Abstract
A deficiency in nitric oxide (NO) generation leads to salt-sensitive hypertension, but the role of increased superoxide (O2−) in such salt sensitivity has not been delineated. We examined the hypothesis that an enhancement in O2− activity induced by high-salt (HS) intake under deficient NO production contributes to the development of salt-sensitive hypertension. Endothelial NO synthase knockout (eNOS KO; total n = 64) and wild-type (WT; total n = 58) mice were given diets containing either normal (NS; 0.4%) or high-salt (HS; 4%) for 2 wk. During this period, mice were chronically treated with a O2− scavenger, tempol (400 mg/l), or an inhibitor of NADPH oxidase, apocynin (1 g/l), in drinking water or left untreated (n = 6–8 per group). Blood pressure was measured by radiotelemetry and 24-h urine samples were collected in metabolic cages. Basal mean arterial pressure (MAP) in eNOS KO was higher (125 ± 4 vs. 106 ± 3 mmHg) compared with WT. Feeding HS diet did not alter MAP in WT but increased it in eNOS KO to 166 ± 9 mmHg. Both tempol and apocynin treatment significantly attenuated the MAP response to HS in eNOS KO (134 ± 3 and 139 ± 4 mmHg, respectively). Basal urinary 8-isoprostane excretion rates (UIsoV), a marker for endogenous O2− activity, were similar (2.8 ± 0.2 and 2.4 ± 0.3 ng/day) in both eNOS KO and WT mice. However, HS increased UIsoV more in eNOS KO than in WT (4.6 ± 0.3 vs. 3.8 ± 0.2 ng/day); these were significantly attenuated by both tempol and apocynin treatment. These data indicate that an enhancement in O2− activity contributes substantially to the development of salt-sensitive hypertension under NO-deficient conditions.
Keywords: superoxide anion, nitric oxide, kidney function, hypertension
an excessive salt intake has been suggested as an important contributor to the high prevalence of cardiovascular disease including hypertension (4, 34, 44, 46). There are several mechanisms controlling sodium balance in the body and a disruption of their physiological functions may lead to the development of salt sensitivity (18, 40, 50). Nitric oxide (NO) is a potent vasodilator that modulates vascular tone and renal function maintaining sodium balance and extracellular fluid volume, thus regulating blood pressure (11, 19). NO is produced in most tissues by three different isoforms of NO synthase (NOS) enzyme; neuronal, inducible, and endothelial (eNOS) isoforms. Nonspecific inhibition of all NOS isoforms leads to the development of salt-sensitive hypertension suggesting that the NO system is of critical importance in the regulation of sodium balance and blood pressure (3, 14, 40, 50). In particular, eNOS is a crucial source of NO that regulates cardiovascular and renal function (19, 23), and mice lacking only the eNOS gene exhibit higher blood pressures compared with wild-type mice (31).
The development of oxidative stress is dependent on the balance between the production and the degradation of oxidant radicals such as superoxide anion (O2−) and other reactive oxygen species (18, 30, 35, 45). O2− is a product of cellular oxidative metabolism and NADPH oxidase is one of the major sources of O2− in living tissues (29, 34, 35, 45, 46). Reactive O2− is rapidly reduced by the enzyme superoxide dismutase (SOD) (21, 45). Recent experiments provide evidence that NO exhibits antioxidative properties by its rapid nonenzymatic reaction with O2− and thus helps to maintain minimal levels of O2− under normal conditions and provides a protective function against the actions of O2− (6, 18, 20, 48). These results suggest that under conditions of inadequate production of NO, there is increased accumulation of O2− leading to the development of oxidative stress in the body (6, 14, 18, 20, 45). Endogenous O2− directly stimulates renal tubular reabsorptive function leading to sodium retention and thus may contribute to the development of salt sensitivity associated with an increase in blood pressure (13, 14, 20, 21).
In the present study, we examined the hypothesis that increased renal O2− activity induced by high-salt (HS) intake during deficient NO production contributes to the development of salt-sensitive hypertension. Accordingly, we examined the role of O2− activity in the development of salt sensitivity in mice lacking the gene for eNOS. These mice were fed either normal- or HS diets for 2 wk. Arterial blood pressure and excretory responses were evaluated with or without treatment of O2− scavenger tempol (4-hydroxy-tetramethylpiperidime-1-oxyl) or NADPH oxidase inhibitor apocynin during the course of the 2-wk experimental period (14, 28, 30, 43).
