Abstract
Macrophages and T-lymphocytes are known to accumulate in the white adipose tissue (WAT) of obese mice and humans, but the factors that cause this infiltration are not yet determined. Chemokines, which attract leukocytes to inflammatory sites, are candidates for this process. Macrophage inflammatory protein-1α (MIP-1α) expression is significantly elevated in WAT of obese mice and humans and positively correlates with fasting plasma insulin, but its potential role in leukocyte recruitment to WAT is unknown. MIP-1α-deficient, heterozygous, and wild-type mice were fed a Western diet (WD) for 16 wk. Plasma lipids, adipose tissue mass, energy expenditure, food intake, liver triglyceride content, and inflammatory cytokine expression were not different among genotypes. Gene expression of macrophage markers F4/80 and CD68, as well as T-lymphocyte marker CD3ε was increased in perigonadal WAT of obese WD-fed mice but was not influenced by MIP-1α expression level. Immunohistochemical analysis of WAT also showed no effect of MIP-1α on macrophage content. Two related chemokines, MIP-1β and RANTES, had reduced expression in obese male MIP-1α-deficient mice compared with wild-type controls (P ≤ 0.05). In mice fed the WD for 6 wk, WAT macrophage content was unchanged; however, CD8+ T-lymphocytes accumulated to a lesser extent in the MIP-1α-null mice. Therefore, expression of MIP-1α, as well as that of MIP-1β and RANTES, increases as a consequence of weight gain, but these chemokines may not be required for the recruitment of monocytes to WAT during diet-induced obesity in mice and may impact T-lymphocyte recruitment only at early time points after WD feeding.
Keywords: chemokine; adipose tissue macrophage; macrophage inflammatory protein-1β; regulated upon activation, normal T cell expressed and secreted
proinflammatory macrophages and t-lymphocytes accumulate in the white adipose tissue (WAT) of obese mice and humans (6, 14, 22, 25, 31, 33–35). Research within this field has focused on multiple topics related to adipose tissue macrophages (ATMs) and T-lymphocytes, but many questions remain unanswered. In particular, although many different genes have been show to be upregulated in obese WAT, the individual contribution of many of these factors to the recruitment of immune cells to WAT has not been determined.
One group of candidates for the recruitment of inflammatory cells into WAT is the family of “chemotactic cytokines,” referred to as chemokines, which induce chemotaxis of leukocytes. According to their classic definition, chemokines are small, 8- to 10-kDa proteins that share structural similarity (1, 18). Chemokines have been implicated in chronic inflammatory diseases such as atherosclerosis and rheumatoid arthritis and, recently, obesity. Many chemokines, including those mentioned here, are within the “CC” chemokine category in reference to a characteristic pair of cysteine residues at the NH2 terminus of the protein.
Macrophage inflammatory protein-1α (MIP-1α), referred to in standard nomenclature as CCL3, is a CC chemokine with increased expression in obese WAT of humans and mice. MIP-1α transcript and protein are significantly elevated in WAT of three different models of obese mice: ob/ob, db/db, and diet-induced obese (10, 35). Expression of MIP-1α and its receptors CCR1 and CCR5 is increased in omental and subcutaneous WAT from obese humans compared with normal-weight individuals (8). Furthermore, expression of MIP-1α and CCR1 in WAT is positively correlated with fasting plasma insulin concentrations in humans (8, 19, 32). Therefore, as multiple reports have shown, WAT MIP-1α transcript and protein are elevated in obesity and correlated with fasting plasma insulin; however, the consequences of this have not been established. Furthermore, whether MIP-1α contributes to the recruitment of leukocytes to WAT is not known.
To determine the role of MIP-1α in the WAT of obese mice, we used a previously generated genetic model, MIP-1α-deficient (MIP-1α−/−) mice (4). Although these mice have been used for immunology studies, to our knowledge, no one has characterized their metabolic phenotype. We hypothesized that secretion of MIP-1α from WAT increases recruitment of monocytes and T-lymphocytes during weight gain and, therefore, that diet-induced obese MIP-1α−/− mice would have fewer macrophages and T-lymphocytes in their WAT as well as lower fasting plasma insulin compared with MIP-1α+/+ mice. Surprisingly, we found that MIP-1α deficiency did not impact ATM accumulation or other metabolic parameters in Western diet (WD)-fed mice.
MATERIALS AND METHODS
Ethics statement.
All procedures were performed according to and with prior approval from the Vanderbilt University Institutional Animal Care and Use Committee.
Mice.
MIP-1α−/− mice on a C57BL/6 background were purchased from Jackson Laboratory (Bar Harbor, ME; strain B6.129P2-Ccl3tm1Unc/J) and crossed with C57BL/6 mice from our colony (originally purchased from Jackson Laboratory) to obtain MIP-1α+/− mice. Mice from this F1 generation were bred to yield MIP-1α+/+, MIP-1α+/−, and MIP-1α−/− littermates, and the colony was maintained by breeding MIP-1α heterozygous mice. We obtained female mice at the expected Mendelian ratio (24% MIP-1α+/+, 49% MIP-1α+/−, and 27% MIP-1α−/−, n = 119), in agreement with the publication in which these mice are first described (4). However, we obtained fewer MIP-1α+/+ male littermates than predicted (17% MIP-1α+/+, 57% MIP-1α+/−, and 26% MIP-1α−/−, n = 132).
