Abstract
XXY men (Klinefelter syndrome) are testosterone deficient, socially isolated, exhibit impaired gender identity, and may experience more homosexual behaviors. Here, we characterize social behaviors in a validated XXY mouse model to understand mechanisms. Sociability and gender preference were assessed by three-chambered choice tasks before and after castration and after testosterone replacement. Metabolomic activities of brain and blood were quantified through fractional synthesis rates of palmitate and ribose (GC-MS). XXY mice exhibit greater sociability than XY littermates, particularly for male mice. The differences in sociability disappear after matching androgen exposure. Intact XXY, compared with XY, mice prefer male mice odors when the alternatives are ovariectomized female mice odors, but they prefer estrous over male mice odors, suggesting that preference for male mice may be due to social, not sexual, cues. Castration followed by testosterone treatment essentially remove these preferences. Fractional synthesis rates of palmitate are higher in the hypothalamus, amygdala, and hippocampus of XXY compared with XY mice but not with ribose in these brain regions or palmitate in blood. Androgen ablation in XY mice increases fractional synthesis rates of fatty acids in the brain to levels indistinguishable from those in XXY mice. We conclude that intact XXY mice exhibit increased sociability, differences in gender preference for mice and their odors are due to social rather than sexual cues and, these differences are mostly related to androgen deficiency rather than genetics. Specific metabolic changes in brain lipids, which are also regulated by androgens, are observed in brain regions that are involved in these behaviors.
Keywords: aneuploidy, Klinefelter syndrome, testosterone, X-inactivation, brain lipids
klinefelter syndrome (KS) was first described in 1942 and its chromosomal etiology identified in 1959 (11, 16). It is the most frequent sex chromosome aneuploidy in human males, with an incidence of 1 in 500 male conceptions and 1 in 800–1,000 live male births (3, 22, 34). KS patients are males with an extra X chromosome (XXY), and the syndrome is typified by reproductive dysfunction such as androgen deficiency and associated sexual dysfunction, small testicular size, gynecomastia, delayed onset of puberty, infertility, and tall stature as well as nonreproductive characteristics, including dyslexia, slowed learning, and social isolation (17, 40, 42). KS males also exhibit higher rates of attention deficit hyperactivity disorder (AD/HD) and psychiatric diseases such as schizophrenia and depression. These clinical and behavioral features of KS could be explained as genetic and/or hormonal (testosterone deficiency). Others have also suggested that KS is a useful model to dissect the genetic bases for complex cognitive and behavioral disorder and that brain-expressed genes that escape X inactivation are plausible candidates (7).
Population-based studies show that men with KS are more likely to complain of decreased sexuality and reduced sexual satisfaction, to engage in homosexual activities, and to exhibit impaired male gender identity (2, 39, 48). Difficulty relating with male peers, social isolation and, in the extreme, clinical autism can occur (2, 15). These data were obtained from double-blinded inquiries that incorporate more detailed individual assessments, but the small sample sizes of XXY men examined (no more than 14 in any one of these studies) are important limitation. Although it has been suggested that there is increased homosexual behavior in KS compared with XY men, the issue is unresolved due to potential ascertainment bias (35). In contrast, social isolation is generally accepted to be a phenotypic characteristic of KS males (4, 26, 45) but also remains difficult to fully characterize through epidemiological strategies. Establishing whether these social behaviors are coincidental with androgen deficiency requires interventional studies that cannot be undertaken systematically in humans. Furthermore, certain tissues such as the brain can rarely be obtained from humans to directly study biochemical changes that may underpin cognitive or behavioral alterations.
To better understand pathophysiological processes in KS, we have developed a XXY mouse model that exhibits androgen deficiency, defective spermatogenesis, and impaired learning, analogous to the hormonal, testicular, and cognitive phenotype of human KS (20). The purpose of this study was to characterize social behaviors and to determine the role of genetic factors and/or systemic testosterone exposure on sex preference and to dissect the mechanisms by which social behaviors (in particular, social isolation) arise. To scrutinize potential biochemical mechanisms underlying the behavioral alterations, we evaluated the metabolomic activity of regions of the brain known to be important (although not exclusively) for sexual preference: namely the hypothalamus, amygdala, and hippocampus. We examined palmitate (C16:0) metabolism in these regions specifically, because this saturated fatty acid is implicated in brain physiology and pathophysiology in a manner that could possibly apply to XXY mammals. Palmitate metabolism is important for neuronal action, neurotransmitter trafficking, cellular energy metabolism, and the release and action of several hormones (8, 12, 24, 43, 46), and its alterations are involved in abnormal brain development and brain disorders (8, 30). Thus, changes in palmitate metabolism could modify any of these parameters that could plausibly alter the neuronal control of behavior and sexual preference. To our knowledge, the metabolism of any brain lipid, including palmitate, has not been examined in the brains of any XXY mammal.
In this study, we hypothesized that XXY mice would exhibit different social behavior than their XY littermates, that these behaviors would be partly regulated by androgens, and that the XXY brain would exhibit altered androgen-regulated neuronal lipid metabolism. To evaluate genotype-phenotype interactions with testosterone on responses to novel social cues and preferences for other male vs. female mice or their odors, we used a three-chamber social approach procedure designed to test sociability and social preference. The alternate female stimulus in the sex experiments were an ovariectomized mouse, odors from ovariectomized mice, and odors from estrous mice in order to probe social (ovariectomized cue), rather than sexual (estrous cue), preferences. To understand cellular metabolism, we studied the fractions of newly synthesized (FNS) palmitate and ribose molecules in the brain and blood using in vivo deuterated water (2H2O) as tracer and gas chromatography-mass spectrometry (GC-MS) mass isotopomer distribution analysis (MIDA).
MATERIALS AND METHODS
Animals.