METHODS
The study was performed on male mice (10–12 wk of age) lacking the gene for eNOS, B6.129P2-NOSIII (eNOS KO; total n = 64), and their genetic background wild-type strain C57BL/6J (WT; total n = 58) purchased from Jackson Laboratories. All of the experiments were approved by the Institutional Animal Care and Use Committees.
Blood pressure monitoring in conscious mice.
After 7 days of acclimatization, radiotransmitters (TA11PA-C10, DSI) were implanted for monitoring the arterial blood pressure (BP) continuously. Mice were anesthetized with a combination of ketamine (90 mg/kg) and xylazine (10 mg/kg) given intraperitoneally. A midline skin incision 2 cm long from chin to manubrium was performed to isolate the common carotid artery (2, 5, 16). A blunt trocar was passed from the neck incision to abdominal region through the lateral aspect under the skin. The catheter of the implant was placed into the common carotid artery. The transmitter body was placed under the skin in the abdominal region. The skin was sutured and topical antiseptic was applied. Mice were placed on a 12:12-h light-dark cycle and received food and water ad libitum throughout the study (2). After 8–10 days of recovery, we began monitoring BP and heart rate continuously using the telemetry data acquisition system (DSI, St. Paul, MN). Only animals giving stable records were randomly divided into experimental groups receiving different diets [normal-salt (NS) 0.4% NaCl or HS 4% NaCl; Harlan-Teklad, Madison, WI]. Antioxidative treatment with the O2− scavenger tempol (Sigma, St. Louis, MO) at a concentration of 400 mg/l or NADPH oxidase inhibitor apocynin at a concentration of 1 g/l was given in drinking water for 2 wk (14, 28, 30, 43). Apocynin was sonicated with cyclodextrin in 2 ml of ethanol and then dissolved in water. In our preliminary study, solution of cyclodextrin (1 g/l) did not affect BP or excretory parameters in mice (Kopkan L and Majid DS, unpublished observation). The drinking water solutions were changed every 1–2 days and filled into covered bottles to minimize degradation by light. BP was recorded in WT and eNOS KO mice that were randomly divided into the following six groups on NS diet: 1) WT-NS untreated (n = 8), 2) WT-NS + tempol (n = 6), 3) WT-NS + apocynin (n = 6), 4) eNOS KO-NS untreated (n = 8), 5) eNOS KO-NS + tempol (n = 8), and 6) eNOS KO-NS + apocynin (n = 6), and six groups on HS diet: 1) WT-HS untreated (n = 7), 2) WT-HS + tempol (n = 6), 3) WT-HS + apocynin (n = 7), 4) eNOS KO-HS untreated (n = 8), 5) eNOS KO-HS + tempol (n = 8), and 6) eNOS KO-HS + apocynin (n = 6).
Urine collection in conscious mice.
Twenty-four-hour urine samples were collected in conscious mice using metabolic cages on the day before the start of the treatment to establish basal excretory parameters and then on the 7th and 13th day of the experiment. A total of 12 groups (n = 6–8 in each group) of mice as classified earlier for BP measurements were used for metabolic cage studies. Animals were housed individually in metabolic cages and urine was collected for 24 h into sterile tubes.
Urine volumes were determined from each urine collection and samples were centrifuged (3,000 rpm/5 min; 4°C) and preserved for analysis. For the measurement of 8-isoprostane concentration, a solution of butylated hydroxytoluene in ethanol was used and a solution of 2-propanol was used for nitrate/nitrite analysis (12, 14).
Analytical methods and statistics.
Urinary concentrations of sodium and potassium were assessed by flame photometry. Concentration of 8-isoprostane (marker for oxidative stress) in urine samples was determined by enzyme immunoassay and nitrate/nitrite concentration in urine was measured using a colorimetric assay (Caymen Chemical, Ann Arbor, MI) (12, 13, 21). Malondialdehyde (MDA; a product of lipid peroxidation, a marker for endogenous O2− activity) in the renal tissue was measured colorimetrically (Cayman Chemical) (14). Kidney tissues were homogenized and total protein concentrations were determined by the BCA assay. Renal tissue nitrotyrosine (marker for peroxynitrite formation) concentration was measured by immunoassay (Oxis International, Foster City, CA, USA). For the protein expression of gp91phox subunit (catalytic membraneous subunit of NADPH oxidase), samples were separated by electrophoresis on a 10% Tris-glycine gel and proteins were transferred electrophoretically to a PVDF membrane (6, 43). The primary antibody used was goat gp91phox polyclonal antibody (1:200; Santa Cruz Biotechnology). The blots were washed and incubated with anti-goat secondary antibody (1:5,000; Santa Cruz Biotechnology) conjugated to horseradish peroxidase. Detection was accomplished using enhanced chemiluminescence Western blotting (ECL; GE Healthcare, Amersham, UK) and blots were exposed to X-ray film (Hyperfilm-ECL, GE Healthcare). Band intensity was measured densitometrically. The values were normalized to β-actin (6, 43).
