Abstract
In visceral leishmaniasis, the draining lymph node (DLN) is the initial site for colonization and establishment of infection after intradermal transmission by the sand fly vector; however, little is known about the developing immune response within this site. Using an intradermal infection model, which allows for parasite visceralization, we have examined the ongoing immune responses in the DLN of BALB/c mice infected with L. infantum. Although not unexpected, at early times post-infection there is a marked B cell expansion in the DLN, which persists throughout infection. However, the characteristics of this response were of interest; as early as day 7 post-infection, polyclonal antibodies (TNP, OVA, chromatin) were observed and the levels appeared comparable to the specific anti-leishmania response. Although B-cell-deficient JHD BALB/c mice are relatively resistant to infection, neither B-cell-derived IL-10 nor B-cell antigen presentation appear to be primarily responsible for the elevated parasitemia. However, passive transfer and reconstitution of JHD BALB/c with secretory immunoglobulins, (IgM or IgG; specific or non-specific immune complexes) results in increased susceptibility to L. infantum infection. Further, JHD BALB/c mice transgenetically reconstituted to secrete IgM demonstrated exacerbated disease in comparison to wild type BALB/c mice as early as 2 days post-infection. Evidence suggests that complement activation (generation of C5a) and signaling via the C5aR (CD88) is related to the disease exacerbation caused by IgM rather than cytokine levels (IL-10 or IFN-γ). Overall these studies indicate that polyclonal B cell activation, which is known to be associated with human visceral leishmaniasis, is an early and intrinsic characteristic of disease and may represent a target for therapeutic intervention.
Keywords: Parasitic Protozoan, Antibody, C5a
INTRODUCTION
Visceral leishmaniasis (VL) is a potentially fatal human disease caused by the intracellular protozoan parasites Leishmania donovani and L. infantum/L. chagasi. The immune response to visceral leishmaniasis is complex and has been shown to be organ-specific, differing significantly dependent upon the site of infection examined (liver versus spleen) [1, 2]. Although the lymph node is thought to be analogous to the spleen, there are considerable developmental as well as structural and functional differences [3, 4]. Reflective of this is the fact that although both spleens and lymph nodes from fatal human cases of VL exhibit destruction of normal architecture, follicular dendritic cells (FDC) and germinal centers (GC) are lost in spleens, while continuing to be present in lymph nodes [5]. However, few experimental studies to date have examined the lymph node responses that occur as a result of infection and have instead focused on the spleen where, akin to observations in humans, the destruction of FDC and GC is evident [6].
Although these observations in the spleen might appear to preclude a role for B cells in disease, other evidence from both human patients and murine model studies indicate that B cell activation leads to disease exacerbation. Mouse model studies of VL have demonstrated that C57BL/6 B cell deficient mice are relatively resistant to intravenous infection [7]. Further, B cell dysfunction as evidenced by hypergammaglobulinemia, and the presence of significant non-specific (polyclonal) and autoimmune antibodies are evident and are hallmarks of human visceral leishmaniasis [8–14].
Moreover in the case of cutaneous leishmaniasis, polyclonal B cell activation has been reported in response to L. major infection [15, 16], however, the antigens recognized were not defined. Further, B cell functions (antibody and antigen presentation) have been shown to lead to disease exacerbation for infection with L. major [17], while IgG production but not B cell antigen presentation promotes infection/disease in the case of L. mexicana complex parasites [18–21]. However, the role of immunoglobulins in leishmaniasis is controversial and may depend on the nature of the antigen presenting cell involved [22, 23]. Therapeutic approaches targeting B cells have been shown to be effective for the treatment of autoimmune diseases [24, 25] through not only the reduction of autoantibody levels but also the modulation of T cell responses. Consequently, the mechanisms by which B cells potentially contribute to disease in VL are of interest and may represent targets for intervention.
In the intradermal murine VL infection model, it has been noted that parasite levels continuously increase with time in the DLN as well as the spleen, while clearing in the liver and skin [26]. Therefore, the lymph node, as well as the spleen, is a site for parasite persistence. In this study, we have focused on the events in the lymph node. Early in infection in the DLN there was a dramatic rise of the B cell population, which persists through chronic infection. Interestingly, the antibody response was polyclonal (specific and non-specific). However, neither B-cell-derived IL-10 nor antigen-presentation was found to be relevant to B-cell mediated disease exacerbation. Secretory IgM as well as IgG (specific and non-specific) were found to contribute to disease susceptibility and appear to act in part through the activation of complement and generation of C5a. These findings extend earlier murine VL studies [7] and indicate that a polyclonal B cell response is an early and intrinsic feature of VL, which helps to establish and maintain infection in the mammalian host.
RESULTS
Histology of infected draining lymph nodes
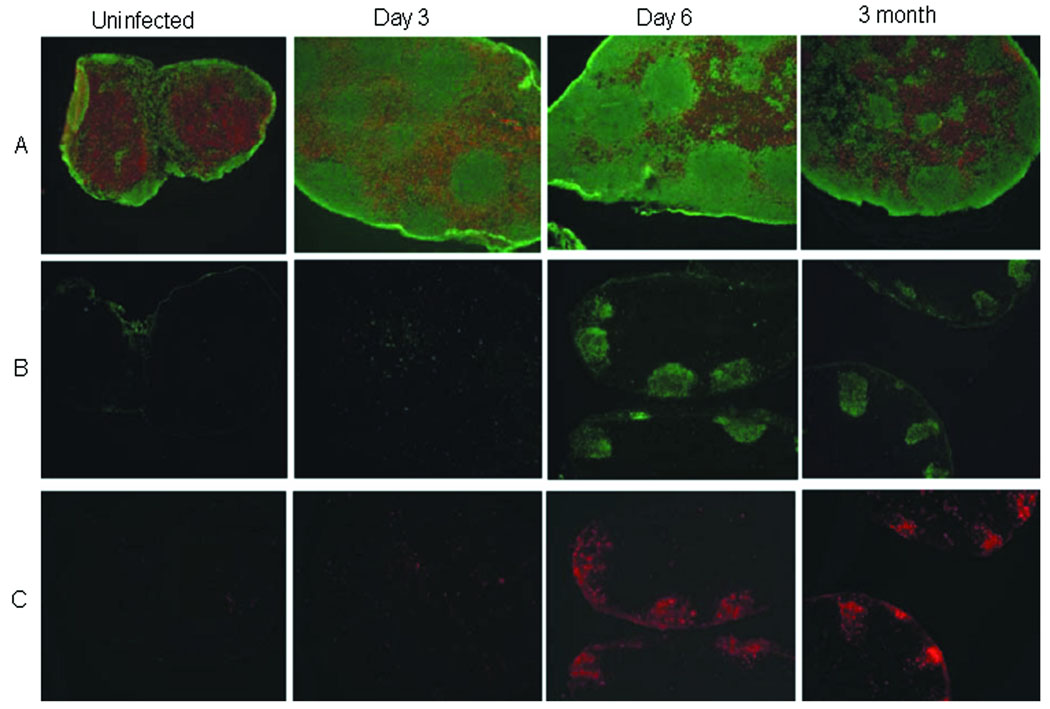
Immunofluorescence staining examining the T and B cell areas/distribution within the DLN of BALB/c mice was utilized to gain an understanding of cellular architecture in response to L. infantum infection. As shown in Figure 1, the DLN become enlarged and a loss of normal architecture is evident as early as 3 days post-infection. Instead of the discrete B and T cell zones observed in normal LN, B cells are found in both T and B cell areas from day 3 post-infection through the chronic phase of the disease (3 months post-infection). As is expected in an ongoing immune response, germinal center formation and the appearance of follicular dendritic cells in the B cell follicle regions are observed by day 6 and maintained throughout infection. Overall, these observations differ from what has been reported in the spleen [2, 6]; however these results support a role for the lymph node in B cell activation and responses during visceral leishmaniasis and potentially the hypergammaglobulinemia that is characteristic of this disease.
Figure 1. Histology of infected lymph nodes.