Breeding pairs of C57BL/6EiJ XY* male and C57BL/6J XX female were initially purchased from The Jackson Laboratory (Bar Harbor, ME). XXY mice (41, XXY) and their littermate XY mice (40, XY) were produced in the fourth generation from our breeding colony as previously described (9, 10, 20). In the first generation, XY* (C57BL/6JEi-Y*) males were mated to normal XX females to produce XY*x females. In the second generation, XY*x females (identified by chromosome analysis; see below) were mated to normal XY males. Because the X and Y*x chromosomes do not segregate properly during female meiosis, sex chromosome aneuploidy occurs frequently among the offspring of XY*x females, so that about one-third of the male offspring are XYY*x males. In the fourth generation, XYY*x males were mated with normal XX female, so that about one-half of all male offspring were XXY males. All opposite-sex mating partners in each generation of this breeding scheme were wild-type C57BL/6J mice from the Jackson Laboratory. The animal breeding colony was established and housed in a standard animal facility, three to four animals per cage, under controlled temperature (22°C) and photoperiod (12-h light, 12-h darkness) with free access to water and mouse chow. Animal breeding, handling, and experimentation were in accordance with the recommendations of the American Veterinary Medical Association and were approved by the Institutional Animal Care and Use Review Committee of Los Angeles Biomedical Institute.
Fibroblast culture and karyotype analysis.
Standard karyotyping was performed on cultured fibroblasts obtained from earclips in adult mice as previously described (20). Briefly, a 1- to 2-mm2 section of tissue was dissected from a piece of ear. The sample was minced and digested with 1.25% trypsin (Gibco, Invitrogen, New York, NY) for 30 min, followed by collagenase (Gibco Invitrogen) for 1.5 h at 37°C. The dispersed cells were suspended in Amino-max-II medium (Gibco, Invitrogen), which supports the growth of anchored fibroblast cells. The cells were placed in flasks and cultured for 5–7 day at 37°C in a CO2 incubator. Once enough colonies were formed, KaryoMAX Colcemid solution (Gibco, Invitrogen) was added to stop mitotic division. Cultured fibroblasts were harvested after a minor digestion with trypsin-EDTA solution (Gibco, Invitrogen). The harvested cells were suspended in 0.075 M potassium chloride solution (Gibco, Invitrogen) and incubated in a water bath (37°C) for 20 min, fixed in a mixture of methanol and acetic acid (3:1 methano-acetic acid), spread on clean glass slides, and air dried for fluorescence in situ hybridization (FISH) analysis. Images were examined with a Zeiss fluorescence microscope using Image-Pro Plus software.
Social approach behavior and sex preference in intact, castrated, and testosterone-substituted mice (experiments 1–4).
We determined the sociability and sex preference of adult 4- to 9-mo-old mice by using a three-chambered choice task to assess social approach behavior as previously described (28, 31). The C57BL/6J background strain has been widely studied using this three-chambered apparatus (29, 32, 49). After a fixed period of habituation to the apparatus, the subject (test) mouse was placed in the center chamber and could choose to explore either the left or right chamber through a single doorway located in each dividing wall (Fig. 1A). All three chambers as well as the external environment can be observed from anywhere within the 20 × 12 × 10.25-in. apparatus because it is entirely constructed of clear Plexiglas. The apparatus was placed in the middle of a symmetrical sound-attenuated room; background white noise was broadcast, and the environment was illuminated with a house light (a light diffuser that was located outside of the operant chamber). All equipment was thoroughly cleaned with water between experiments and with alcohol at the end of each day, as previously described (38).
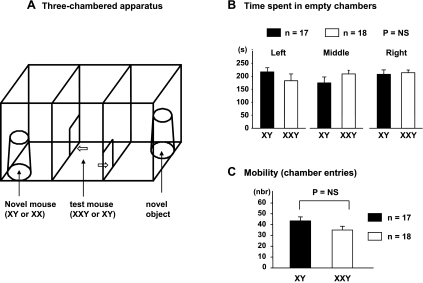
Fig. 1.
A: three-chambered social approach task. An unfamiliar stranger mouse and a novel object (plastic brick) are each enclosed in separate aerated enclosures which are then placed in the left and right main chambers, respectively. The separate enclosures are weighed down to prevent escape. The subject (test) mouse is in the middle chamber and can enter either chamber through a single doorway in each dividing wall. Time spent in each chamber (B) and locomotor activity (C) did not significantly differ between XXY and XY mice during the habituation phase (i.e., when all chambers were empty) when assessed by ANOVA. NS, not significant.
The experiments were conducted in the afternoon (i.e., during the light phase of a 12:12-h, 8 AM to 8 PM light-dark cycle) by one of three technicians certified to handle mice and who followed a written protocol. Social approach behaviors assessed by a three-chambered apparatus yield identical results irrespective of whether testing is performed during light or dark phase (49). Once the video recording commenced, the technician left the room. Exploratory behavior was videotaped using Sony DCR-DVD92 Handycam digital Camcorder w/20x Optical Zoom, ×800 digital zoom Carl Zeiss (Japan). These digital recordings were analyzed by two independent pairs of observers blinded to the identity of the mice who quantified time in each chamber. The mouse entered a chamber when all four paws were in one chamber (31).
For experiment 1, test mice were studied intact, and for experiment 2, another group of mice was studied 2 wk after castration and 2 wk after testosterone replacement. For experiments 3 and 4, the same test mice were studied intact 2 wk after castration and 2 wk after testosterone replacement. Routine surgical castration and implantation of a fixed subcutaneous dose (1-cm Silastic capsule) of testosterone were performed under standard isoflurane anesthesia, as previously described by our laboratory (13). The testosterone capsules were implanted 3 wk after surgical castration and results in plasma testosterone concentrations were comparable with those of intact young wild-type mice (see results).
Adult female mice were ovariectomized under standard isoflurane anesthesia and in sterile conditions. Briefly, a 5-mm incision to expose the pelvic cavity was made between the lowest rib cage and the proximal end of the anterior hindlimb. Muscle fibers were separated by blunt dissection in a dorsal-ventral direction, and the ovary was exteriorized by grasping the fat pad. A ligature was placed at the junction of the oviduct and uterus to allow removal of the ovary and oviduct. The uterus was then returned to the abdominal cavity, the abdominal muscle layer sutured, and the skin incision closed with wound clips. The same procedure was performed on the contralateral side.
