Abstract
Bacterial infection and sepsis are associated with renal tubule dysfunction and dysregulation of systemic electrolyte balance but the underlying mechanisms are incompletely understood. Recently, we demonstrated that HCO3− absorption by the medullary thick ascending limb (MTAL) is inhibited by gram-negative bacterial LPS through activation of Toll-like receptor 4 (TLR4). Here, we examined whether MTAL transport is altered by activation of TLR2, the receptor predominantly responsible for recognizing gram-positive bacteria. Confocal immunofluorescence showed expression of TLR2 in the basolateral membrane domain of rat and mouse MTALs. The functional role of TLR2 was examined in perfused MTALs using Pam3CSK4, a bacterial lipoprotein analog that specifically activates TLR2. Adding Pam3CSK4 to the bath decreased HCO3− absorption by 25%. The inhibition by Pam3CSK4 was eliminated in MTALs from TLR2−/− mice. HCO3− absorption was also inhibited by the TLR2 agonists lipoteichoic acid and peptidoglycan, two cell wall components of gram-positive bacteria. The MEK/ERK inhibitor U0126 eliminated inhibition of HCO3− absorption by bath LPS but had no effect on inhibition by Pam3CSK4. The inhibition by Pam3CSK4 was eliminated by the protein kinase C inhibitors chelerythrine Cl and bisindolylmaleimide. Moreover, the inhibition by Pam3CSK4, lipoteichoic acid, and peptidoglycan was additive to inhibition by LPS. Thus, agonists of basolateral TLR2 and TLR4 inhibit HCO3− absorption independently through distinct signaling pathways. We conclude that bacterial components act directly through TLRs to modify the transport function of renal tubules. During polymicrobial sepsis, gram-positive bacterial molecules acting through TLR2 and gram-negative LPS acting through TLR4 can function through parallel signaling pathways to impair MTAL transport. The inhibition of luminal acidification may impair the ability of the kidneys to correct systemic acidosis that contributes to sepsis pathogenesis.
Keywords: acid-base transport, sepsis, kidney, LPS, gram-positive bacteria
bacterial infection is associated frequently with kidney dysfunction and dysregulation of systemic electrolyte balance, and the development of renal insufficiency increases morbidity and predicts higher mortality in patients with sepsis or urinary tract infection (19, 24, 28, 43, 48, 56, 62, 67). Impaired renal tubule function accentuates the pathogenesis and lethality of sepsis through the loss of metabolic, fluid, and electrolyte homeostasis (26, 43, 67), including the development of systemic metabolic acidosis that contributes to multiple organ dysfunction (12, 22, 44, 59, 67) and is an independent risk factor for mortality in septic patients (30, 45). Acute endotoxemia induces a number of functional defects within the nephron, including a urinary concentrating defect, increased fractional excretion of sodium and glucose, and reduced excretion of ammonium (10, 36, 65–67, 80). At present, the immunopathologic mechanisms that underlie renal tubule dysfunction during sepsis are poorly understood. Recently, we demonstrated that absorption of HCO3− by the medullary thick ascending limb (MTAL) is inhibited by lipopolysaccharide (LPS), the dominant cell wall molecule of gram-negative bacteria responsible for initiating innate immune and inflammatory responses (34). The inhibition by LPS is mediated through activation of its cell surface receptor Toll-like receptor 4 (TLR4) (34). These studies established that bacterial molecules act directly through TLRs to modify the transport function of renal tubules, identifying a new pathophysiological mechanism that can contribute to renal tubule dysfunction during sepsis.
Gram-negative and gram-positive bacteria are responsible for the majority of sepsis cases (3, 7, 14, 22, 50, 63). A mixture of gram-negative and gram-positive organisms accounts for a significant fraction (15–25%) of these infections, including those involving the digestive tract, respiratory tract, and burn injury (3, 7, 14, 21, 50, 55, 63). As noted above, TLR4 plays a major role in mediating cellular responses to gram-negative bacteria through recognition of LPS (2, 22, 40). Conversely, TLR2 is predominantly responsible for the detection of gram-positive bacteria (2, 15, 40, 73). TLR2 recognizes a variety of bacterial components, including lipoprotein, lipoteichoic acid, and peptidoglycan of gram-positive bacteria (2, 4, 5, 17, 20, 40, 54, 57, 69, 73–75, 87). TLR2 is constitutively expressed in proximal and distal nephron segments of rat, mouse, and human kidney (1, 23, 25, 42, 46, 49, 70, 84) and mediates lipoprotein-induced production of proinflammatory cytokines and chemokines in cultured renal tubule epithelial cells (20, 79). Recent evidence supports a role for TLR2 in mediating inflammatory responses that lead to kidney injury during ischemia-reperfusion, exposure to nephrotoxic drugs, and obstructive nephropathy (1, 25, 42, 46, 47, 49, 70, 84). At present, however, the subcellular location, signaling mechanisms, and functions of TLR2 in renal tubules are largely undefined. Whether activation of TLR2 modulates renal epithelial transport and the possible role of TLR2 in mediating renal tubule dysfunction during bacterial infection have not been investigated.