Immunohistochemical analyses of sections from the DLN of uninfected mice and L. infantum-infected mice (days 3 and 6, and at 3 months post-infection). DLN were stained with (A) CD3 (red; T cells) and B220 (green; B cells), (B) peanut agglutinin (germinal centers), or (C) FDC-M1 (follicular dendritic cells). There is an early and persistent B-cell expansion and apparent loss of T cells in the DLN. Germinal centers (GC) and follicular dendritic cells (FDC) are evident at day 6 and persist, through the chronic stage of the disease (3 months). Data are representative of at least 3 experiments.
Given the increase in B cells observed in the DLN, it was of interest to determine whether this was the result of increased proliferation. At various times post-infection with L. infantum promastigotes, wild type BALB/c mice were evaluated for cellular proliferation in the DLN using BrdU incorporation (Figure 2). Both T and B cell populations initially expand in response to infection. However, B cells continue to proliferate, whereas the level of T cell proliferation was reduced to levels found in naïve mice by 30 days post-infection. The reduction in T cell responsiveness in the DLN is similar to recent observations in the spleen, where the expression of B7-H1 was related to the decreased T cell responsiveness [27]; however, the mechanisms involved in the DLN remain to be determined. The overall increase in B cells (14-fold at 30 days post-infection) is much more prominent than what is observed for the T cell populations, which display a more modest 3.5-fold increase. Overall, these increases in lymphocyte populations (Table 1) result in a change in the B to T cell ratio in the DLN, from 0.29 in the uninfected LN to 0.86 in infected LN by 14 days post-infection. FACS analyses indicated that the B cell populations were activated, expressing the early activation marker CD69+. Although CD69 expression decreased in later stages of infection, it remained above background. Thus constant local stimulation and expansion of B cells occurs, and represents a continued source of new B cells in the DLN. However, this does not exclude the possibility that selective B cell recruitment and retention may also contribute to the increased B cell numbers within the DLN.
Figure 2. Lymph node responses to L. infantum infection.
Lymph node T and B lymphocyte responses of BALB/c mice intradermally infected with L.infantum. (A) Cellular proliferation was evaluated using BrdU incorporation. BrdU was injected intraperitoneally 4 hours prior to obtaining cells at the indicated times for FACS analysis. Results are shown as the % of BrdU+ T cells (CD3+) and % of BrdU+ B cells (CD19+) cells over time. B) Increases in cell numbers present in the DLN (total lymph node cells, B cells (CD19+), T cells (CD3+)). Data show mean ± SEM (n= 3–4/time point) and are representative of at least 2 experiments.
Table 1.
B cell activation and expansion in the draining lymph node as a response to infectiona)
| Days Post- Infection |
B cell /T Cell Ratio |
%CD69+ Bcells |
|---|---|---|
| Uninfected | 0.29 | 16.3±2.1 |
| 2 | 0.62 | 79.5.±8.7** |
| 5 | 0.76 | 50.1±6.6** |
| 14 | 0.86 | 24.9±3.8* |
| 37 | 1.02 | 26.2±7.5+ |
The ratio of B cells/T cells found in the DLN at various times post-infection with L. infantum. An early B cell activation (CD69+) is evident, which decreases but remains above background. The increase in B cell CD69 expression precedes increasing BrdU incorporation (Figure 2); the kinetics is consistent with B-cell activation followed by proliferation. Data are representative of 2 experiments and show mean ± SE (n=3) per time point.
p<0.001,
p=0.03,
p=0.09;
Student’s t-test.
The expansion and maintenance of B cells throughout the DLN (T and B cell areas), in addition to the increasing parasitemia, allude to aberrant B cell function and a potential B cell contribution to parasite persistence. Consequently, we examined the nature of this B cell response and mechanisms by which these cells might modulate L. infantum infection.
B cell activation leads to polyclonal antibody production
Hypergammaglobulinemia and non-specific antibody responses are hallmarks of visceral leishmaniasis normally noted in the chronic stage of the disease [8, 12–14, 28]. Given the level of B cell activation observed early after intradermal infection, we were interested in the nature and specificity of this B cell response. To determine this, BALB/c mice were infected with L. infantum and at various times post-infection serum was recovered. Serum antibody levels (IgG and IgM-total) and specificity were assessed by ELISA (Figure 3). These results indicate, as expected, an increase in antibody production against Leishmania antigen (SLA), which is detectable at 7 days after infection and increases with time post-infection. In addition, significant antibody responses were also observed to non-leishmanial antigens (TNP, ovalbumin (OVA), chromatin).
Figure 3. Early serum antibody responses in BALB/c mice infected with L. infantum are polyclonal.
Sera were collected from mice at days 7, and 14 post-infection and assayed for antibody levels (total Ig (IgG and IgM)) to leishmanial (SLA) and non-leishmanial antigens ((TNP)15-BSA, ovalbumin, chromatin). Statistical significance is indicated and was determined using Student’s t-test in comparison to uninfected controls for each antigen. Data show mean ± SEM (n=5 per group) and are representative of 2 experiments.
To further evaluate the polyclonal responses, ELISPOT analyses for antigen-specific antibody-producing cells (Total Ig: IgG + IgM) were performed (Table 2). These data confirmed serological analyses and indicated the fold-increases in the overall antibody responses (leishmanial and non-specific). The polyclonal response, measured by ELISPOT, in the DLN was observed earlier (day 7) than in the spleen (day 14). Overall, the anti-SLA response and the polyclonal antibody responses (OVA and chromatin) appear later in the spleen than the DLN and appears to reflect the relatively lower level of parasites present [26]. Interestingly, the increase of B cells producing anti-ovalbumin and/or anti-TNP-BSA was comparable to or greater than that found for B cells producing anti-Leishmania antibodies. These data suggest that overall the polyclonal response (non-specific) was comparable to the anti-parasite response. Therefore, polyclonal B cell activation is an early, intrinsic and significant response to infection.
Table 2.
Early antibody responses in BALB/c mice infected with L. infantum are polyclonala)
| Antigen | ELISPOT Lymph Node (Fold Increase) |
ELISPOT Spleen (Fold Increase) |
|
|---|---|---|---|
| Day7 Post-infection |
OVA | 4.3±0.3* | 1.0 |
| TNP(15)-BSA | 27.0±1.6*** | 1.0 | |
| SLA | 4.1±0.6** | 1.0 | |
| Day 14 Post-infection |
OVA | 13.1±0.6*** | 8.1±0.2*** |
| TNP(15)-BSA | 9.2±0.8** | 4.7±0.21*** | |
| SLA | 7.6±0.3*** | 3.48±0.5* | |
| Chromatin | 4.2±0.04*** | 4.1±0.2*** |
ELISPOT analyses of antigen-specific antibody producing cells were determined at days 7, and 14 post-infection. The number of cells secreting antibodies to soluble Leishmania antigen (SLA) as well as non-specific antigens (ovalbumin, (TNP)15-BSA, chromatin) were quantified. Data represent the fold-increase in the number of antibody-producing cells over background levels (determined using cells from uninfected mice). Data are representative of 2 experiments and show mean ± SE (n=5 mice per group). Background levels of antibody producing cells/106 total cells were: in the spleen: TNP, 1728±72; OVA, 372±8 ; SLA, 1860±252.; chromatin, 222±10; and in the draining lymph node: TNP, 616±40; OVA, 238±38; SLA, 556±28; and chromatin, 44±4.
p≤0.001,
p=0.002,
0.002≤p≤0.05;
Student’s t-test.
B cells do not contribute to disease susceptibility through IL-10 production or antigen presentation
Given the persistent and polyclonal activation of B cells in response to intradermal infection, the impact of B cells on infection was further examined. Initially, B cell deficient BALB/c JHD and WT BALB/c mice were infected with L. infantum; parasite burdens were determined (DLN, spleens, livers) at one and two months post-infection. These experiments (data not shown) revealed that BALB/c JHD mice had reduced parasite numbers at all tissue sites. Overall these results are similar to those reported for intravenous infection of C57BL/6-µMT mice [7]; however, the mechanisms by which B cells contribute to increased parasitemia were not established. Consequently, the effects of B cell antigen presentation, IL-10 production, and secretory immunoglobulin (IgG and IgM) on infection were examined.