At 8 wk of age, male and female littermates were switched from a rodent chow diet (CD; Lab Diets, St. Louis, MO) to the WD (TD.88137; 42% kcal from fat, 42.7% kcal from carbohydrates, 0.15% added cholesterol; Harlan Teklad, Madison, WI), which they were fed for a total of 16 wk. A second cohort of mice was kept on CD throughout the duration of the study. In both the WD- and CD-fed groups, mice were weighed weekly for a 16-wk period and were bled at 24 wk of age. Their total lean and fat mass was measured every 4 wk, from weeks 8 to 24 using nuclear magnetic resonance (NMR) with a Bruker Minispec (Woodlands, TX) housed in Vanderbilt University's Mouse Metabolic Phenotyping Center (MMPC). At 24 wk of age, mice were euthanized with isoflurane followed by cervical dislocation and were perfused with PBS. Tissues were then harvested, weighed, frozen immediately in liquid nitrogen, and stored at −70°C. In a separate study, male and female MIP-1α+/+ and MIP-1α−/− mice were maintained on the WD for 6 wk.
Genotyping.
Mice were genotyped using DNA extracted from ear tissue. The PCR protocol was obtained from Jackson Laboratory's website, and the following primers were used: 5′-ctt ggg tgg aga ggc tat tc-3′ (mutant forward), 5′-agg tga gat gac agg aga tc-3′ (mutant reverse), 5′-atg aag gtc tcc acc act gc-3′ (wild-type forward), and 5′-agt caa cga tga att ggc g-3′ (wild-type reverse).
Plasma analyses.
Following a 5-h fast, mice were anesthetized with isoflurane and bled retroorbitally using heparinized glass capillary tubes. Fasting glucose was measured on whole blood using a One-Touch glucometer (Lifescan, Milpitas, CA). Plasma was separated by centrifugation (12,000 rpm at 4°C), aliquotted, and frozen at −20°C for short-term storage or −70°C for long-term storage. Plasma total cholesterol, triglycerides (TGs), and nonesterified fatty acids (NEFAs) were measured by enzymatic colorimetric assays according to manufacturers' directions, using kits from Cliniqa (San Marco, CA) or Wako [NEFA-HR (2) kit, Richmond, VA]. Plasma fasting insulin was measured by radioimmunoassay by Vanderbilt University's MMPC Hormone Assay Core.
Energy expenditure.
After 8 wk on WD, representative male MIP-1α−/−, MIP-1α+/−, and MIP-1α+/+ mice were housed individually in OxyMax cages (in Vanderbilt's MMPC) and kept on a 12:12-h light-dark cycle. After an acclimation period of ∼15 h, oxygen consumption (V̇o2) data were recorded for a 24-h period, and energy expenditure (kcal/h) was calculated based on the (V̇o2 rate)/(V̇co2 rate) ratio using software provided by Columbia Instruments. Mice were weighed before and after being housed individually, and the initial body mass was used for energy expenditure/body mass calculations. The food in each cage was weighed at the beginning and end of the experiment to calculate food consumption per mouse.
Staining of tissue sections.
Perigonadal WAT was harvested from mice and weighed, and a portion was fixed overnight in 10% formalin, transferred to 70% ethanol, and paraffin embedded. Tissue was cut into 7-μm sections (HM325 microtome, Microm) and stained with 0.1% wt/vol toluidine blue solution (Newcomer Supply, Middleton, WI). Representative sections are shown. Images were taken at ×100 magnification. Liver sections were stained with Oil red O and hematoxylin as previously described (3). Immunohistochemistry was performed using antibody to F4/80 (purchased from Abcam, Cambridge, MA) at a 1:50 dilution.
Hepatic TG content.
After mice were killed, livers were weighed and frozen in liquid nitrogen. Measurement of TG content was conducted by the Lipid Core Lab of Vanderbilt's MMPC as previously described (26).
Real-time RT-PCR.
RNA was isolated from tissues using the RNeasy Mini Kit (Qiagen, Valencia, CA), and RNA concentration was quantified by measuring optical density at 260 nm. Bio-Rad's (Hercules, CA) iScript cDNA Synthesis kit was used to synthesize cDNA. An iQ5 thermal cycler was used in conjunction with iQ Supermix (Bio-Rad) and individual Assay-on-Demand (Applied Biosystems, Foster City, CA) primer/probe sets for each gene. The following genes were assessed: 18S (4352930E), Ccl2 (Mm00441242_m1), Ccl3 (Mm00441258_m1), Ccl4 (Mm00443111_m1), Ccl5 (Mm01302428_m1), Emr1 (Mm00802530_m1), Cd68 (Mm00839636_g1), Cd3e (Mm00599683_m1),Saa1 (Mm00656927_g1), Tnfa (Mm00443258_m1), Il-10 (Mm99999062_m1), Arg1 (Mm01190441_g1), Adipoq (Mm00456425_m1), and Foxp3 (Mm00475156_m1). Relative expression was calculated by the ΔΔCT method using 18S expression to normalize the results.
Flow cytometry.
The stromal vascular fraction was collected from WAT of 6-wk WD-fed mice by collagenase digestion, filtration through 100-μm nylon membranes, and centrifugation. Red blood cells were lysed using ACK buffer, and the cells were then blocked with Fc block. Antibody against F4/80 was purchased from AbD Serotec (Raleigh, NC), and remaining antibodies were purchased from BD Biosciences (San Jose, CA). Antibody against F4/80 was used at a 1:20 dilution; antibody against CD4 was used at a 1:50 dilution; and antibodies against CD3 and CD8 were used at a 1:200 dilution. Samples were processed on a 5 Laser LSRII machine in the Vanderbilt Flow Cytometry Core, and data were analyzed using FlowJo software. Compensations were performed on stromal vascular fractions of pooled WAT from a separate group of wild-type mice.