Experiment 1: assessment of sociability in intact mice.
The original experiment was performed with 17 XXY and 18 XY adult littermates (including a habituation period during the first 10 min) to assess sociability (experiment 1, second 10 min), as previously validated (28, 31). At the beginning of all phases, the test mouse was returned to the center (empty) chamber (Fig. 1A). For the sociability testing (experiment 1, 10 min), an unfamiliar C57BL/6J mouse (stranger male or stranger ovariectomized female) from a separate colony with no prior contact with the subject mice was placed in the left chamber, a novel inanimate object (plastic brick) was placed in the right chamber, and the middle chamber remained empty. As a minor modification of the original description (and following discussion with an experienced mouse behavior scientist, J. D. Jentsch), an inanimate object was used as the control for novelty rather than an entirely empty chamber. Whether a male or female stranger mouse was introduced was determined from a randomization list generated in a balanced fashion. Seven XXY and eight XY mice were exposed to a male stranger and 10 other XXY and 10 other XY mice were exposed to a female stranger.
The stranger mice were individually housed in truncated, conical, transparent plastic enclosures that contained holes to allow nose contact and circulation of air (and odors) but prevented fighting or sexual consummatory activity. The enclosures had floors and roofs without holes, so that odors could not be transferred to the walls of the three-chambered apparatus. The stranger mice were adult males or ovariectomized females that had been previously habituated to placement into these enclosures. The inanimate object was also housed analogously.
Subsequent experiments were performed with new sets of mice to further characterize sociability and sex preference after analysis of the original experiment showed increased sociability and possible differences in sex preference between intact XY and XXY mice (see results). Multiple stranger mice, objects, and odors from mice were available and exposures carefully coordinated, so that test mice were always exposed to a novel stimulus even with repeated testing.
Experiment 2: influence of testosterone on sociability.
In experiment 2, seven XXY and seven XY adult littermates were allowed to choose between a stranger male mouse and an unfamiliar inanimate object. This experiment was analogous to experiment 1, except that the test mice were studied 2 wk after castration and 2 wk after testosterone replacement. This experiment was designed to determine whether the increased sociability (preference for a novel mouse instead of a novel inanimate object) exhibited by intact XXY mice (see experiment 1, results) was due to karyotype per se or coincident androgen deficiency by testing mice after matching testosterone exposure in a low (castrated) or high (testosterone-treated) state.
Experiment 3: sex preference of intact, castrated, and testosterone-replaced mice.
In experiment 3, five XXY and six XY adult littermates were allowed to choose between a stranger male and an unfamiliar ovariectomized female mouse. Test mice were studied intact 2 wk after castration and 2 wk after testosterone substitution, and behaviors were recorded for 5 min following a report that this shorter assessment period was sufficient (31).
Experiment 4: odor stimuli for sex preference: dissecting social from sexual preference.
In experiment 4, six XXY and eight XY littermate mice were given a 5-min opportunity to cohabitate a chamber with 1) male odors or ovariectomized female odors, followed by another 5-min opportunity to choose 2) male odors or estrous female odors. Sequential 5-min opportunities are sufficient for this purpose (31). The novel male and the novel ovariectomized female odors from experiment 4a were replaced with estrous female odors and different novel male odors during experiment 4b, respectively. For odor collection, cotton wool (original size of 10 × 5 × 3 cm for each cage) was spread under the standard bedding material and collected 24 h later from four cages. Two cages separately housed four adult males and four adult ovariectomized females (used for experiment 4a), and two other cages housed five other adult males and five estrous females (used for experiment 4b). The estrous state was determined from vaginal smears. All cotton wool from one cage was used in each odor experiment. These experiments were designed to determine the extent to which sex preference behaviors were triggered by olfactory signals alone and to separate social (ovariectomized) from sexual (estrous) cues.
Metabolomic experiments: GC-MS MIDA and FNS determination.
Three separate age-matched groups (n = 4 each group) of adult 4- to 5-mo-old male mice were studied: 1) XXY-mice, 2) their littermate XY controls, and 3) XY mice without testicular androgen production and action. Androgen deprivation was achieved with a single subcutaneous injection of long-acting gonadotropin-releasing hormone antagonist (GnRH-A), acyline [20 mg/kg body wt (BW), kindly provided by Dr. Richard P. Blye, Contraceptive and Reproductive Health Branch, National Institute of Child Health and Human Development, National Institutes of Health] in combination with an antiandrogen, flutamide, in the form of subcutaneously implanted pellets (25 mg; Innovative Research, Sarasota, FL) as previously described by our laboratory (47). The mice were given deuterated water, 2H2O, tracer for 5 days (concurrently with androgen ablation in the androgen deprivation group), after which blood and regions of the brain were sampled (18). Mice were injected with heparin (130 IU/100 g BW ip) 15 min before being killed by cervical dislocation followed by immediate decapitation, and harvested tissues were snap-frozen in liquid nitrogen. Study procedures (including androgen deprivation and tissue harvesting) were performed in this manner to minimize potential effects of anesthetics on brain lipid synthesis. The brain region of interest, i.e., amygdala, hippocampus, or hypothalamus, was dissected following standard anatomic landmarks. The entire region of interest, rather than any subregion, was collected to ensure that inadvertent collection and comparison of smaller subregions could not occur. Furthermore, the left and right amygdalas of an individual mouse were pooled to avoid lateralization as a potential confounder.
GC-MS MIDA was used to measure the changes in the masses (resulting from incorporation of the deuterium atoms, instead of hydrogen ones, into the newly synthesized molecules) and the proportions of the newly synthesized (or FNS) palmitate (c16:0) and ribose molecules (18). Briefly, lipids and ribose were separated from brain tissues and blood by TRIzol extraction (19) and derivatized, and all measurements were performed in triplicate with appropriate standards for each run using Hewlett-Packard (Palo Alto, CA) model 5973 Mass Selective Detectors connected to model 7683 injectors and model 6890 gas chromatographs (18).
Assays.