One of the mechanisms by which B cells potentially contribute to pathogenesis is through production of immunomodulatory cytokines, such as IL-10. IL-10 has been implicated in disease progression and severity in human patients and in mice [29–31]. Previous studies, using intravenous infection, indicate that mice deficient in IL-10 are able to resolve infection [32]. Further, flow cytometric analyses of intradermally infected mice indicated that in the early DLN response to infection (day 5) B cells produce IL-10 (Figure 4A), suggesting that early IL-10 production and in particular B-cell-derived IL-10 could be important for infection and parasite survival. Initially the role of IL-10 in the course of intradermal infection in the lymph node and other tissues was examined using BALB/c mice deficient in IL-10. As seen in Table 3, IL-10 deficient mice have reduced parasite levels; a reduction in parasite levels in the DLN is observed as early as day 3 post-infection. Notably, the effect of IL-10 upon infection was observed at all tissue sites and appears to increase with time post-infection. Therefore, IL-10 appears to contribute to both the early and later phases of disease.
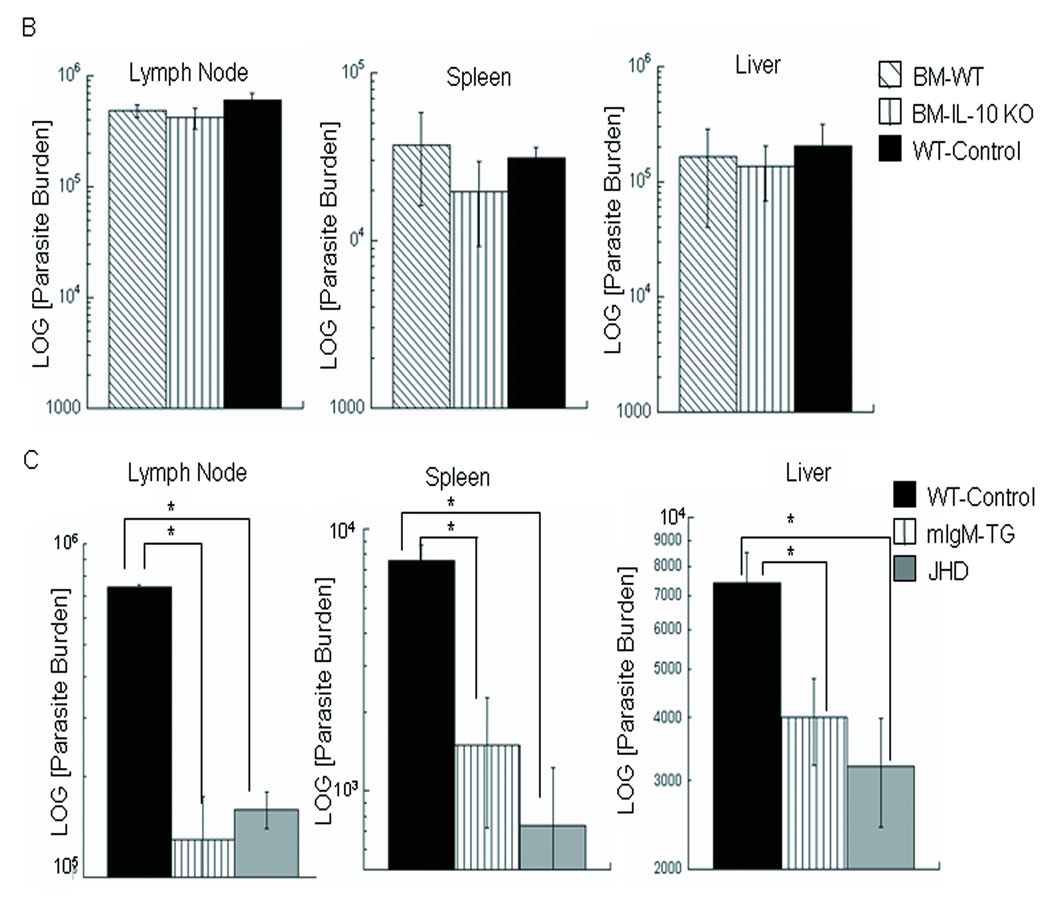
Figure 4. B-cell-derived IL-10 is insufficient to restore susceptibility.
A) FACS analyses of the DLN cells from WT BALB/c mice (CD3+ T cells or CD19+ B cells) at 5 days post-infection with L. infantum. B cells and T cells contribute to IL-10 production early in infection. B) Bone marrow chimeric JHD mice were employed to evaluate the effect of B-cell-derived IL-10 on disease progression. Shown are the parasite burdens 1 month after infection with L. infantum (DLN, spleen, liver) of JHD mice reconstituted with B cells not producing IL-10 (20% IL-10 deficient + 80% JHD bone marrow cells) or with IL-10-producing B cells (20% WT + 80% JHD bone marrow cells). WT BALB/c mice were employed as controls. Data show mean ± SE (n=5 mice per group). No statistical differences were found between various groups of mice (p>0.2). C) Parasite burdens (LN, spleen, liver) and immune responses (Table 4) of BALB/c wild-type, JHD and mice transgenically altered to have functional B cells but no circulating antibody (mIgM/JHD transgenic mice) were compared at one month post-infection. Data are representative of 3 experiments and show mean ± SE (n=3 mice per group). *p=0.01, Student’s t-test.
Table 3.
IL-10 deficiency leads to lower parasite numbers and disease ameliorationa)
| DLN | Spleen | Liver | ||
|---|---|---|---|---|
|
3 Days Post-infection |
BALB/c-WT | 1.4±0.3 ×104 | 1.0±0.9×101 | 2.6±2.0×102 |
| IL-10−/− BALB/c |
5.7±0.2 × 103* | 1.0± 0.9×101 | 1.2±0.4×102 | |
|
15 Days Post-infection |
BALB/c-WT | 6.9±0.6×104 | 6.2±0.2×104 | 3.1±0.9×105 |
| IL-10−/− BALB/c |
3.4±0.01×104*** | 4.6±1.1×103**** | 1.1±0.2×104** | |
|
1 Month Post-infection |
BALB/c-WT | 2.7±0.1×105 | 7.3±0.2 ×104 | 8.8±0.6×104 |
| IL-10−/− BALB/c |
2.6±0.01×104**** | 4.5±0.2 ×103**** | 3.2+0.5×103*** |
The effects of IL-10 upon the course of infection in the LN, spleen and liver of BALB/c mice intradermally infected with L. infantum. BALB/c IL-10-deficient and WT mice were infected with L. infantum and parasite burdens determined. Data are representative of three experiments and show mean ± SE (n=3–4 mice/group).
p= 0.01,
p=0.005,
p=0.0001,
p≤10−6;
Student’s t-test. The parasite levels at day 3 in the liver and spleen were not statistically different between the BALB/c WT and IL-10-deficient mice.
However, in order to ascertain the direct consequence of B-cell-derived IL-10 on disease, bone marrow chimeras were employed. Chimeric mice were produced using BALB/c JHD, in which the B cells were derived from either IL-10 deficient or wild type BALB/c mice. The JHD bone marrow recipients, along with BALB/c wild-type mice, were then intradermally infected with L. infantum. Given the more effective control observed in IL-10 deficient mice at one month post-infection, parasite levels were determined (DLN, spleens, livers) at 1 month post-infection (Figure 4B). JHD mice receiving B cells from either wild type or IL-10 deficient mice have parasite burdens that are comparable to wild-type mice. Overall, these results indicate that although IL-10 can promote parasite survival, B-cell-derived IL-10 is not essential for increased disease susceptibility observed in wild type versus B cell deficient mice.
