Abstract
Fatty acids, acetylcholine, and GLP-1 enhance insulin secretion in a glucose-dependent manner. However, the interplay between glucose, fatty acids, and the neuroendocrine regulators of insulin secretion is not well understood. Therefore, we studied the acute effects of PA (alone or in combination with glucose, acetylcholine, or GLP-1) on isolated cultured mouse islets. Two different sets of experiments were designed. In one, a fixed concentration of 0.5 mM of PA bound to 0.15 mM BSA was used; in the other, a PA ramp from 0 to 0.5 mM was applied at a fixed albumin concentration of 0.15 mM so that the molar PA/BSA ratio changed within the physiological range. At a fixed concentration of 0.5 mM, PA markedly inhibited acetylcholine-stimulated insulin release, the rise of intracellular Ca2+, and enhancement of cAMP production but did not influence the effects of GLP-1 on these parameters of islet cell function. 2-ADB, an IP3 receptor inhibitor, reduced the effect of acetylcholine on insulin secretion and reversed the effect of PA on acetylcholine-stimulated insulin release. Islet perfusion for 35–40 min with 0.5 mM PA significantly reduced the calcium storage capacity of ER measured by the thapsigargin-induced Ca2+ release. Oxygen consumption due to low but not high glucose was reduced by PA. When a PA ramp from 0 to 0.5 mM was applied in the presence of 8 mM glucose, PA at concentrations as low as 50 μM significantly augmented glucose-stimulated insulin release and markedly reduced acetylcholine's effects on hormone secretion. We thus demonstrate that PA acutely reduces the total oxygen consumption response to glucose, glucose-dependent acetylcholine stimulation of insulin release, Ca2+, and cAMP metabolism, whereas GLP-1's actions on these parameters remain unaffected or potentiated. We speculate that acute emptying of the ER calcium by PA results in decreased glucose stimulation of respiration and acetylcholine potentiation of insulin secretion.
Keywords: glucagon-like peptide-1, fatty acids
the effects of free fatty acids (FFA) on glucose-stimulated insulin secretion have been investigated intensely and widely discussed in the literature (2, 3, 6, 25–28, 31, 32, 42). It is considered that fatty acids (FA) alter β-cell function by two different mechanisms: by signaling through the FFA G protein-coupled receptor 40 (19) or by entering into intermediary metabolism of β-cells, where they are either oxidized via the β-oxidation pathway for energy production when the glucose level is low or converted to complex lipids when the glucose level is high (31). It is hypothesized that a transient physiological increase in cytosolic long-chain acyl-CoA leads to stimulation of insulin secretion either directly, affecting exocytosis, or indirectly via complex lipid formation (diacylglycerol, phosphatidic acid, lysophosphatidic acid) that acts distally by modulating key enzymes such as protein kinase C (31, 42). However, chronic exposure of the β-cells to elevated FFA results in inhibition of insulin secretion, as shown by in vitro studies (with the isolated perfused pancreas and isolated islets) (25, 36, 45). This phenomenon has been termed lipotoxicity (30, 31). In contrast, corresponding in vivo studies in man demonstrated that 48-h elevation of plasma FFA potentiated glucose-stimulated insulin secretion at glucose levels clamped at 8.6 mM (3). These latter findings provided the basics for a novel hypothesis that insulin secretion enhanced by FFA serves to compensate for the peripheral insulin resistance induced by FA, preventing the development of type 2 diabetes in the majority of obese individuals (3).
In addition to nutrients, insulin secretion is stimulated by many hormones and neurotransmitters. Acetylcholine, the neurotransmitter of the parasympathetic nervous system, plays a significant role in the regulation of insulin secretion in pancreatic β-cells (1, 13, 38). Mutant mice selectively lacking the M3 muscarinic acetylcholine receptor subtype in pancreatic β-cells display impaired glucose tolerance and greatly reduced insulin release (12, 44). In contrast, transgenic mice overexpressing M3 receptors in pancreatic β-cells show a pronounced improvement in glucose tolerance and insulin secretion and are resistant to diet-induced glucose intolerance and hyperglycemia (11). The secretory response of β-cells to fuel stimulation is also markedly enhanced by the gut hormone glucagon-like peptide-1 (GLP-1), a member of the glucagon family, which is released into the portal circulation when a meal is digested (17). Both GLP-1 and acetylcholine greatly increase the sensitivity of β-cells to nutrients, but their effects on secretion depend entirely on availability, levels, and compositions of metabolic substrates. However, to our knowledge, little is known about the interplay between FA metabolism and the neuroendocrine regulation of insulin secretion. Therefore, the goal of the present study was to evaluate the acute action of palmitic acid (PA; present alone or in combination with glucose and/or acetylcholine or GLP-1) on isolated mouse islets. The data demonstrate that PA interferes with the cholinergic regulation of β-cell function, resulting in inhibition of glucose-dependent acetylcholine but not GLP-1 stimulation of insulin secretion.
RESEARCH DESIGN AND METHODS
Animals.
Our research was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (protocol no. 077603). B6D22F1 mice were used throughout. The mice were maintained on a 12:12-h light-dark cycle and were fed a standard rodent chow diet.
Islet isolation.
Mouse islets were isolated using collagenase (EC 3.4.24.3 Serva, 17449) digestion in Hanks' buffer, followed by separation of islets from exocrine tissue in a Ficoll (F-9378; Sigma) gradient. Isolated islets were cultured for 3–4 days in RPMI 1640 medium (Sigma) containing 10% fetal bovine serum, 10 ml/l penicillin-streptomycin-amphotericin B solution (GIBCO-BRL), and 10 mM glucose.
Preparation of sodium palmitate solution.
A 5 mM stock solution of sodium palmitate (Sigma-Aldrich) was prepared by dissolving the FA salt in 10% bovine serum albumin (BSA; fraction V, FA free; Sigma-Aldrich) in Krebs buffer by stirring continuously for ∼4 h in a 37°C water bath. The stock solution was then diluted by Krebs buffer to obtain the final concentration of 0.5 mM sodium palmitate and 1% BSA.
Perifusion of islets for measurement of insulin release.