Results are expressed as means ± SE. Statistical analysis Using GraphPad Prism software (Graph Pad Software, San Diego, CA), statistical analysis within groups were conducted by the use of the repeated-measures ANOVA and Dunnett multiple comparisons test. Statistical comparisons between the groups were performed by two-way ANOVA, followed by Newman-Keuls test. P < 0.05 is considered as significant.
RESULTS
BP responses in WT and eNOS KO mice.
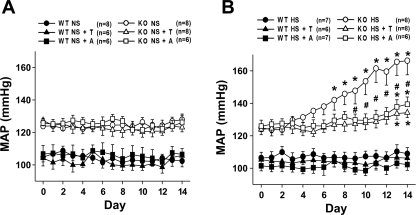
The basal level of mean arterial pressure (MAP) was significantly higher in eNOS KO compared with WT mice (123 ± 2 vs. 105 ± 3 mmHg), as shown in Fig. 1. In WT mice, HS intake did not cause any significant changes in MAP during the 2-wk experimental period (109 ± 3 to 112 ± 4 mmHg). Neither tempol nor apocynin treatment altered MAP in WT mice on NS (108 ± 5 to 101 ± 2 mmHg and 109 ± 3 to 102 ± 4 mmHg, respectively) or HS intake (105 ± 3 to 106 ± 4 mmHg and 101 ± 2 to 100 ± 3 mmHg, respectively).
Fig. 1.
Mean arterial pressure (MAP) in wild-type (WT) and endothelial nitric oxide synthase (eNOS)-deficient mice (KO) untreated and treated with tempol (T) or apocynin (A) during normal (NS; A)- or high-salt (HS; B) intake. Basal MAP was significantly higher in KO mice compared with WT mice (P < 0.05). *P < 0.05 vs. basal. #P < 0.05 vs. KO HS.
In eNOS KO mice, MAP was not altered during NS intake for 2 wk (123 ± 2 to 125 ± 3 mmHg). Treatment with tempol or apocynin along with NS intake in eNOS KO mice did not alter MAP (127 ± 3 to 123 ± 2 and 126 ± 2 to 125 ± 4 mmHg; Fig. 1A). However, HS intake in eNOS KO mice caused a significant increase in MAP (125 ± 2 to 166 ± 9 mmHg; P < 0.05) as shown in Fig. 1B. Antioxidant treatment with tempol or apocynin significantly attenuated the increase in MAP in eNOS KO mice during HS intake (124 ± 2 to 134 ± 3 and 126 ± 3 to 139 ± 4 mmHg; P < 0.05; Fig. 1B).
In agreement with previous studies (15, 29), eNOS KO mice not only exhibit higher BP but these mice display lower heart rate (HR) compared with WT mice (541 ± 12 vs. 613 ± 18 beats/min). There were no significant changes in HR during the 2-wk experimental period in both WT and eNOS KO mice with HS intake (626 ± 13 to 608 ± 10 and 539 ± 18 to 518 ± 19 beats/min, respectively). Furthermore, tempol treatment did not alter HR in WT or in eNOS KO mice with NS intake (612 ± 17 to 598 ± 15 and 548 ± 16 to 523 ± 18 beats/min, respectively) or HS intake (621 ± 18 to 594 ± 14 and 541 ± 21 to 518 ± 15 beats/min, respectively). Apocynin administration also did not alter HR during the experimental periods in WT or eNOS KO mice with NS intake (628 ± 12 to 623 ± 14 and 544 ± 19 to 538 ± 16 beats/min, respectively) as well as with HS intake (616 ± 17 to 613 ± 18 and 551 ± 14 to 548 ± 13 beats/min, respectively).
Renal excretory responses in WT and eNOS KO mice.