Consequently, we examined whether B cell antigen presentation might be critical to the development of disease. Although controversial, it was possible that B cell antigen presentation could drive a Th2-like response (IL-10 and IL-4) [17, 33, 34] and thereby potentially could skew ongoing T cell responses to prevent healing. To examine this possibility, BALB/c wild type, JHD, and mIgM/JHD mice genetically altered to contain functional B cells (surface IgM but producing no circulating antibody)[35, 36], were infected with L. infantum. Parasite burdens (DLN, spleens, livers) at one month post-infection (Figure 4C) clearly indicate that mIgM/JHD mice were comparable to JHD mice in their resistance to infection. Therefore, B cell antigen presentation fails to reconstitute mice to WT susceptibility.
Interestingly, the mIgM/JHD mice were comparably resistant to infection as the JHD mice, and yet had IL-10 levels higher than the more susceptible WT mice (Table 4). Further, the overall IL-10/IFN-γ ratio did not appear to correlate with the disease susceptibility observed. Therefore, evidence from the mIgM/JHD mice confirms the bone marrow chimera results indicating that B-cell- derived IL-10 is not critical for disease exacerbation.
Table 4.
Cytokine responses in the draining lymph node of B cell deficient and transgenetically reconstituted BALB/c micea)
| Time post- Infection |
Mouse | IL-10 [pg/ml] | IFNγ [pg/ml] | Ratio: IL-10/IFNγ |
|---|---|---|---|---|
| 6 Days | BALB/c [WT] |
187±5*** | 683±41*** | 0.27 |
| mIgM-JHD- BALB/c |
505 ± 17***, ‡‡‡ | 1672 ± 87***,‡ | 0.30 | |
| JHD-BALB/c | 0.0 ±4.0 | 183 ± 23 | 0.00 | |
| 16 Days | BALB/c [WT] |
30± 10*** | 1842 ± 88*** | 0.016 |
| mIgM-JHD- BALB/c |
381 ±50***,‡ | 6132 ± 221***,‡‡‡ | 0.060 | |
| JHD-BALB/c | 0 ±1.7 | 79 ± 16 | 0.00 | |
| 2 Months | BALB/c [WT] |
10 ±18ns | 339 ± 32** | 0.029 |
| mIgM-JHD- BALB/c |
5 ± 8**, ‡ | 78 ± 16*, ns | 0.064 | |
| JHD-BALB/c | 56 ± 10 | 268 ±85 | 0.21 |
Cells from the DLN of uninfected BALB/c and L. infantum-infected mice at 6, 16 days and 2 months post-infection were isolated and stimulated with SLA. Levels of IL-10 and IFN-γ were determined. No correlation susceptibility to infection with IL-10 or IFN-γ levels or IL-10/IFN-γ is evident. Data show mean ± SE (n=3 mice/group) for each time post-infection. Shown are the p values for comparisons of JhD (to either WT BALB/c or IgM-Tg):
p≤0.02,
p≤0.002,
p≤0.0002;
comparisons of WT BALB/c to IgM-Tg:
p≤0.02,
p≤0.002,
p≤0.0002,
ns= not significant; Student’s t-test.
Secretory immunoglobulin IgM causes disease exacerbation
Having determined that mice reconstituted for B cells (antigen presentation, IL-10 production) but lacking circulating antibody exhibit decreased susceptibility to infection with L. infantum, the effect of secretory immunoglobulin was examined. Initially, BALB/c (wild-type) and transgenic mice whose B cells can only express surface and secretory IgM antibody ({m+s}IgM/JHD) [36, 37], were infected with L. infantum. As shown in Figure 5 and in contrast to what was observed for mice expressing only receptor/membrane IgM (mIgM/JHD mice), the {m+s}IgM/JHD) mice exhibited exacerbated infection. Disease exacerbation was evident as early as 2 days post-infection and persisted throughout infection. Parasite levels in the DLN, spleens, and livers of {m+s}IgM/JHD mice were 5- to 25-fold higher than those observed for wild-type mice.
Figure 5. Secretory IgM causes disease exacerbation.
Parasite burdens in mice transgenically altered to produce membrane and secretory IgM ({m+s}IgM/JHD mice) were compared to that of wild type mice by limiting dilution analysis at A) 2 days and B) 1 month after infection with Leishmania infantum. Parasite burdens are shown for the DLN, spleen and liver, tissue sites. Data are representative of at least 2 experiments and are similar to what was found at other times post-infection (days 6, 10, 14, and 2 months). (Minimum of n=3 mice per group). *0.04≥p≥0.02; **0.002≥p≥0.001; Student’s t-test. C) In response to infection, B-cell activation and IgM production in {m+s}IgM/JHD mice is elevated in comparison to WT mice. Data show summary of FACS analyses examining IgM+ (intracellular and extra-cellular), CD69+, CD19+ cells at five days post-infection. Data are representative of 2 experiments and show mean + SE (n=3).
Although B cell activation in response to infection is evident in WT BALB/c mice (Figure 1; Table 1) at early times post-infection, the level of IgM produced in the {m+s}IgM/JHD mice was significantly higher (Figure 5C) than that in WT mice. Notably, IgM antibodies are commonly observed during active chronic VL [8, 9, 12, 38]. Taken together, these results suggest that secretory IgM can potentially contribute to the establishment and maintenance of infection in visceral leishmaniasis. This hypothesis was further examined through antibody transfer experiments.
Reconstitution of JHD mice with immunoglobulin (IgG or IgM) restores wild type susceptibility to infection
To further examine the role of immunoglobulins in disease exacerbation, JHD mice were passively reconstituted with serum from chronically infected wild type or {m+s}IgM/JHD mice or mice immunized with amastigote membrane preparations or normal mouse serum (controls). The transfer of immune serum to the JHD mice was found to restore susceptibility (parasitemia) to wild type levels. Passive transfer of normal mouse serum into JHD mice, however, failed to modulate disease resistance. Consequently, antibodies present during infection contributed to disease susceptibility in VL. These results (data not shown) were similar to those previously observed for cutaneous leishmaniasis (caused by L. major, L. mexicana and L. amazonensis) [18–22] where antibody contributes to disease progression. However, these results differ from those previously reported for murine VL [7] and also studies where the opsonized L. major organisms were found to stimulate dendritic cell function (IL-12 and antigen presentation) leading to parasite containment [23]. Therefore, to further validate that disease exacerbation is a direct result of circulating immunoglobulins (IgM and/or IgG) in murine VL, and to examine whether non-specific as well as specific antibodies are capable of promoting disease, passive antibody transfer experiments were performed.
Purified immunoglobulins (from either OVA immunized BALB/c mice (IgG), or naïve or chronically infected wild type (IgG) or {m+s}IgM/JHD BALB/c mice (IgM)) were employed to reconstitute JHD mice. The transfer of purified antibody from naive (normal) mouse serum (IgG from wild type or IgM from {m+s}IgM/JHD), respectively) did not lead to increases in parasite levels (Figure 6). In contrast, the transfer of purified immunoglobulins from chronically infected wild type (IgG) or {m+s}IgM/JHD mice (IgM) led to significantly increased parasite levels in the DLN, spleen and liver (Figure 6). Further, the transfer of purified anti-OVA IgG to JHD mice, which were then given OVA antigen at the time of infection, led to increased parasite levels in the spleen and liver. These results indicate that non-leishmanial antigen-antibody complexes could also impact on disease progression and are of interest, given the early polyclonal activation observed. Although the transfer of IgG appeared somewhat more effective in enhancing parasitemia (in the LN, spleen and liver) than IgM, this may be due, in part, to the known differences in half-life of these two immunoglobulins (6–8 days versus 2 days, respectively)[39]. However, overall these results clearly indicate that antigen-antibody complexes (IgG or IgM; specific and non-specific) can contribute to overall disease pathogenesis in murine VL.
Figure 6. Reconstitution of JHD mice with IgG or IgM antibodies restores wild type susceptibility to infection.
To assess the role of antibody or antigen-antibody complexes, isolated IgG or IgM from naïve or chronically L. infantum-infected WT or ({m+s}IgM/JHD Tg mice was employed. Additionally, IgG was isolated from BALB/c mice immunized with OVA. Mice were given 600 µg of purified antibody intraperitoneally, one day pre-infection, and subsequently, every 3 days for IgM, and every 7 days for IgG. OVA antigen was injected together at the time of infection in mice receiving anti-OVA antibody to create nonspecific antigen-antibody complexes. Parasite burdens were determined 3 weeks post-infection. Data show mean ± SE (n=3–5 mice per group). *0.05≥p≥0.01, **p≤0.002 in comparison to JHD mice; Student’s t-test.