Cultured islets (130 islets) were placed on a nylon filter in a plastic perifusion chamber (Millipore, Bedford, MA) and were perfused with a flow rate of 2 ml/min. The perifusion apparatus consisted of a computer-controlled fast-performance HPLC system (Waters 625 LC System) that allowed programmable rates of flow and glucose concentration in the perfusate, a water bath (37°C), and fraction collector (Waters Division of Millipore). The perifusate was Krebs buffer (pH 7.4) containing 114 mmol/l NaCl, 5 mmol/l KCl, 24 mmol/l NaHCO3, 1 mmol/l MgCl2 6H2O, 2.2 mmol/l Ca2+, 1 mM Pi, 10 mmol/l HEPES (pH 7.4), 1% BSA (fraction V, FA free; Sigma-Aldrich) equilibrated with 20% O2, and 5% CO2 balanced with N2.
Perifusion of islets for simultaneous measurement of oxygen consumption and insulin release.
The islets (600 islets) were loaded into the 250-μl chamber using a P200 pipette and gel-loading tip and were allowed to settle for 1 min before the flow was resumed at 80 μl/min. The perifusion apparatus consisted of a peristaltic pump, a water bath (37°C), a gas exchanger (artificial lung; medium flowed through the thin-walled silastic tubing loosely coiled in a glass jar that contained 20% O2 and 5% CO2 balanced with N2), and a fraction collector (Waters Division of Millipore) (9). In this set of experiments, the Krebs buffer (pH 7.4) containing 1% BSA (fraction V, fatty acid free) was used.
Oxygen consumption measurements.
Oxygen partial pressure was measured by the phosphorescence quenching method, using a new oxygen-sensitive phosphorescent porphyrin-dendrimer Oxyphor G3 (palladium-tetrabenzoporphyrin, encapsulated inside gen 2 poly-arylglycine dendrimer) (22). The periphery of the dendrimer is modified with oligoethyleneglycol residues, which makes the probe water soluble and biologically inert. Oxyphor G3 does not require serum albumin in the perifusion medium, and thus there are no competitive interactions between dye and FA for binding to albumin.
The measured phosphorescence lifetime may be converted to oxygen pressure using the Stern-Volmer relationship 1/τ0 = 1/τ0 + kQ × [Po2], where τ0 is the phosphorescence lifetime in the absence of oxygen, τ is the lifetime at oxygen pressure Po2, and kQ is a constant describing the efficiency of quenching.
The inflow oxygen tension was measured in the absence of islets in the chamber before and after each experiment. Oxygen consumption by the islets was calculated from the difference in oxygen partial pressure between the influent and the effluent (oxygen extraction) and the rate at which medium flowed through the chamber.
Islet batch incubation studies.
Islets were isolated as above, hand-picked, and cultured for 3 days. Batches of 50 islets were loaded into 12 × 75 mm disposable glass culture tubes and preincubated in 1 ml of oxygenated glucose-free Krebs-Ringer bicarbonate buffer (KRBB) at 37°C for 40 min, followed by a 45-min exposure to different stimuli. After incubations, islets and culture medium were transferred to 1.5-ml microcentrifuge tubes and spun at low speed. The supernatant was used for insulin measurements. Pelleted islets were washed two times with cold glucose-free Hanks' buffer and then homogenized with 100 μl of 1% Triton in Tris-EDTA buffer. Protein concentration was measured in islet lysates using the Bradford method.
cAMP content determination.
One-hundred cultured islets were exposed to different treatments for 30 min in the presence of 0.1 mmol/l isobutylmethylxanthine. After incubation, islets were quickly washed two times by cold glucose-free Hanks' buffer. cAMP was measured in islet lysates by an enzyme-linked immunosorbent assay (GE Healthcare).
Ca2+ measurement.
Mouse islets were loaded with fura 2-AM during a 40-min pretreatment at 37°C in 2 ml of KRBB supplemented with 5 mmol/l fura 2-AM (Molecular Probes, Eugene, OR). The loaded islets were transferred to the perifusion chamber and placed on the homeothermic platform of an inverted Zeiss microscope. Islets were perfused with KRBB at 37°C at a flow rate of 2 ml/min, and various treatments were applied to the islets. The intracellular Ca2+ was determined by the ratio of the excitation of fura at 334 and 380 nm. Emission was measured at 520 nm by an Zeiss charge-coupled device camera and calibrated using the Attofluor Ratio Vision Software.
Insulin measurements.
Insulin in the effluent was measured by radioimmunoassay (15).
Statistical analysis.
Data are presented as the mean ± SE of three to four experiments. In appropriate cases, significant differences between groups were determined by ANOVA with post hoc analysis using Dunnett's multiple comparison test. P ≤0.05 was considered significant. IGORPro data analysis software (Wavemetrics, Lake Oswego, OR) was used to measure the total insulin secretion by calculating the integral under the insulin curve (presented in relative units).
RESULTS
Effects of PA on glucose- and acetylcholine-stimulated insulin release in mouse islets.
To examine the effects of PA on islet function, initial experiments utilized a fixed concentration of 0.5 mM of PA bound to 0.15 mM BSA. The PA/BSA molar ratio was 3.3, lower compared with most other studies (29, 33, 43) but moderately higher than that observed in humans (between 0.5 and 2, and rarely exceeding 4 in obese patients) (10, 14).
