Abstract
Instability of tissue protein biomarkers is a critical issue for molecular profiling. Pre-analytical variables during tissue procurement, such as time delays during which the tissue remains stored at room temperature, can cause significant variability and bias in downstream molecular analysis. Living tissue, ex vivo, goes through a defined stage of reactive changes that begin with oxidative, hypoxic and metabolic stress, and culminate in apoptosis. Depending on the delay time ex vivo, and reactive stage, protein biomarkers, such as signal pathway phosphoproteins will be elevated or suppressed in a manner which does not represent the biomarker levels at the time of excision. Proteomic data documenting reactive tissue protein changes post collection indicate the need to recognize and address tissue stability, preservation of post-translational modifications, and preservation of morphologic features for molecular analysis. Based on the analysis of phosphoproteins, one of the most labile tissue protein biomarkers, we set forth tissue procurement guidelines for clinical research. We propose technical solutions for (i) assessing the state of protein analyte preservation and specimen quality via identification of a panel of natural proteins (surrogate stability markers), and (ii) using multi-purpose fixative solution designed to stabilize, preserve and maintain proteins, nucleic acids, and tissue architecture.
Keywords: Biomarker, Fixation, Histology, Phosphoprotein, Preservation
1 Tissue protein biomarkers: Emerging opportunities for diagnosis and prognosis
Protein tissue biomarkers offer great promise to provide clinical diagnostic and prognostic information that cannot be obtained from genomics or serum biomarkers. The phosphorylation, or activation state, of kinase-driven signal networks contains important information concerning both disease pathogenesis and the ongoing state of kinase-associated therapeutic targets [1-3]. Modulation of ongoing cellular kinase activity represents one of the most rapidly growing arenas in new drug discovery. Identification of specific phosphoprotein signaling aberrations is leading to the development of targeted therapies for patients with lung, breast and colon cancer [4-8]. Profiling the tumor phosphoproteome and the transcriptome, using human tumor biopsy specimens, is a critical component of the perceived upcoming revolution of individualized cancer therapy [9]. Thus tissue protein biomarkers offer a means for individualizing therapy by revealing how an activated or amplified proteomic signal pathway drives an individual patient’s tumor cells. If the revealed pathway contains the drug target of a specific therapeutic, then this information could be used by the physician to select the optimal therapy for this patient.
In the past, the discovery and clinical implementation of tissue biomarkers has been delayed by several hurdles including tissue heterogeneity and the lack of sensitive technology to identify and measure proteins in small volumes of human biopsy tissue. These hurdles have been largely overcome by the wide availability of laser microdissection technology, protein microarrays, and advances in mass spectroscopy [10-12]. Nevertheless, the problem of protein biomarker instability and perishability within the tissue aspirate or biopsy is a very serious issue that has not been adequately studied or solved. If this problem is not resolved in the real world of clinical practice, then the future promise of tissue biomarkers will never be realized. The present opinion piece sets forth the need for collecting data concerning the stability of tissue protein biomarkers and proposes goals for novel technologic solutions to address the problem of biomarker stability in tissue. An emphasis is placed on tissue phosphorylation, since this highly labile class of tissue biomarkers contains information about the state of kinase drug targets, and the vast majority of emerging therapeutics target kinases and kinase pathways.
2 Protein biomarker stability in tissue: A critical unmet need
There is a critical need to develop standardized protocols and novel technologies that can be used in the routine clinical setting for seamless collection and immediate preservation of tissue biomarker proteins, particularly those that have been post-translationaly modified such as phosphoproteins (Fig. 1). This vital need transcends the large research hospital environment and extends most acutely to the private practice, where most patients receive therapy. Although molecular profiling offers tremendous promise to change the practice of oncology, the fidelity of the data obtained from a diagnostic assay applied to tissue must be monitored and quality controlled; otherwise, a clinical decision may be based on incorrect molecular data. To date, clinical preservation practices routinely rely on protocols that are decades old, such as formalin fixation, and are designed to preserve specimens for histologic examination. Under the current standard of care, tissue is procured for pathologic examination in three main settings: (i) surgery in a hospital-based operating room, (ii) biopsy conducted in an outpatient clinic, and (iii) image-directed needle biopsies or needle aspirates conducted in a radiologic suite. In a typical tissue procurement research protocol, tissue is often frozen in dry ice or liquid nitrogen to preserve the tissue proteome. Unfortunately, however, in a busy clinical setting, it will be impossible to immediately preserve procured tissue in liquid nitrogen. Moreover even if the tissue is preserved by freezing, it may remain at room temperature awaiting pathologic gross examination, for an indefinite period prior to freezing. The time delay from patient excision to pathologic examination and molecular analysis is often not recorded and may vary from 30 min to many hours depending on the time of day, the length of the procedure and the number of concurrent cases. During this variable waiting period the tissue remains living and reactive. For this reason preanalytical variables can have a major impact on tissue biomarker fidelity and bias [13-15].
Figure 1.
