., 2004). In a meta-analysis aerobic exercise training was shown to improve executive control, control processing, speed, and visualspatial function in older adults without cognitive impairment (Colcombe & Kramer, 2003). In another meta-analysis (Heyn, Abreu, & Ottenbacher, 2004), structured exercise training was found to enhance cognitive performance as measured by standard instruments such as the Mini-Mental State Examination (MMSE), even in persons with dementia. Another meta-analysis of nine observational studies (in which the sample size ranged from 469 to 4,615) showed that leisure-time physical activity earlier in life is associated with fewer occurrences of dementia and Alzheimer’s disease (AD; Fratiglioni, Paillard-Borg, & Winblad, 2004). Leisure-time physical activity of at least twice a week in midlife was related to reduced risk for dementia (odds ratio = .48) and AD (odds ratio = .38) 21 years later in a sample of 1,449 persons aged 65–79 years (Rovio et al., 2005). Similar findings were reported in a study indicating that of the 1,740 participants, those who exercised three or more times per week in 1994 had significantly less dementia in 2003 (13/1,000 person years developed dementia) than those who did not engage in as much exercise (19.7/1,000 persons years developed dementia; Larson et al., 2006). However, others have failed to demonstrate a relationship between leisure-time physical activity and reduced AD risk (Verghese, Lipton, & Katz, 2003). It is also unclear how leisure-time physical activity relates to verbal memory and inductive reasoning, because few researchers have directly measured these variables. It is possible that individuals with higher fluid intelligence are more likely to be more physically active and to participate in a range of activities that enhance brain health.
Leisure-time cognitive activity (e.g., puzzles) is another potential protective factor for cognition. A longitudinal study (N = 801) found that a 1-point increase in leisure-time cognitive activity score was associated with a 64% reduction in the odds of developing AD, controlling for education and occupation (Wilson et al., 2002). Leisure-time cognitive activity was associated with reduced AD risk even after controlling for subgroups of early AD manifestation, apolipoprotein E alleles 4, medical condition, and depression (Wilson et al., 2002). A meta-analysis of 22 studies with than 29,000 individuals showed robust evidence that leisure-time cognitive activity is associated with a decreased risk of dementia (Valenzuela & Sachdev, 2006). In six of these studies, the relationship was longitudinally examined. The researchers found a 50% lower incidence of dementia. Frequent participation in leisure-time cognitive activity is thought to place a high demand on the brain and improve brain reserve by stimulating neuronal activation and by buffering against neural degeneration (Valenzuela & Sachdev). Despite the encouraging findings, the association of leisure-time cognitive activity with verbal memory and inductive reasoning is not well understood.
Self-directed work at employment/occupation, including work complexity (complexity with data processing and people interaction), work control (autonomous, low supervisor control), and work routine (lack of repetitive job tasks), is posited to improve cognition (Andel, Kareholt, Parker, Thorslund, & Gatz, 2007; Schooler, 1984; Schooler, Mulatu, & Oates, 2004). Similar to the mechanism of action for leisure-time cognitive activity, these work conditions stimulate an individual to perform cognitively demanding tasks on a daily basis. In a sample of 233 individuals (including married couples), more complex work was associated with better cognition 20 years later (Schooler, Mulatu, & Oates, 1999). In contrast, lower work complexity -- characterized by lower mental and higher physical occupational demands -- was associated with increased risk for AD after controlling for race, sex, age, and education (N = 357; Smyth et al., 2004). However, the effect of self-directed work on cognition might be more complex and mediated by confounding variables such as higher occupational status (Andel et al.). For example, after controlling for age and education, higher occupational status (professional, white collar jobs) was associated with better fluid intelligence 10 years later in a sample of 6,073 British workers from the Whitehall II study, accounting for 27% and 52% of the variance in inductive reasoning in men and women, respectively (Brunner, 2005). After controlling for occupational status, age, sex, and childhood socioeconomic status, work complexity remained significantly and positively correlated with MMSE scores in a cross-sectional analysis of 386 participants from a nationally representative Swedish sample (Andel et al.). Whether the association of self-directed work with verbal memory and inductive reasoning found in this cross-sectional study will hold in longitudinal designs is unknown.
An inverse relationship has been found between blood pressure and cognition (Elias, Robbins, Schultz, & Pierce, 1990; Elias, Schultz, Robbins, & Elias, 1989; Hertzog, Schaie, & Gribbin, 1978). Uncontrolled hypertension was related to deficits in fluid intelligence above and beyond those attributable to age alone (N = 357; Brady, Spiro, & Gaziano, 2005). In a 5-year longitudinal study of 46 adults, systolic blood pressure at follow-up correlated with white matter hyperintensities in those with hypertension and with a decline in fluid intelligence such as reasoning (Raz, Rodrigue, Kennedy, & Acker, 2007).