Statistics.
GraphPad Prism software (version 4.01) was used for all statistical analyses. Data were analyzed with two-way ANOVA and Bonferroni's post hoc test or by one-way ANOVA followed by Tukey's multiple comparison post hoc test, as indicated. Two-way ANOVA was used to assess the relative contribution of multiple effects and to check for interactions between different conditions. The OxyMax study was performed in a subset of seven to nine male mice from each group. For all other parameters studied, all mice that underwent the WD feeding were included for each analysis, and outliers were removed from the data for each individual parameter if outside the range of the mean ± 2SD. P ≤ 0.05 was considered significant.
RESULTS
Metabolic phenotype of diet-induced obese mice.
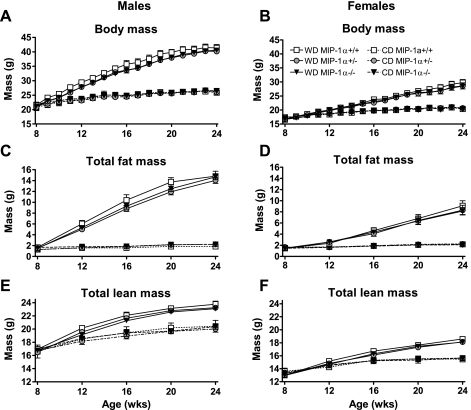
At 8 wk of age, MIP-1α+/+, MIP-1α+/−, and MIP-1α−/− littermates were placed on WD for 16 wk. Total body mass, fat mass, and lean mass significantly increased during WD feeding (diet effect in males and females was P < 0.0001 for each at 24 wk of age), but MIP-1α expression level did not affect these parameters in either sex (Fig. 1). Perigonadal and perirenal WAT, liver, kidneys, and spleen masses were unaffected by MIP-1α genotype in male and female mice on either diet (Table 1 and Supplemental Table 1; supplemental materials are found with the online version of this paper at the journal's website).
Fig. 1.
Body mass and total fat and lean mass in macrophage inflammatory protein (MIP)-1α+/+, MIP-1α+/−, and MIP-1α−/− mice. Mice were either started on Western diet (WD; solid lines) at wk 8 or kept on chow diet (CD; dashed lines). Data shown are for the 16-wk study period: body mass for male (A) and female (B) mice, total fat mass measured by NMR for male (C) and female (D) mice, and total lean mass measured by NMR in male (E) and female (F) mice. Open square, MIP-1α+/+; gray circle, MIP-1α+/−; closed triangle, MIP-1α−/−. Nos. of animals per group were as follows: CD-fed males: 4 MIP-1α+/+, 15 MIP-1α+/−, 6 MIP-1α−/−; WD-fed males: 15 MIP-1α+/+, 32 MIP-1α+/−, 23 MIP-1α−/−; CD-fed females: 6 CD MIP-1α+/+, 15 CD MIP-1α+/−, 6 CD MIP-1α−/−; WD-fed females: 20 MIP-1α+/+, 23 CD MIP-1α+/−, 24 CD MIP-1α−/−.
Table 1.
Tissue masses in mice at 24 wk of age
| Perigonadal WAT Mass |
Liver Mass |
|||
|---|---|---|---|---|
| Genotype | CD | WD | CD | WD |
| Males | ||||
| MIP-1α+/+ | 0.32 ± 0.02 | 2.18 ± 0.10 | 1.13 ± 0.09 | 2.83 ± 0.25 |
| MIP-1α+/− | 0.34 ± 0.02 | 2.02 ± 0.06 | 1.12 ± 0.02 | 2.68 ± 0.14 |
| MIP-1α−/− | 0.33 ± 0.02 | 1.98 ± 0.05 | 1.15 ± 0.03 | 3.07 ± 0.13 |
| Females | ||||
| MIP-1α+/+ | 0.27 ± 0.05 | 1.17 ± 0.14 | 0.85 ± 0.03 | 1.46 ± 0.08 |
| MIP-1α+/− | 0.26 ± 0.03 | 0.98 ± 0.12 | 0.93 ± 0.03 | 1.41 ± 0.08 |
| MIP-1α−/− | 0.23 ± 0.02 | 0.98 ± 0.09 | 0.92 ± 0.05 | 1.45 ± 0.06 |
Data shown are means ± SE in grams. MIP-1α, macrophage inflammatory protein-1α; WAT, white adipose tissue; CD, chow diet; WD, Western diet.
To compare the metabolic phenotype of MIP-1α−/− mice with those of MIP-1α+/+ and MIP-1α+/− mice more comprehensively, we measured energy expenditure of individually housed WD-fed male mice (n = 7–9 mice per genotype) after 8 wk of WD feeding. As expected, energy expenditure was elevated during the dark hours relative to the light hours (P < 0.0001, light effect). Consistent with the absence of differences in body mass and fat mass, no significant differences in energy expenditure were detected among the mice with varying MIP-1α expression levels (Supplemental Fig. S1). Likewise, daily food consumption was similar among MIP-1α−/−, MIP-1α+/−, and MIP-1α+/+ mice (Supplemental Fig. S2).