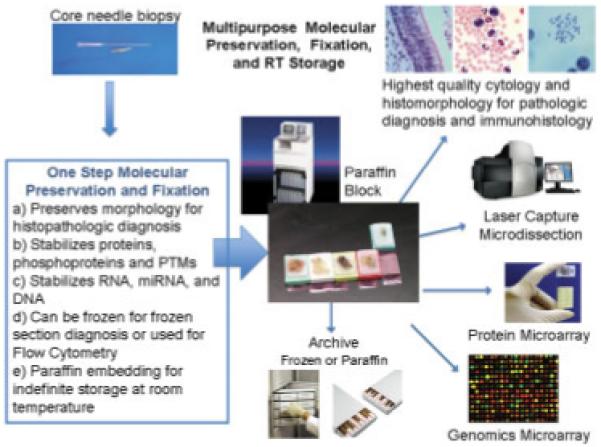
Ideal tissue stabiliziation/preservation scheme for biomarker analysis. A core needle biospy is immediately placed in a multi-purpose stabilization/preservation solution at room temperature. The fixed biopsy specimen could subsequently undergo cryosectioning, processing in standard formalin or ethanol tissue processors, or flow cytometric analysis. The fixed samples could be archived as frozen sections/blocks or paraffin-embedded blocks. The samples would be compatible with standard protein/nucleic acid methodologies.
Two categories of variable time periods define the stability intervals for human tissue procurement. A primary starting time, time point A, is defined as the moment that tissue is excised from the patient and becomes available ex vivo for analysis and processing. The post excision delay time, is the time from time point A to the time that the specimen is placed in a stabilized state, time point B, e.g. immersed in fixative or snap-frozen in liquid nitrogen. Given the complexity of patient-care settings, during the excision delay time the tissue may reside at room temperature in the operating room or on the pathologist’s cutting board, or it may be refrigerated in a specimen container. The second variable time period is the processing delay time. At the beginning of this interval the tissue is immersed in a preservative solution or stored in a freezer. At the end of this interval, the tissue is subject to processing for molecular analysis. In addition to the uncertainty about the length of these two time intervals, a multitude of known and unknown variables can influence the stability of tissue molecules (Table 1). These include (i) temperature and pH fluctuations, hypoxia and dehydration, prior to fixation or freezing, (ii) choice of preservative chemistry and rate of tissue penetration, (iii) size of the tissue specimen, (iv) extent of handling, cutting and crushing of the tissue, (v) fixation and staining prior to microdissection, and (vi) the introduction of phosphatases, RNAases or proteinases from the environment, or from dying cells, at any time. Given current practices, in the face of these uncertainties it would appear virtually impossible to develop a standardized procedure for routine clinical profiling. Even if a strict protocol is followed, there is no ultimate assurance that processing variables are free from compromise up to the time that the molecular profile data are collected.
Table 1.
Tissue preservation/stabilization variables associated with biomarker analysis
| Variables | Possible reasons | Potential solutions |
|---|---|---|
| Pre-analytical | ||
| Tissue evaluation time (grossing) |
Delay in transport | Transport and process tissues as soon as possible post excision |
| Time delay to preservation |
Heavy work load or back-log of cases | Communicate tissue processing requirements with clinical and laboratory staff |
| Phosphatase, kinase and proteinase activity in tissue |
Type of preservative/fixation | Use rapid preservative method compatible with downstream analysis |
| Lack of inhibitors/stabilization in transport medium |
Use preservative/fixative containing phosphatase, kinase, proteinase inhibitors or with inhibitor activity |
|
| Delay in transport/preservation/ stabilization |
Transport, preserve and process tissues as soon as possible post excision |
|
| Storage temperature | Store tissue at appropriate temperature for preservation solution |
|
| Introduction of phosphatases/ proteinases from the environment |
Maintain clean tissue processing/grossing areas | |
| Degree of fixative cross- linking |
Preservative/fixative containing formalin, gluteraldehyde or other cross-linker |
Use minimal amount of cross-linking agent to achieve adequate fixation |
| Excessive tissue size in relation to fixative volume |
Reduce tissue size. Increase volume of fixative | |
| Penetration time of preservative solution |
Reduce tissue size. Use preservative with permeabilization reagents |
|
| Elevated storage temperature | Store tissue at appropriate temperature for preservation solution. Reduce storage temperature |
|
| Tissue hydration state | Low ambient humidity. Open specimen container |
Transport tissue in a closed container |
| Delay in transport/preservation | ||
| Post-analytical | ||
| Time delay to analysis | Lack of proper equipment, reagents, storage conditions |
Prepare reagents in advance. Plan procedure prior to retrieving samples |
| Malfunctioning equipment | Develop a plan to use an alternate source of equipment | |
| Poor/variable quality protein and/or nucleic acids results |
Delay in stabilizing tissue sample | Transport/preserve/fix tissue as soon as possible post excision |
| Elevated ambient temperature during tissue transport/processing |
Place samples on wet ice or in chilled container | |
| Inadequate fixation/preservation | Follow directions for type of fixation/tissue | |
| Use of post-mortem tissue samples | Limit ischemic conditions. Procure samples as soon as possible post-mortem |
|
| Differences in sample handling and processing |
Stabilize/preserve tissue as soon as possible post excision (time <20 min) |
|
| Obtain highly pure cell populations (>75%) | ||
| Freeze tissue in such a method as to ensure rapid, uniform cooling (<5 min). | ||
| Use RNAse-free precautions: wear gloves, use nucleic acid free plastic ware, clean work area with RNAse decontamination solutions | ||
| Add protease inhibitors to appropriate reagents | ||
| Extensive cross-linking due to fixation | Determine type of fixation, volume or size of tissue sample, depth of tissue within the tissue block |
|
| Inadequate removal of paraffin from formalin-fixed paraffin embedded tissue |
De-paraffinize in two changes of xylene for 5–15 min each | |
3 Protein stability may be unrelated to RNA transcript stability
Several studies have been conducted concerning the stability of RNA in tissue ex vivo [16-20]. These studies indicate that refrigeration is superior to room temperature and the addition of RNAase inhibitors may be useful as RNA preservatives. Although this information is applicable to gene array profiling, it may have little bearing on protein stability in general or phosphoprotein stability specifically. Chemical conditions favoring protein stability may be completely different from those for RNA stability.