The purpose of the present study was to determine whether activation of TLR2 by bacterial components alters HCO3− absorption by the MTAL. The results demonstrate that TLR2 is expressed in the basolateral membrane domain of the MTAL and that TLR2-specific agonists inhibit HCO3− absorption. The inhibition of HCO3− absorption by bacterial components acting through TLR2 is blocked by inhibitors of protein kinase C (PKC) and is additive to inhibition by LPS acting through TLR4. These results establish a role for TLR2 in the regulation of renal tubule ion transport and show that gram-positive and gram-negative bacterial molecules function independently through distinct receptor signaling pathways to impair the transport function of the MTAL. The ability of bacterial molecules to directly inhibit HCO3− absorption may contribute to and/or impair the ability of the kidneys to correct systemic acidosis during sepsis.
METHODS
Animals.
Male Sprague-Dawley rats (50–90 g body wt) were purchased from Taconic (Germantown, NY). Male C57BL/6J (wild-type) and B6.129-Tlr2tmlKir/J (TLR2−/−) mice (6 to 8 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.129-Tlr2tmlKir/J mice do not produce TLR2 protein due to targeted disruption of the Tlr2 gene (85). Animals were maintained under pathogen-free conditions in microisolator cages and received standard rodent chow (NIH 31 diet, Ziegler) and distilled water up to the time of experiments. Body weight did not differ in wild-type and TLR2−/− mice (22 ± 1 g wild-type vs. 23 ± 1 g TLR2−/−). All protocols in this study were approved by the Institutional Animal Care and Use Committee of The University of Texas Medical Branch.
Tubule perfusion and measurement of net HCO3− absorption.
MTALs were isolated and perfused in vitro as previously described (31, 35, 82). Tubules were dissected from the inner stripe of the outer medulla at 10°C in control bath solution, transferred to a bath chamber on the stage of an inverted microscope, and mounted on concentric glass pipettes for perfusion at 37°C. The tubules were perfused and bathed in control solution that contained (in mM) 146 Na+, 4 K+, 122 Cl−, 25 HCO3−, 2.0 Ca2+, 1.5 Mg2+, 2.0 phosphate, 1.2 SO42−, 1.0 citrate, 2.0 lactate, and 5.5 glucose (equilibrated with 95% O2-5% CO2, pH 7.45 at 37°C). Experimental agents were added to the bath solution as described in results. Solutions containing experimental agents were prepared as described (32, 33, 83). Bisindolylmaleimide I, HCl was purchased from Calbiochem. The synthetic bacterial lipopeptide N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-[R]-cysteinyl-[S]-seryl-[S]-lysyl-[S]-lysine × 3 HCl (Pam3CSK4), purified lipoteichoic acid from Staphylococcus aureas, and ultra pure Escherichia coli K12 LPS were purchased from InvivoGen. Peptidoglycan from Staphylococcus aureas was purchased from Sigma. Pam3CSK4 and lipoteichoic acid were studied at 1 μg/ml and peptidoglycan at 10 μg/ml because 1) these concentrations have been used typically to induce TLR2-dependent innate immune responses in other biological systems, including classic immune cells (4, 5, 17, 53, 54, 57, 69, 72–75, 87) and renal epithelial cell lines (20, 79), and 2) they induce highly reproducible and reversible changes in MTAL transport (see results).
The protocol for study of transepithelial HCO3− absorption was as described (31, 35). Tubules were equilibrated for 20–30 min at 37°C in the initial perfusion and bath solutions and the luminal flow rate (normalized per unit tubule length) was adjusted to 1.5–1.9 nl·min−1·mm−1. One to three 10-min tubule fluid samples were then collected for each period (initial, experimental, and recovery). The tubules were allowed to reequilibrate for 5–10 min after an experimental agent was added to or removed from the bath solution. The absolute rate of HCO3− absorption (JHCO3−; pmol·min−1·mm−1) was calculated from the luminal flow rate and the difference between total CO2 concentrations measured in perfused and collected fluids (31). An average HCO3− absorption rate was calculated for each period studied in a given tubule. When repeat measurements were made at the beginning and end of an experiment (initial and recovery periods), the values were averaged. Single tubule values are presented in Figs. 2–7. Mean values ± SE (n = number of tubules) are presented in the text.
