Abstract
This commentary aims to integrate or interrelate the available data with the current study by Chiga and coworkers [1], which defines an important influence of aldosterone in the phosphorylation and thus activation of NCC in response to changes in NaCl intake, and implicates the involvement of SPAK/OSR1 kinases and WNKs.
An impaired ability of the kidney to excrete NaCl plays a critical pathophysiological role for a long-term increase in blood pressure. The aldosterone-sensitive distal nephron is of primary importance in the regulation of renal NaCl excretion and thus body NaCl homeostasis. The stimulatory effect of aldosterone on the epithelial sodium channel ENaC is well known and primarily localized to the late distal convoluted tubule (DCT) and connecting tubule (CNT). Less appreciated is the regulation of the Na+-Cl−-cotransporter NCC in the DCT by aldosterone and other regulators. Moreover, whereas much has been learned about the molecular pathways involved in the regulation of ENaC, relatively little is still known about the determinants of NCC activity. The study by Chiga and coworkers in this issue [1] provides new in vivo evidence in this regard as they indicate an important role of aldosterone in the regulation of NCC phosphorylation by dietary salt intake. Moreover, evidence is provided that the signaling cascade involves With-No-Lysine kinase (WNK) and the mammalian STE20 (sterile 20)-like kinases SPAK (STE20/SPS1-related proline/alanine-rich kinase) and OSR1 (oxidative stress-responsive kinase-1).
SPAK and OSR1 were discovered through their ability to interact with, phosphorylate and stimulate the activity of the Na+-K+-2Cl−-cotransporter (NKCC1), a member of a superfamily of electroneutral cation coupled chloride cotransporters (SLC12)(for review see [2]). The Na+-driven members of this superfamily include NKCC1 and NKCC2 as well as NCC. Fragments of these cotransporters containing a cluster of conserved threonine residues are phosphorylated in vitro by the SPAK/OSR1 kinases. The SPAK and OSR1 enzymes themselves are phosphorylated and activated by the WNK1 and WNK4 protein kinases (for review see [2]). These findings suggest that a signaling cascade including WNK1, WNK4 and SPAK/OSR1 could be involved in the regulation of the Na+-driven members of the SLC12 superfamily including NCC.
WNK1 and WNK4 are mutated in patients with pseudohypoaldosteronism type II (PHAII), an autosomal-dominant disorder that is characterized by hyperkalemia and hypertension (for review see [3]). Importantly, patients with PHAII are rather sensitive to pharmacological inhibition of NCC by thiazide diuretics. Moreover, transgenic mice or gene knockin mice carrying the PAHII mutation on the Wnk4 gene have their PHAII phenotypes corrected when treated with thiazides or when crossed with mice lacking NCC. These studies indicated that NCC is a primary effector of WNK4 mutations in the pathogenesis of PHAII. Overactivation of NCC in PAHII enhances NaCl reabsorption in the DCT and impairs the ability of the kidney to excrete NaCl and thus increases blood pressure. Enhanced NCC-mediated reabsorption of Na+ in the DCT limits the Na+ available for Na+/K+ exchange in the distal nephron thereby impairing the renal ability to excrete K+. Mutations in WNK1 and WNK4 may in addition affect other renal transport systems that can contribute to the PHAII phenotype [3].
Importantly, the enhanced phosphorylation and activation of NCC in Wnk4 PAHII knockin mice was associated with enhanced phosphorylation of SPAK/OSR1 kinases [10], implicating a potential role of WNK/SPAK/OSR1 kinases in NCC phosphorylation and activation in the PAHII phenotype. In accordance, more recent studies demonstrated that SPAK and OSR1 kinases phosphorylate human NCC at three conserved residues (Thr46, Thr55 and Thr60)[7]. Moreover, activation of the WNK1-SPAK/OSR1 signaling pathway by treatment of HEK293 or mpkDCT-derived cells with hypotonic low-chloride conditions induced phosphorylation of NCC at the residues phosphorylated by SPAK/OSR1 kinases. Efficient phosphorylation of NCC required docking interactions between NCC and SPAK/OSR1. Finally, mutation of Thr60 in NCC markedly inhibited phosphorylation of Thr46 and Thr55 as well as NCC activation in response to hypotonic low-chloride treatment of HEK293 cells. These studies implicated the WNK1-SPAK/OSR1 signaling pathway in the phosphorylation and activation of human NCC [7].