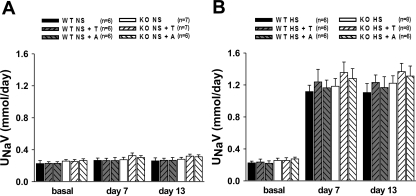
Twenty-four-hour urine samples were collected from mice that were implanted with radiotransmitters as well as nonimplanted mice. There were no significant differences in excretory parameters between implanted and nonimplanted mice. Thus, results are combined for the presentation of mean data in groups (n = 6–8 in each group). There were no differences in sodium excretion among the groups on NS intake (Fig. 2A). HS intake increased sodium excretion significantly in all groups of mice as expected (Fig. 2B). In both WT and eNOS KO mice fed a NS diet, chronic tempol as well as apocynin treatment did not cause any significant alterations in daily sodium excretion.
Fig. 2.
Urinary sodium excretion (UNaV) in WT and eNOS-deficient mice (KO) untreated and treated with T or A during NS (A) or HS (B) intake. Compared with basal values, HS intake significantly increased UNaV in all goups (P < 0.05).
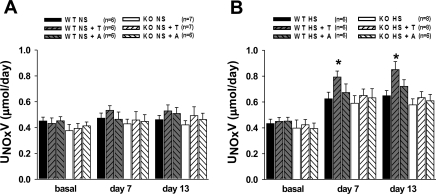
Figure 3 illustrates the daily urinary excretion rate of NO metabolites, nitrate/nitrite (UNOxV). Basal UNOxV was not significantly different between WT and eNOS KO mice (0.45 ± 0.03 and 0.38 ± 0.05 μmol/day) and NS intake did not alter UNOxV in either group. HS intake for 2 wk induced similar increases in UNOxV in both WT and eNOS KO mice (0.65 ± 0.04 and 0.58 ± 0.05 μmol/day, respectively; Fig. 3B). However, chronic tempol in WT fed a HS diet caused further increases in UNOxV (0.85 ± 0.06 μmol/day), but this increase was not seen in eNOS KO fed a HS diet (Fig. 3B). Apocynin treatment did not significantly affect UNOxV in either WT or eNOS KO mice.
Fig. 3.
Urinary nitrate/nitrite excretion (UNOxV) in WT and KO untreated and treated with T or A during NS (A) or HS (B) intake. Compared with basal values, HS intake significantly increased UNOxV in all goups (P < 0.05). *P < 0.05 vs. WT HS.
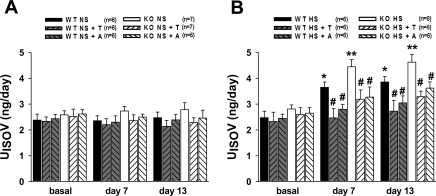
As shown in Fig. 4, basal levels of 24-h urinary 8-isoprostane excretion rates (UISOV) were not significantly different in WT and eNOS KO mice (2.4 ± 0.3 and 2.6 ± 0.2 ng/day). Tempol and apocynin did not alter UISOV in either WT (2.1 ± 0.2 and 2.4 ± 0.2 ng/day, respectively) or eNOS KO mice (2.3 ± 0.2 and 2.5 ± 0.3 ng/day, respectively) during NS intake. HS intake increased UISOV in both WT and KO mice; however, the magnitude was markedly higher in eNOS KO mice compared with WT mice (4.6 ± 0.3 vs. 3.8 ± 0.2 ng/day; Fig. 4B). Chronic tempol or apocynin treatment attenuated UISOV in both WT (2.7 ± 0.4 and 3.0 ± 0.3 ng/day, respectively) and eNOS KO mice (3.3 ± 0.2 and 3.6 ± 0.2 ng/day, respectively) in response to HS intake (Fig. 4B).
Fig. 4.
Urinary 8-isoprostane excretion (UISOV) in WT and KO untreated and treated with T or A during NS (A) or HS (B) intake. *P < 0.05, **P < 0.001 vs. basal, #P < 0.05 vs. corresponding untreated groups.
Renal tissue analysis in WT and eNOS KO mice.
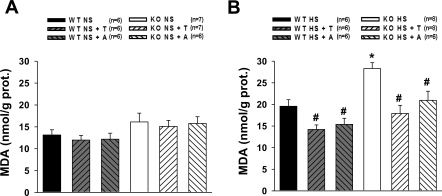
Concentration of MDA was measured in the renal tissues of WT and eNOS KO mice collected at the end of experimental period. As shown in Fig. 5A, MDA level was similar in all groups of mice in NS-fed mice. Increases in MDA by HS intake were markedly greater in eNOS KO mice compared with WT (Fig. 5B). Both tempol and apocynin caused significant reduction in the tissue level of MDA during HS intake (Fig. 5B).