Blocking the action of C5a reduces parasitemia in BALB/c (WT) mice
Although IgG has been shown to promote Leishmania infection through ligation to macrophage FcR [18, 19, 21, 22] and subsequent production of IL-10 (upon TLR activation), the contribution of IgM to disease exacerbation was unexpected. Further, the early effect observed (2 days post-infection), lack of a correlation of parasite burdens with IL-10 levels (Table 3; data not shown) as well as the ability of non-specific immune complexes to cause disease exacerbation suggested an alternate mechanism might be involved. Given the early time course of disease exacerbation in {m+s}IgM/JHD mice and the effect of IgM as well as IgG, the role of complement was considered. Consistent with the activation of complement, as a consequence of L. infantum infection, there is a heightened cellular (macrophages, neutrophils, dendritic cells) response in the DLN. Moreover, cells from the DLN of infected mice expressed reduced levels of C5aR (CD88) (data not shown), suggesting down-regulation and activation through this receptor during infection. As C5a is known to be involved in the chemotaxis and recruitment of both monocytic, dendritic, and neutrophil cell populations [40, 41] critical to leishmaniasis [42–45], we examined the involvement of C5a receptor (CD88 signaling) on infection.
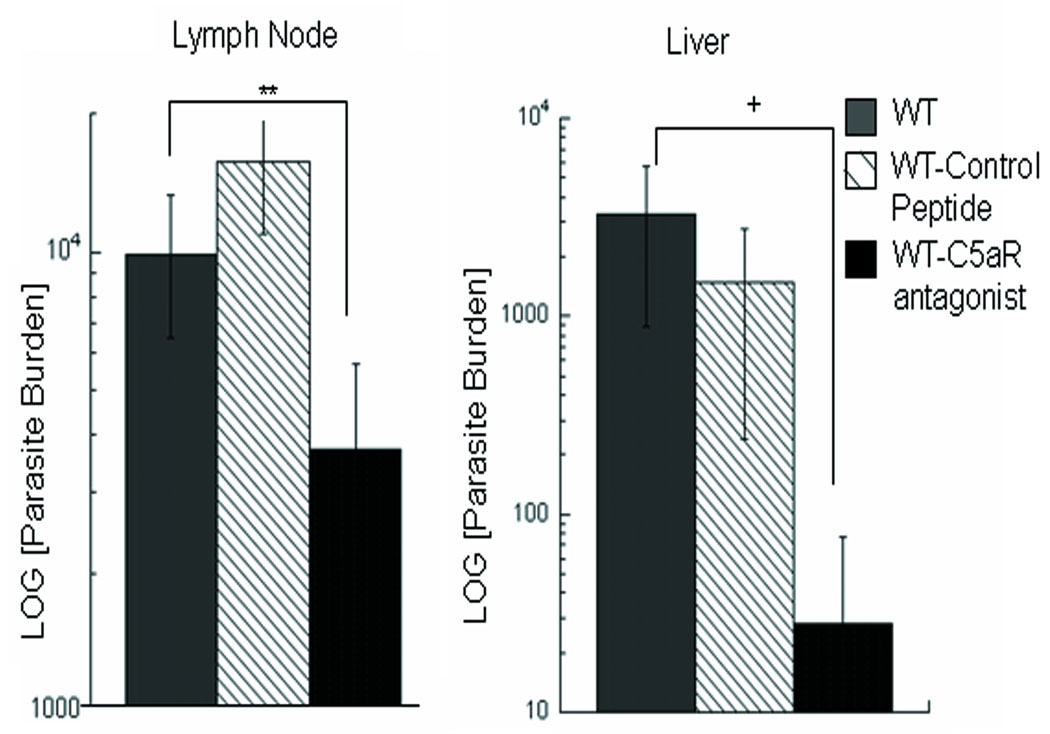
To evaluate the role of complement/C5a on infection, the effect of treatment with a C5a receptor antagonist on infection of WT BALB/c mice was examined. Mice were treated with the C5a receptor antagonist (Ac-Phe-[Orn-Pro-dCha-Trp-Arg]), and parasite burdens in these mice were evaluated and compared to those receiving a control peptide (Ac-Phe-[Orn-Pro-dCha-Ala-D-Arg]) and to control (untreated) mice. As shown in Figure 7, blocking the C5a receptor reduces the level of infection in the liver, and DLN as early as 6 days post-infection. Comparative analyses of the ongoing immune response indicated that treatment with C5aR antagonist does not lead to the preferential development of a Th1-like response. Comparable levels of IFN-γ and IL-10 were found in the treated mice. The levels of IFN-γ found in response to SLA for the control infection (PBS); control peptide and C5aR antagonist groups were respectively,: 61.5, 50.9 and 67.7ng/ml; the respective levels of IL-10 were: 1.8, 2.25 and 2.38 ng/ml. These results concerning the cytokines levels differ from reports of C5aR deficient mice [46], where heightened IFN-γ responses were found to limit cutaneous infection with L. major; however, this may reflect the short duration of treatment as well as possibly differences in the mouse genetic background.
Figure 7. C5aR Antagonist peptide treatment ameliorates disease in wild type BALB/c and {m+s}IgM/JHD Tg mice.
BALB/c mice were treated with a C5a receptor antagonist C5aRa (Ac-Phe-[Orn-Pro-dCha-Trp-Arg]) or control peptide (Ac-Phe-[Orn-Pro-dCha-Ala-D-Arg]) on days -1 and 0, pre-infection and days 2 and 4 post-infection intradermally with 5×105 L. infantum promastigotes. Parasite burdens were evaluated at day 6 post-infection in the DLN and liver. Parasite numbers were too low in the spleen at this early time point for evaluation. P values are for mice treated with C5a receptor antagonist in comparison to control mice. Data are representative of three independent experiments and show mean ± SE (n=3-5). **p=0.005, +p=0.06; Student’s t-test.
Overall, these results suggest that immunoglobulins can contribute to disease severity through the activation of complement, production of C5a, and consequent signaling via its receptor (CD88).
DISCUSSION
Although immunoglobulins can provide protection against infectious organisms, antibodies are also known to contribute to the disease pathology associated with infection (viral, bacterial, and parasitic [47–51]) as well as autoimmune diseases [52–54]. Hypergammaglobulinemia and polyclonal B cell responses are hallmarks of visceral leishmaniasis [8–14]. Elevated levels of total antibody (IgG; IgM) have been shown to correlate with disease pathology of human [8, 55, 56] and canine visceral leishmaniasis [28, 57]. Notably, visceral leishmaniasis patients display detectable antibody titers against antigens unrelated to the parasite such as haptens (DNP, FITC), protein antigens (KLH), and autoantigens (e.g. DNA, IgG, and smooth muscle), in addition to anti-leishmanial antibodies [8–14]. However, while abnormal B cell responses are evident in visceral leishmaniasis, the mechanisms by which B cells may contribute to disease in VL are not fully understood.
In the murine intradermal model for visceral leishmaniasis, a pronounced and early B cell response is observed in the DLN, which is sustained in response to infection. Although a B cell response to infection is not unusual, this B cell response, as found in human disease, was notably polyclonal in nature, with the production of immunoglobulins against non-specific as well as leishmanial antigens. The early onset of the polyclonal B cell response suggests an intrinsic response to infection and prompted an investigation into the mechanisms by which B cells might contribute to disease.