In the current literature, cognitive impairment is frequently operationalized as a diagnosis of AD or dementia. Research findings support a possible association between reduced dementia risk and the modifiable factors of leisure-time physical activity, leisure-time cognitive activity, self-directed work, and normal blood pressure. There is, however, a lack of research that examines the associations of these same factors with verbal memory and inductive reasoning by using longitudinal designs in healthy populations. Moreover, little work has been done to discern the interactive effects among these factors. Understanding these relationships over time has the potential to promote successful cognitive aging by facilitating early recognition and diagnosis of cognitive impairment and by informing the investigation and application of preventive interventions. Thus, this study was to examine the association of four modifiable factors -- leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension -- with changes in verbal memory and inductive reasoning in a sample of adults followed for 14 years.
Methods
The purpose of this study was to examine the association of four modifiable factors (leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension) with changes in verbal memory and inductive reasoning, controlling for established covariates (age, sex, education, and income) in a sample of adults who were followed for 14 years. MLM was used for data analysis.
Data Source
This study was a secondary analysis of data from the Seattle Longitudinal Study (SLS; Schaie, 2005). The SLS used a successive cohort design, with the first cohort recruited in 1956 from a health maintenance organization (HMO) based in the Pacific Northwest. Follow-ups occurred every 7 years, and new samples were added at each follow-up (wave). Participants were assessed at each wave by trained research staff in small groups and individually with a series of surveys and cognitive tests. Participants were also given a set of surveys to complete on their own and mail back when finished. Medical diagnosis data were retrieved from HMO medical records (Schaie).
Approvals from the Institutional Review Board for the SLS study were obtained by coauthor KWS, the principal investigator for the SLS, from the HMO, the source of study participants; and from the Pennsylvania State University, the study grantee throughout the data collections. Written informed consent was obtained from all participants. Data were identified only by an ID number. All secondary data analyses were conducted in co-author KWS’s laboratory.
The SLS data are unique for several reasons. First, the SLS uses the superior successive cohort design. This design allows for the distinct assessment of cohort, cross-sectional, and longitudinal effects. Second, SLS participants are healthy adults with high socioeconomic status. Having a sample of healthy, highly educated adults allowed us to investigate risk and protective factors in a group homogeneous for positive life circumstances. Third, the SLS is one of the major studies conducted in the past 50 years that followed participants’ cognitive development from young adulthood to advanced old age (Schaie, 2005). Thus, important factors, including leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension, could be examined in one study while controlling for common covariates (age, sex, education, and income). Last, the SLS data set includes repeated measures of verbal memory and inductive reasoning. This enabled us to study the association of postulated risk/protective factors with changes in fluid intelligence -- a unique addition to the literature. The repeated measures of fluid intelligence also warrant the use of MLM. This analytic technique can be applied to examine both intra- and inter-individual differences longitudinally, is sensitive to time, accommodates a large age range in a sample, and can easily distinguish cross-sectional, cohort, and longitudinal effects (Singer & Willett, 2003).
Sample
We included SLS participants who met two criteria: (a) had completed three waves of data collection (in 1984in 1991, and 1998), thus allowing longitudinal examination of differences in the dependent variables of interest (verbal memory and inductive reasoning); and (b) had no missing data for the independent variables (leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension) and covariates (age, sex, education, and income). This stipulation keeps the MLM models equivalent for model testing as independent variables are added.
The first criterion generated 703 participants. In that group, 77 participants were missing data for one or more independent variables. There were no missing data for covariates. As a result, our study had a final sample of 626 participants, representing 38% of the total SLS sample in 1984 (N = 1647).
The average age of the sample in 1984 was 53.20 (SD = 12.76; range = 23 – 82), and the mean years of education was 15.01 (SD = 2.66; range = 7 – 20). Ninety-six percent were white, 56% were female, 50% had an annual family income of ≥ $28,000 (equal to $73,360 in 2008 dollars), and 30% worked in semi-professional or professional occupations that required a graduate degree in 1984. Compared to the SLS total sample in 1984, the study sample was younger, but otherwise relatively equivalent (see Table 1).
Table 1.