Blood was collected at the time of death by cardiac puncture, and serum was stored at −20°C until analyses. Testosterone concentrations in plasma were measured by RIA using a kit form DPC (Coat-a-Tube, Torrance, CA), as reported previously (20). The minimal detection limit in the assay was 0.25 ng/ml, and the intra- and interassay coefficients of variations were ∼10%.
Statistical analysis.
The difference in time spent in one chamber compared with the other represents relative time preference for that chamber and was assessed by a one-sample Student's t-test against a null hypothesis of zero difference. Relative preference for that chamber between XY and XXY mice was assessed by two-sample Student's t-test. Relative preference was first adjusted for baseline chamber preference determined during the habituation phase to remove any potential influence of chamber lateralization. Repeated-measures ANOVA (mixed models with compound symmetrical covariance structures) was also utilized to determine interaction and overall effects in those experiments where the same test mice were studied on more than one occasion (see below).
We assessed sociability by comparing the relative amount of time XY and XXY mice cohabited a chamber containing a newly introduced mouse, using two-sample Student's t-test as previously described (28, 31). Experiments 2, 3 and 4 included repeated measurements in the same test mice under different conditions and was therefore also analyzed by repeated-measures ANOVA using models that included main effects, as well as other models that explored interactions. For statistical comparisons of the metabolomic data (GC-MS MIDA, FNS), data obtained from the replicate experiments (means ± SE) were analyzed by ANOVA followed by Bonferroni-adjusted two-tailed t-tests. Analyses were performed with SAS v. 9.1 (SAS Institute, Cary, NC) and statistical significance construed from a two-tailed P < 0.05. Data are means ± SE.
RESULTS
XXY mice exhibit increased social interest toward stranger males compared with inanimate objects (experiment 1).
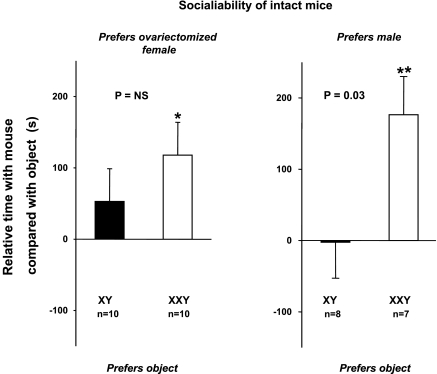
The time spent in each chamber (Fig. 1B) and the locomotor activity (Fig. 1C) did not significantly differ between XXY and XY mice during the habituation phase (i.e., when all the chambers were empty). During the second 10 min (sociability assessment), XXY mice chose to occupy the chamber containing an introduced stranger mouse vs. an inanimate object for a longer duration of time than their XY littermates. A statistically significant difference was unveiled only if the introduced stranger mouse was male rather than an ovariectomized female (Fig. 2).
Fig. 2.
Sociability test (experiment 1). Relative time preference of XXY (open bars) or XY (filled bars) subject for a stranger mouse or an inanimate object is shown. Data are means and SE of difference in time spent in the chamber containing the stranger mouse vs. time spent in the chamber containing the novel inanimate object, stratified by the sex of the stranger mouse (OVX female, left; male, right). Positive differences indicate preference for the mouse, whereas negative differences indicate preference for the object. Significant nonzero differences were assessed by one-sample Student's t-test and are indicated by * or ** for P < 0.05 or P < 0.01, respectively. Whether these time differences differed between XXY and XY mice was assessed by two-sample Student's t-test as shown by P values.
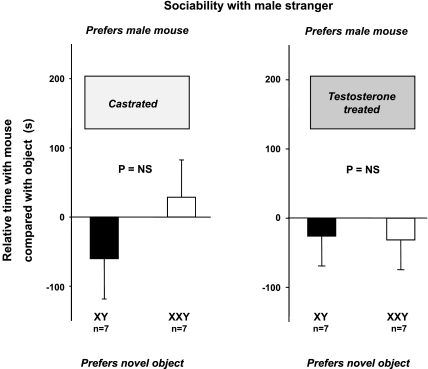
Castration followed by testosterone replacement removes increased social interest toward stranger males previously exhibited by intact XXY mice (experiment 2).
XXY and XY mice were studied after castration and then after treatment with testosterone. Plasma hormone concentrations were measured to confirm equivalent systemic hormone exposure. We have previously shown (20) that plasma testosterone in intact XXY mice is less than for XY mice (P < 0.05): 2.6 ± 0.8 (n = 7) and 5.7 ± 0.8 ng/dl (n = 7). Plasma testosterone concentrations after castration were undetectable in both groups (XYs and XXYs). Plasma testosterone concentrations after testosterone implantation were increased to intact adult male mice levels, and equivalently: 5.9 ± 3.0 and 7.2 ± 2.6 ng/dl in XXY (n = 5) and XY (n = 8) mice, respectively (P = 0.46).
Castration (Fig. 3, left) followed by testosterone treatment (Fig. 3, right) removed the increased interest toward stranger male mouse compared with the inanimate object that XXY mice exhibited compared with XY littermates (P > 0.05 by unpaired t-test). Repeated-measures ANOVA, using models of karyotype (XXY vs. XY), treatment (castration vs. testosterone), and with or without the interaction, confirmed these findings.
Fig. 3.
Sociability test after castration (left) and then after testosterone replacement (right) (experiment 2). Relative time preference of XXY (open bar) or XY (filled bar) subject mouse for a novel male stranger mouse or an inanimate object. Data are means and SE of the difference in time spent in the chamber containing the mouse vs. time spent in the chamber containing the novel inanimate object, stratified on whether the subject mouse was castrated (left) or castrated and then testosterone treated (right). Hence, positive differences indicate preference for the mouse, whereas negative differences indicate preference for the object. Significant nonzero differences were assessed by one-sample Student's t-test and are indicated by * or ** for P < 0.05 or P < 0.01, respectively. Whether these time differences differed between XXY and XY mice was assessed by two-sample Student's t-test as shown by P values.