Fig. 5.
Renal malondialdehyde (MDA) concentration in WT and KO untreated and treated with T or A during NS (A) or HS (B) intake. *P < 0.05 vs. corresponding WT groups. #P < 0.05 vs. corresponding untreated groups.
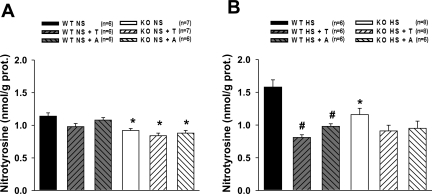
In NS intake state, nitrotyrosine concentration in the renal tissue was slightly lower in eNOS KO mice compared with WT (Fig. 6A). HS intake induced increases in nitrotyrosine level only in WT mice but not in eNOS KO (Fig. 6B). Tempol and apocynin prevented the rise in nitrotyrosine level in HS-fed WT mice but no changes were observed in HS-fed eNOS KO mice.
Fig. 6.
Renal nitrotyrosine concentration in WT and KO untreated and treated with T or A during NS (A) or HS (B) intake. *P < 0.05 vs. corresponding WT groups. #P < 0.05 vs. untreated WT group.
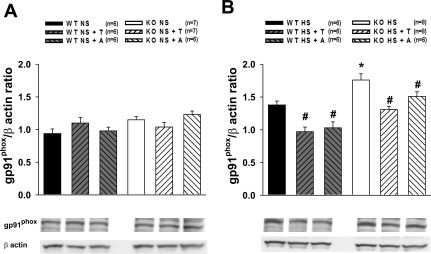
As shown in Fig. 7A, protein expression of gp91phox subunit in the renal tissue was similar in WT and eNOS KO mice. However, HS intake induced protein expression of gp91phox subunit of NADPH oxidase, the magnitude of which is greater in eNOS KO mice compared with that in WT mice (Fig. 7B). Both tempol and apocynin prevented this enhancement in the gp91phox protein expression in HS-fed WT as well as eNOS KO mice (Fig. 7B).
Fig. 7.
Protein expression of gp91phox subunit in the kidney tissue in WT and KO untreated and treated with T or A during NS (A) or HS (B) intake. *P < 0.05 vs. WT groups. #P < 0.05 vs. corresponding untreated group.
DISCUSSION
The present study demonstrates that chronic HS intake for 2 wk causes a substantial increase in arterial BP in eNOS KO mice, but not in WT mice. Furthermore, this study shows for the first time that the increase in BP during HS intake was markedly attenuated during treatment with the O2− scavenger tempol or NADPH oxidase inhibitor apocynin in eNOS KO mice. Previous studies also showed that antioxidant treatment significantly attenuates BP in several hypertensive models, particularly those associated with salt sensitivity (1, 30, 39, 43, 47, 51). In an earlier study in rats (14), we also demonstrated that the enhanced O2− activity caused by chronic administration of a nonspecific inhibitor of NOS contributes to the development of salt sensitivity. The present findings indicate that the development of salt-sensitive hypertension in eNOS KO mice is critically dependent on an associated enhancement in O2− activity induced by HS intake. Although HS also induces O2− production in WT mice, an oxidative balance is tightly maintained by the optimal production of NO, which serves an important antioxidative function. However, in eNOS KO mice, HS intake tilted this balance due to a lack of NO generation by eNOS and thus resulted in increased oxidative stress. Previous data from our laboratory (13, 14, 20, 21) as well as from others (1, 29, 30, 39, 45) suggested that such alterations in the oxidative balance modulate renal function, contributing to the pathophysiology of salt sensitivity.