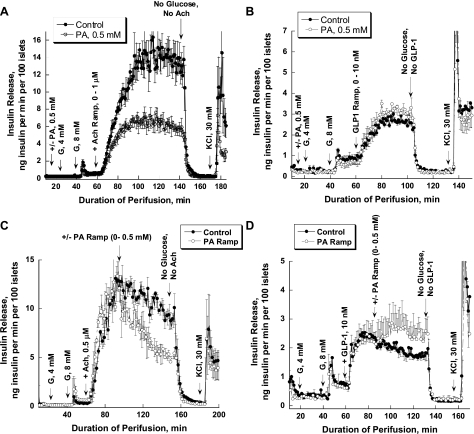
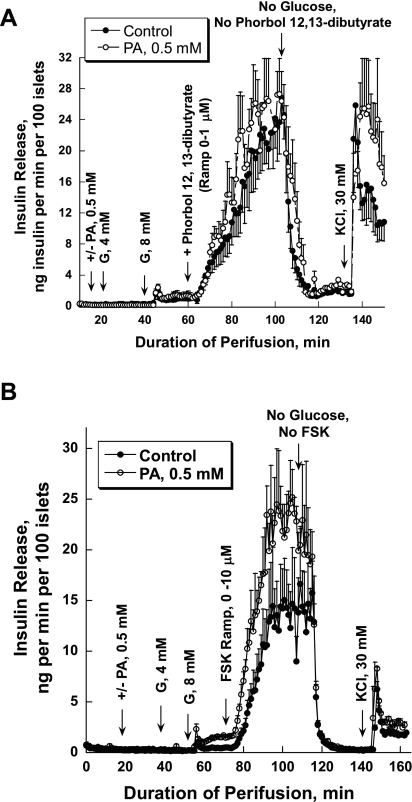
Islets were preperfused in the absence of glucose for 30 min, and then 4 and 8 mM glucose were added for 20 min. Afterward, acetylcholine or GLP-1 ramps were applied for 80 and 40 min, respectively, in the presence of 8 mM glucose. It should be noted that ramp protocols are unable to characterize time-dependent changes at a specific concentration of the secretagogue. Finally, 30 mM KCl was added after 30 min of a washout period with glucose-free medium. In one set of experiments, 0.5 mM of PA was included in the perfusate during the entire period. PA did not significantly change glucose-stimulated insulin release but markedly reduced the stimulation of insulin secretion by increasing concentrations of acetylcholine (Fig. 1A). Total insulin release during acetylcholine stimulation was reduced to one-half in the presence of 0.5 mM PA (917 ± 49.75 vs. 458.71 ± 37.35, P = 0.0003). The glucose-dependent GLP-1 stimulation of insulin release was not affected by PA (104.98 ± 2.65 vs. 119.15 ± 4.19, P = 0.06; Fig. 1B). In both sets of experiments, KCl produced a sharp biphasic increase in insulin release. It should be noted that insulin release during GLP-1 stimulation is only one-fifth compared with acetylcholine-induced secretion.
Fig. 1.
Effects of palmitic acid (PA) on acetylcholine (Ach)- and glucagon-like peptide-1 (GLP-1)-stimulated insulin release. A: an Ach ramp from 0 to 1 μM (12.5 nM increment/min) was applied after islet preperfusion with 0, 4, and 8 mM glucose (G) for 30, 20, and 20 min, respectively (●). In 1 set of experiments, PA was added after 20 min of preperfusion with no glucose added and then was present during the entire experiment (○). PA significantly affected Ach potentiation of insulin release (from 69 to 144 min of perifusion, P < 0.05) starting at Ach concentration of 62.5 nM. B: a GLP-1 ramp from 0 to 10 nM was applied using the same experimental design as presented in A. C: insulin release was stimulated initially with 4 and 8 mM G, followed by stimulation with Ach (0.5 μM). Afterward, a PA ramp from 0 to 0.5 mM (12.5 μM increment/min; ●) was applied for 40 min. In control experiments, islets were perfused with 0.5 μM of Ach (○) for the same duration as the PA ramp. PA significantly affected Ach potentiation of insulin release (from 97 to 154 min of perifusion, P < 0.05), starting at a PA concentration of 50 μM. D: GLP-1 (10 nM) instead of Ach was used to potentiate insulin secretion with the same experimental design as that presented in C. Note that here and in all other experiments, 10 μM neostigmine, a cholinesterase inhibitor, and 100 μM valine-pyrrolidide, a dipeptidyl peptidase-4 inhibitor, were used to prevent breakdown of Ach and GLP-1, respectively. Each curve represents the mean ± SE of 3–4 perfusions.
To evaluate the concentration dependence of PA's effect on acetylcholine-stimulated insulin release, a PA ramp from 0 to 0.5 mM (12.5 μM increment/min with BSA fixed at 0.15 mM) was applied after islet stimulation with 0.5 μM of acetylcholine in the presence of 8 mM glucose (Fig. 1C). The molar PA/BSA ratio changed progressively from 0 to 3.3. In control experiments, islets were perfused with 0.5 μM of acetylcholine for the same duration as the PA ramp. The effect of acetylcholine on insulin release declined linearly with time (−0.053 ng insulin·min−1·100 islets−1), possibly reflecting desensitization of muscarinic receptors. In contrast, a marked exponential decrease in insulin release was seen when the PA ramp was applied. The slopes at the beginning and at the end of the ramp were −0.18 and 0.10 ng insulin·min−1·100 islets−1. A significant difference between the slopes was already evident at 50 μM PA (P = 0.043). In contrast to the acetylcholine effect, glucose-dependent GLP-1 stimulation of insulin release was potentiated by PA (Fig. 1D).
Oxygen consumption.
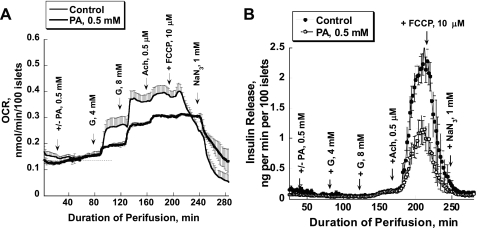
PA caused a small, gradual increase (∼15%) in basal oxygen consumption rate (OCR) of perifused mouse islets (Fig. 2A) and did not change basal insulin release (Fig. 2B) in the absence of glucose. Glucose-stimulated insulin release occurred at 8 mM, and this effect was not potentiated by PA. The increment of the OCR due to low glucose (4 mM) was reduced by two-thirds in the presence of PA (P ≤ 0.05, time interval from 94 to 126 min). Glucose at 8 mM produced the same stimulation of the OCR in both cases; however, the maximal OCR was significantly lower in the presence of PA (P ≤ 0.05, time interval from 128 to 160 min). In contrast to dramatic stimulation of insulin release, acetylcholine affected oxygen consumption only a little, suggesting that the energy requirement for exocytosis is very small. As seen in the previous sets of experiments, PA inhibited glucose-dependent acetylcholine stimulation of insulin release >50% (45.67 ± 6.38 vs. 18.43 ± 3.65, P = 0.009; Fig. 2B). Uncoupling mitochondrial respiration and oxidative phosphorylation by FCCP (5 μM) blocked insulin release. The effect of FCCP on OCR was biphasic: a small stimulation followed by inhibition. It should be noted that FCCP may change the intracellular pH because it is a protonophore. We have shown previously that stimulation of respiration by FCCP in mouse islets occurs only when the perifusion medium is supplemented by a mixture of amino acids (9) or glutamine (23). It should be noted that, in this set of experiments, 600–700 islets were perfused with a flow rate of 80 μl/min. This resulted in a significantly lower absolute rate of insulin release compared with that in experiments where 130 islets were perfused with a flow rate of 2 ml/min. Higher number of islets and lower flow rate may lead to higher insulin and glucagon concentration within the islet extracellular space and the closely surrounding medium.