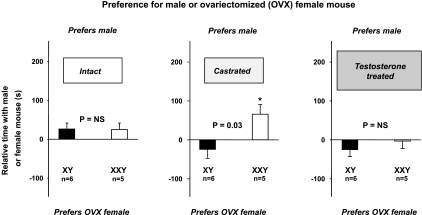
Castrated XXY mice, compared with castrated XY littermates, prefer male mice over ovariectomized female mice when mice of different sex are presented at the same time (experiment 3).
Whereas the preceding experiments examined social approach behaviors, these following experiments were designed to directly assess sex preference for simultaneously introduced and therefore equally novel mice of the opposite sex. Castrated XXY mice, compared with castrated XY littermates, significantly prefer male mice over female mice (P = 0.03; Fig. 4). However, no significant differences in sex preference were observed between XXY and XY mice in the intact or castrated with testosterone replacement (P > 0.05, for each by unpaired Student's t-test; Fig. 4). Repeated-measures ANOVA showed a significant impact of karyotype (P = 0.04) but no impact of treatment (P = 0.12), and a nonsignificant interaction effect (P = 0.08). Over all, XXY mice significantly (P = 0.04) spent more time with male instead of ovariectomized female mice (relative time: 29 ± 12 s) compared with XY mice (−8 ± 11 s).
Fig. 4.
Castrated XXY mice prefer male mice over OVX female mice when mice of different sex are presented at the same time (experiment 3). Data from intact (left) castrated (middle), and then testosterone-treated (right) mice are shown. Mice had to choose between a male mouse or an OVX female mouse. Data are means and SE of the difference in time spent in the chamber containing the male mouse stimulus vs. time spent in the chamber containing the female stimulus. Hence, positive differences indicate preference for the male mouse stimulus, whereas negative differences indicate preference for the female mouse stimulus. Significant nonzero differences were assessed by one-sample Student's t-test and are indicated by * or ** for P < 0.05 or P < 0.01, respectively. Whether these time differences differed between XXY (open bar) and XY (filled bar) mice was assessed by two-sample Student's t-test as shown by P values.
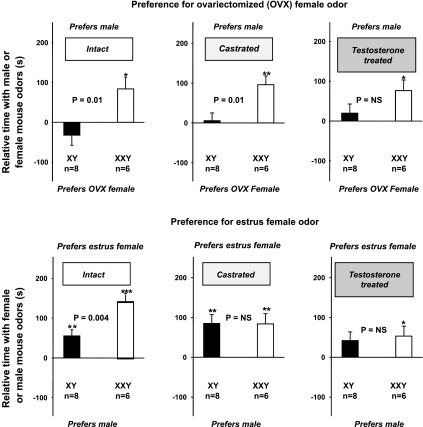
Intact and castrated XXY mice prefer male odors to odors from ovariectomized, but not estrous, female mice; this preference is removed by testosterone treatment (experiment 4).
When choosing between odors from male or ovariectomized female mice, intact (P = 0.01) and castrated (P = 0.01) XXY mice preferred odors from male mice at the expense of ovariectomized female mice compared with XY littermates (Fig. 5, top left and top middle). These differences between XXY and XY mice essentially disappeared with testosterone treatment (P > 0.05; Fig. 5, top right).
Fig. 5.
Preference for odors from male mice instead of odors from OVX female mice (top) or from estrous female mice (bottom) (experiment 4). Mice had to choose between odors from male or OVX female mice (top) and then odors from different male or different estrous female mice (bottom). Data from intact (left), castrated (middle), and then testosterone-treated (right) mice are shown. Top: data are means and SE of relative time spent in the chamber containing the male odor, vs. time spent in the chamber containing odors from OVX female mice. Bottom: data are means and SE of relative time spent in the chamber containing the estrous female odor vs. time spent in the chamber containing male odors. Significant nonzero differences were assessed by one-sample Student's t-test and are indicated by * or ** for P < 0.05 or P < 0.01, respectively. Whether these time differences differed between XXY (open bar) and XY (filled bar) mice was assessed by two-sample Student's t-test as shown by P values.
When choosing between odors from male or estrous female mice, intact XXY mice preferred odors from estrous female mice at the expense of male mice (P = 0.004) compared with XY littermates (Fig. 5, bottom left). These differences between XXY and XY mice all disappeared with castration or testosterone treatment (all P > 0.05, unpaired Student's t-tests; Fig. 5, bottom middle and right).
Repeated-measures ANOVA confirmed the above relationships. Main effects considered were karyotype (XXY vs. XY), treatment (intact, castrated, or testosterone treated), and female odor (from ovariectomized or estrous mice). Full factorial, main effects, and reduced models were examined. The reduced model was constructed by progressively removing nonsignificant main effects and interaction terms. All models showed highly significant differences depending on karyotype (P < 0.0051, from all models) and female odor (P < 0.015, from all models) but not treatment (P > 0.4). The only significant interaction was between karyotype and female odor (P = 0.045 from reduced model). Post hoc Bonferroni-adjusted testing showed that 1) XXY mice, compared with XY littermates, prefer male odors over odors from ovariectomized mice (P = 0.0092), and 2) XY mice are more strongly attracted to estrous mice (over male mice) than male mice (over ovariectomized mice) (P = 0.0137), whereas XXY mice are equally attracted to estrous mice (over male mice) compared with male mice (over ovariectomized mice).
XXY mice exhibit altered palmitate, but not ribose, metabolism in the brain.
FNS palmitate was higher in XXY than in XY mice in brain regions important for behavior and sexual preference (amygdala, hypothalamus, and hippocampus) but not in blood (Figs. 6 and 7). Androgen deprivation in XY mice significantly increased fractional synthesis rates to levels that were not statistically different from those of intact XXY mice in brain regions but again, not in blood (Figs. 6 and 7).
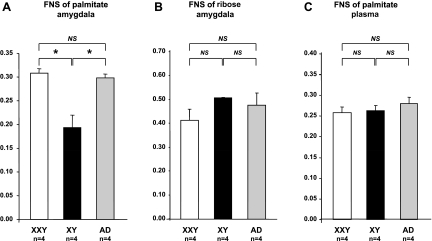
Fig. 6.