Previous studies (14, 20, 35, 36, 47, 51) validated that changes in plasma or urinary 8-isoprostane levels are closely correlated with endogenous O2− activity and thus UISOV serves as a reliable marker for oxidative stress. Although UISOV was increased due to HS intake in both strains of mice, the eNOS KO mice exhibited greater UISOV than WT mice. Furthermore, renal tissue levels of MDA as a product of lipid peroxidation were markedly elevated in HS-fed eNOS KO mice compared with HS-fed WT mice suggesting the enhanced local activity of O2− in the kidney. Thus, the present findings indicate that O2− activity is significantly enhanced in eNOS KO mice during HS intake. It should be noted that only eNOS KO mice showed the salt sensitivity and the increment in BP in response to HS intake. These data indicate that functional eNOS activity is essential to offset the effects of HS-induced increase in O2− activity particularly via NADPH oxidase, a phenomenon that appears necessary for the maintenance of appropriate sodium balance and normal BP. We observed that both tempol and apocynin treatment attenuated the increase in UISOV in response to HS intake in both strains. However, the basal level of oxidative stress or O2− production in mice on NS diet is maintained at minimal levels due to efficient regulation by endogenous antioxidant mechanisms and thus no further reduction of O2− activity would be expected during the treatment with tempol or apocynin. In agreement, previous studies with chronic administration of tempol (14, 36, 47) and apocynin (33) in rats on NS diets did not cause any significant decreases in urinary excretion or plasma levels of 8-isoprostane.
The pharmacological inhibition of NO production in various studies in dogs (20), rats (14, 24, 30, 37, 40, 51), and mice (41) caused changes in UNOXV levels that correlate with NO production. In the present study, mice fed a HS diet had higher UNOxV than the mice fed a NS diet, indicating that HS increases NO formation as reported previously (7, 8, 30, 37). The fact that UNOxV increased in eNOS KO mice fed on HS diet suggests that NOS isoforms other than eNOS also contributed to the NO generation in response to HS intake (22, 25, 37, 48). Although the activation of other NOS isoforms by HS intake has been demonstrated in the kidney indicating their role in the regulation of sodium balance (7, 11, 22, 23), this compensatory mechanism seems to be inadequate in eNOS KO mice to protect against oxidative stress and salt sensitivity in these mice. Moreover, tempol treatment during HS intake caused an increase in UNOxV in WT but not in eNOS KO mice, indicating that eNOS-induced NO generation may be more involved in mediating its antioxidative function against endogenous O2− activity (17, 32). This finding also indicates that the attenuation of the hypertensive response by tempol or apocynin in eNOS KO mice is not due to an increase in NO bioavailability, but rather to a decrease in O2− activity. It is likely that in the renal tubules, the eNOS-induced NO generation is involved in reducing HS induced O2− generation that enhances sodium reabsorptive function (8, 38) and thus, constitutes an important mechanism of salt sensitivity as observed in HS-fed KO mice. Thus, the present results further support an important role of eNOS-derived NO in providing protection against HS-induced increases in O2− activity which contribute to the development of salt sensitivity and hypertension (14, 16).
The BP responses to HS intake in the eNOS KO mice could be attributed to the effects of enhanced O2− activity modulating the function of many organs including the kidney (7, 14, 20, 32, 45). Apart from direct vascular effects (13, 17, 21), enhanced renal O2− activity stimulates tubular sodium reabsorptive function leading to sodium retention (13, 20, 21, 38), thus contributing to the development of salt-sensitive hypertension. In earlier studies in dogs (20) and in hypertensive NO-deficient rats (13), acute administration of tempol into the kidney caused increases in sodium excretion during NO inhibition supporting the notion that enhanced O2− activity under conditions of NO deficiency modulates renal excretory function leading to sodium retention. An augmentation of sympathetic activity due to enhanced endogenous O2− activity may also contribute to this hypertensive response to HS intake (49). However, we observed that HR was not significantly altered in the eNOS KO mice fed a HS diet, indicating a minimal influence of altered sympathetic activity in these hypertensive mice.
The exact mechanism by which a deficiency in eNOS isoform/activity leads to development of oxidative stress induced by HS intake or how eNOS activity limits NADPH oxidase activity remained unresolved. In the present study, we found that the renal tissue protein expression of gp91phox subunit of NADPH oxidase was enhanced by HS intake which was more pronounced in eNOS KO mice compared with WT mice. Importantly, both tempol and apocynin prevented such enhancement in gp91phox protein expression. This would indicate that the activation of NAD(P)H oxidase by HS intake is one of the major sources of O2−, at least in the kidney, as shown previously (10, 29, 30, 45). In agreement, we also observed that the inhibitor of NADPH oxidase apocynin attenuated the development of salt-sensitive hypertension in eNOS KO mice. Although apocynin may not be regarded as a highly specific inhibitor of vascular NADPH oxidases (9), it is to be noted that the currently available more specific inhibitors cannot be utilized in in vivo studies, although they can be used in an in vitro experiment. Thus, apocynin is still the drug of choice as an orally active NADPH oxidase inhibitor. Moreover, it has been demonstrated that the upregulation of NADPH oxidase proteins in renal tissue from rats given HS diet alone (30, 39) or in combination with angiotensin II administration (33) was ameliorated by chronic apocynin treatment. Thus, it seems likely that an inhibition of NADPH oxidase in the renal tissue due to apocynin treatment is linked to the amelioration of salt-sensitive hypertension in eNOS KO mice.