Fig. 2.
Effect of PA on glucose and Ach-stimulated oxygen consumption (A) and insulin release (B) in mouse islets. Oxygen consumption and insulin release were stimulated by 4 and then 8 mM G, followed by stimulation with Ach (0.5 μM). Each intervention lasted 40 min. FCCP (5 μM) was added for 30 min to uncouple the respiration and oxidative phosphorylation. NaN3 (1 mM) was used at the end of each experiment to block respiration. In 1 set of experiments, PA (0.5 mM) was added at 0 mM G and was then present during the whole experiment [bold line (A) or ○ (B)]. There was a 4-min delay in the islet response to Ach in the presence of PA. Starting from 182 min of perifusion, the changes in insulin release were significant; P < 0.05. In these experiments, a new water-soluble generation of oxygen-sensitive dye Oxyphor G3 (Pd-tetrabenzoporphyrin, encapsulated inside a 2 polyarylglycine dendrimer) was used (see research design and methods) (22). Each curve represents the mean of 3–4 perfusions. Note that 8 mM G stimulates insulin release 3- to 4-fold but that the effect is dwarfed by the high efficacy of Ach. OCR, oxygen consumption rate.
The sequence of adding PA.
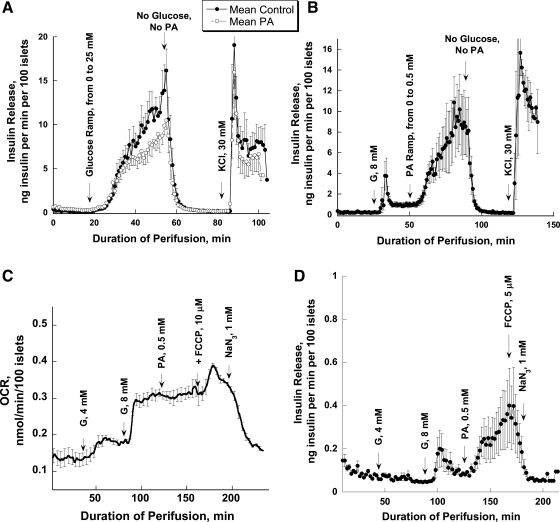
The absence in our experiments of PA potentiation of insulin release by 8 mM glucose is in disagreement with previous studies (6, 31). To clarify these differences, three sets of experiments were performed. In one, PA was added prior to a glucose ramp from 0 to 25 mM (0.6 mM increment/min; Fig. 3A); in the second, a PA ramp from 0 to 0.5 mM (12.5 μM increment/min) was applied after preperfusion with 8 mM glucose (Fig. 3B); in the third, batch incubation was used to evaluate the effect of PA on insulin secretion (Table 1). In the first scenario, PA did not significantly affect the glucose-stimulated insulin release, although a trend toward decreasing glucose-stimulated insulin release was seen at high concentrations of glucose (>8 mM; Fig. 3A). In contrast, a marked stimulation of insulin release by a PA ramp was evident following islet preperfusion with 8 mM glucose (Fig. 3B), with the effect of PA on insulin release already significant at 50 μM PA (0.97 ± 0.19 vs. 2.65 ± 0.54, P = 0.026).
Fig. 3.
The sequence of adding PA determines its effect on glucose-stimulated insulin release and oxygen consumption. A: PA was added 20 min before a glucose ramp from 0 to 25 mM (0.6 mM increment/min; n = 3). B: a PA ramp from 0 to 0.5 mM (12.5 μM increment/min) was applied after islet preperfusion with 4 and then 8 mM G (n = 4). PA starting from a concentration of 50 μM significantly potentiated insulin release (P < 0.05). C: effect of PA on oxygen consumption. PA (0.5 mM) was added after islet preperfusion with 4 and then 8 mM G; D: insulin release for the experiments presented in C (n = 3). Note that 120 islets and a 1.8 ml/min flow rate were used in experiments presented in A and B; 700 islets and an 80-μl flow rate were used in perfusions presented in C and D. The difference in number of islets and flow rate may result in a large difference in the absolute rate of insulin release in the 2 sets of experiments for reasons that remain to be determined. Also note that the O2 pressure decreases by only 10–13%, such that anoxia cannot be a problem.
Table 1.
Insulin secretion of mouse pancreatic islets cultured for 45 min
| Stimuli | Control | PA |
|---|---|---|
| 0 mM G | 2.26 ± 0.17 | |
| 8 mM G | 3.49 ± 0.41 | 10.01 ± 1.44* |
| 8 mM G + 10 μM Go 6976 | 4.28 ± 1.44 | 2.46 ± 0.36* |
| 8 mM G + 100 μM 2-ADB | 4.29 ± 2.55 | 3.06 ± 1.65 |
| 8 mM G + 1 μM Ach | 27.26 ± 0.80 | 13.08 ± 2.11* |
| 8 mM G + 1 μM Ach + 10 μM Go 6976 | 14.15 ± 0.83† | 13.87 ± 1.12 |
| 8 mM G + 1 μM Ach + 100 μM 2-ADB | 7.81 ± 1.01† | 9.72 ± 1.42 |
Values are means ± SE of 4 experiments. PA, palmitic acid; G, glucose; Go 6976, 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazolemodel; 2-ADB, 2-aminoethyl diphenylborinate; Ach, acetylcholine. Batches of 50 islets were preincubated in oxygenated glucose-free Krebs-Ringer bicarbonate buffer at 37°C for 40 min, followed by a 45-min exposure to different stimuli. Data are presented as ng insulin·μg protein−1·45 min−1.
P ≤ 0.05 compared with control of the same experimental design;
P ≤ 0.05 compared with 8 mM glucose + Ach.
When, instead of perfusion, batch incubation was used, then PA significantly potentiated insulin release at 8 mM glucose in the course of 45-min incubation (see Table 1).