Plasma parameters.
Mean fasting blood glucose was significantly higher in MIP-1α−/− and MIP-1α+/− male mice relative to their MIP-1α+/+ littermates after 16 wk on WD (P < 0.05), but among CD-fed mice, glucose concentrations did not differ (Table 2). Fasting plasma insulin concentrations for the WD-fed male and female mice were similar among all genotypes (Table 2). Plasma NEFA concentrations in MIP-1α−/− and MIP-1α+/− male mice were significantly lower than plasma NEFAs of MIP-1α+/+ mice (P < 0.05 relative to MIP-1α+/+ mice; Table 2). The absence of MIP-1α did not alter plasma cholesterol or TGs in males or females on either diet (Table 2).
Table 2.
Plasma parameters in mice at 24 wk of age
| Genotype | n | Glucose, mmol/l | Insulin, ng/ml | TC, mmol/l | TG, mmol/l | NEFA, mEq/l |
|---|---|---|---|---|---|---|
| Chow Diet | ||||||
| Males | ||||||
| MIP-1α+/+ | 4^ | 5.66 ± 0.44 | ND | 1.86 ± 0.18 | 0.55 ± 0.02 | 1.19 ± 0.19 |
| MIP-1α+/− | 16 | 5.77 ± 0.22 | ND | 1.68 ± 0.05 | 0.54 ± 0.01 | 0.79 ± 0.10 |
| MIP-1α−/− | 7 | 5.44 ± 0.33 | ND | 1.71 ± 0.05 | 0.57 ± 0.03 | 1.05 ± 0.11 |
| Females | ||||||
| MIP-1α+/+ | 6 | 5.22 ± 0.44 | ND | 1.24 ± 0.08 | 0.50 ± 0.02 | 0.74 ± 0.06 |
| MIP-1α+/− | 15^ | 5.05 ± 0.28 | ND | 1.30 ± 0.03 | 0.52 ± 0.01 | 0.77 ± 0.05 |
| MIP-1α−/− | 6 | 5.05 ± 0.17 | ND | 1.30 ± 0.08 | 0.55 ± 0.02 | 0.80 ± 0.07 |
| Western Diet | ||||||
| Males | ||||||
| MIP-1α+/+ | 14–15 | 6.83 ± 0.28 | 4.24 ± 0.82 | 4.82 ± 0.28 | 0.50 ± 0.02 | 0.73 ± 0.06 |
| MIP-1α+/− | 29–32 | 7.70 ± 0.22* | 3.29 ± 0.32 | 4.71 ± 0.13 | 0.54 ± 0.01 | 0.49 ± 0.03† |
| MIP-1α−/− | 22–24 | 7.66 ± 0.17* | 3.42 ± 0.36 | 5.13 ± 0.16 | 0.53 ± 0.02 | 0.55 ± 0.04* |
| Females | ||||||
| MIP-1α+/+ | 18–20 | 6.55 ± 0.22 | 1.06 ± 0.10 | 2.86 ± 0.13 | 0.44 ± 0.02 | 0.45 ± 0.04 |
| MIP-1α+/− | 22–23 | 6.77 ± 0.22 | 0.81 ± 0.08 | 2.77 ± 0.10 | 0.41 ± 0.02 | 0.42 ± 0.02 |
| MIP-1α−/− | 22–24 | 6.77 ± 0.22 | 0.91 ± 0.10 | 2.84 ± 0.10 | 0.43 ± 0.02 | 0.49 ± 0.03 |
Data shown are means ± SE, Mice were 8 wk old when started on WD. Data were analyzed using 1-way ANOVA with Tukey's multiple comparison test.
TC, total cholesterol; TG, triglyceride; NEFA, nonesterified fatty acid; ND, not determined. Variation in n is due to removal of outliers (see materials and methods).
P < 0.05 and
P < 0.001 vs. WD-fed MIP-1a+/+ males.
Blood glucose measurements were obtained for only 3 (male MIP-1α+/+) or 14 (female MIP-1α+/−) mice.
Gene expression in WAT.
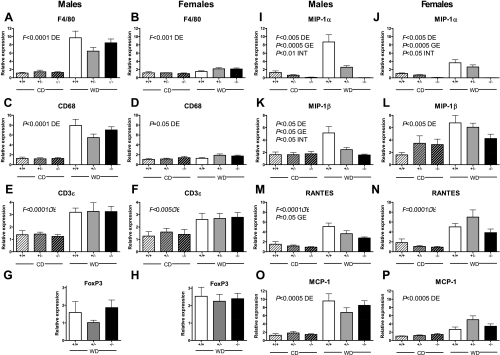
Gene expression of macrophage markers F4/80 and CD68 were significantly elevated by WD feeding in both males and females (P ≤ 0.05). However, gene expression of these macrophage markers was not influenced by MIP-1α genotype (Fig. 2, A–D). Expression of CD3ε, a general T-lymphocyte marker, was significantly increased in WD-fed compared with CD-fed mice, but this was not altered by MIP-1α deficiency (Fig. 2, E and F; P < 0.005, diet effect). T-regulatory lymphocyte infiltration of obese WAT, as measured by FoxP3 gene expression, was also not influenced by the absence of MIP-1α (Fig. 2, G and H). Regardless of diet, gene expression of MIP-1α was absent in MIP-1α−/− mice and highest in MIP-1α+/+ mice, and its expression was increased with WD feeding (Fig. 2, I and J; P < 0.005 for genotype effect and diet effect, P < 0.05 for diet × genotype interaction).