NO and O2− are continuous products of cellular metabolism and interact with each other in biological tissues (11, 18, 34). Normally, tissue O2− levels are kept at minimal levels by the anti-oxidative function of NO as well as SOD (20, 21, 30, 45, 48). However, when NO production is diminished, this balance is altered, allowing accumulation of O2− in the tissue because of its inadequate removal by NO (14, 17, 20, 21). Accordingly, in the present study, we observed that HS intake caused an increase in renal tissue MDA and UISOV that was greater in eNOS KO mice compared with WT, indicating that there was an increase in O2− accumulation when NO production is deficient. Many previous studies from our laboratory (14, 18, 20, 24) as well as others (7, 15, 17, 32, 42, 48) have also postulated a protective role of NO-O2− interaction in the regulation of renal and vascular function. The possible formation of peroxynitrite due to NO-O2− interaction in WT mice during HS intake also seems to exert a protective role in maintaining systemic arterial pressure. We demonstrated in a recent study in rats (24) that peroxynitrite at a low infusion rate produced renal vasodilation, but higher infusion rates caused vasoconstriction. The vasodilatory action of peroxynitrite may be due to reverse formation of NO from peroxynitrite by the action of NOS that acts as nitrate reductase in this case as suggested earlier (24). In the present study, we determined the concentration of nitrosylated protein, nitrotyrosine, in the renal tissue as a marker for peroxynitrite activity. It was observed that under normal condition, eNOS KO mice exhibit lower concentration of nitrotyrosine compared with that in WT mice. Moreover, HS intake increased nitrotyrosine in WT mice but not in eNOS KO mice (Fig. 6). Thus, it is likely that in WT mice during HS intake, peroxynitrite was formed at a level that serves a protective function in the regulation of systemic arterial pressure. However, such protective function was not present in eNOS KO mice where a lower peroxynitrite production has been suggested in the kidney (27).
It could be argued that changes in overall oxidative stress may possibly be due secondarily to enhanced renin-angiotensin system (RAS) in these eNOS KO mice which may also be influenced or inhibited by superoxide scavenging/NADPH oxidase inhibition. In the present study, we have not measured plasma renin content or angiotensin II concentration in plasma or renal tissue. However, a previous study (26) reported that increasing the salt intake from a low to a high level for 3 days caused similar decreases in plasma renin concentration (PRC) in both eNOS KO and WT mice. Nevertheless, it might be possible that a prolonged intake of HS diet for 2 wk induced a different response on PRC or kidney angiotensin II level that influenced the hypertensive response in eNOS KO mice in the present study. Further studies would be needed to examine the possible contribution of RAS components in the responses to prolonged HS intake in these eNOS KO mice. However, it was also reported that chronic treatment with tempol or apocynin lowered oxidative stress but not the enhanced plasma and kidney angiotensin II levels in hypertensive transgenic ren2 rats (12). Thus, it was unlikely that the reduction in BP in response to tempol/apocynin treatment might be related to any reduction in RAS components in the present study.
In conclusion, these data demonstrate that an enhanced O2− activity induced by HS intake under deficient NO production by eNOS enzyme contributes substantially to the development of salt-sensitive hypertension.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grants HL-66432 and HL-18426 and Louisiana Board of Regents and Tulane Enhancement fund. L. Kopkan is also supported by Grant KJB-502030801 from Grant Agency of the Academy of Sciences of the Czech Republic and Grant NS/9699-4 awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic. Z. Husková is supported by Grant No. 305/08/P053 awarded by Czech Science Foundation. L. Cervenka is currently supported by institutional finance support from the Institute for Clinical and Experimental Medicine (no. MZO 00023001) and Center for Cardiovascular Research (no. 1M6798582302). Some financial support from European Union (Operational Program Prague Competitiveness project-CEVKOON; no. CZ.2.16/3.1.00/22126) was also used in this study. A. Hess was a medical student supported by American Heart Association summer student research fellowship program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the excellent technical help provided by A. Castillo.