BALB/c mice lacking B cells (JHD) intradermally infected with L. infantum were found, in fact, to have significantly reduced parasitemia (all tissue sites- liver, spleen, lymph node). Potentially several B cell-mediated mechanisms (APC function, cytokine (IL-10) and/or immunoglobulin production) could lead to the increased disease severity. Although IL-10 contributes to susceptibility and parasitemia in murine VL, B cell-derived IL-10 appeared to have little, if any effect on parasite levels at the various tissue sites. Studies with bone marrow chimera mice indicated that B cells deficient in IL-10 were comparable to WT B cells in reconstituting susceptibility of JHD mice. Further BALB/c mice that were transgenetically reconstituted [35, 36] for surface IgM expression (and also antigen presentation; IL-10 production) displayed comparable levels of resistance to infection to JHD mice. Notably, similar results were obtained for mIgM transgenetic mice infected with the New World species, L. pifanoi or L. amazonensis [18, 19]. In these studies, infected mIgM transgenetic mice developed comparable cytokine responses to infected wild type mice (IL-10, IL-4, no IFN-γ), yet had significantly lower parasite burdens. However, our observations do differ from recent observations for L. major, where the APC function of B cells can contribute to disease; nonetheless, this contribution varied and was dependent upon the infecting strain [17, 22]. Consequently, the mechanisms of B cell modulation of infection may vary and be dependent upon the species/strain of Leishmania. Overall, our results indicate neither B cell antigen presentation nor B cell derived IL-10 is the primary B cell mechanism leading to increased parasitemia and disease in murine VL.
In contrast, B cell-derived immunoglobulins (IgM and IgG) were found to play a critical role in disease progression in visceral leishmaniasis. Passive antibody transfer experiments indicate that IgG, IgM, and non-specific antigen-antibody complexes restored disease susceptibility to JHD mice. These observations differ with previous studies of murine visceral leishmaniasis using a high dose intravenous infection model of VL and mice that lack mature B cells (µMT; C57BL/6), where the transfer of serum from chronically infected WT mice failed to restore susceptibility to infection for the µMT mice [7]. It is unclear whether the differences in Leishmania species (L. donovani versus L. infantum), infection model and/or the use of serum versus purified antibodies contribute to these different observations.
Interestingly, disease exacerbation (in comparison to WT BALB/c mice) was evident for ({m+s}IgM/JHD) BALB/c mice, which are genetically reconstituted to secrete only IgM, indicating for the first time that IgM can facilitate parasite survival. Further, IgM antibody from infected {m+s}IgM/JHD BALB/c mice transferred into JHD BALB/c mice exacerbated infection. Importantly, disease exacerbation was evident as early as 2 days post-infection, and sustained throughout infection. The early effect of IgM as well as the observation that the cytokine responses of {m+s}IgM/JHD mice appeared to be comparable to WT mice, suggested that alternate mechanisms contributed to the observed enhanced susceptibility to infection.
Immunoglobulins may act through the activation of complement. Although complement activation can lead to the lysis of Leishmania parasites, it is well established that infective metacyclic promastigotes as well as intracellular amastigotes are resistant to complement-mediated lysis [58]. Further leishmanial promastigotes subvert the complement system by utilizing CR3 for macrophage entry and to down-regulate host oxidative burst and IL-12 responses, essential for parasite containment [59, 60]. The results herein for IgM differ from vaccine studies [61] where IgM from B-1 B cells was found to enhance protection induced to repetitive antigen HASPB1. However, the situation in naïve animals may differ from vaccinated mice. A selective expansion of B-1 B cells in the DLN during infection was not observed in the DLN of infected mice [62]; consequently, the level of antibody from B-1 B cells may be obscured in the context of the ongoing response to infection.
In addition to cell lysis, the activation of complement results in the generation of cellular mediators (C3a, C5a) that can lead to cellular recruitment and the modulation of the host immune response [40, 63]. Notably as a consequence of L. infantum infection, there is a heightened cellular response in the lymph node; in addition to the increases in B cells, increases are observed in the numbers of macrophages, neutrophils, and dendritic cells [62]. Further, a reduced level of expression of CD88 on lymph node cells of L. infantum infected mice was observed, suggesting an ongoing generation, binding and uptake of C5a during infection. Significantly, treatment with a C5a receptor antagonist causes a reduction in parasitemia in wild type BALB/c mice in the lymph node, and liver. These effects were noted early in infection (day 6). Notably, treatment with the C5aR antagonist peptide did not appear to lead to changes in the overall cytokine responses (IL-10; IFN-γ). These results are consistent with the response to infection of IgM transgenetically reconstituted mice, where cytokine responses do not correlate with disease. However, the effect of C5aR antagonist treatment on the mouse immune response appears to differ from those reported for C5aR−/− C57BL/6 mice infected with L. major. These mice have been shown to up-regulate IL-12 expression, promoting a heightened Th1 response and parasite control [46]. Nonetheless, the lack of an effect on the ongoing immune response by the C5aR antagonist peptide in comparison to the immunodeficient mice may reflect pharmakinetics and/or short duration treatment with the C5aR antagonist peptide. Although the mechanisms responsible for disease modulation by the C5aR antagonist peptide remain to be determined, it is possible that changes in cellular recruitment may contribute. Additionally, C5a is known to up-regulate CR3 expression on macrophages [64]; thus, increased infection of host macrophages and parasite survival [59] through C5a modulation of CR3 could contribute to disease.
Overall, observations suggest that B cell activation and the production of IgM as well as IgG contribute to disease pathogenesis in murine visceral leishmaniasis. These findings in light of the polyclonal B cell responses observed for human VL are noteworthy, and reveal that the apparently early and continuous B cell expansion/activation is not only an intrinsic feature of the initial parasite-host interaction but is important for overall disease severity. The fact that there is a lack of a requirement for antibody specificity (parasite) in pathogenesis, would suggest that antibody-antigen complexes, in general, are detrimental to the host. IgM responses are known to persist throughout the course of human visceral leishmaniasis [8, 55]. Therefore, B cell derived immunoglobulins (IgM and IgG), immune complexes and consequent complement activation, with the generation of C5a, potentially may contribute to disease and parasite persistence throughout infection. Overall, these results indicate a new mechanism of disease pathogenesis in visceral leishmaniasis and suggest that intervention in the action of C5a could potentially be considered for immunotherapeutic approach to alleviate disease.
MATERIALS AND METHODS
Mice
BALB/c mice were purchased from the NCI or from Taconic Farms Inc. Breeding pairs of BALB/c JHD mice, VH186.2-{m+s}IgM/JHD/JHD transgenic mice ({m+s}IgM/JHD Tg; produce membrane and soluble IgM), and VH186.2-mIgM/JHD/JHD transgenic mice (mIgM/JHD Tg; functional B cells with surface IgM) [35, 36, 65] were kindly provided by Dr. Shlomchik (Yale University). Breeding pairs of IL-10 deficient BALB/c mice were originally provided by Dr. Coffman (DNAX) through Drs. Kullberg, and Sher (NIH). All experiments using mice were reviewed by the Yale University Animal Care and Use Committee. All procedures were conducted in accordance with U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training. Yale University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Parasites, Infection and parasite burden analyses
Mice were infected with isolated late stationary-growth-phase promastigotes (9–12 days of culture) of L. infantum (MHOM/ES/92/LLM-320; isoenzyme typed MON-1), expressing the P-8 PGLC (an amastigote associated molecule) as previously described [26]. Mice were infected intradermally either in ear pinnae or rear hind feet, using 5–10 ×106 organisms unless otherwise indicated. Parasite burden analyses for the lymph node, spleen, and liver were performed as previously described using 3–5 mice/group [26]; under these conditions, the cloning efficiency for this strain was ≥90%.
Immunofluorescence staining of lymph nodes and spleens
Organs were frozen in O.C.T. compound; sections (7µm) were fixed in acetone, blocked with TNB buffer (3% Casein in PBS; NEN Life Science Products) containing 5% normal donkey serum and then treated with Avidin-Biotin block (Vector Laboratories) and endogenous peroxidase activity was quenched. Using antibodies from BD Biosciences, slides were stained with rat IgG anti-mouse CD45R/B220, hamster IgG1k anti- mouse CD3ε, or rat anti-mouse FDC (Clone FDC-M1), followed by biotin-conjugated donkey anti-rat IgG or biotin-conjugated anti-hamster antibody. Germinal centers (GC) were stained with biotin-labeled peanut lectin followed by streptavidin-HRP conjugate. The antigens were detected using tyramide-FITC or tyramide-tetramethylrhodamine (NEN Life Science Products). Slides were mounted with Fluoromount-G (Southern Biotechnology Associates, Inc.) and analyzed by fluorescence microscopy (Leitz Orthoplan 2).