Comparison of the Study Sample and The Total SLS Sample in 1984
| Variables (Mean [SD]) | Study Sample (N = 626) | Total SLS Sample (N = 1647) |
|---|---|---|
| Age, years | 53.20 (12.76) | 59.16 (15.92) |
| Education, years | 15.01 (2.66) | 14.26 (2.95) |
| % Female | 56.41% | 55.17% |
| % Racial minority | 3.55% | 3.16% |
| Median household annual | $28,000 | $26,000 |
| income | (= $73,360 in 2008) | (= $68,120 in 2008) |
The average yearly attrition rate for the study sample was 2.71%. The annual attrition rate was computed by dividing the sample size (n = 626) by the total SLS sample in 1984 (N = 1647), and again by 14 for each year of the study.
T-tests were used to identify differences between the sample (n = 626) and those excluded due to missing data on independent variables (n = 77). The excluded participants were significantly older and had lower scores on verbal memory and inductive reasoning in 1984 (data not shown).
Measures
The two dependent variables were verbal memory and inductive reasoning. The four independent variables were leisure-time physical activity, leisure-time cognitive activity, self-directed work, and hypertension. Age, sex, education, and income were included as covariates.
Dependent Variables
Verbal memory refers to the ability to encode, store, and recall meaningful language units ( Schaie et al., 2005). Verbal memory was computed as a composite score from three instruments: (a) word fluency (Thurstone & Thurstone, 1949), (b) immediate recall (Zelinski, Gilewski, & Schaie, 1993), and (c) delayed recall (Zelinski et al.). The instruments were administered by trained research personnel through in-person interviews, and their test-retest reliabilities were measured 2 weeks apart (Schaie, Willis, Hertzog, & Schulenberg, 1987). Word fluency requires participants to recall as many words as possible based on a lexical rule over a 5-minute period (test-retest reliability = .89, 7-year stability = .78). Immediate recall requires participants to examine a list of words for 3½ minutes and provides an equivalent period of time to recall as many as possible (test-retest reliability = .83, 7-year stability = .72). Delayed recall requires participants to recall the same words from the immediate recall measure after an hour has passed (test-retest reliability = .95, 7-year stability = .77). The formula used for deriving the composite score for verbal memory was based on SLS previous study findings and is as follows: Verbal Memory = .005 (Word Fluency) + .500 (Immediate Memory) + .495 (Delayed Memory). The composite scores were converted to t-scores to reflect a mean of 50 with a standard deviation of 10 for comparison across waves. The mean and standard deviation were based on the total SLS sample, including participants not targeted in the present study.
Inductive reasoning refers to a person’s ability to identify novel relationships in serial patterns and to assess the use of principles and rules for determining further serial patterns. The composite score for inductive reasoning was derived from four instruments: (a) Primary Mental Abilities -- PMA -- reasoning measure (Thurstone & Thurstone, 1949); (b) Adult Development and Enrichment Project letter series - ADEPT-letter (Blieszner, Willis, & Baltes, 1981); (c) word series (Schaie, 2005); and (d) Educational Testing Service number series (Ekstrom, French, Harman, & Derman, 1976). The instruments were administered by trained research personnel through in-person interviews, and their test-retest reliabilities were measured 2 weeks apart (Schaie et al., 1987). The PMA-reasoning measure provides participants with a series of letters and requires the identification of the next letter in the series (test-retest reliability = .88, 7-year stability = .84). The ADEPT-letter is a test that parallels the PMA reasoning measure and requires participants to identify the next letter in a series of letters (test-retest reliability = .84, 7-year stability = .82). The word series measure provides a series of words and requires the participants to identify the next word in the series (test-retest reliability = .85, 7-year stability = .63. The ETS-number requires participants to identify the next number in a series (test-retest reliability = .83, 7-year stability = .74). The formula used for deriving the composite score was based on the SLS previous study findings and is as follows: Inductive Reasoning = .280 (PMA Reasoning) + .272 (ADEPT Letter Series) + .259 (Word Series) + .189 (ETS Number Series). The composite scores were then converted to t-scores to reflect a mean of 50 with a standard deviation of 10 for comparison across waves. The mean and standard deviation were based on the total SLS sample, including participants not targeted in the present study.
Independent Variables
Leisure-time physical activity was a proportion operationalized as the number of weekly hours spent participating in sports, physical fitness, and outdoor hobbies, divided by the total weekly hours for all leisure-time activities. Data for leisure-time physical activity were abstracted from items on the self-report survey: Life Complexity Inventory – LCI (Gribbin, Schaie, & Parham, 1980). Participants completed the LCI questionnaires as homework after their initial SLS interview. Each leisure-time activity was measured with an open-ended question about how many hours per week the individual participated in the activity. The LCI leisure activities had been analyzed using both exploratory and confirmatory factor analysis in randomly split halves of the 1977 sample and confirmed in the 1984 sample. Six meaningful leisure and eight lifestyle factors were identified and replicated over 7 years (Schaie, 2005). The six leisure factors included household, social, educational/cultural, fitness, solitary, and communication factors. The eight lifestyle factors were social status, subjective dissatisfaction with life status, noisy environment, family dissolution, semi-passive engagement with the environment, disengagement from interaction with the environment, maintenance of acculturation, and female homemaker role (Schaie). A longitudinal factor analysis showed stability in those factors. Data for leisure-time physical activity were mean centered for analysis.