It was of interest whether such stimulation of insulin secretion by PA in the presence of 8 mM glucose would lead to an increased OCR. Therefore, a similar design was used in OCR experiments. In contrast to markedly elevated insulin release (Fig. 3D), PA did not significantly alter oxygen consumption (Fig. 3C).
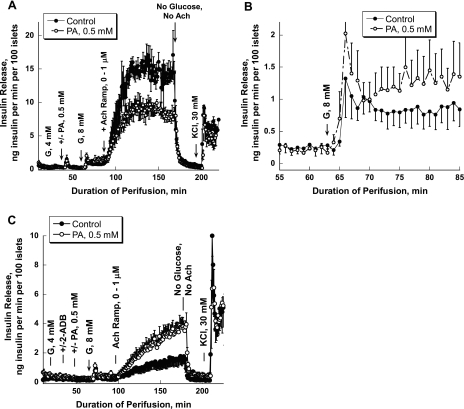
We have also tested whether the sequence of adding PA (in the absence or presence of glucose) would change the acetylcholine effect on insulin release. Therefore, in one set of experiments, 0.5 mM of PA was added after islets were preperfused with 4 mM glucose. As in previous experiments, PA did not increase insulin release at a basal glucose concentration (Fig. 4A) but potentiated the second phase of insulin release during stimulation with 8 mM glucose (Fig. 4B). However, the sequence of adding the PA (at 0 or 4 mM glucose) did not affect the inhibitory action of PA on acetylcholine-stimulated insulin release [1,028 ± 64.07 vs. 636.84 ± 74.74, P = 0.017; Fig. 4A (also see Fig. 1A)].
Fig. 4.
Effect of 2-aminoethyl diphenylborinate (2-ADB), an inositol triphosphate (IP3) receptor inhibitor, on Ach-stimulated insulin release in the presence and absence of PA. A: the sequence of adding PA does not affect its inhibitory action on Ach-stimulated insulin. PA was added after islet preperfusion with 4 mM G and then was present for the duration of the experiment. Potentiation of insulin release by Ach was reduced significantly in the presence of PA (106–165 min of perifusion, P < 0.05). B: a magnified view of 1 section (55–85 min) of the perfusion experiments is presented to clearly show the potentiating effect of PA on glucose-stimulated insulin release. C: effect of 2-ADB, an IP3 receptor inhibitor, on Ach-stimulated insulin secretion. Each curve represents the mean ± SE of 3 perfusions.
The role of inositol triphosphate receptors, PKC, and cAMP.
It is known that acetylcholine activates PLC, resulting in the generation of diacylglycerol, a potent PKC activator, and inositol triphosphate (IP3), which causes a rapid mobilization of Ca2+ from the endoplasmic reticulum (ER) (13). Both PKC and Ca2+ activate adenylyl cyclase (types 2, 3, 5, and 7) and increase cAMP (39). Therefore, the next set of experiments was designed to evaluate the involvement of PKC, cAMP, and IP3-induced Ca2+ mobilization in the inhibitory effect of PA on acetylcholine-stimulated insulin secretion.
Gradual increases in concentration of the phorbol 12,13-dibutyrate, an activator of PKC, from 0 to 1 μM in the presence of 8 mM glucose greatly augmented insulin release (Fig. 5A). The presence of PA in the perfusate did not reduce insulin release; on the contrary, a trend for potentiating insulin secretion at the higher concentrations was evident (641.49 ± 129.16 vs. 775.12 ± 136.38, P = 0.5). In the next set of experiments, an inhibitor of PKC, Go 6976 {12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazolemodel; specific for α (IC50 = 2.3 nM) and β1 (IC50 = 6.2 nM) isozymes}, was used, and insulin secretion was evaluated in batch incubation (Table 1). The PKC inhibitor reduced to one-half the acetylcholine effect at 8 mM glucose, supporting the general view that PKC is the major intracellular target for acetylcholine action. At this condition, PA did not significantly affect the acetylcholine effect on insulin secretion. Both sets of data suggest that the inhibitory effect of PA on acetylcholine-stimulated insulin release is upstream of PKC.
Fig. 5.
The role of PKC and cAMP in the action of PA on insulin release. A: phorbol 12,13-dibutyrate (ramp from 0 to 1 μM for 40 min) was used to stimulate insulin release in the presence of 8 mM G. B: forskolin (FSK; ramp from 0 to 10 μM for 40 min) was used to stimulate insulin release. Each curve represents the mean ± SE of 3 perfusions.
In another set of perifusion experiments, forskolin (ramp from 0 to 10 μM) was used to gradually increase the level of intracellular cAMP. PA did not significantly affect the forskolin-stimulated insulin secretion (432.26 ± 121.19 vs. 725.79 ± 106.1, P = 0.143), although the insulin secretion profile was shifted to the left (Fig. 5B).
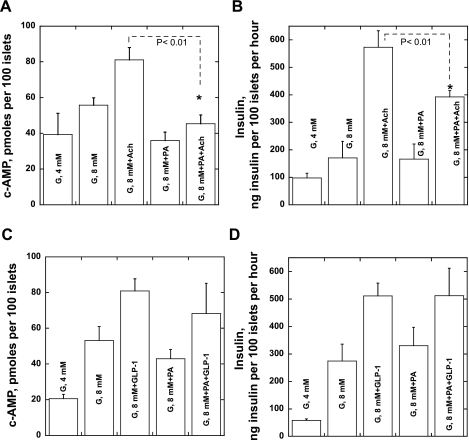
In the next set of experiments, we tested whether intracellular cAMP levels are modulated by acute exposure to PA. Both high glucose and acetylcholine elevated the intracellular cAMP concentration and enhanced insulin release in batch incubation experiments (Fig. 6, A and B). PA prevented the rise of cAMP during acetylcholine stimulation in the presence of 8 mM glucose and significantly reduced the stimulation of insulin release by acetylcholine. In contrast, the effects of GLP-1 on both cAMP concentrations and insulin release were not influenced by FA administration (Fig. 6, C and D). Thus, the acetylcholine-induced changes in intracellular cAMP level are altered by PA. Since the changes in cAMP are downstream of intracellular Ca2+, we tested whether inhibition of the IP3-mediated Ca2+ mobilization from the ER by 2-aminoethyl diphenylborinate (2-ADB) would change the PA effect on acetylcholine-induced insulin secretion. 2-ADB greatly reduced acetylcholine's effect on insulin secretion and, surprisingly, partially reversed the effect of PA on acetylcholine stimulation of hormone release from inhibition to stimulation (Fig. 4C).