Fluorescence-activated cell sorter (FACS) analyses
DLN were excised and single cell suspensions were prepared and cells were stained for CD3, CD19, or CD69; all antibodies were from BD Biosciences. To determine lymphocyte populations expanding in response to infection, mice were injected with 2 mg BrdU intraperitoneally. Four hours later, DLN were excised and scells were stained with cell surface markers (CD3 or CD19). After washing, cells were fixed and permeabilized with BD Cytofix/Cytoperm Buffers (BD Biosciences). Cells were treated with DNase to expose incorporated BrdU, and stained with FITC-conjugated anti-BrdU. For intracellular detection of IgM, cells were fixed with 2% paraformaldehyde after surface staining, permeabilized with 0.05% saponin and stained with anti-IgM (eBiosciences). For intracellular cytokine staining of IL-10, cells were stimulated with soluble leishmania antigen (SLA) at 25 ug/ml in RPMI medium (supplemented with 10% FBS, 2-mercaptoethanol, glutamine and antibiotics) for 72 hours followed by 6 hours of culture with PMA, ionomycin, and monensin and stained using anti-IL-10 or an isotype control (PE-labeled; eBiosciences). Data were acquired by using FACSCalibur and were analyzed by using FlowJo software (Tree Star, Inc.).
Determination of antibody levels and antibody-producing cells by ELISA and ELISPOT
For ELISA and ELISPOT analyses, plates were initially coated with 10 µg of either TNP15-BSA, chicken egg albumin (OVA; Sigma Chemical Co.), chromatin, or soluble Leishmania antigen in PBS overnight at 4°C. For ELISAs, after overnight antigen incubation, plates were blocked (3% BSA in PBS). Serial dilutions of serum from uninfected and infected mice incubated overnight at 4°C, followed by incubations with biotinylated anti-mouse Ig antibody (BD Biosciences). Plates were then incubated with streptavidin-HRP, followed by development using TMB substrate. Plates were analyzed at 450 nm. Statistical significance was measured using a T-test for infected mice compared to uninfected controls.
For ELISPOT analysis, after overnight antigen incubation (as indicated above), plates were blocked (3% BSA in PBS). Single cell suspensions of lymph node or splenic cells (serial dilutions) were then added to wells and incubated at 37°C. Subsequently, plates were incubated with biotinylated anti-mouse Ig, followed by phosphatase-labeled streptavidin and developed using. SigmaFast BCIP/NBT as substrate. Plates were examined microscopically. Statistical significance was measured (T-test) for each antigen in comparison to uninfected controls
Passive antibody transfer experiments
To assess the role of antigen-antibody complexes, isolated IgG or IgM from naïve or chronically L. infantum-infected WT or ({m+s}IgM/JHD Tg mice or IgG from OVA-immunized BALB/c mice were used. Antibody was isolated by ammonium sulfate precipitation followed (in the case of IgG) by protein G column purification. All antibody preparations were then fractionated over polymyxin B columns. All preparations were endotoxin-free, as assessed using a Limulus amebocyte lysate assay (Cambrex). SDS-PAGE was used to assess antibody purity. Mice were injected intraperitoneally with 600 µg of purified antibody, one day pre-infection, and subsequently, every 5 days for IgM, and every 7 days for IgG. Ovalbumin (50 µg) was injected at the time of infection in mice receiving anti-OVA antibody to create OVA-antibody complexes.
Bone marrow chimeras
BALB/c JHD mice were reconstituted as previously described after irradiation with 350 rads [66], using a mixture of either 20% WT/80% JHD bone marrow cells, or 20% IL-10 deficient bone marrow/80% JHD bone marrow cells (9×106 cells/mouse). Reconstitution of B cells was confirmed by FACS.
C5aR antagonist treatment
To examine the role of complement and specifically C5a on infection, mice were treated with either C5a receptor antagonist (C5aRa; Ac-Phe-[Orn-Pro-dCha-Trp-Arg]) or control peptide (Ac-Phe-[Orn-Pro-dCha-Ala-D-Arg]), according to described methods [67]. Additionally a group of untreated mice were used as infection controls. Briefly, mice were injected intraperitoneally with 1 mg/kg body weight of peptide suspended in PBS at days -1 and 0 pre-infection, and subsequently, every other day post-infection [67, 68]. Mice were infected using 5×105 L. infantum parasites.
Statistics
A Student’s t-test was used to determine statistical significance in all experiments.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Sholmchik for providing breeding pairs of BALB/c JHD, VH186.2-{m+s}IgM/JHD/JHD and VH186.2-mIgM/JHD/JHD transgenic mice, and Drs. Craft, Ruddle and Mamula for helpful discussions. This work was supported by NIH grants AI 45044 and AI27811 to (DMcP) and GM-62134 and AI-068730 (to J.L.) and AI-43603 (MJS); ED was supported in part on an NIH Training Grant, T32 AI07404 and a grant from Fort Dodge Animal Health.
Abbreviations
- L
Leishmania
- CL, VL
cutaneous and visceral leishmaniasis
- DLN
DLN
- IC
immune complex
- GC
germinal center
- FDC
follicular dendritic cell
- OVA
ovalbumin
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- 2.Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 3.Mueller SN, Ahmed R. Lymphoid stroma in the initiation and control of immune responses. Immunol Rev. 2008;224:284–294. doi: 10.1111/j.1600-065X.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 4.Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veress B, Omer A, Satir AA, El Hassan AM. Morphology of the spleen and lymph nodes in fatal visceral leishmaniasis. Immunology. 1977;33:605–610. [PMC free article] [PubMed] [Google Scholar]
- 6.Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–3821. [PubMed] [Google Scholar]
- 7.Smelt SC, Cotterell SE, Engwerda CR, Kaye PM. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 8.Ghose AC, Haldar JP, Pal SC, Mishra BP, Mishra KK. Serological investigations on Indian kala-azar. Clin Exp Immunol. 1980;40:318–326. [PMC free article] [PubMed] [Google Scholar]
- 9.Casato M, de Rosa FG, Pucillo LP, Ilardi I, di Vico B, Zorzin LR, Sorgi ML, Fiaschetti P, Coviello R, Lagana B, Fiorilli M. Mixed cryoglobulinemia secondary to visceral leishmaniasis. Arthritis Rheum. 1999;42:2007–2011. doi: 10.1002/1529-0131(199909)42:9<2007::AID-ANR30>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Pontes De Carvalho LC, Badaro R, Carvalho EM, Lannes-Vieira J, Vinhaes L, Orge G, Marsochi MC, Galvao-Castro B. Nature and incidence of erythrocyte-bound IgG and some aspects of the physiopathogenesis of anaemia in American visceral leishmaniasis. Clin Exp Immunol. 1986;64:495–502. [PMC free article] [PubMed] [Google Scholar]
- 11.Louzir H, Belal-Kacemi L, Sassi A, Laouini D, Ben Ismail R, Dellagi K. Natural autoantibodies, IgG antibodies to tetanus toxoid and CD5+ B cells in patients with Mediterranean visceral leishmaniasis. The Leishmania Study Group. Clin Exp Immunol. 1994;95:479–484. doi: 10.1111/j.1365-2249.1994.tb07022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvao-Castro B, Sa Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin Exp Immunol. 1984;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Bohme MW, Evans DA, Miles MA, Holborow EJ. Occurrence of autoantibodies to intermediate filament proteins in human visceral leishmaniasis and their induction by experimental polyclonal B-cell activation. Immunology. 1986;59:583–588. [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnaire MH, Galambrun C, Stephan JL. Hemophagocytic syndrome: A misleading complication of visceral leishmaniasis in children--a series of 12 cases. Pediatrics. 2000;106:E58. doi: 10.1542/peds.106.4.e58. [DOI] [PubMed] [Google Scholar]
- 15.Lohoff M, Matzner C, Rollinghoff M. Polyclonal B-cell stimulation by L3T4+ T cells in experimental leishmaniasis. Infect Immun. 1988;56:2120–2124. doi: 10.1128/iai.56.8.2120-2124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessner A, Will A, Vieth M, Schroppel K, Rollinghoff M. Stimulation of B-cell lymphopoiesis by interleukin-7 leads to aggravation of murine leishmaniasis. Immunology. 1995;84:416–422. [PMC free article] [PubMed] [Google Scholar]