Leisure-time cognitive activity was a proportion operationalized as the number of weekly hours spent on socializing, educational activities, and cultural activities, divided by the total weekly hours for all leisure-time activities. Leisure-time cognitive activity was also abstracted from the LCI and mean centered for analysis (O'Hanlon, 1993; Schaie, 2005).
Self-directed work included 3 components and was operationalized in three ways as perceived autonomy, work control, and innovation. Each was measured by a scale of the Work Environment Inventory (Moos, 1981). The autonomy scale assesses an employee’s perceptions of being encouraged to be self-sufficient and make his/her own decisions. The work control scale assesses an employee’s perceptions of control in his/her work environment. The innovation scale assesses an employee’s perception of whether variety, change, and new approaches in work are emphasized. Each scale included five items rated on a 5-point Likert scale from 1 to 5 with the sum score for each scale ranging from 5 to 25. The 1-month test-retest reliabilities for the scales are .77 for perceived autonomy, .77 for work control, and .75 for innovation (Moos). Data from each scale were assessed in 1998 only and were mean centered for analysis.
Hypertension was dichotomized as present or absent from 1992 to 1998, where 0 = no diagnosis and 1 = diagnosed. Diagnosis of hypertension was made according to established criteria of systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg. The diagnosis was retrieved from participants’ HMO records for every year they participated in the study. While diagnosis data were available in the SLS for the 1984 and 1991 waves, there were high proportions of missing data (44% missing in 1984 and 56% missing in 1991). Thus, the current study only included data for the 1998 wave.
Covariates
Age, sex, education, and income were included as control variables. They have been established as factors associated with cognition (Brady et al., 2005; Tyas et al., 2007). Data for covariates were abstracted from the LCI. Income, education, and age were assessed at all three waves, while sex was assessed in 1984 and included as a time invariant control. Education was centered at the mean (12 years, representing a high school education). Age was centered at 60, because significant population-level declines in fluid intelligence begin after 60 years of age (Finkel et al., 2003; Schaie, 2005). Income was mean centered, and sex was coded as 0 = male, 1 = female. Race was not included, because only 3.55% of the sample was non-white.
A subsample of the SLS participants over age 60 were administered a comprehensive neuropsychological battery to screen for dementia (Schaie et al., 2005). In our study sample (n = 626), 429 of the 502 participants over 60 years of age were given the neuropsychological battery. Of those 429 participants, 3.7% were identified as having probable or definite dementia in 1998, representing only 2.2% of the study sample. Thus, the use of dementia diagnosis as a covariate was not warranted.
Analysis
Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). To check violations of statistical assumptions, skewness and kurtosis were assessed on all variables included in the present sample with SAS proc univariate. Although none was perfectly normally distributed, all dependent variables, independent variables, and covariates had ≤ 1.0 (+/−) on skewness or kurtosis. Thus, repeated-measures MLM were specified following four steps.
First, unconditional means models (empty models) estimating the effects of random intercepts on each dependent variable were specified to calculate intraclass correlations.
Second, unconditioned growth curve models testing fixed and random effects of age and time-in-study for each dependent variable were specified. Time-in-study refers to the length of time a participant was in the present study. This ranged from 0 – 14 years (1984 as time 0). Change in time-in-study perfectly correlates with change in age, but when included in the model with age, it indicates the presence/absence of cohort differences on the outcome in the sample. Random slope-intercept covariance for age and time-in-study was estimated and reported when significant slopes were identified.
Third, conditioned growth curve models adding tests of fixed effects of the independent variables were specified.
Fourth, conditioned growth curve models including other covariates (income, education, and sex) were specified. Each independent variable and covariate was tested individually for significance, and if significant, was retained to identify the most parsimonious model for each dependent variable. Interactions between age and time with significant independent variables were tested individually for factors with significant main effects on the dependent variables. All models were estimated using SAS proc mixed to examine the patterns of intra-and inter-individual differences in dependent variables across three waves (14 years). Restricted maximum likelihood (REML) was used to report the parameter estimates and assess random effects. Degrees of freedom were estimated with the Satterthwaite method (Satterthwaite, 1941).