REFERENCES
- 1.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Cervenka L, Vanecková I, Husková Z, Vanourková Z, Erbanová M, Thumová M, Skaroupková P, Opocenský M, Malý J, Chábová VC, Tesar V, Bürgelová M, Viklický O, Teplan V, Zelízko M, Kramer HJ, Navar LG. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. J Hypertens 26: 1379–1389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol 32: 400–409, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr 25, 3Suppl: 247S–255S, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 53: 351–355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison CB, Drummond GR, Sobey CG, Selemidis S. Evidence that nitric oxide inhibits vascular inflammation and superoxide production via a p47-dependent mechanism in mice. Clin Exp Pharmacol Physiol 37: 429–434, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 290: F1279–F1284, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Herrera M, Silva G, Garvin JL. A high-salt diet dissociates NO synthase-3 expression and NO production by the thick ascending limb. Hypertension 47: 95–101, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an oxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol 24: 299–315, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kopkan L, Husková Z, Vanourková Z, Thumová M, Skaroupková P, Malý J, Kramer HJ, Dvorák P, Cervenka L. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol 51: 175–181, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension 47: 568–572, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salamon M, Grayson J, Lefer AM. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest 99: 684–691, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard AM, Chafe LL, Montani JP, Van Vliet BN. Increased salt sensitivity in endothelial nitric oxide synthase-knockout mice. Am J Hypertens 19: 1264–1269, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Majid DS, Kopkan L. Nitric oxide and superoxide interaction in the kidney and its implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol 34: 946–952, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens 14, Suppl 3: 74S–82S, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal reponses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Manning RD, Jr, Hu L, Tan DY, Meng S. Role of abnormal nitric oxide systems in salt-sensitive hypertension. Am J Hypertens 14, Suppl 3: 68S–73S, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Massion PB, Balligand JL. Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): lessons from genetically modified mice. J Physiol 546: 63–75, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matavelli LC, Kadowitz PJ, Navar LG, Majid DS. Renal hemodynamic and excretory responses to intra-arterial infusion of peroxynitrite in anesthetized rats. Am J Physiol Renal Physiol 296: F170–F176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattson DL, Maeda CY, Bachman TD, Cowley AW., Jr Inducible nitric oxide synthase and blood pressure. Hypertension 31: 15–20, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Mattson DL, Meister CJ. Sodium sensitivity of arterial blood pressure in l-NAME hypertensive but not eNOS knockout mice. Am J Hypertens 19: 327–329, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Mendoza MG, Castillo-Henkel C, Medina-Santillan R, Jarillo Luna RA, Robles HV, Romo E, Rios A, Escalante B. Kidney damage after renal ablation is worsened in endothelial nitric oxide synthase −/− mice and improved by combined administration of l-arginine and antioxidants. Nephrology 13: 218–227, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med 34: 93–102, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 43: 841–848, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol 15: 306–315, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol 284: R628–R638, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Pech V, Sikka SC, Sindhu RK, Vaziri ND, Majid DS. Oxidant stress and blood pressure responses to angiotensin II administration in rats fed varying salt diets. Am J Hypertens 19: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Romero JC, Reckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 34: 943–949, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 295: R1858–R1865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int 46: 230–236, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya K, Sakai H, Suzuki N, Iwashima F, Yoshimoto T, Shichiri M, Hirata Y. Chronic blockade of nitric oxide synthesis reduces adiposity and improves insulin resistance in high fat-induced obese mice. Endocrinology 148: 4548–4556, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Uppu RM, Nossaman BD, Greco AJ, Fokin A, Murthy SN, Fonseca VA, Kadowitz PJ. Cardiovascular effects of peroxynitrite. Clin Exp Pharmacol Physiol 34: 933–937, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 22: 535–542, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wilcox CS. Special feature: cardiovascular-kidney interactions in health and disease. Am J Physiol Regul Integr Comp Physiol 290: R34–R36, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Williams JM, Pollock JS, Pollock DM. Arterial pressure response to the antioxidant tempol and ETB receptor blockade in rats on a high-salt diet. Hypertension 44: 770–775, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 3: 203–213, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Fink GD, Galligan JJ. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in DOCA-salt rats. Am J Physiol Heart Circ Physiol 283: H885–H892, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Yamada SS, Sassaki AL, Fujihara CK, Malheiros DM, De Nucci G, Zatz R. Effect of salt intake and inhibitor dose on arterial hypertension and renal injury induced by chronic nitric oxide blockade. Hypertension 27: 1165–1172, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R903–R908, 2005 [DOI] [PubMed] [Google Scholar]