Fig. 6.
Effects of PA on cAMP levels and insulin release during islet stimulation with glucose, Ach, and GLP-1. A: changes in cAMP due to glucose (4 and 8 mM) and Ach (0.5 μM). B: insulin release for the experiments presented in A. C and D: GLP-1 (10 nM) instead of Ach was used to stimulate cAMP (C) and insulin release (D) in the same experimental design as presented in A and B. Results are presented as means ± SE of 4 experiments.
Changes in intracellular Ca2+ concentration in response to PA.
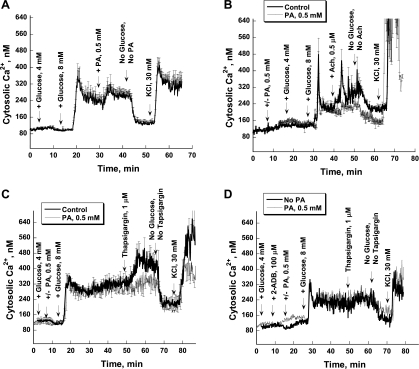
Fura-2 fluorescence techniques were used to evaluate whether intracellular changes in Ca2+ contribute to altered insulin release in the presence of PA. Eight millimolar glucose caused biphasic changes in Ca2+ (a spike of Ca2+ followed by higher basal levels; Fig. 7A). PA, when applied after preperfusion with 8 mM glucose, significantly increased intracellular Ca2+ concentrations (Fig. 7A). Acetylcholine also increased Ca2+ and induced serial Ca2+ oscillations in the majority of control islets (Fig. 7B). PA (0.5 mM) did not modify the effect of 8 mM glucose; however, it reduced the acetylcholine-stimulated rise in Ca2+ (31,528 vs. 18,847, P = 0.042; Fig. 7B).
Fig. 7.
Effects of PA on the intracellular Ca2+ concentration in mouse islets. A: islets were stimulated initially with 4 and then with 8 mM glucose. Afterward, PA (0.5 mM) was added to perfusate. B: in control experiments, the Ach (0.5 μM) was added after islet stimulation with 4 and 8 mM glucose (bold line). In another set of experiments, PA was added after 10 min of preperfusion with no glucose and then was present during the entire experiment (thin line). C: effect of PA on calcium capacity of the endoplasmic reticulum (ER). D: effect of 2-ADB on thapsigargin-induced Ca2+ release from ER. At the end of each experiment, KCl (30 mM) was added after a 15-min washout period with no secretagogues to test the maximal islet Ca2+ response.
In the next set of experiments, thapsigargin was used to indirectly measure the calcium storage capacity of the ER when acutely influenced by PA (Fig. 7C). It was found that 35–40 min of islet incubation with PA in the presence of 8 mM glucose significantly reduced the calcium storage capacity of the ER. Blocking IP3 receptors by 2-ADB decreased thapsigargin's effect on Ca2+ release and abolished the Ca2+-depletive effect of PA on ER but decreased the magnitude of the thapsigargin response (Fig. 7D). Based on the mechanism well described in the literature that acetylcholine acts through IP3-mediated Ca2+ release from ER, it is proposed that the emptying of ER calcium by PA interferes with acetylcholine effects on islet function.
DISCUSSION
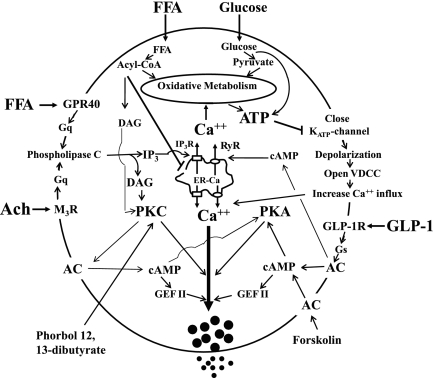
Nutrient-induced insulin secretion is potentiated by many hormones and neurotransmitters. Acetylcholine, the parasympathetic neurotransmitter, is released by intrapancreatic vagal nerve endings during the preabsorptive and absorptive phases of feeding, and it acts mainly by binding to β-cell muscarinic M3 receptors. It enhances glucose-stimulated insulin secretion (13) by the following mechanism: activation of PLC, an increase in IP3 that causes mobilization of Ca2+ from intracellular stores, and formation of diacylglycerol, which activates PKC, resulting in increased efficiency of Ca2+ on exocytosis during glucose-stimulated insulin release (Fig. 8).
Fig. 8.
Signaling in pancreatic β-cells. Free fatty acid (FFA), glucose, Ach, and GLP-1 signaling pathways in pancreatic β-cells are depicted. The main intracellular targets are PKC, PKA, Ca2+, ATP, adenylate cyclase (AC), PLC, IP3, diacylglyceride (DAG), cAMP-guanydine nucleotide exchange factor II (GEFII), and ER. GPR40, G protein-coupled receptor for FFA; M3R, muscarinic receptor type 3; GLP-1R, glucagon-like peptide-1 receptor; VDCC, voltage-dependent Ca2+ channels; IP3R, IP3 receptor; RyR, ryanodine receptor.
Despite a crucial role of acetylcholine in the regulation of insulin release, information on the influence of FFA on this regulation is lacking. Results of the present study demonstrate that PA at physiological levels acutely inhibits acetylcholine-induced stimulation of insulin secretion. This inhibition does not depend on the sequence of adding PA (at 0 or 4 mM glucose) and is also evident when a PA ramp is applied after enhanced insulin secretion is initiated by acetylcholine in the presence of moderately high glucose. In the latter case, the suppression of acetylcholine-stimulated insulin release is evident at very low-concentration PA (50 μM albumin bound). The inhibitory effect of PA is specific for acetylcholine and does not occur when glucose-stimulated insulin release is potentiated by GLP-1.
Experimental conditions that may affect the fatty acids' effect on glucose and acetylcholine-stimulated insulin secretion.