Results
The mean composite scores for verbal memory and inductive reasoning were both ~50, with an average change of −2 at each wave (SD for verbal memory = 7.1, SD = 4.2 for inductive reasoning). Both verbal memory and inductive reasoning remained stable over the 7 years from Time 0 to Time 1, and then began to decline over the 7 years from Time 1 to Time 2 (see Table 2). The range of change over this 14-year period is from −29 to +21 for verbal memory and from −35 to +11 for inductive reasoning.
Table 2.
Description of Study Variables (N = 626)
| Variables (Means [SD]) | Time 0 (1984) | Time 1 (1991) | Time 2 (1998) |
|---|---|---|---|
| Dependent Variables | |||
| Verbal Memory | 50.46 (8.83) | 50.54 (9.03) | 48.58 (9.87) |
| Inductive Reasoning | 51.84 (7.81) | 51.49 (8.16) | 49.85 (8.80) |
| Independent Variables | |||
| Leisure-time Physical Activity | .06 (.06) | .05 (.06) | .05 (.06) |
| Leisure-time Cognitive Activity | .05 (.06) | .05 (.05) | .05 (.05) |
| Perceived Autonomy | -- | -- | 20.12 (4.26) |
| Work Control | -- | -- | 16.63 (5.09) |
| Innovation | -- | -- | 16.62 (4.31) |
| Hypertension (% with Diagnosis) | -- | -- | 29.72% |
Verbal Memory
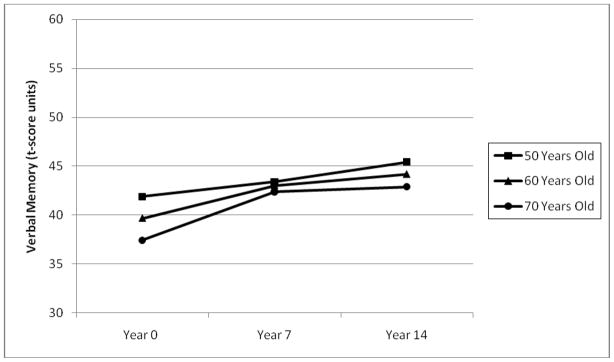
The unconditional means models showed an interclass correlation coefficient of .72, indicating that 72% of the variance in verbal memory across the three waves was between persons. Unconditioned growth curve models testing fixed effects of age and time-in-study indicated significant cohort effects, thus both age and time-in-study were retained. Time-in-study changed in concert with age. Both were included in the model to allow testing of cohort differences in the relationship between time and verbal memory. A small but significant interaction between age and time-in-study was found and included in the unconditioned growth curve model (see Figure 1). Random slopes for age and time-in-study were tested but were not significant. The fixed linear term for time-in-study (p < .001) indicated an average gain in verbal memory with increasing years in the study. However, the fixed effect of age was negative (p < .001), showing that verbal memory declines with increasing age.
FIGURE 1.
Interaction of age and time-in study in the prediction of verbal memory in a sample of 50, 60, and 70 year olds (at the first occasion).
In the conditioned growth curve model, relative to a 60-year-old, every extra year older was associated with a .21 decline in verbal memory (see Table 3). Further, work control was the only significant factor associated with verbal memory (p < .05). After adding covariates, work control remained significant. Every increased unit of work control at the third wave was associated with a .13 increase in verbal memory (p < .05). In addition, having more years of education (p < .001), being female (p < .001), and having a higher income (p < .01) were also associated with gains in verbal memory (see Table 3).
Table 3.
The Final Multilevel Model for Verbal Memory (N = 626)
| Parameter | Final Model |
||
|---|---|---|---|
| Estimate | SE | p-value | |
| Fixed Effects: | |||
| Intercept | 39.658 | 1.218 | < .001 |
| Time | 0.163 | 0.031 | < .001 |
| Linear Age | −0.211 | 0.024 | < .001 |
| Age X Time Interaction | −0.009 | 0.002 | < .001 |
| Work Control | 0.148 | 0.056 | < .05 |
| Education | 0.489 | 0.104 | < .001 |
| Female | 4.303 | 0.566 | < .001 |
| Income | 0.097 | 0.041 | < .05 |
| Variance Components: | |||
| Individual Variance | 39.171 | 2.689 | < .001 |
| Residual Variance | 21.947 | 0.905 | < .001 |
| Individual Level Pseudo R2 | .64 | ||
| Model Fit: | |||
| REML Deviance | 11854 | ||
| AIC | 11858 | ||
| BIC | 11867 | ||
Note. AIC is Akaike Information Criterion. BIC is Bayesian Information Criterion. REML is Restricted Estimate Maximal Likelihood. SE is Standard Error .