4 Phosphoprotein stability: The balance between kinases and phosphatases
Phosphoproteins offer a unique minute-by-minute record of ongoing signal pathway events of high-functional relevance to therapeutic target selection and the prediction of toxicity. At any point in time within the tissue cellular microenvironment, the phosphorylated state of a protein is a function of the local stoichiometry of associated kinases and phosphatases specific for the phosphorylated residue. Protein phosphatases have been classified into three distinct categories: (i) serine/threonine (Ser/Thr)-specific [21], (ii) tyrosine-specific [22], and (iii) dual specificity phosphatases. Protein tyrosine phosphatases remove phosphate groups from phosphorylated tyrosine residues of proteins. A variety of chemical- and protein-based inhibitors of phosphatases exist [23, 24]. An important long-term need for the clinical implementation of phosphoprotein biomarkers will be the design of stabilizers for the preservation of phosphoproteins without the need for freezing. Optimally the stabilizing chemistry should arrest both sides of the kinase/phosphatase balance, in order to prevent positive or negative fluctuations in phosphorylation events as the living-excised tissue reacts to the ex vivo conditions [13].
5 Recognition that tissue is alive and reactive following procurement
Although investigators have worried about the effects of vascular clamping and anesthesia, prior to excision, a much more significant and underappreciated issue is the fact that excised tissue is alive and reacting to ex vivo stress [13]. The instant a tissue biopsy is removed from a patient, the cells within the tissue react and adapt to the absence of vascular perfusion, ischemia, hypoxia, acidosis, accumulation of cellular waste, absence of electrolytes, and temperature changes [13]. In as little as 30 min post excision drastic changes can occur in the protein signaling pathways of the biopsy tissue as the tissue remains in the operating room suite or on the pathologist’s cutting board. In response to wounding cytokines, vascular hypotensive stress, hypoxia, and metabolic acidosis, it would be expected that a large surge of stress related, hypoxia related, and wound repair-related protein signal pathway proteins and transcription factors will be induced in the tissue immediately following procurement [25-28].
During the ex vivo time period, as the tissue cells are alive and reactive, phosphorylation of certain kinase substrates may transiently increase due to the persistence of functional signaling, activation by hypoxia, or some other stress–response signal [13, 29-31]. The excised tissue is hypotensive and wounded, triggering a cascade of further reactive changes, including the activation of kinase pathways, which may predominate close to the cut surfaces. Forensic wound evaluation and wound vitality studies further support the persistence of functional protein signaling. Immunohistochemical analysis of cytokines (TGFα, TGFβ, IL1-β, IL-6, and TNFα) from human skin wounds demonstrated increases in cytokine levels at wound sites in as little as 10 min post wounding, with additional increases noted over 30–60 min [29-31]. Although these reactive changes would be expected to increase protein phosphorylation, the availability of ubiquitous cellular phosphatases would be expected to ultimately destroy phosphosphorylation sites, given enough time [13, 25, 26]. This will significantly distort the molecular signature of the tissue compared with the state of the markers in vivo. The manner of distortion will be dependent on the time delay following procurement, with reactive increases in phosphorylation at early times, and loss of phosphorylation at later times. Moreover the degree of ex vivo protein fluctuation could be quite different between tissue types and influenced by the pathologic microenvironment. This physiologic fact must be taken into consideration as we plan to implement tissue protein biomarker analysis in the real world of the clinic, where the living, reacting tissue may remain in the collection basin for hours.
6 Timecourse of post-translationally modified proteins post excision
We present the following example of supravital tissue fluctuations post excision. Uterine tissue (leiomyoma (myometrium)) was obtained under informed consent following an IRB-approved protocol in a community hospital (Inova Fairfax Hospital, Falls Church, VA, USA). Tissue was excised in the surgical suite following standard of care guidelines. Tissue was transported at room temperature to the surgical frozen section room. A board-certified pathologist performed gross examination of each tissue sample and provided non-diseased tissue that was not required for diagnosis. The tissue was cut with a scalpel into eight relatively homogeneous pieces. One piece of tissue, designated as time zero (10 min post excision), was immediately processed by embedding in a cryomold and freezing on dry ice. The remaining tissue pieces were incubated at room temperature for 21, 40, 70, 100, 130, 160, and 190 min post excision and processed as described above. Cryosections (8 μm) were cut from the tissue block at a depth of 1.0–2.0 mm from the surface to mimic conditions found at the center of a core needle biopsy specimen. The cryosections were fixed, stained, and analyzed by reverse phase protein microarray (RPPA) as described previously [27, 28].