Palmitate (A) and ribose (B) synthesis rates in amygdala and palmitate synthesis rates in blood (C) in XXY, XY, and androgen-deprived (AD) XY mice. Mice received 2H2O tracer for 5 days, after which amygdala and blood samples were collected for GC-MS MIDA. In amygdala (A), which is a region important for sexual preference, the rate of newly synthesized (fractions of newly synthesized, FNS) palmitate was significantly higher in XXY and AD mice than in controls. No significant differences were observed in ribose FNS in amygdala (B) or palmitate FNS in blood (C). Each value represents a mean of 3 replicate experiments in 4 mice ± SE. Nos. in brackets indicate nos. of mice in experimental setting. *P < 0.05.
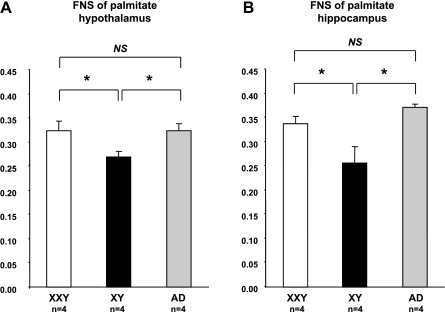
Fig. 7.
Palmitate (C16–0) turnover in hypothalamus and hippocampus of XXY, XY, and AD XY mice. Mice received 2H2O tracer for 5 days, after which hypothalamus and hippocampus regions were collected for GC-MS MIDA. In brain regions important for sexual preference, hypothalamus (A), and hippocampus (B), rates of FNS palmitate were significantly higher in XXY and AD mice than in controls. Each value represents a mean of 3 replicate experiments in 4 mice ± SE. Nos. in brackets indicate nos. of mice in experimental setting. *P < 0.05.
The FNS of ribose did not differ among XXY, XY, and androgen-deficient XY mice in amygdala (P > 0.05; Fig. 6). Identical FNS of ribose findings were observed in the hypothalamus and hippocampus (P > 0.05; data not shown).
DISCUSSION
Here, we introduce the behavioral phenotype of the XXY mouse to study social behaviors (sociability), sexual preference, and the underlying mechanisms. For the behavior experiments, we used a three-chamber social approach paradigm. The apparatus has been utilized previously in the context of examining mouse models of autism (27, 28) and to assess partner preference (33). This is because social approach behaviors, and not only consummatory behaviors, are important components of sexual preference (33). It is for this reason that an ovariectomized mouse, or odors from such mice, were chosen as the primary female stimulus to probe attractions modulated by social rather than sexual preferences predominantly modulated by consummatory attractions. We reasoned that this three-chambered approach-avoidance paradigm to assess social behaviors (sociability) would also have direct relevance in extrapolating whether or not the psychosexual behavior of KS humans is related to social, rather than sexual, reasons. Furthermore, understanding social behaviors in mice is highly relevant, because autism is the extreme expression of social isolation in men with KS, and these investigative paradigms have both been used and validated in mouse models of autism. For example, the hypothesis that X-linked genes may be important in autism has also been examined using XO mice as a model of human Turner's syndrome (21), as have other mouse models implicating X gene dosage or parental imprinting in social behaviors, aggression, parenting, habit formation, and nociception (1). However, to our knowledge, no previous study has specifically investigated the social approach behaviors (sociability) of XXY mice.
We show, somewhat surprisingly, that intact XXY mice, compared with XY littermates exhibit increased social interest when presented with a male mouse vs. an inanimate object and no difference in interest for an ovariectomized female compared with an inanimate object. Since XXY mice (and most KS humans) are androgen deficient (20), we investigated whether the heightened sociability for male mice exhibited by XXY mice was due to androgen deficiency or due to genes that escape X-inactivation. We therefore reassessed sociability under matched hormonal conditions and showed that these differences between XXY and XY mice disappeared after castration (induced androgen deficiency) followed by testosterone replacement (with testosterone implantation). However, castration of XY mice (which ostensibly would be more XXY-like after castration) did not increase preference for male mice. This points to the possibility that karyotype and/or differences in early life exposure to testosterone due to karyotype may modulate subsequent behavioral responses to testosterone manipulation. The latter is plausible since the castration experiments were performed in adulthood, whereas androgen deficiency occurs at least by the time of puberty in XXY mice. We also demonstrated that XXY mice exhibit altered lipid metabolism in those brain regions that are important for behavior and sex preference (i.e., hypothalamus, amygdala, and hippocampus). These alterations in palmitate turnover were also regulated by androgens. Together, these data clearly show that social approach behaviors are regulated by androgens.
A novel finding is that the social interest (i.e., sociability) of intact mice was modulated by the sex of the stranger mouse (Fig. 2). Although this was not observed by other researchers in XY C57BL/6J mice (38), no other study has examined sociability in XXY mice generated on the C57BL/6J background, as demonstrated here. To explore this further, we designed additional experiments to understand whether the increased interest for male mice exhibited by XXY mice was due to olfactory signals and its specificity for ovariectomized or estrous mice. We used olfactory stimuli from estrous mice in this context to understand whether female sex preference was due to social (ovariectomized) or sexual (estrous) interest.
Our first set of experiments led us to perform further experiments using odor only (cotton wool) to deconstruct the critical components of these interactions, since it allows separation of olfactory from other cues, such as visual ones (an animate actual mouse) (14). Previous research supports this approach (14)(38). For example, others have shown that olfactory with visual cues are required to elicit social transmission of food preference in C57BL/6J mice, but that olfactory cues alone can elicit social approach behaviors (38). This is consistent with the premise that olfactory cues alone in mice are critical for many aspects of reproductive physiology, social and sexual behavior (14). Here we confirm after castration and following testosterone replacement that XXY and XY mice display equivalent interest in an actual male mouse, but that intact and castrated XXY mice show heightened interest in odors from male mice. Furthermore, the reduced interest of XXY mice for female mice and their odors appears to be specific for ovariectomized mice, because intact XXY mice show an increased interest for odors also from estrus mice compared with XY mice. Hence, the choices made depend on the alternatives (for example, male mice vs. inanimate object, male vs. ovariectomized females mice and male odors vs. female estrus odors). These findings suggest that social behaviors (sociability) modulated by gender can be elicited solely by olfactory cues, although not necessarily exclusively so; that XXY mice find odors from ovariectomized female mice to be less interesting for presumed social rather than sexual reasons (since attraction for odors from estrus mice was heightened compared with that of XY littermates); and that XY, but not XXY, mice have heightened interest in odors from estrus female mice compared with odors from ovariectomized mice when the alternative choice are odors from male mice. Together, these point to social, rather than sexual, cues being altered in XXY mice.