Fig. 2.
White adipose tissue (WAT) gene expression in 24-wk-old CD- and WD-fed mice. Relative gene expression is shown for male (A, C, E, G, I, K, M, O) and female (B, D, F, H, J, L, N, P) MIP-1α+/+, MIP-1α+/−, and MIP-1α−/− mice. Mice were either fed CD for 24 wk or switched from CD to WD at 8 wk of age (for a total of 16 wk of WD feeding). RNA was isolated from perigonadal WAT and used to synthesize cDNA, which was used for real-time PCR. Data were analyzed by 2-way ANOVA. DE, diet effect; GE, genotype effect; INT, diet × genotype interaction.
The expression pattern of MIP-1β was influenced by the expression level of MIP-1α such that male MIP-1α−/− mice had lower MIP-1β expression than MIP-1α+/+ and MIP-1α+/− mice (Fig. 2K). In fact, both diet and genotype significantly affected MIP-1β expression in male mice (P < 0.05 for diet effect, genotype effect, and diet × genotype interaction). MIP-1β expression was increased by WD feeding in female mice (P < 0.005), but the genotype effect was not significant (Fig. 2L). The CC chemokine RANTES was also elevated with obesity (Fig. 2, M and N; P < 0.0005), and in male mice its expression was significantly affected by MIP-1α expression level (P = 0.05). Gene expression of monocyte chemoattractant protein-1 (MCP-1), another CC chemokine, was elevated with obesity but unaffected by the level of MIP-1α expression (Fig. 2, O and P; P < 0.0005, diet effect). The proinflammatory cytokine TNFα and the insulin-sensitizing adipokine adiponectin as well as the anti-inflammatory markers arginase 1 and IL-10 [which are expressed by M2 polarized ATMs (16, 17, 37)] were expressed to a similar extent in MIP-1α−/−, MIP-1α+/−, and MIP-1α+/+ WD-fed mice (Supplemental Fig. S3).
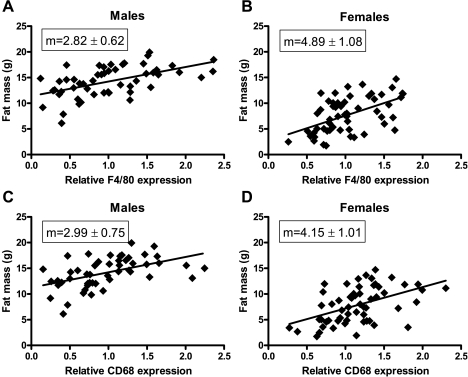
In males, after 16 wk on WD, gene expressions of F4/80 and CD68 were on average at least sixfold greater than they were in their lean counterparts. In females, F4/80 and CD68 expression in WAT was significantly elevated relative to CD-fed females, but the differences were only about twofold or less. To address the reason for these sex differences, we plotted linear regression curves of total fat mass vs. macrophage marker gene expression for males and females (Fig. 3). The slopes were not statistically different for males compared with females for either F4/80 or CD68 expression, which implies that the males had a greater fold difference in ATM recruitment than the females due to their larger fat mass.
Fig. 3.
Correlation between total fat mass and macrophage markers. Relative gene expression of F4/80 (A and B) and CD68 (C and D) are plotted vs. total fat mass of individual mice after 16 wk on WD. Data from MIP-1α−/−, MIP-1α+/−, and MIP-1α+/+ mice were analyzed together. The slope (m) of each linear regression line is shown. Slopes in A and B and in C and D do not differ significantly from each other.
WAT morphology.
WAT tissue was sectioned and stained with toluidine blue O to compare the morphology of WAT from MIP-1α−/− mice with that of MIP-1α+/+ and MIP-1α+/− mice. Adipocytes tended to be larger, and crown-like structures were more frequently observed in male mice; however, the absence of MIP-1α did not appear to influence WAT morphology in either males or females, and crown-like structures were observed in all six groups of mice (Supplemental Fig. S4, A–F). In addition, immunostaining against the macrophage surface marker F4/80 demonstrated increased numbers of crown-like structures in male mice, without MIP-1α genotype effects (Supplemental Fig. S4, G–L).
Liver phenotype.
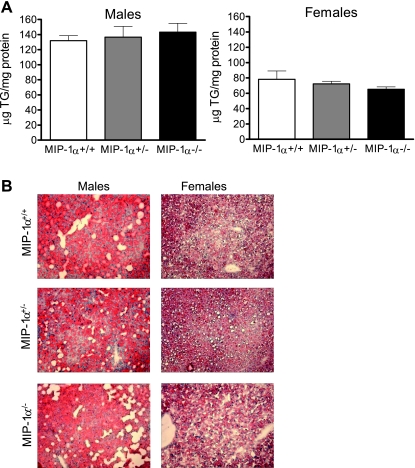
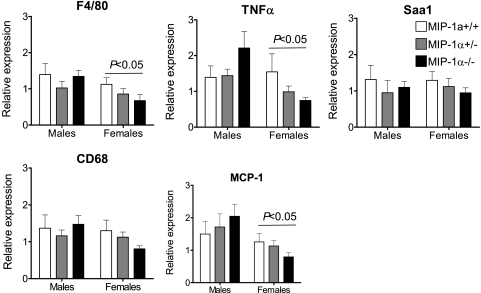
Since the liver plays a critical role in lipid metabolism and hepatic steatosis is often present in obese mice, we measured hepatic TG concentrations and visualized hepatic neutral lipid accumulation with Oil red O staining of liver sections. MIP-1α deficiency did not influence the TG content of the liver, although males had significantly more hepatic TGs than females after 16 wk on WD (Fig. 4A; P < 0.0001 for sex effect), which was also reflected by Oil red O staining (Fig. 4B). As shown in Fig. 5, absence of MIP-1α did not impact liver gene expression of F4/80, CD68, or proinflammatory serum amyloid A1, TNFα, and MCP-1. Expression of F4/80, TNFα, and MCP-1 were significantly lower in females than in males (P < 0.05).