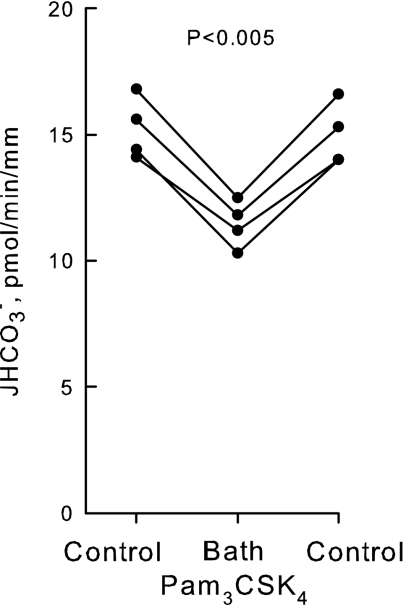
Fig. 2.
Pam3CSK4 inhibits HCO3− absorption in the MTAL. MTALs from rats were isolated and perfused in vitro in control solution, and then Pam3CSK4 (1 μg/ml) was added to and removed from the bath solution. Absolute rates of HCO3− absorption (JHCO3−) were measured as described in methods. Data points are average values for single tubules. Lines connect paired measurements made in the same tubule. P value is for paired t-test. Mean values are given in results.
Fig. 7.
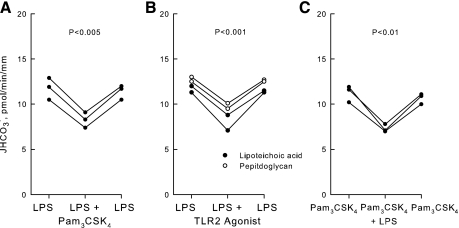
Inhibition of HCO3− absorption by TLR2 agonists is additive to inhibition by LPS. A and B: MTALs were bathed with LPS (500 ng/ml), and then Pam3CSK4, lipoteichoic acid, or peptidoglycan was added to and removed from the bath solution. C: MTALs were bathed with Pam3CSK4, and then LPS was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 2. Mean values are given in results.
Confocal immunofluorescence microscopy.
MTALs were studied by confocal microscopy as previously described (33, 34, 81). Rat and mouse MTALs were microdissected and mounted on Cell-Tak-coated coverslips at 10°C. The tubules were then incubated for 15 min at 37°C in a flowing bath using the same control solution as in HCO3− transport experiments. Following incubation, the tubules were washed with PBS and fixed and permeabilized in ice-cold acetone for 10 min. The tubules were incubated in Image-iT FX signal enhancer (Invitrogen) for 30 min at room temperature, washed, and blocked in 10% normal donkey serum in PBS for 1 h at room temperature. The tubules were then incubated overnight at 4°C with a 1:100 dilution of goat anti-mouse TLR2 polyclonal antibody (D-17, Santa Cruz Biotechnology), washed, and then incubated for 1 h at room temperature in Alexa 488-conjugated donkey anti-goat IgG antibody (1:100; Invitrogen) in blocking buffer. In some experiments, the anti-TLR2 antibody was incubated in the absence and presence of a fivefold excess by weight of blocking peptide (Santa Cruz Biotechnology) for 2 h at room temperature before tubule staining. Fluorescence staining was examined using a Zeiss laser-scanning confocal microscope (LSM510 UV META), as described (33, 81). Tubules were imaged longitudinally and z-axis optical sections (0.4 μm) were obtained through a plane at the center of the tubule, which provides a cross-sectional view of cells in the lateral tubule walls. For individual experiments, two to four tubules from the same kidney for each experimental condition, or from wild-type and TLR2−/− mice, were fixed and stained identically and imaged in a single session at identical settings of illumination, gain, and exposure time.
RESULTS
TLR2 is expressed in the basolateral membrane domain of the MTAL.
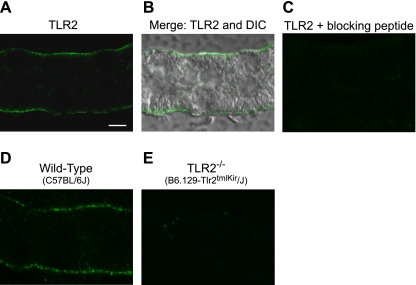
To localize TLR2 in the MTAL, tubules microdissected from normal rats (Fig. 1, A–C) and from wild-type control (C57BL/6J) and TLR2−/− (B6.129-Tlr2tmlKir/J) mice (Fig. 1, D and E) were stained with anti-TLR2 antibody and analyzed by confocal immunofluorescence microscopy. Staining for TLR2 was observed selectively in the basolateral membrane domain in both rat and mouse MTALs (Fig. 1, A, B, and D). The TLR2 staining was absent in the presence of specific blocking peptide (Fig. 1C) and in MTALs from the TLR2−/− mice (Fig. 1E). These results indicate that TLR2 is expressed in the MTAL and exhibits a selective basolateral location.
Fig. 1.