- 17.Ronet C, Voigt H, Himmelrich H, Doucey MA, Hauyon-La Torre Y, Revaz-Breton M, Tacchini-Cottier F, Bron C, Louis J, Launois P. Leishmania major-specific B cells are necessary for Th2 cell development and susceptibility to L. major LV39 in BALB/c mice. J Immunol. 2008;180:4825–4835. doi: 10.4049/jimmunol.180.7.4825. [DOI] [PubMed] [Google Scholar]
- 18.Colmenares M, Constant SL, Kima PE, McMahon-Pratt D. Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody. Infect Immun. 2002;70:6597–6605. doi: 10.1128/IAI.70.12.6597-6605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanasen N, Xin L, Soong L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int J Parasitol. 2008;38:417–429. doi: 10.1016/j.ijpara.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas BN, Buxbaum LU. FcgammaRIII mediates immunoglobulin G-induced interleukin-10 and is required for chronic Leishmania mexicana lesions. Infect Immun. 2008;76:623–631. doi: 10.1128/IAI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008;127:280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Blank M, Shoenfeld Y. B cell targeted therapy in autoimmunity. J Autoimmun. 2007;28:62–68. doi: 10.1016/j.jaut.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect Immun. 2003;71:401–410. doi: 10.1128/IAI.71.1.401-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida MA, Jesus EE, Sousa-Atta ML, Alves LC, Berne ME, Atta AM. Clinical and serological aspects of visceral leishmaniasis in northeast Brazilian dogs naturally infected with Leishmania chagasi. Vet Parasitol. 2005;127:227–232. doi: 10.1016/j.vetpar.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Ghalib HW, Piuvezam MR, Skeiky YA, Siddig M, Hashim FA, el-Hassan AM, Russo DM, Reed SG. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karp CL, el-Safi SH, Wynn TA, Satti MM, Kordofani AM, Hashim FA, Hag-Ali M, Neva FA, Nutman TB, Sacks DL. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peruhype-Magal V, Martins-Filho OA, Prata A, Silva Lde A, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimaraes-Carvalho SF, Ferrari TC, Van Weyenbergh J, Correa-Oliveira R. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral leishmaniasis due to Leishmania chagasi infection. Clin Exp Immunol. 2006;146:124–132. doi: 10.1111/j.1365-2249.2006.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 34.Deng J, Dekruyff RH, Freeman GJ, Umetsu DT, Levy S. Critical role of CD81 in cognate T-B cell interactions leading to Th2 responses. Int Immunol. 2002;14:513–523. doi: 10.1093/intimm/14.5.513. [DOI] [PubMed] [Google Scholar]
- 35.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossbacher J, Shlomchik MJ. The B cell receptor itself can activate complement to provide the complement receptor 1/2 ligand required to enhance B cell immune responses in vivo. J Exp Med. 2003;198:591–602. doi: 10.1084/jem.20022042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casali P, Lambert PH. Purification of soluble immune complexes from serum using polymethylmetacrylate beads coated with conglutinin or C1q. Application to the analysis of the components of in vitro formed immune complexes and of immune complexes occurring in vivo during leishmaniasis. Clin Exp Immunol. 1979;37:295–309. [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 40.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Gutzmer R, Kother B, Zwirner J, Dijkstra D, Purwar R, Wittmann M, Werfel T. Human plasmacytoid dendritic cells express receptors for anaphylatoxins c3a and c5a and are chemoattracted to c3a and c5a. J Invest Dermatol. 2006;126:2422–2429. doi: 10.1038/sj.jid.5700416. [DOI] [PubMed] [Google Scholar]
- 42.Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting facto. Immunobiology. 2008;213:183–191. doi: 10.1016/j.imbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J Immunol. 2006;176:5486–5493. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 45.McFarlane E, Perez C, Charmoy M, Allenbach C, Carter KC, Alexander J, Tacchini-Cottier F. Neutrophils contribute to the development of a protective immune response during the onset of infection with Leishmania donovani. Infect Immun. 2007 doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Daniel-Ribeiro C, de Oliveira-Ferreira J, Banic DM, Galvao-Castro B. Can malaria-associated polyclonal B-lymphocyte activation interfere with the development of anti-sporozoite specific immunity? Trans R Soc Trop Med Hyg. 1989;83:289–292. doi: 10.1016/0035-9203(89)90476-8. [DOI] [PubMed] [Google Scholar]
- 48.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 49.Spera JM, Ugalde JE, Mucci J, Comerci DJ, Ugalde RA. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc Natl Acad Sci U S A. 2006;103:16514–16519. doi: 10.1073/pnas.0603362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevceva L, Yoon V, Anastasiades D, Poznansky MC. Immune responses to HIV Gp120 that facilitate viral escape. Curr HIV Res. 2007;5:47–54. doi: 10.2174/157016207779316396. [DOI] [PubMed] [Google Scholar]
- 51.Sangster MY, Topham DJ, D'Costa S, Cardin RD, Marion TN, Myers LK, Doherty PC. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 52.Nikbin B, Bonab MM, Khosravi F, Talebian F. Role of B cells in pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:13–42. doi: 10.1016/S0074-7742(07)79002-5. [DOI] [PubMed] [Google Scholar]
- 53.Nagy G, Koncz A, Perl A. T- and B-cell abnormalities in systemic lupus erythematosus. Crit Rev Immunol. 2005;25:123–140. doi: 10.1615/critrevimmunol.v25.i2.30. [DOI] [PubMed] [Google Scholar]
- 54.van den Berg WB, van Lent PL, Joosten LA, Abdollahi-Roodsaz S, Koenders MI. Amplifying elements of arthritis and joint destruction. Ann Rheum Dis. 2007;66 Suppl 3:iii45–iii48. doi: 10.1136/ard.2007.079830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anam K, Afrin F, Banerjee D, Pramanik N, Guha SK, Goswami RP, Saha SK, Ali N. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclass antibodies in Indian kala-azar patients after chemotherapy. Infect Immun. 1999;67:6663–6669. doi: 10.1128/iai.67.12.6663-6669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elassad AM, Younis SA, Siddig M, Grayson J, Petersen E, Ghalib HW. The significance of blood levels of IgM, IgA, IgG and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin Exp Immunol. 1994;95:294–299. doi: 10.1111/j.1365-2249.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reis AB, Teixeira-Carvalho A, Vale AM, Marques MJ, Giunchetti RC, Mayrink W, Guerra LL, Andrade RA, Correa-Oliveira R, Martins-Filho OA. Isotype patterns of immunoglobulins: hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol. 2006;112:102–116. doi: 10.1016/j.vetimm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Puentes SM, Da Silva RP, Sacks DL, Hammer CH, Joiner KA. Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J Immunol. 1990;145:4311–4316. [PubMed] [Google Scholar]
- 59.Mosser DM, Edelson PJ. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- 60.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stager S, Alexander J, Kirby AC, Botto M, Rooijen NV, Smith DF, Brombacher F, Kaye PM. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9:1287–1292. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 62.Deak E. The role of the lymph node and B cells in visceral Leishmania pathogenesis. Yale University: 2008. p. xiv. 160 leaves. [Google Scholar]
- 63.Kohl J, Wills-Karp M. Complement regulates inhalation tolerance at the dendritic cell/T cell interface. Mol Immunol. 2007;44:44–56. doi: 10.1016/j.molimm.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 65.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 66.Tsitoura DC, Yeung VP, DeKruyff RH, Umetsu DT. Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol. 2002;14:659–667. doi: 10.1093/intimm/dxf032. [DOI] [PubMed] [Google Scholar]
- 67.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 68.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]