Fig. 4.
Hepatic triglyceride (TG) content in WD-fed male and female MIP-1α+/+, MIP-1α+/−, and MIP-1α−/− mice. Quantitative analysis of hepatic TGs in mice after 16 wk of WD feeding (A) and representative Oil red O stained liver sections (B) are shown. Hepatic TGs were measured as described in materials and methods.
Fig. 5.
Hepatic gene expression in male and female mice after 16 wk on WD. RNA was isolated from liver and used to synthesize cDNA, which was used for real-time PCR. Data from males and females were analyzed by 2-way ANOVA using the same real-time PCR threshold. P < 0.05 for sex effect as indicated. Saa1, serum amyloid A1.
Six-week study.
A second cohort of male and female MIP-1α+/+ and MIP-1α−/− mice (n = 8–9 females and 4 males per group) were placed on WD for 6 wk to determine whether macrophage or T-lymphocyte infiltration into WAT was altered at an earlier time point. As in the 16-wk WD-fed mice, there were no differences in adiposity, fasting blood glucose, or plasma total cholesterol, TGs, or NEFA (data not shown). Real-time RT-PCR analysis of WAT (Supplemental Fig. S5) demonstrated no difference in expression of the macrophage markers F4/80 and CD68 or the T-lymphocyte marker CD3ε. MIP-1α was not detected in the MIP-1α−/− mice, and MCP-1 was not different between groups. In contrast to the 16-wk WD-fed mice, there was no decrease in MIP-1β or RANTES expression in the 6-wk WD-fed mice; in fact, RANTES was significantly increased in the female MIP-1α−/− mice compared with MIP-1α+/+ controls.
We also quantified macrophage and T-lymphocyte populations in the stromal vascular fraction of male mice fed the WD for 6 wk by flow cytometry (Supplemental Fig. S6). We detected no differences in F4/80-positive macrophages or CD3+ T-lymphocytes between the MIP-1α+/+ and MIP-1α−/− mice. However, after gating on the CD3+ population, the MIP-1α−/− mice had a significantly lower percentage of CD8+ cytotoxic T cells (P < 0.05).
DISCUSSION
The elevation of MIP-1α in WAT of obese humans and mice is well established. Recruitment of macrophages (27, 31, 35) and T-lymphocytes (6, 14, 22, 25, 34) to WAT has been shown to play an important role in obesity-associated insulin resistance. On the basis of its known ability to chemoattract monocytes, macrophages, and T-lymphocytes, we hypothesized that MIP-1α has an important role in the recruitment of these cells into WAT during diet-induced weight gain. Therefore, we predicted that MIP-1α−/− mice would have less accumulation of macrophages and T-lymphocytes in their WAT. However, F4/80, CD68, and CD3ε were expressed to the same degree in MIP-1α−/− mice as in MIP-1α+/+ mice when they were fed a high-fat WD for 16 wk. This suggests that MIP-1α does not contribute to the recruitment of monocytes and T-lymphocytes to WAT in mice after a long course of WD feeding. However, we did note a modest decrease in CD8+ T-lymphocytes of the 6-wk WD-fed MIP-1α−/− mice, indicating that MIP-1α may impact early recruitment of cytotoxic T cells to WAT. Chemokines are considered to play a major role in recruitment of immune cells to WAT in obesity; thus, our findings have important implications for whether MIP-1α specifically, and chemokines in general, are truly driving this process, as will be discussed below.
Although our overall conclusion is that MIP-1α deficiency does not significantly impact macrophage and T-lymphocyte recruitment and accumulation in WAT, the reduced percentage of CD8+ cells in the 6-wk WD-fed male mice implies the potential for a minor role for MIP-1α in shifting T-lymphocyte populations in WAT. This is of potential relevance, as CD8+ T-lymphocytes have been demonstrated to precede macrophage recruitment to WAT and to increase the inflammatory nature of the ATMs (22). Even so, over a longer time course, lack of MIP-1α did not impact any of the metabolic parameters we tested, leading us to conclude that absence of MIP-1α does not significantly impact metabolism in mice.