The role of olfactory stimuli (pheromones) in mammalian chemical communication has long been recognized, but further studies will be required to determine whether these signals are mediated through the main olfactory epithelium and/or the vomeronasal organ in XXY mice specifically (5). Indeed, some studies in rodents show that vomeronasal stimulation is important for overall gender identification (25), whereas others point to the importance of the main olfactory epithelium stimulation in discriminating male from female conspecifics (14). Sexual preference (attraction and avoidance) was reported to be mediated predominantly by the main olfactory epithelium by some investigators (25) but by the vomeronasal organ by others (14). These seemingly contradictory data will require direct clarification in XXY mice.
Male sexual behavior in rodents is regulated primarily by the preoptic area of the anterior hypothalamus through neural networks that are also necessary for olfaction (33, 44). The amygdala and hippocampus also receive projections from the olfactory system (14, 41), and these regions are also important for sex-specific behavior (6) as well as general learning and cognition (41). Mechanistically, odors have been shown previously to promote neurogenesis, and these neuronal changes are important for certain behaviors (23). Metabolomic assessment of lipid synthesis rates in the brain is important because fatty acids play major functional and structural roles in the brain, and alterations in key areas of the brain that control social behavior due to alterations in lipid turnover could plausibly alter sexual preference, but this has not been examined before in XXY mice. Interestingly, our data consistently show that XXY mice exhibit increased fractional synthesis rates of palmitate in these three key brain regions (hypothalamus, amygdala, and hippocampus) compared with XY littermates. These alterations are likely to be specific for lipids in the brain, as FNS of ribose in the brain is not changed, nor is the FNS of palmitate in plasma. Induced androgen deficiency in XY mice increases fractional synthesis rates to levels indistinguishable from those of XXY littermates. These novel data are consistent with our behavioral experiments showing that XXY and XY mice differ in sociability and sexual preference and that these differences are explained, at least partly, by systemic androgen exposure but do not prove a causal relationship between metabolomic activity and behavior. We also utilized two validated and widely used methods to induce androgen deficiency (surgical as well as chemical castration), but again we cannot exclude the possibility that chemical vs. surgical castration could yield different changes in behavioral responses or metabolomic activity. Nevertheless, this seems unlikely, since our findings from the behavior and metabolomic experiments are consistent. Thus the particular androgen-regulated changes in fatty acid metabolism may play a role in the XXY brain (dys)function and/or serve as important markers of altered brain metabolism in KS.
Human interactions are bidirectional and complex and require rapid and accurate processing of visual, olfactory, and auditory signals. Unraveling the genetic, hormonal, and metabolic bases of human behavior is difficult. Here, we show in a validated XXY mouse model that sociability and sexual preference differ between XXY mice and their XY littermates and that these differences are mostly explained as being because intact XXY mice are relatively androgen deficient. The implications of these findings to human KS are speculative but support existing clinical recommendations that androgen replacement in humans with KS should be adequate and age appropriate and partly explains why early diagnosis of KS is associated with improved outcome (26, 36, 37).
GRANTS
P. Y. Liu was supported by a postdoctoral fellowship from the Lalor Foundation and Career Development Award 511929 from the National Health and Medical Research Council of Australia. K. Erkkila was supported by the Foundation for Pediatric Research (Finland), Jalmari and Rauha Ahokas, Paulo and Juselius Foundations (Finland). This study was supported by Endocrinology, Metabolism and Nutrition Training Grant T32 DK-007571 and research funds from the Los Angeles Biomedical Research Institute.
DISCLOSURES
No conflicts of interest are reported by the authors.
ACKNOWLEDGMENTS
We thank V. Antienza (animal care), A. Leung (assays), K. Ma, R. Ruiz, and M. Rantakari for technical assistance, M. Ferrini for the mice brain dissections, and L. G. Boros, S. Bassilian, N. Lee, and S. Lim for mass spectrometry.