The time zero sample data were designated as 100%, assuming that any changes during incubation would be reflected in deviations from the time zero sample. Room temperature incubation of tissue revealed significant (±20% from the time zero sample) augmentation, as well as decreases, of phosphoprotein levels over time, independent of post-translational modification and protein sub-cellular location (Fig. 2A). Although some individual proteins participating in stress/inflammation, hypoxia, proliferation/survival, and cell cycle signaling pathways showed increases over time ex vivo, other protein levels remained stable over time. This is in keeping with the physiologic concept that excised tissue is alive and reactive prior to processing/fixation [13].
Figure 2.
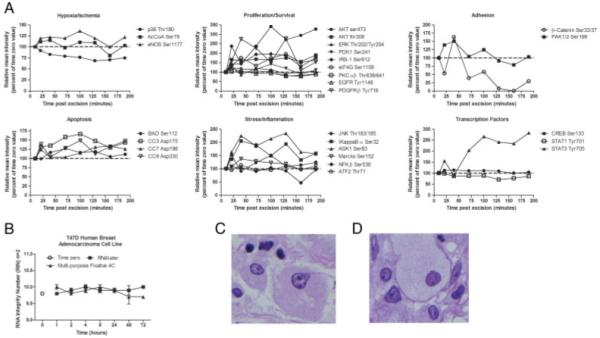
A multi-purpose chemical solution for stabilization and preservation of proteins and RNA, and maintenance of histomorphology. (A) Frozen uterine leiomyoma tissue reveals on-going phosphoproteomic changes post excision. A reverse phase protein microarray analysis of tissue analyzed from a deep area of the tissue block (1.0–2.0 mm from surface) showed reactive protein changes as compared with the time zero sample (100% value, 10 min post excision, outermost tissue surface). Reactive proteins constituted a variety of molecular cascades: apoptotic pathway, stress/inflammation pathway, hypoxia/ischemia pathway, transcription factors, proliferation/survival pathway, and adhesion/cytoskeleton proteins. (B) RNA stability time course of T47D-cultured cells. T47D human mammary adenocarcinoma cell lines were cultured at 37°C, 5% CO2. Medium was removed and cells washed three times with cold phosphate-buffered saline. The cells were scraped from the flasks, combined and aliquots were incubated with RNAlater (Qiagen) (black square) or our multi-purpose fixative (black triangle) at 4°C for 1, 2, 4, 8, 24, 48, and 72 h. An untreated cell culture aliquot was used as the time zero control (open circle). RNA was extracted using the RNA Mini Kit (Qiagen) and RIN were determined using a Bioanalyzer 2100 (Agilent) for duplicate samples at each time point. (C and D) Tissue stabilized at room temperature in a multi-purpose chemical solution yields histomorphology similar to formalin fixed tissue. (C) Human breast tumor epithelium fixed in a multi-purpose stabilization solution containing phosphatase and kinase inhibitors, alcohol, and a permeation enhancer, was processed via a standard histology technique. H&E staining showed well-delineated nuclear membranes and chromatin clumping comparable to an adjacent tissue sample fixed in 5% formalin (D). (UltraLight Histology™ processing courtesy of Dr. Thomas Donndelinger, Bi-Biomics).
Based on the emerging published data [13, 29-33] revealing that excised tissue is reactive, and showing how this state can introduce sources of variability for diagnostic molecular endpoints, we can propose guidelines for the reduction of pre-analytical variables.
Tissue procurement guidelines for molecular analysis:
Tissue procurement protocols must recognize the fact that excised tissue is alive and reactive to ex vivo stress. Kinase pathways are active and reactive until the tissue cells are stabilized.
Reactive changes occurring in tissue post excision can generate false elevation as well as false declination in protein and nucleic acid analytes. This may be a significant source of bias in the analysis of protein or nucleic acid as potential biomarkers.
Kinase pathway stabilization methods should block both sides of the kinase/phosphatase kinetic reaction. Blocking only phosphatases can cause false elevation of an analyte’s phosphorylation level.
Tissue should be stabilized as soon as possible after excision. Taking into consideration the average time for procurement in a community hospital, the recommended maximum elapsed time is 20 min from excision to stabilization (e.g. flash freezing, thermal denaturation, or chemical stabilization).
Tissue stabilization and preservation methods should be compatible with the intended downstream analysis. Preservation of tissue histology and morphology is essential for the verification of tissue type and cellular content.
Documentation of the sample excision/collection time, elapsed time to preservation/stabilization, and length of fixation time are critical data elements for sample quality assessments.