The current study by Chiga and coworkers [1] elucidated the potential physiological relevance of WNK4 and SPAK/OSR1 in the regulation of NCC by dietary salt. They showed that in wild-type mice the phosphorylation status of SPAK and OSR1 as well as of NCC (at Ser71, Thr53 and Thr58) is inversely related to NaCl intake, and immunofluorescence evidence was provided that most of the p-SPAK/OSR1 expression was confined in the NCC-expressing nephron segments. Moreover and intriguingly, application of aldosterone to wild-type mice on a high-salt diet increased phosphorylation of SPAK/OSR1 as well as NCC. Vice versa, blocking aldosterone receptors by spironolactone in wild-type mice fed a low-salt diet decreased the phosphorylation status [1]. Previous studies have shown that mutations of rat NCC Thr53, Thr58 and Ser71 completely reduce NCC activation in oocytes following hypotonic low chloride treatment [6]. Moreover, Thr55 and Thr60 in human NCC correspond to Thr53 and Thr58 in mouse NCC. Thus, a low salt diet or exogenous aldosterone in mice as well as exposure of DCT-derived cells to a hypotonic low-chloride medium phosphorylate NCC at residues that correspond to the human NCC residues phosphorylated by SPAK/OSR1. Together, these studies provide very strong evidence for a role of SPAK/OSR1 in the phosphorylation and activation of NCC in response to a low NaCl diet and aldosterone.
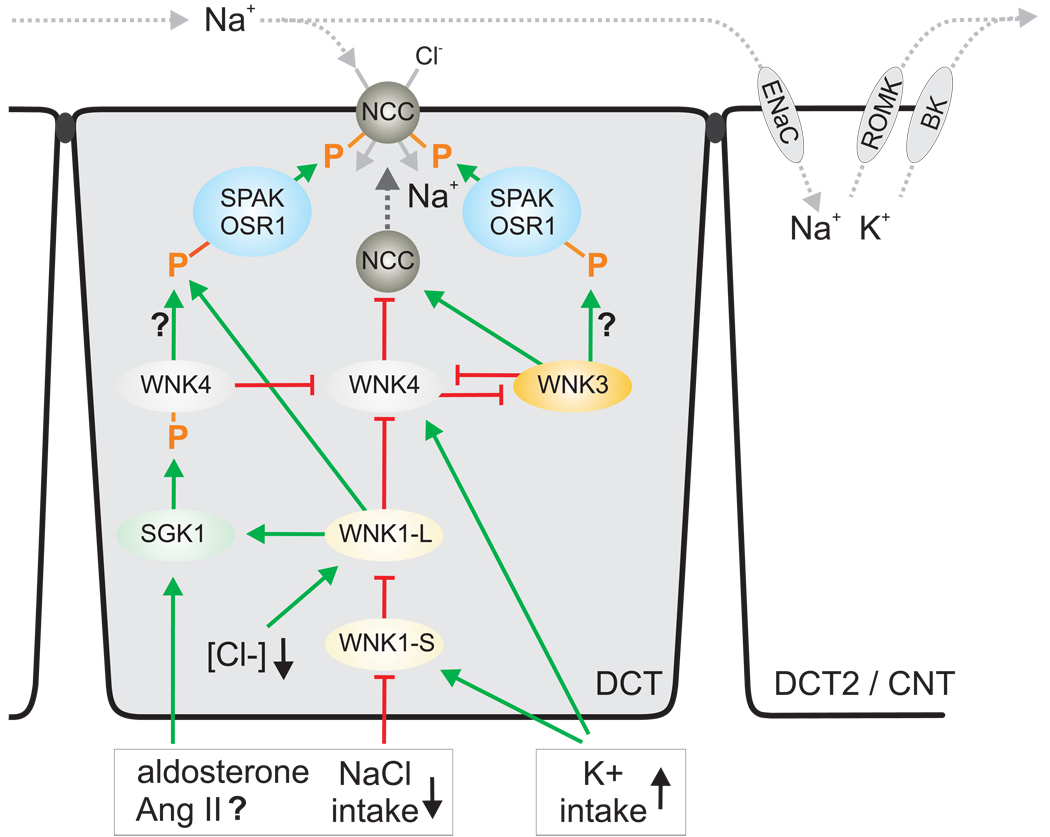
What do we know about the regulation of WNK1 and WNK4 in the kidney and their effects on NCC? In the kidney, a 5'-truncated kinase-deficient WNK1-short isoform (WNK1-S) predominates, while full-length WNK1-long (WNK1-L) is ubiquitously expressed at low levels. Moreover, mRNA expression of WNK1-S and WNK4 is strongest in DCT / CNT and drops sharply in the collecting duct [5], i.e. the expression is highest in the segments expressing NCC and the greatest activity of ENaC. WNK4 is in addition expressed in thick ascending limb and the macula densa. Importantly, a high K+ intake upregulated WNK1-S and WNK4, and low intake of K+ or NaCl reduced WNK1-S in mouse kidney [5]. Notably, the responses to changes in K+ intake were much more pronounced than the responses to changes in NaCl intake. This