Inductive Reasoning
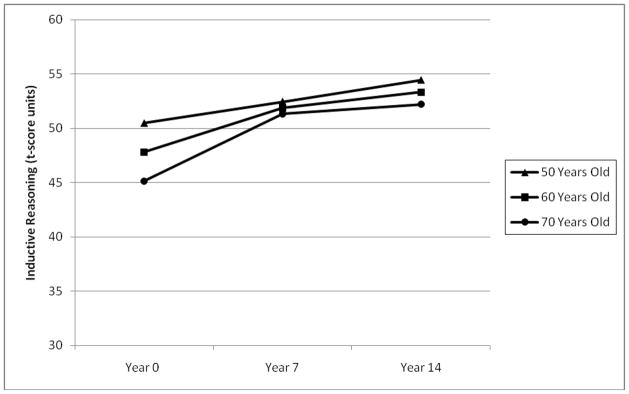
The unconditioned means models showed an interclass correlation coefficient of 0.87, indicating that 87% of the variance in inductive reasoning across the three waves was due to between-person differences. In the unconditioned growth curve model, fixed effects of both age and time-in-study were significant, indicating significant cohort differences in inductive reasoning changes. The fixed linear effect of time-in-study was positive and significant (p < .001), suggesting that the average score in inductive reasoning increased over time, whereas the fixed linear age effect was significant and negative, indicating that with increasing age, individuals had diminished inductive reasoning ability. Similar to the model for verbal memory, a significant interaction between age and time was tested and improved the model fit, indicating different rates of change in inductive reasoning over time by age (see Figure 2). Finally, after testing random slopes of both linear age and time, a significant effect of linear age was discovered. This indicates significant inter-individual differences in the rates of intra-individual change in inductive reasoning.
FIGURE 2.
Interaction of age and time-in study in the prediction of inductive reasoning in a sample of 50, 60, and 70 year olds (at the first occasion).
After individually testing independent variables in the conditioned growth curve models, work control was the only significant factor associated with inductive reasoning. After adding other covariates, work control remained significant. Every increased unit of work control at the third wave was associated with a .14 t-score unit increase in inductive reasoning (p < .05). In addition, higher income (p <. 001) and education (p < .001) were also significantly associated with gains in inductive reasoning (see Table 4).
Table 4.
The Final Multilevel Model for Inductive Reasoning (N = 626)
| Parameter | Final Model |
||
|---|---|---|---|
| Estimate | SE | p-value | |
| Fixed Effects: | |||
| Intercept | 47.811 | 0.488 | <.001 |
| Time | 0.203 | 0.023 | < .001 |
| Linear Age | −0.266 | 0.022 | < .001 |
| Age X Time Interaction | −0.008 | 0.001 | < .001 |
| Work Control | 0.154 | 0.051 | < .01 |
| Education | 0.374 | 0.087 | < .001 |
| Income | 0.085 | 0.023 | < .001 |
| Variance Components: | |||
| Individual Variance | 35.152 | 2.228 | < .001 |
| Age Variance | 0.015 | 0.004 | < .001 |
| Individual-age Covariance | 0.040 | 0.062 | = .52 |
| Residual Variance | 5.584 | 0.305 | < .001 |
| Individual Level Pseudo R2 | 0.86 | ||
| Model Fit: | |||
| REML Deviance | 10264 | ||
| AIC | 10272 | ||
| BIC | 10290 | ||
Note. AIC is Akaike Information Criterion. BIC is Bayesian Information Criterion. REML is Restricted Estimate Maximal Likelihood. SE is Standard Error .
Discussion
A number of factors should be considered when interpreting our findings. First, this sample had high levels of education, health status, and income, and lacked racial and ethnic diversity, thus limiting the generalizability of the findings. Second, while the SLS afforded us a rich, longitudinal database, the use of this data set limited the questions that we could ask and the analyses we could perform. Third, some of the independent variables examined (e.g., leisure-time physical activity and leisure-time cognitive activity) were based on subjective reports and therefore prone to recollection errors. Those data, however, were factor-analyzed and found to be stable over time (O'Hanlon, 1993). Last, while MLM allows one to examine multiple variables longitudinally and is robust for dealing with missing data on dependent variables, it excludes subjects with missing data on independent variables. The 77 excluded participants were significantly older and performed worse on verbal memory and inductive reasoning measures in 1984. The results might have been different had these participants been included in the analysis.