7 Technologies for the future: Surrogate markers of stability and multi-purpose fixative
Surrogates of tissue protein biomarker stability
An immediate technologic approach to the preservation of the tissue proteome is a mean to qualify the state of protein analyte preservation in a tissue, prior to molecular analysis. In order to address the uncertainty of protein and phosphoprotein analyte quality within an individual patient’s biopsy, the authors propose the concept of endogenous protein surrogate markers for tissue preservation: the identification of a panel of natural proteins and phosphorylated amino acids that are highly labile and constitute an early warning of a compromised preservation state [34]. Such surrogates would be selected from a variety of classes of phosphoproteins, including those from specific pathways; classes of residues (e.g. tyrosine, serine, or threonine); and nuclear, cytoplasmic, and cell membrane compartments [13, 34]. For a tissue sample of unknown or questionable state of preservation, the panel of endogenous surrogate markers would be measured before the tissue could be cleared for molecular diagnostic analysis. If values of the surrogate markers fall within cut points defining tissue that has been preserved adequately (e.g. frozen in less than 30 min), then the tissue would pass initial quality control assessments for further analysis. The choice of the surrogates must of course be different than the diagnostic protein analyte that would be measured in the same piece of tissue. Developing a list of tissue protein stability surrogate markers is an important goal for molecular profiling research.
8 Non-formalin fixation chemistries for molecular analysis
Formalin fixation may be unsuitable for quantitative protein biomarker analysis in tissue
Although it is now possible to extract proteins from formalin-fixed tissue [35], formalin penetrates tissue at a variable rate, reported to be within the range of millimeters per hour [36-38]. During this time delay period the portion of the living tissue deeper than several millimeters would be expected to undergo significant fluctuations in regards to phosphoprotein analytes. When one considers the volume of a typical 16-gauge core needle biopsy (7 mm × 1.6 mm (volume = 17.9 mm3)) it is clear that the cellular molecules in the depth of the tissue will have significantly degraded by the time formalin permeates the tissue [36, 39]. In addition, penetration rate is not synonymous with fixation. In aqueous solution formaldehyde becomes hydrated, forming methylene glycol [36, 38]. The small percentage of formaldehyde in solution forms the actual covalent cross-links with proteins and nucleic acids. Methylene glycol penetrates the tissue, yet it is the carbonyl formaldehyde component that causes tissue fixation [36, 38]. Formalin cross-linking, the formation of methylene bridges between amide groups of protein, blocks analyte epitopes as well as decreases the yield of proteins extracted from the tissue. Since the dimensions of the tissue and the depth of the block from which samples are prepared are unknown variables, formalin fixation would be expected to cause significant variability in protein and phosphoprotein stability for molecular diagnostics [36, 40, 41].
Although new fixatives have been developed for preservation and/or extraction of RNA from formalin-fixed tissue, there is an awakening recognition that new chemistries are needed for preserving proteins and post-translationally modified proteins [20, 42, 43]. Rapid fixation chemistries and formalin alternatives are being developed but as yet have not been thoroughly evaluated as a timecourse analysis of phosphoproteins or other post-translationally modified proteins [39, 42-47]. Thermal/pressure inactivation of protein kinases and phosphatases has been developed as an effective, rapid protein stabilization/inactivation method [48]. Rapid thermal inactivation of enzymes ensures stabilization of kinetic reactions but fails to maintain the tissue morphology. Although this technique has shown utility for mass spectrometric analysis it is not compatible with histomorphology including paraffin embedding, cryosectioning, or laser capture microdissection, which are critical components of the clinical/translational research tool kit. Ultra-sound rapid fixation [39, 44, 45, 47, 49] and non-formalin-based fixatives [42, 46] processed with or without microwave assistance, are technologies that were developed with the goal of preserving diagnostic macromolecules during tissue processing for subsequent histopathologic analysis. Nevertheless, the contribution of pre-analytical variables (prior to immersion in the non-formalin fixative or delay in processing) and the preservation of phosphoproteins, must still be addressed before these technologies gain widespread clinical utility. Moreover, microwave, infrared heating, and ultrasound technologies proposed for tissue preservation may be expensive and may not be available to the community hospital or out-patient clinic.
The paramount requirement of any human tissue procurement is an accurate histopathologic diagnosis. The pathologic diagnosis is the determining factor for irrevocable clinical decisions about mode and extent of surgery, the successful attainment of clean surgical margins, and the administration of toxic therapies. Surgeons, pathologists, and IRB review boards, are frequently concerned that tissue procured for molecular diagnostics, or for exploratory research, will compromise the accuracy of the histopathologic diagnosis. Imagine the scenario that one peice of tissue from a patient’s surgical specimen is procured for research, while an adjacent region of tissue is used to make the pathologic diagnosis. The research tissue sample is frozen and pulverized to extract proteins and RNA. Imagine further that the tissue that is removed for research purposes contains invasive cancer cells or reveals an aggressive neoplastic morphology, while the tissue used for diagnosis contains only benign tissue. The unfortunate outcome is a missed cancer diagnosis because the processing of the research specimen precludes an accurate histologic diagnosis.