stresses the importance of the system for K+ balance. It also indicates that potential secondary effects on K+ homeostasis have to be considered when effects of a given maneuver on changes in WNK expression are studies (including the responses to exogenous aldosterone or spironolactone). Expression studies in Xenopus oocyte implicated that WNK4 inhibits NCC-mediated NaCl reabsorption by reducing NCC membrane expression, while WNK1-L may in turn inhibit WNK4 (for review see [3;4]). Kinase-deficient WNK1-S was proposed to inhibit the inhibitory influence of WNK1-L on WNK4 thereby increasing NCC expression in the membrane. WNKs are active kinases as well as scaffolds that can have unexpected effects when expressed at high levels. As a consequence, caution is needed when interpreting studies with overexpression. Nevertheless, the outlined findings could be integrated as follows and illustrated in Figure 1: low NaCl intake inhibits the expression of WNK1-S thereby disinhibiting WNK1-L. The latter through effects on WNK4 stimulates NCC membrane expression, whereas WNK1-L-mediated phosphorylation of SPAK/OSR1 phosphorylates and activates NCC. Both will help to increase NCC activity and conserve NaCl. In comparison, a high K+ diet increases WNK1-S and WNK4 expression. WNK1-S inhibits WNK1-L thereby inhibiting the membrane expression of NCC by disinhibition of WNK4. In addition, lowering WNK1-L reduces SPAK/OSR1-mediated phosphorylation and activation of NCC. Both changes will reduce NCC activity and thus enhance the Na+ delivery for Na+/K+ exchange via ENaC, ROMK, and the BK-channel and increase K+ excretion. Preliminary data in mice show that phosphorylation of NCC Ser71 is inversely related to dietary K+ intake (Vallon, Schroth, Lang, Kuhl, Uchida; unpublished observation).
Figure 1.
Model for the activation of NCC by increasing membrane expression and phosphorylation in the distal convoluted tubule (DCT). Effects are stimulatory (green arrows) or inhibitory (red breaks). In addition, a cell of the late DCT (DCT2) / connecting tubule (CNT) is shown to illustrate the indirect effect of inhibiting NCC-mediated Na+ reabsorption by high K+ intake on ROMK- and BK-channel-mediated K+ excretion. Ang II, angiotensin II.