REFERENCES
- 1.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30: 1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft J, Axworthy D, Ratcliffe S. The personality and psycho-sexual development of boys with 47 XXY chromosome constitution. J Child Psychol Psychiatry 23: 169–180, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 88: 622–626, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Boone KB, Swerdloff RS, Miller BL, Geschwind DH, Razani J, Lee A, Gonzalo IG, Haddal A, Rankin K, Lu P, Paul L. Neuropsychological profiles of adults with Klinefelter syndrome. J Int Neuropsychol Soc 7: 446–456, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature 444: 308–315, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol 501: 904–915, 2007 [DOI] [PubMed] [Google Scholar]
- 7.DeLisi LE, Maurizio AM, Svetina C, Ardekani B, Szulc K, Nierenberg J, Leonard J, Harvey PD. Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. Am J Med Genet B Neuropsychiatr Genet 135: 15–23, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Huang K, El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr Opin Neurobiol 15: 527–535, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Hunt PA, Eicher EM. Fertile male mice with three sex chromosomes: evidence that infertility in XYY male mice is an effect of two Y chromosomes. Chromosoma 100: 293–299, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Hunt PA, Worthman C, Levinson H, Stallings J, LeMaire R, Mroz K, Park C, Handel MA. Germ cell loss in the XXY male mouse: altered X-chromosome dosage affects prenatal development. Mol Reprod Dev 49: 101–111, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Jacobs PA, Strong JA. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183: 302–303, 1959 [DOI] [PubMed] [Google Scholar]
- 12.Jensen MD, Martin ML, Cryer PE, Roust LR. Effects of estrogen on free fatty acid metabolism in humans. Am J Physiol Endocrinol Metab 266: E914–E920, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Johnson C, Jia Y, Wang C, Lue YH, Swerdloff RS, Zhang XS, Hu ZY, Li YC, Liu YX, Hikim AP. Role of caspase 2 in apoptotic signaling in primate and murine germ cells. Biol Reprod 79: 806–814, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelliher KR, Wersinger SR. Olfactory regulation of the sexual behavior and reproductive physiology of the laboratory mouse: effects and neural mechanisms. Ilar J 50: 28–42, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kielinen M, Rantala H, Timonen E, Linna SL, Moilanen I. Associated medical disorders and disabilities in children with autistic disorder: a population-based study. Autism 8: 49–60, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Klinefelter HGJ, Reifenstein ECJ, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without a-Leydigism and increased excretion of follicle-stimulation hormone. J Clin Endocrinol Metab 2: 615–627, 1942 [Google Scholar]
- 17.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet 364: 273–283, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lee WN, Bassilian S, Lim S, Boros LG. Loss of regulation of lipogenesis in the Zucker diabetic (ZDF) rat. Am J Physiol Endocrinol Metab 279: E425–E432, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein JM, Brunengraber H, Wadke M. Measurement of rates of lipogenesis with deuterated and tritiated water. Methods Enzymol 35: 279–287, 1975 [DOI] [PubMed] [Google Scholar]
- 20.Lue Y, Jentsch JD, Wang C, Rao PN, Hikim AP, Salameh W, Swerdloff RS. XXY mice exhibit gonadal and behavioral phenotypes similar to Klinefelter syndrome. Endocrinology 146: 4148–4154, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lynn PM, Davies W. The 39,XO mouse as a model for the neurobiology of Turner syndrome and sex-biased neuropsychiatric disorders. Behav Brain Res 179: 173–182, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Maclean N, Harnden DG, Brown WM, Bond J, Mantle DJ. Sex-chromosome abnormalities in newborn babies. Lancet 13: 286–290, 1964 [DOI] [PubMed] [Google Scholar]
- 23.Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci 10: 1003–1011, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids 71: 298–303, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Garcia F, Martinez-Ricos J, Agustin-Pavon C, Martinez-Hernandez J, Novejarque A, Lanuza E. Refining the dual olfactory hypothesis: pheromone reward and odour experience. Behav Brain Res 200: 277–286, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Money J, Annecillo C, Van Orman B, Borgaonkar DS. Cytogenetics, hormones and behavior disability: comparison of XYY and XXY syndromes. Clin Genet 6: 370–382, 1974 [DOI] [PubMed] [Google Scholar]
- 27.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry 13: 4–26, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3: 287–302, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res 191: 118–129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet 36: 725–731, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 3: 303–314, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci USA 106: 1989–1994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paredes RG. Evaluating the neurobiology of sexual reward. Ilar J 50: 15–27, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Paulsen CA, De Souza A, Yoshizumi T, Lewis BM. Results of a buccal smear survey in noninstitutionalized adult males. J Clin Endocrinol Metab 24: 1182–1187, 1964 [DOI] [PubMed] [Google Scholar]
- 35.Ratcliffe SG. The sexual development of boys with the chromosome constitution 47,XXY (Klinefelter's syndrome). Clin Endocrinol Metab 11: 703–716, 1982 [DOI] [PubMed] [Google Scholar]
- 36.Robinson A, Bender BG, Linden MG. Summary of clinical findings in children and young adults with sex chromosome anomalies. Birth Defects Orig Artic Ser 26: 225–228, 1990 [PubMed] [Google Scholar]
- 37.Rovet J, Netley C, Keenan M, Bailey J, Stewart D. The psychoeducational profile of boys with Klinefelter syndrome. J Learn Disabil 29: 180–196, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res 193: 235–242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiavi RC, Theilgaard A, Owen DR, White D. Sex chromosome anomalies, hormones, and sexuality. Arch Gen Psychiatry 45: 19–24, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Simpson JL, de la Cruz F, Swerdloff RS, Samango-Sprouse C, Skakkebaek NE, Graham JM, Jr, Hassold T, Aylstock M, Meyer-Bahlburg HF, Willard HF, Hall JG, Salameh W, Boone K, Staessen C, Geschwind D, Giedd J, Dobs AS, Rogol A, Brinton B, Paulsen CA. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med 5: 460–468, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Slotnick B. Animal cognition and the rat olfactory system. Trends Cognitive Sci 5: 216–222, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Smyth CM, Bremner Klinefelter, syndrome WJ. Arch Intern Med 158: 1309–1314, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Stanislaus D, Janovick JA, Brothers S, Conn PM. Regulation of G(q/11)alpha by the gonadotropin-releasing hormone receptor. Mol Endocrinol 11: 738–746, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Swaab DF, Gooren LJ, Hofman MA. Gender and sexual orientation in relation to hypothalamic structures. Horm Res 38, Suppl 2: 51–61, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Swanson DW, Stipes AH. Psychiatric aspects of Klinefelter's syndrome. Am J Psychiatry 126: 814–822, 1969 [DOI] [PubMed] [Google Scholar]
- 46.Vannucci S, Hawkins R. Substrates of energy metabolism of the pituitary and pineal glands. J Neurochem 41: 1718–1725, 1983 [DOI] [PubMed] [Google Scholar]
- 47.Vera Y, Erkkila K, Wang C, Nunez C, Kyttanen S, Lue Y, Dunkel L, Swerdloff RS, Sinha Hikim AP. Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol 20: 1597–1609, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Witkin HA, Mednick SA, Schulsinger F, Bakkestrom E, Christiansen KO, Goodenough DR, Hirschhorn K, Lundsteen C, Owen DR, Philip J, Rubin DB, Stocking M. Criminality in XYY and XXY men. Science 193: 547–555, 1976 [DOI] [PubMed] [Google Scholar]
- 49.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci 1: 1, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]