Future tissue preservation technology must take into consideration the requirement for an accurate histopathologic diagnosis, and recognize limitations in the availability of specialized processing instruments, or liquid nitrogen, in the typical community hospital or clinic. In the face of the aforementioned limitations and requirements, the ideal future technology is a new class of multipurpose fixative chemistries (Fig. 1). At the time of tissue procurement in the OR or clinic the tissue would be immediately immersed in the stabilizing chemistry to arrest all reactive fluctuations in protein and nucleic acid macromolecules.
This proposed chemistry would also maintain tissue arcitecture and morphology, and be compatible with cryosectioning techniques and paraffin embedding for standard of care histopathologic diagnosis. Such a multipurpose chemistry would be the starting point for processing all pathologic specimens into a standard paraffin block. Multiple sections from the same paraffin block could be cut and distributed for all the following uses: (i) histopathologic diagnosis, (ii) immunohistochemistry, (iii) microdissection, (iv) proteomic analysis, and (v) nucleic acid analysis. Importantly, the tissue with its preserved macromolecules could be stored indefinitely at room temperature as a paraffin block in the standard fashion of an anatomic pathology archive.
Espina et al. [13] have described an ethanol-based fixative chemistry that contains phosphatase and kinase inhibitors to successfully arrest the reactive kinase pathways activated ex vivo following tissue procurement. This previously described chemical composition preserves phosphoproteins while maintaining tissue histomorphology for frozen sectioning and paraffin embedding (Fig. 2A). Human breast cancer tissue immersed in either the multi-purpose stabilization solution or the 5% formalin presented similar cellular morphology post processesing in formalin via UltraLight Histology, a method which provides nucelar detail at a resolution between electron microscopy and light microscopy (Fig. 2C and D).
In order to achieve the full purpose, one step chemistry proposed in Fig. 1, RNA preservation must be added as a necessary functionality. Since chemical solutions designed to preserve phosphoproteins may not be compatible with RNA preservation we tested this candidate chemistry for the ability to preserve RNA in a T47D human mammary adenocarcinoma cell line model (Fig. 2B). RNA integrity was maintained over a 72-h timecourse as compared with RNAlater-(Qiagen) preserved cells. RNA integrity numbers (RIN) for the cells preserved in RNAlater and our multi-purpose fixative were not statistically different over the 72-h timecourse, indicating that the multi-purpose chemical stabilization solution containing phosphatase and kinase inhibitors was indeed compatible with nucleic acid preservation.
9 Concluding remarks
Clinical standard of care guidelines and surgical techniques subject most tissue samples, prior to excision, to some degree of anesthesia exposure (local or systemic), potential ischemia due to clamping of blood vessels, wounding via incisions or cauterization, and exposure to dyes, stains, or contrast media. Although these sources of cellular stresses may affect potential biomarkers, the extent of tissue reactivity post excision has been greatly underappreciated by the biomarker community. Although it may be on a microscopic scale, there is a life and death struggle playing out in the living tissue cells that are reacting to hypoxia, nutrient deprivation, wounding, and metabolic acidosis [13]. Interpretation of biomarker data will require the acknowledgement of this struggle and the pre-analytical variables affecting molecular biomarkers.
Acknowledgments
The authors thank Drs. Lucia Pastore, James Mize, Geetha Menezes and Diane Rice, Pathologists at Inova Fairfax Hospital, for their assistance in specimen procurement and gross tissue evaluation. They also thank Stacey Banks and Barbara Merritt, Inova Fairfax Cancer Center, for assistance with patient consent and specimen procurement. This work was supported in part by George Mason University, Inova Fairfax Hospital, the Istituto Superiore di Sanita, Italy, and a National Institutes of Health grant to LAL (R21CA125698-01A1) from the National Cancer Institute program “Innovations in cancer sample preparation”.
Aspects of the technologies and discoveries discussed in this manuscript are the subject of US Government and University assigned patents of which the authors are co-inventors. Under US Law they would be allowed to receive royalties on any licenses taken. VE, EFP and LAL are shareholders and/or consultants for Theranostics Health, LLC, which is a licensee for the technology described herein.