Despite these limitations, our findings have important implications for practice and research. We found that in a sample of participants with high income and education, changes in verbal memory and inductive reasoning -- two aspects of fluid intelligence -- were related to both modifiable and non-modifiable factors. Higher work control was associated with higher verbal memory and inductive reasoning. This finding is similar to what others have reported about the relationship between cognitive stimulation and cognitive function (Andel et al., 2007; Churchill et al., 2002; Schooler, 1984; Schooler et al., 2004). Specifically, individuals who engage in occupations that provide a high degree of work control and complexity and low degree of supervision show less decline in their fluid intelligence than individuals who are engaged in less intellectually demanding jobs. Our data, and that of others reported in the literature, are supported by significant experimental work in animal models that indicates exposure to stimulating physical and cognitive environments promotes neurogenesis and better cognitive performance (Lambert et al., 2005; Leggio et al., 2005; Rampon & Tsien, 2000). Performing cognitively demanding tasks on a daily basis may stimulate neuronal activation and buffer against neural degeneration in humans (Churchill et al.).
Our study further confirms the independent relationship between work control and fluid intelligence over and above education, income, and age. Schooler and colleagues (1984; 1999) reported that high work complexity, high work control, and low work routine were especially associated with high cognitive flexibility. Thus, those domains of the work environment seem to be important for maintaining fluid intelligence in adults. The lack of intra-individual differences in verbal memory and inductive reasoning suggest that retooling work control, particularly in early adulthood and midlife, might be salient for protecting fluid intelligence in older adulthood.
The proportion of weekly hours spent on leisure-time physical and cognitive activity was not related to fluid intelligence in this study. This might be attributable to the fact that there was little variance in the data for these two factors. Other epidemiologic studies have shown that frequency and regularity of physical activity (≥ 3 times a week over an extended period of time) plays a robust role in delaying dementia onset and decreasing the risk for AD (Fratiglioni et al., 2004; Larson et al., 2006). In our study, participants’ leisure-time physical activity levels remained stable over 14 years; that is, their habits of physical activity formed in middle age were carried over to late age. This finding suggests that increasing physical activity in middle age might be key to continued physical activity participation in old age, and better cognitive performance could result from that participation.
The lack of an association between hypertension and fluid intelligence in the present study supports some of the current literature. However, our finding might be explained by the low occurrence of hypertension in our study sample: after 14 years, only 29.72% of the sample was hypertensive. Also we had no measure of hypertension severity. People with poorly controlled hypertension show more frontal-lobe-mediated cognitive deficits with concomitant greater frontal lobe atrophy and increased white matter volume on structural magnetic resonance imaging (Raz et al., 2007). It is likely that our participants had better access to medical care and management for their hypertension because of their higher education and income levels, and consequently better fluid intelligence. The relationship of hypertension severity and management effectiveness with fluid intelligence needs further investigation.
Future studies are needed to confirm our findings. Limited research has been done exploring self-directed work and its impact on fluid intelligence. Although leisure-time physical and cognitive activities have been associated with delayed dementia onset, future studies need to focus on their relationship to fluid intelligence. These findings are likely to have significant implications for successful aging and dementia prevention. Work control was associated with better verbal memory and inductive reasoning over 14 years. With the increasing complexity and the incorporation of technology at work, system-level redesigns of work control might help employees maintain fluid intelligence.
Acknowledgments
This research was supported in part by a grant from the National Institute on Aging (R37 AG08055) to K. Warner Schaie. Dr. Yu was supported by a NIH K12 Career Development Award (NIH RR023247-01) and Ryan was supported by a National Institute of Mental Health T32 training grant (#T32 MH18904).
Contributor Information
Fang Yu, Assistant Professor, University of Minnesota School of Nursing.
Lindsay H. Ryan, Post-doctoral Fellow, University of Michigan.
K. Warner Schaie, Evan Pugh Professor of Human Development and Psychology, The Pennsylvania State University.
Sherry L. Willis, Professor of Human Development, Department of Human Development and Family Studies and the Gerontology Center.
Ann Kolanowski, Professor of School of Nursing, The Pennsylvania State University.