Both WNK1-L and WNK4 can phosphorylate SPAK/OSR1 in vitro [2]. WNK1-L-induced phosphorylation of SPAK/OSR1 and activation of NCC fits with the outlined physiological scenarios. WNK4-mediated phosphorylation and activation of SPAK/OSR1/NCC could be conflicting with the observed increase in WNK4 expression and proposed inhibition of NCC activity in response to high K+ intake (Figure 1). The current study by Chiga and coworkers reported that the increased phosphorylation of SPAK/OSR1/NCC in Wnk4 PHAII knockin mice was unresponsive to changes in NaCl intake [1], implicating a role of Wnk4 in the phosphorylation of SPAK/OSR1. One may speculate that the Wnk4 PHAII mutant itself phosphorylates NCC or that the mutant enhances WNK1-L activity. In addition or alternatively, the Wnk4 PHAII mutant may disinhibit WNK3, another member of the WNK family that more recently has been found to stimulate NCC activity and to interact with WNK4 and WNK1-S in vitro (for review see [4])(Figure 1). The physiological relevance of WNK3 and its role in OSR1/SPAK/NCC signaling remains to be determined.
Aldosterone levels increase in response to low NaCl and high K+ intake. The opposing effects of these diets on NCC phosphorylation and activity implicate additional regulators (Figure 1). WNK4 has been reported to be phosphorylated by the serum- and glucocorticoid regulated kinase SGK1 [8], which is expressed and activated in response to aldosterone. Notably, SGK1 phosphorylation of WNK4 alleviates the inhibitory effects of WNK4 on ENaC [3] and possibly on NCC (Figure 1). Whether SGK1 phosphorylation of WNK4 affects phosphorylation of OSR1/SPAK/NCC or the membrane expression of NCC remains to be determined. Mice lacking sgk1 have an impaired renal ability to conserve NaCl and excrete K+ [9]. Preliminary data indicate that phosphorylation of NCC Ser71 in response to a low NaCl diet is attenuated in mice lacking sgk1; in comparison, a high K+ diet induced hyperkalemia in these mice associated with an exaggerated suppression of NCC Ser71 phosphorylation, which may serve to deliver more Na+ to the distal segments of impaired Na+/K+ exchange in the absence of sgk1 (Vallon, Schroth, Lang, Kuhl, Uchida; unpublished observation).
In summary, the Na+-Cl−-cotransporter NCC in the distal convoluted tubule plays a critical role in NaCl and, indirectly, in K+ homeostasis. Recent studies implicated its regulation by WNK kinases. The current in vivo study by Chiga and coworkers has defined an important influence of aldosterone in the phosphorylation and activation of NCC in response to changes in NaCl intake, and implicates the involvement of SPAK/OSR1 kinases and WNKs.
Acknowledgements
These studies and comments were supported with funds provided by the National Institutes of Health (DK56248, DK28602, GM66232), the American Heart Association (0655232Y), and from the Research Service of the Department of Veterans Affairs.
Reference List
- 1.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. The WNK4-OSR1/SPAK-NCC phosphorylation cascade is a novel aldosterone-dependent system controlling sodium balance. Kidney Int. 2008 [Google Scholar]
- 2.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem.J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 3.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu.Rev.Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 4.McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease: an integrated view. Hypertension. 2008;51:588–596. doi: 10.1161/HYPERTENSIONAHA.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J.Am.Soc.Nephrol. 2006;17:2402–2413. doi: 10.1681/ASN.2005111197. [DOI] [PubMed] [Google Scholar]
- 6.Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J.Biol.Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 7.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J.Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 8.Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc.Natl.Acad.Sci.U.S.A. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallon V, Wulff P, Huang DY, Loffing J, Volkl H, Kuhl D, Lang F. Role of Sgk1 in salt and potassium homeostasis. Am J Physiol Regul Integr Comp Physiol. 2005;288:R4–R10. doi: 10.1152/ajpregu.00369.2004. [DOI] [PubMed] [Google Scholar]
- 10.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]