Abbreviation
- RIN
RNA integrity number
10 References
- [1].Wilker E, Lu J, Rho O, Carbajal S, et al. Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol. Carcinog. 2005;44:137–145. doi: 10.1002/mc.20132. [DOI] [PubMed] [Google Scholar]
- [2].Sun SY, Rosenberg LM, Wang X, Zhou Z, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- [3].Shah A, Swain WA, Richardson D, Edwards J, et al. Phospho-akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin. Cancer Res. 2005;11:2930–2936. doi: 10.1158/1078-0432.CCR-04-1385. [DOI] [PubMed] [Google Scholar]
- [4].Calvo E, Rowinsky EK. Effect of epidermal growth factor receptor mutations on the response to epidermal growth factor receptor tyrosine kinase inhibitors: target-based populations for target-based drugs. Clin. Lung Cancer. 2004;6:S35–S42. doi: 10.3816/clc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- [5].Huang S, Armstrong EA, Benavente S, Chinnaiyan P, et al. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- [6].Lee SH, Lopes de Menezes D, Vora J, Harris A, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin. Cancer Res. 2005;11:3633–3641. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- [7].Normanno N, De Luca A, Maiello MR, Campiglio M, et al. The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell Physiol. 2006;207:420–427. doi: 10.1002/jcp.20588. [DOI] [PubMed] [Google Scholar]
- [8].Yokoi K, Sasaki T, Bucana CD, Fan D, et al. Simultaneous inhibition of EGFR, VEGFR, and platelet-derived growth factor receptor signaling combined with gemcita-bine produces therapy of human pancreatic carcinoma and prolongs survival in an orthotopic nude mouse model. Cancer Res. 2005;65:10371–10380. doi: 10.1158/0008-5472.CAN-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bild AH, Yao G, Chang JT, Wang Q, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- [10].Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, et al. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- [11].Liotta LA, Espina V, Mehta AI, Calvert V, et al. Protein microarrays: meeting analytical challenges for clinical applications. Cancer Cell. 2003;3:317–325. doi: 10.1016/s1535-6108(03)00086-2. [DOI] [PubMed] [Google Scholar]
- [12].Petricoin EF, III, Bichsel VE, Calvert VS, Espina V, et al. Mapping molecular networks using proteomics: a vision for patient-tailored combination therapy. J. Clin. Oncol. 2005;23:3614–3621. doi: 10.1200/JCO.2005.02.509. [DOI] [PubMed] [Google Scholar]
- [13].Espina V, Edmiston KH, Heiby M, Pierobon M, et al. A portrait of tissue phosphoprotein stability in the clinical tissue procurement process. Mol. Cell Proteomics. 2008;7:1998–2018. doi: 10.1074/mcp.M700596-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Imbeaud S, Graudens E, Boulanger V, Barlet X, et al. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res. 2005;33:e56. doi: 10.1093/nar/gni054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Imbeaud S, Auffray C. The 39 steps’ in gene expression profiling: critical issues and proposed best practices for microarray experiments. Drug Discov. Today. 2005;10:1175–1182. doi: 10.1016/S1359-6446(05)03565-8. [DOI] [PubMed] [Google Scholar]
- [16].Almeida A, Paul Thiery J, Magdelenat H, Radvanyi F. Gene expression analysis by real-time reverse transcription polymerase chain reaction: influence of tissue handling. Anal. Biochem. 2004;328:101–108. doi: 10.1016/j.ab.2004.02.004. [DOI] [PubMed] [Google Scholar]
- [17].Micke P, Ohshima M, Tahmasebpoor S, Ren ZP, et al. Biobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimens. Lab. Invest. 2006;86:202–211. doi: 10.1038/labinvest.3700372. [DOI] [PubMed] [Google Scholar]
- [18].Mutter GL, Zahrieh D, Liu C, Neuberg D, et al. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004;5:88. doi: 10.1186/1471-2164-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ohashi Y, Creek KE, Pirisi L, Kalus R, et al. RNA degradation in human breast tissue after surgical removal: a time-course study. Exp. Mol. Pathol. 2004;77:98–103. doi: 10.1016/j.yexmp.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [20].Perlmutter MA, Best CJ, Gillespie JW, Gathright Y, et al. Comparison of snap freezing versus ethanol fixation for gene expression profiling of tissue specimens. J. Mol. Diagn. 2004;6:371–377. doi: 10.1016/S1525-1578(10)60534-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klumpp S, Krieglstein J. Serine/threonine protein phosphatases in apoptosis. Curr. Opin. Pharmacol. 2002;2:458–462. doi: 10.1016/s1471-4892(02)00176-5. [DOI] [PubMed] [Google Scholar]
- [22].Stone RL, Dixon JE. Protein-tyrosine phosphatases. J. Biol. Chem. 1994;269:31323–31326. [PubMed] [Google Scholar]
- [23].Goldstein BJ. Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J. Clin. Endocrinol. Metab. 2002;87:2474–2480. doi: 10.1210/jcem.87.6.8641. [DOI] [PubMed] [Google Scholar]
- [24].Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- [25].Li J, Gould TD, Yuan P, Manji HK, et al. Post-mortem interval effects on the phosphorylation of signaling proteins. Neuropsychopharmacology. 2003;28:1017–1025. doi: 10.1038/sj.npp.1300112. [DOI] [PubMed] [Google Scholar]