Nonesterified long-chain FA circulate in the plasma, complexed to serum albumin. Albumin has one very strong binding site with an equilibrium constant in the range of 10−7 M and two sites with constants in the range of 10−6 M. Five additional sites having constants ranging from 10−5 to 10−4 M have also been detected. The diffusion affinity of the first site for various FFA is palmitic > palmitoleic > oleic > stearic > linoleic > myristic (37). The concentration of albumin in the plasma is 4%, and the concentration of PA observed in moderately obese patients is 0.5 mM (20). In the majority of studies, BSA is used experimentally as a FFA carrier or acceptor. In man, the FFA/albumin molar ratio rarely exceeds 4 and, for the most part, is between 0.5 and 2 (10, 14). Only the free (unbound) palmitate determines insulin secretion. In the majority of studies exploring the effects of FA on insulin secretion, the concentrations of albumin is ≤1%. According to Prentki et al. (33), the free concentration of PA at 1% albumin and 0.5 mM PA is 0.9 μM, and the threshold for stimulation of insulin release in HIT cells at 10 mM glucose is ≈4 μM. The FFA concentration at the physiological concentration of 4% albumin extrapolates to much lower than those proposed thresholds. Therefore, it remains unresolved how FA might stimulate insulin release in vivo. One possibility that must be considered is that serum albumin, in addition to a passive role as a carrier of long-chain fatty acids, has a direct stimulatory effect on FA uptake through interactions with the cell surface (40). In the present study, two different experimental designs were applied. In the first, a fixed concentration of 0.5 mM of PA bound to 0.15 mM BSA was used; in the second, a PA ramp from 0 to 0.5 mM was applied at a fixed albumin concentration of 0.15 mM such that the molar PA/BSA ratio changed from 0 to 3.33. It is remarkable that, in the ramp study, PA significantly potentiated insulin release in the presence of 8 mM glucose starting at the very low concentration of 50 μM. In the same conditions, oxygen consumption did not change, suggesting that the potentiation effect of PA on insulin release may be due to the formation of acyl-CoA or complex lipids that act directly by modulating PKC or the exocytotic machinery rather than by an increased oxidative metabolism and ATP synthesis. Completely different results were observed when PA was added after 30-min preperfusion with glucose-free medium. In this case, PA did not potentiate the effect of glucose (glucose concentration was progressively increased from 0 to 25 mM) on insulin secretion. On the contrary, at high glucose concentrations (beginning from 8 mM) a moderate suppression of insulin release was seen in the presence of PA. We speculate that at low glucose concentrations PA is oxidized, resulting in increased NADH production. Elevated NADH may then decrease islet pyruvate dehydrogenase activity (due to an increase in pyruvate dehydrogenase kinase activity) (34, 35), resulting in reduced glucose oxidation and conversion of pyruvate into acetyl-CoA. However, when the glucose level rises in β-cells, FA oxidation is decreased and glucose oxidation fulfills a larger part of the cellular energy needs.
Possible mechanisms of PA inhibition of acetylcholine effect on insulin secretion.
The results of the present paper suggest that PKC, the major subcellular target of acetylcholine action (39), may not be involved in the inhibition of acetylcholine-stimulated insulin release. PA does not inhibit insulin release when stimulated by a PKC activator. Inhibition of α and β1 isozymes of PKC reduced the effect of acetylcholine and completely abolished the inhibitory effect of PA on acetylcholine-stimulated insulin release. The results also indicate that the inhibitory effect of PA may be upstream of cAMP, because the stimulation of insulin release by forskolin, an activator of adenylyl cyclase, was not reduced by PA. However, the acetylcholine-induced rise in intracellular cAMP was clearly reduced by PA. Again, this effect of PA is specific for acetylcholine and does not occur during β-cell stimulation with GLP-1. It is known that GLP-1 directly stimulates the membrane-bound adenylyl cyclase and cAMP production, leading to PKA and Epac (16, 18) activation. However, acetylcholine increases cAMP through the activation of PKC and/or rise in intracellular Ca2+ concentration (39). Both acetylcholine and GLP-1 stimulate Ca2+ release from the ER, but they employ different mechanisms. Acetylcholine stimulates ER Ca2+ release via IP3 receptors (13). GLP-1 stimulates cAMP-guanydine nucleotide exchange factor II-mediated Ca2+ mobilization through ryanodine receptors (18). Both acetylcholine and GLP-1 at high glucose promote entering of extracellular Ca2+ by causing additional depolarization of β-cell (in case of acetylcholine) (13) and by increasing levels of cytosolic and mitochondrial ATP (in the case of GLP-1) (41). Based on these data, it is proposed that alteration in Ca2+ may play a role in reduced cAMP and insulin secretion in response to acetylcholine in the presence of PA. Indeed, the acetylcholine-induced rise in intracellular Ca2+ was reduced significantly in the presence of PA. The latter effect may be due to the emptying of ER calcium stores by PA. It has been shown that saturated and, to a lesser extent, unsaturated FFAs trigger β-cell ER stress and apoptosis (4, 7, 21) through changes in ER Ca2+ handling and secondary protein malfolding. A 24-h exposure to oleate and, to a greater extent, palmitate depleted ER Ca2+ by 25 and 40%, respectively, as tested by thapsigargin-induced Ca2+ release (7). The present study indicates that such an effect of PA on ER Ca2+ release is evident even after 35–40 min of incubation with PA. Blocking IP3 receptors abolished the effect of PA on the ER and therefore also on acetylcholine stimulation of insulin secretion. It has also been shown that the GLP-1 agonists exendin-4 and forskolin protected primary β-cells and INS-1E cells against oleate- and palmitate-induced (14–24 h) ER stress and apoptosis (8). These literature data may explain the difference in the effects of PA on acetylcholine and GLP-1 potentiation of insulin secretion. Thus, the data suggest that acute emptying of ER Ca2+ stores by PA interferes with acetylcholine's action on islet function.
Effects of PA on oxygen consumption.
Current knowledge about effects of fatty acid on oxygen consumption is limited. Conget et al. (5) have shown that PA failed to affect O2 uptake at 6 mM glucose during 10- to 15-min exposure to this fatty acid. Palmitate and oleate failed to affect the oxidation of d-[U-14C]glucose or its conversion to 14C-labeled amino acids (5). Our data suggest that PA increases oxygen consumption only slightly at 0 mM glucose, but it decreased stimulation of oxygen consumption by 4 mM glucose, resulting in a lower total response to glucose (0 → 4 → 8 mM). It is known that cytosolic Ca2+ levels affect intramitochondrial free Ca2+ concentration and activity of several matrix dehydrogenases, resulting in a change of Krebs cycle turnover (24). On the basis of these data, we speculate that the emptying of ER calcium by FFA may contribute to the reduced total oxygen consumption response to glucose.