One interesting finding in our study was the observation that MIP-1α had a gene-dosage effect on MIP-1β and RANTES expression in WAT in the 16-wk WD-fed male mice. The significant reduction in RANTES and MIP-1β gene expression in the MIP-1α-null mice was detected only in male mice after 16 wk of WD-feeding, indicating that there may be diet- and/or sex-specific mechanisms by which MIP-1α regulates the expression of other chemokines. It is possible that MIP-1α regulates the expression of MIP-1β, as previous in vitro reports have established some precedence for one chemokine influencing the secretion of another chemokine in neutrophils (23, 24). In particular, MIP-1α can induce secretion of TNFα (23), which can in turn stimulate chemokine secretion, and MIP-2/CXCL2 can induce MIP-1α secretion (24). Due to the proximity of the MIP-1α gene to the MIP-1β and RANTES genes (0.01 cM from MIP-1β gene and 0.2 cM from RANTES gene on chromosome 11), distal promoter or enhancer elements of the MIP-1β and RANTES genes might have been disrupted when a portion of the MIP-1α gene was deleted to generate MIP-1α−/− mice (4). An alternative explanation is that MIP-1β and RANTES might be expressed at lower levels in the original 129 background strain and that, even after 10 back-crosses, the genomic sequences close to MIP-1α are still of the 129 origin. Regardless of the mechanisms by which MIP-1β and RANTES were reduced in MIP-1α−/− mice, these data extend the importance of our observations, because complete absence of MIP-1α along with significant reductions of MIP-1β and RANTES still had no effect on ATM accumulation in mice.
Increased expression of chemokines in obese WAT has often been assumed to indicate a role for chemokines in the recruitment of macrophages to WAT. A recent report showed that RANTES expression in human WAT positively correlates with the presence of inflammatory macrophages (13). Those authors also provided in vitro evidence for a role of RANTES in monocyte adhesion and transmigration through endothelial cell layers and that RANTES protects macrophages against free cholesterol-induced apoptosis. Other chemokines, such as CCL20, CXCL14, CXCL5, and keratinocyte-derived chemokine, have also been reported to be upregulated in obese WAT and to influence leukocyte recruitment (2, 5, 20, 21) and reviewed in Ref. 29. In contrast, the role of MCP-1 in recruitment of monocytes to WAT has been controversial (9, 11, 12, 15, 36). For example, three studies have shown that MCP-1 contributes to macrophage accumulation in WAT (11, 12, 36), whereas two studies have found no effect of MCP-1 deficiency on ATM numbers (9, 15). In our study, relative gene expression of both F4/80 and CD68 significantly and positively correlated with MCP-1 expression level in the WAT (Supplemental Fig. S7; r2 = 0.79 and =0.77, respectively, P < 0.0001 for the slope's deviation from zero). Therefore, our data are in agreement with a correlation between MCP-1 expression in WAT and ATM numbers. However, the question of whether chemokines play an active role in macrophage recruitment to WAT remains. Based on the published literature and our current results, what can be determined is that elevation of any particular chemokine in WAT in obesity, or correlation of chemokine expression with ATM numbers, does not necessarily indicate that its absence will prevent or even reduce macrophage and T-lymphocyte recruitment to WAT. It is also important to remember that absence of an effect of a chemokine in mice does not preclude that chemokine from playing an important role in ATM accumulation and insulin resistance in humans.
Upregulation of MIP-1α is likely a consequence of the changes that occur in obese WAT such as excess exposure to lipids and inflammatory factors. Both murine and human macrophages express MIP-1α in vitro upon stimulation with very-low-density lipoprotein and proinflammatory stimuli (26, 37). In addition, administration of free fatty acids upregulates MIP-1α release by 3T3-L1 adipocytes via NF-κB, a transcription factor that regulates inflammatory genes (10). Therefore, we believe that initial changes in the WAT during the onset of obesity secondarily induce MIP-1α production by immune cells. In agreement with this, MIP-1α protein is elevated in WAT of mice after 20 wk of high-fat diet feeding but not after 1 wk (10). In contrast, other chemokines, including MCP-1, MCP-2, MCP-3, and MRP-2, are produced in WAT in response to 1 wk of high-fat diet feeding (10). In addition, a high-fat diet induces early infiltration of T-lymphocytes into WAT in mice (22). Taken within the context of the literature, our data indicate that elevated MIP-1α in WAT is a consequence of obesity, but its contribution to WAT inflammation in obese mice remains unknown. Furthermore, a role for MIP-1α in ATM accumulation and insulin resistance in humans cannot be ruled out.
We predicted that MIP-1α−/− mice would have lower fasting glucose and plasma insulin concentrations based on the improved insulin and glucose tolerance observed in other genetic models of chemokine deficiency, specifically in MCP-1−/−, CCR2−/−, and CXCL14−/− mice (12, 20, 30) and the fact that expression of MIP-1α in WAT is positively correlated with fasting plasma insulin concentrations in humans (8, 19, 32). However, after 16 wk on WD, male MIP-1α−/− and MIP-1α+/− mice had higher fasting blood glucose levels than male MIP-1α+/+ mice, and blood glucose concentrations did not differ among female mice. The cause of elevated blood glucose, but not plasma insulin, in male MIP-1α−/− and MIP-1α+/− mice is not known. It is possible that MIP-1α−/− and MIP-1α+/− mice have minor pancreatic dysfunction, which could inhibit a corresponding upregulation in insulin production when challenged with WD and that estrogen in the female mice protects against this effect. In that case, elevated circulating glucose could subsequently increase the percentage of NEFAs that are reesterified in the WAT, driving down the plasma NEFAs, as was observed in male MIP-1α−/− and MIP-1α+/− mice. Alternatively, the lipolytic rate may be decreased in male MIP-1α−/− and MIP-1α+/− mice, in which case not only NEFAs but also plasma glycerol concentrations would be decreased. Future experiments will be necessary to determine the source of these differences in plasma glucose and NEFAs.