References
- Andel R, Kareholt I, Parker MG, Thorslund M, Gatz M. Complexity of primary lifetime occupation and cognition in advanced old age. Journal of Aging and Health. 2007;19(3):397–415. doi: 10.1177/0898264307300171. [DOI] [PubMed] [Google Scholar]
- Blieszner R, Willis SL, Baltes PB. Training research in aging on the fluid ability of inductive reasoning. Journal of Applied Developmental Psychology. 1981;2(3):247–265. [Google Scholar]
- Brady CB, Spiro A, 3rd, Gaziano JM. Effects of age and hypertension status on cognition: The Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19(6):770–777. doi: 10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Brunner EJ. Social and biological determinants of cognitive aging. Neurobiology of Aging. 2005;26S (Suppl 1):S17–S20. doi: 10.1016/j.neurobiolaging.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a function of age. Psychology & Aging. 1999;14(3):365–379. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiology of Aging. 2002;23(5):941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Craik FI, Bialystok EB. Cognition through the lifespan: Mechanisms of change. Trends in Cognitive Sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA. The handbook of aging and cognition. 3. New York, NY, US: Psychology; 2008. [Google Scholar]
- Ekstrom RB, French JW, Harman G, Derman D. Fit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Elias MF, Robbins MA, Schultz NA, Pierce TW. Is blood pressure an important variable in research on aging and neuropsychological test performance? Journal of Gerontology: Psychological Sciences. 1990;45(4):128–135. doi: 10.1093/geronj/45.4.p128. [DOI] [PubMed] [Google Scholar]
- Elias MF, Schultz NR, Robbins MA, Elias PK. A longitudinal study of neuropsychological performance by hypertensives and normotensives : A third measurement point. Journal of Gerontology: Psychological Sciences. 1989;44(1):25–28. doi: 10.1093/geronj/44.1.p25. [DOI] [PubMed] [Google Scholar]
- Finkel DG, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Psychology and Aging. 2003;39(3):535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Gribbin K, Schaie KW, Parham IA. Complexity of lifestyle and maintenance of intellectual abilities. Journal of Social Issues. 1980;36(2):47–61. [Google Scholar]
- Hebert LE, Scherr PA, Bienjas JL, Bennett DA, Evans DA. Alzheimer's disease in the US population: Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Schaie KW, Gribbin K. Cardiovascular diseases and changes in intellectual functioning from middle to old age. Journal of Gerontology. 1978;33(6):872–883. doi: 10.1093/geronj/33.6.872. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine & Rehabilitation. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hogan M. Physical and cognitive activity and exercise for older adults: A review. International Journal of Aging & Human Development. 2005;60(2):95–126. doi: 10.2190/PTG9-XDVM-YETA-MKXA. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiology of Learning & Memory. 2005;83(3):206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P. Exercise is associated with reduced risk for incident dementia among person 65 years of age and older. Annals of Internal Medicine. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behavioral Brain Research. 2005;163(1):78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Moos RH. Work environment scale manual. Palo Alto, CA: Consulting Psychologists Press; 1981. [Google Scholar]
- O'Hanlon A. Unpublished paper. The Pennsylvania State University; 1993. Inter-individual patterns of intellectual aging: The influence of environmental factors. [Google Scholar]
- Rampon C, Tsien JZ. Genetic analysis of learning behavior-induced structural plasticity. Hippocampus. 2000;10(5):605–609. doi: 10.1002/1098-1063(2000)10:5<605::AID-HIPO11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurology. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Satterthwaite FE. Synthesis of variance. Psychometrika. 1941:309–316. [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle longitudinal study. New York: Oxford University Press; 2005. [Google Scholar]
- Schaie KW, Caskie GIL, Revell AJ, Willis SL, Kazniak AW, Teri L. Extending neuropsychological assessment into the primary mental ability space. Aging, Neuropsychology and Cognition. 2005;12(3):1–33. doi: 10.1080/13825580590969343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Hertzog C, Schulenberg JE. Effects of cognitive training upon primary mental ability structure. Psychology and Aging. 1987;2:233–242. doi: 10.1037//0882-7974.2.3.233. [DOI] [PubMed] [Google Scholar]
- Schooler C. Psychological effects of complex environments during the lifespan: A review and theory. Intelligence. 1984;8(4):259–281. [Google Scholar]
- Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology & Aging. 1999;14(3):483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. Occupational self-direction, intellectual functioning, and self-directed orientation in older workers: Findings and implications for individuals and societies. American Journal of Sociology. 2004;110(1):161–197. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Smyth KA, Fritsch T, Cook TB, McClendon MJ, Santillan CE, Friedland RP. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63(3):498–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Nun S. Healthy aging and dementia: Findings from the nun study. Annals of Internal Medicine. 2003;139(5[Pt 2]):450–454. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG. Examiners manual, SRA Primary Mental Abilities Test, intermediate form. Chicago: Science Research Associates; 1949. [Google Scholar]
- Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS, et al. Transitions to mild cognitive impairments, dementia, and death: Findings from the nun study. American Journal of Epidemiology. 2007;165(11):1231–1238. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychological Medicine. 2006;36(8):1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: The FINE study. Neurology. 2004;63(12):2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348(25):2508–2616. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Three-year longitudinal memory assessment in older adults: Little change in performance. Psychology and Aging. 1993;8(2):176–186. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]