- [26].Li X, Friedman AB, Roh MS, Jope RS. Anesthesia and post-mortem interval profoundly influence the regulatory serine phosphorylation of glycogen synthase kinase-3 in mouse brain. J. Neurochem. 2005;92:701–704. doi: 10.1111/j.1471-4159.2004.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petricoin EF, III, Espina V, Araujo RP, Midura B, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007;67:3431–3440. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- [28].VanMeter AJ, Rodriguez AS, Bowman ED, Jen J, et al. Laser capture microdissection and protein microarray analysis of human non-small cell lung cancer: differential epidermal growth factor receptor (EGPR) phosphorylation events associated with mutated EGFR compared with wild type. Mol. Cell. Proteomics. 2008;7:1902–1924. doi: 10.1074/mcp.M800204-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grellner W, Vieler S, Madea B. Transforming growth factors (TGF-alpha and TGF-beta1) in the determination of vitality and wound age: immunohistochemical study on human skin wounds. Forensic Sci. Int. 2005;153:174–180. doi: 10.1016/j.forsciint.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [30].Grellner W. Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci. Int. 2002;130:90–96. doi: 10.1016/s0379-0738(02)00342-0. [DOI] [PubMed] [Google Scholar]
- [31].Grellner W, Madea B. Demands on scientific studies: vitality of wounds and wound age estimation. Forensic Sci. Int. 2007;165:150–154. doi: 10.1016/j.forsciint.2006.05.029. [DOI] [PubMed] [Google Scholar]
- [32].Ohshima T. Forensic wound examination. Forensic Sci. Int. 2000;113:153–164. doi: 10.1016/s0379-0738(00)00269-3. [DOI] [PubMed] [Google Scholar]
- [33].Oehmichen M. Vitality and time course of wounds. Forensic Sci. Int. 2004;144:221–231. doi: 10.1016/j.forsciint.2004.04.057. [DOI] [PubMed] [Google Scholar]
- [34].Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- [35].Becker KF, Schott C, Hipp S, Metzger V, et al. Quantitative protein analysis from formalin-fixed tissues: implications for translational clinical research and nanoscale molecular diagnosis. J. Pathol. 2007;211:370–378. doi: 10.1002/path.2107. [DOI] [PubMed] [Google Scholar]
- [36].Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J. Histochem. Cytochem. 1985;33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- [37].Helander KG. Kinetic studies of formaldehyde binding in tissue. Biotech. Histochem. 1994;69:177–179. doi: 10.3109/10520299409106282. [DOI] [PubMed] [Google Scholar]
- [38].Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nassiri M, Ramos S, Zohourian H, Vincek V, et al. Preservation of biomolecules in breast cancer tissue by a formalin-free histology system. BMC Clin. Pathol. 2008;8:1. doi: 10.1186/1472-6890-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Devireddy RV. Predicted permeability parameters of human ovarian tissue cells to various cryoprotectants and water. Mol. Reprod. Dev. 2005;70:333–343. doi: 10.1002/mrd.20209. [DOI] [PubMed] [Google Scholar]
- [41].He Y, Devireddy RV. An inverse approach to determine solute and solvent permeability parameters in artificial tissues. Ann. Biomed. Eng. 2005;33:709–718. doi: 10.1007/s10439-005-1511-x. [DOI] [PubMed] [Google Scholar]
- [42].Belief V, Boissiére F, Bibeau F, Desmetz C, et al. Proteomic analysis of RCL2 paraffin-embedded tissues. J. Cell Mol. Med. 2008;12:2027–2036. doi: 10.1111/j.1582-4934.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Delfour C, Roger P, Bret C, Berthe ML, et al. RCL2, a new fixative, preserves morphology and nucleic acid integrity in paraffin-embedded breast carcinoma and microdissected breast tumor cells. J. Mol. Diagn. 2006;8:157–169. doi: 10.2353/jmoldx.2006.050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nadji M, Nassiri M, Vincek V, Kanhoush R, et al. Immunohistochemistry of tissue prepared by a molecular-friendly fixation and processing system. Appl. Immunohistochem. Mol. Morphol. 2005;13:277–282. doi: 10.1097/01.pai.0000146544.51771.79. [DOI] [PubMed] [Google Scholar]
- [45].Chu WS, Furusato B, Wong K, Sesterhenn IA, et al. Ultrasound-accelerated formalin fixation of tissue improves morphology, antigen and mRNA preservation. Mod. Pathol. 2005;18:850–863. doi: 10.1038/modpathol.3800354. [DOI] [PubMed] [Google Scholar]
- [46].Stanta G, Mucelli SP, Petrera F, Bonin S, et al. A novel fixative improves opportunities of nucleic acids and proteomic analysis in human archive’s tissues. Diagn. Mol. Pathol. 2006;15:115–123. doi: 10.1097/00019606-200606000-00009. [DOI] [PubMed] [Google Scholar]
- [47].Vincek V, Nassiri M, Nadji M, Morales AR. A tissue fixative that protects macromolecules (DNA, RNA, and protein) and histomorphology in clinical samples. Lab. Invest. 2003;83:1427–1435. doi: 10.1097/01.lab.0000090154.55436.d1. [DOI] [PubMed] [Google Scholar]
- [48].Svensson M, Skold K, Nilsson A, Falth M, et al. Neuropeptidomics: MS applied to the discovery of novel peptides from the brain. Anal. Chem. 2007;79:15–16. 18–21. doi: 10.1021/ac071856q. [DOI] [PubMed] [Google Scholar]
- [49].Morales AR, Nassiri M, Kanhoush R, Vincek V, et al. Experience with an automated microwave-assisted rapid tissue processing method: validation of histologic quality and impact on the timeliness of diagnostic surgical pathology. Am. J. Clin. Pathol. 2004;121:528–536. doi: 10.1309/ACK8-AHV0-1T47-QR53. [DOI] [PubMed] [Google Scholar]