Similar to a recent report by Clegg et al. (7), our data point to a sex difference in response to high-fat diet feeding. Male mice gained more total body mass and adipose tissue mass than female mice (Fig. 1, A–D). Furthermore, 16 wk of WD feeding induced more dramatic increases in ATM numbers, plasma cholesterol, plasma insulin, and hepatic steatosis in males than in females. Thus, female mice seem to be relatively protected from the adverse metabolic effects of high-fat feeding compared with male mice. This is likely due to the protective effect of estrogen, as Clegg's group also showed that inflammatory gene expression and crown-like structures in WAT of ovariectomized females increased compared with sham-operated females (7).
We have recently used another model of MIP-1α deficiency to investigate its role in atherosclerosis. In this model, we lethally irradiated low-density lipoprotein receptor-deficient (LDLR−/−) mice and then transplanted them with bone marrow from MIP-1α−/− or MIP-1α+/+ mice. This yielded mice with MIP-1α deficiency only in their bone marrow-derived cells (MIP-1α−/−→LDLR−/−) and mice capable of expressing MIP-1α in all of their cells (MIP-1α+/+→LDLR−/−). Similar to the WAT phenotype we observed in MIP-1α−/− mice, diet-induced obese MIP-1α−/−→LDLR−/− mice did not differ from MIP-1α+/+→LDLR−/− mice in their expression of macrophage and T-lymphocyte markers in their WAT (unpublished data). Thus, two different models of MIP-1α deficiency in diet-induced obese mice indicate that MIP-1α is not required for monocyte and T-lymphocyte accumulation in WAT.
One caveat to our study is the use of WD, which is rich in cholesterol. Dietary cholesterol has been shown to increase ATM accumulation (28). Thus, the increased cholesterol content of the WD used in our studies most likely accelerated the ATM accumulation. It is possible that an effect of MIP-1α deficiency would have been detected in a high-fat diet that did not contain cholesterol. However, even if this were the case, the question still remains of how important MIP-1α is to ATM recruitment if effects are only observed under certain dietary conditions.
In conclusion, our data demonstrate that simultaneously lowering the expression of three different CC chemokines, MIP-1α, MIP-1β, and RANTES, does not impact macrophage accumulation in WAT of diet-induced obese mice, has only mild effects on T-lymphocyte populations, and does not alter whole body metabolic phenotype. This implies that, although these proinflammatory chemokines are elevated in obese WAT, their absence can be compensated for by other factors that promote the accumulation of ATMs, leading to the downstream metabolic consequences of diet-induced obesity.
GRANTS
This work was funded by a Career Development Award from the American Diabetes Association (1-07-CD-10) to A. H. Hasty. B. K. Surmi was also supported by a predoctoral fellowship from the American Heart Association (0815231E). Insulin assays were performed in the Analytical Services Core of the Diabetes Research and Training Center/Mouse Metabolic Phenotyping Center (DK-20593/DK-59637). Hepatic TG analyses were performed in the Lipid Core of the MMPC. Flow cytometric analysis was performed in the Flow Cytometry Core of the Vanderbilt University Medical Center's Digestive Disease Research Center supported by National Institutes of Health Grant P30 DK-058404.
DISCLOSURES
No conflicts of interest are reported by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to members of the Hasty laboratory for their thoughtful comments on this manuscript and for the technical assistance of Kirk Gerrald and Yan Cui.
REFERENCES
- 1.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610–621, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J, Annicotte JS, Schmidt J, Mataki C, Yamamoto H, Sanches R, Guma A, Stich V, Vitkova M, Jardin-Watelet B, Renard E, Strieter R, Tuthill A, Hotamisligil GS, Vidal-Puig A, Zorzano A, Langin D, Fajas L. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab 9: 339–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coenen KR, Hasty AH. Obesity potentiates development of fatty liver and insulin resistance, but not atherosclerosis, in high-fat diet-fed agouti LDLR-deficient mice. Am J Physiol Endocrinol Metab 293: E492–E499, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 269: 1583–1585, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun 384: 482–485, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 34: 989–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab 93: 3215–3221, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56: 2242–2250, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 58: 104–115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602–26614, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keophiphath M, Rouault C, Divoux A, Clément K, Lacasa D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol 30: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28: 1304–1310, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes 57: 1254–1261, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57: 3239–3246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 338: 436–445, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Murdolo G, Hammarstedt A, Sandqvist M, Schmelz M, Herder C, Smith U, Jansson PA. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J Clin Endocrinol Metab 92: 2688–2695, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Nara N, Nakayama Y, Okamoto S, Tamura H, Kiyono M, Muraoka M, Tanaka K, Taya C, Shitara H, Ishii R, Yonekawa H, Minokoshi Y, Hara T. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem 282: 30794–30803, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Neels JG, Badeanlou L, Hester KD, Samad F. Keratinocyte-derived chemokine in obesity: expression, regulation, and role in adipose macrophage infiltration and glucose homeostasis. J Biol Chem 284: 20692–20698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol 78: 167–177, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4). Eur J Immunol 36: 2025–2034, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res 47: 1406–1415, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56: 2910–2918, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 28: 685–691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol 52: 27–36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westerbacka J, Corner A, Kolak M, Makkonen J, Turpeinen U, Hamsten A, Fisher RM, Yki-Järvinen H. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am J Physiol Endocrinol Metab 294: E841–E845, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15: 921–929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115: 1029–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 14: 1353–1362, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 31: 1420–1428, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.