In conclusion, PA interferes acutely with acetylcholine stimulation of pancreatic β-cell metabolism and secretion, as evidenced by attenuated elevations of insulin release, intracellular cAMP, and Ca2+. In addition, PA reduces the effect of low glucose on oxygen consumption; however, it greatly potentiates insulin secretion in the presence of high glucose dose dependently. In contrast to acetylcholine, the GLP-1 effects on these parameters remain unchanged or potentiated. The results of the present study suggest that acute emptying of ER calcium by PA interferes with cholinergic stimulation of insulin secretion.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-22122 (to F. M. Matschinsky) and by the Institute for Diabetes, Obesity, and Metabolism (via NIDDK Grant DK-19525).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Heather Collins for insulin measurements and Habiba Najafi for excellent technical assistance. We also thank Drs. Adam Resnick and Karen Teff for helpful advice and comments. We are indebted to Paul Kosterin for assistance in the statistical analysis of data.
REFERENCES
- 1.Ahren B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43: 393–410, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Chen X. Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes 48: 577–583, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44: 1239–1242, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem 282: 3989–3997, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Conget I, Rasschaert J, Sener A, Leclercq-Meyer V, Villanueva-Penacarrillo M, Valverde I, Malaisse WJ. Secretory, biosynthetic, respiratory, cationic, and metabolic responses of pancreatic islets to palmitate and oleate. Biochem Med Metab Biol 51: 175–184, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Corkey BE, Deeney JT, Yaney GC, Tornheim K, Prentki M. The role of long-chain fatty acyl-CoA esters in beta-cell signal transduction. J Nutr 130: 299S–304S, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 121: 2308–2318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha DA, Ladriere L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, Marchetti P, Eizirik DL, Cnop M. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes 58: 2851–2862, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doliba NM, Qin W, Vatamaniuk MZ, Buettger CW, Collins HW, Magnuson MA, Kaestner KH, Wilson DF, Carr RD, Matschinsky FM. Cholinergic regulation of fuel-induced hormone secretion and respiration of SUR1−/− mouse islets. Am J Physiol Endocrinol Metab 291: E525–E535, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Fredrickson DS, Gordon RS., Jr Transport of fatty acids. Physiol Rev 38: 585–630, 1958 [DOI] [PubMed] [Google Scholar]
- 11.Gautam D, Han SJ, Duttaroy A, Mears D, Hamdan FF, Li JH, Cui Y, Jeon J, Wess J. Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes Metab 9, Suppl 2: 158–169, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 3: 449–461, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22: 565–604, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Havel RJ, Ekelund LG, Holmgren A. Kinetic analysis of the oxidation of palmitate-1-14C in man during prolonged heavy muscular exercise. J Lipid Res 8: 366–373, 1967 [PubMed] [Google Scholar]
- 15.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 25: 1375–1384, 1965 [DOI] [PubMed] [Google Scholar]
- 16.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53: 5–13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept. Trends Biochem Sci 17: 388–393, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holz GG, Heart E, Leech CA. Synchronizing Ca2+ and cAMP oscillations in pancreatic β-cells: a role for glucose metabolism and GLP-1 receptors? Focus on “Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach”. Am J Physiol Cell Physiol 294: C4–C6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V. Inhibition of insulin gene expression by long-term exposure of pancreatic beta cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metabolism 49: 532–536, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145: 5087–5096, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lebedev AY, Cheprakov AV, Sakadziæ S, Boas DA, Wilson DF, Vinogradov SA. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Appl Mater Interfaces 1: 1292–1304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Matter A, Kelly A, Petty TJ, Najafi H, MacMullen C, Daikhin Y, Nissim I, Lazarow A, Kwagh J, Collins HW, Hsu BY, Nissim I, Yudkoff M, Matschinsky FM, Stanley CA. Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J Biol Chem 281: 15064–15072, 2006 [DOI] [PubMed] [Google Scholar]
- 24.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 25.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51: 7–18, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin Sci (Lond) 112: 27–42, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 55, Suppl 2: S16–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Nolan CJ, Prentki M. The islet beta-cell: fuel responsive and vulnerable. Trends Endocrinol Metab 19: 285–291, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Parker SM, Moore PC, Johnson LM, Poitout V. Palmitate potentiation of glucose-induced insulin release: a study using 2-bromopalmitate. Metabolism 52: 1367–1371, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143: 339–342, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes 51, Suppl 3: S405–S413, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67: 1185–1248, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267: 5802–5810, 1992 [PubMed] [Google Scholar]
- 34.Randle PJ, Priestman DA, Mistry S, Halsall A. Mechanisms modifying glucose oxidation in diabetes mellitus. Diabetologia 37, Suppl 2: S155–S161, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem 55, Suppl: 1–11, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Sako Y, Grill VE. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology 127: 1580–1589, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Spector AA, Fletcher JE, Ashbrook JD. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry 10: 3229–3232, 1971 [DOI] [PubMed] [Google Scholar]
- 38.Teff KL, Townsend RR. Early phase insulin infusion and muscarinic blockade in obese and lean subjects. Am J Physiol Regul Integr Comp Physiol 277: R198–R208, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Tian Y, Laychock SG. Protein kinase C and calcium regulation of adenylyl cyclase in isolated rat pancreatic islets. Diabetes 50: 2505–2513, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Trigatti BL, Gerber GE. A direct role for serum albumin in the cellular uptake of long-chain fatty acids. Biochem J 308: 155–159, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J 369: 287–299, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaney GC, Corkey BE. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia 46: 1297–1312, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Yaney GC, Korchak HM, Corkey BE. Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal beta-cells. Endocrinology 141: 1989–1998, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Zawalich WS, Zawalich KC, Tesz GJ, Taketo MM, Sterpka J, Philbrick W, Matsui M. Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem Biophys Res Commun 315: 872–876, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 80: 1584–1590, 1995 [DOI] [PubMed] [Google Scholar]