Toll-like receptor 2 (TLR2) is expressed in the basolateral membrane domain of the medullary thick ascending limb (MTAL). MTALs dissected from rats (A–C) and from wild-type (D) and TLR2−/− (E) mice were stained with anti-TLR2 antibody and analyzed by confocal immunofluorescence as described in methods. B: merged differential interference contrast image of the tubule in A to establish cell boundaries for the TLR2 localization. Images are z-axis sections (0.4 μm) taken through a plane at the center of the tubule, which shows a cross-sectional view of cells in the lateral tubule walls (33, 81). TLR2 staining was observed selectively in the basolateral membrane domain. The TLR2 staining was absent in the presence of blocking peptide and in MTALs from TLR2−/− mice. Images are representative of at least 4 tubules of each type. Scale bar = 5 μm.
Pam3CSK4 inhibits HCO3− absorption in the MTAL.
To examine a possible role for TLR2 in modulating MTAL transport, we examined the effect on HCO3− absorption of Pam3CSK4, a synthetic bacterial lipopeptide that activates cells as a specific TLR2 ligand (5, 17, 20, 57, 74, 75). Addition of Pam3CSK4 (1 μg/ml) to the bath decreased HCO3− absorption by 25%, from 15.2 ± 0.6 to 11.4 ± 0.5 pmol·min−1·mm−1 (Fig. 2). The inhibition by Pam3CSK4 is rapid (<15 min), sustained for up to 60 min, and reversible. These results demonstrate that a TLR2 agonist directly alters the transport function of the MTAL.
Inhibition by Pam3CSK4 is eliminated in MTALs from TLR2−/− mice.
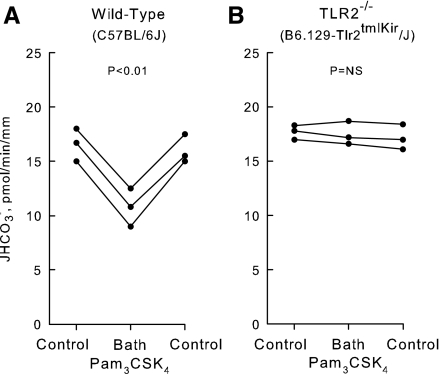
To establish the functional significance of TLR2 for ion transport regulation, the effects of Pam3CSK4 on HCO3− absorption were examined in MTALs from wild-type control and TLR2−/− mice (Fig. 3). The basal rate of HCO3− absorption was similar in MTALs from wild-type and TLR2−/− mice. Similar to results obtained in the rat, addition of Pam3CSK4 to the bath decreased HCO3− absorption in MTALs from wild-type mice from 16.4 ± 0.7 to 10.8 ± 1.0 pmol·min−1·mm−1 (Fig. 3A). In contrast, addition of Pam3CSK4 to the bath had no effect on HCO3− absorption in MTALs from TLR2−/− mice (17.4 ± 0.5 pmol·min−1·mm−1, control vs. 17.5 ± 0.6 pmol·min−1·mm−1, Pam3CSK4; Fig. 3B). These results identify a role for TLR2 in the regulation of renal tubule transport and support the conclusion that TLR2 is the signaling receptor that mediates inhibition of HCO3− absorption by Pam3CSK4.
Fig. 3.
Inhibition of HCO3− absorption by Pam3CSK4 is eliminated in MTALs from TLR2−/− mice. MTALs from wild-type control (A) and TLR2−/− (B) mice were perfused in vitro in control solution, and then Pam3CSK4 was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 2. NS, not significant. Mean values are given in results.
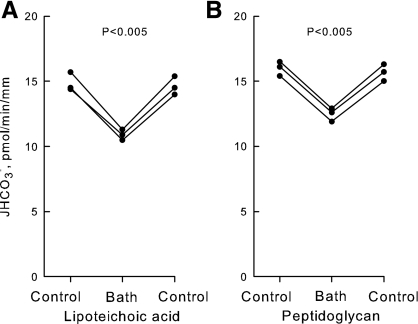
Lipoteichoic acid and peptidoglycan inhibit HCO3− absorption in the MTAL.
To assess further the significance of TLR2 for MTAL transport regulation, we examined the effects of the TLR2 agonists lipoteichoic acid and peptidoglycan. Lipoteichoic acid and peptidoglycan are components of the cell wall of gram-positive bacteria and function as TLR2 ligands structurally distinct from bacterial lipoproteins (2, 4, 40, 54, 57, 69, 72, 87). Addition of lipoteichoic acid (1 μg/ml) to the bath decreased HCO3− absorption by 26%, from 14.7 ± 0.3 to 10.9 ± 0.2 pmol·min−1·mm−1 (Fig. 4A). Similarly, addition of peptidoglycan (10 μg/ml) to the bath decreased HCO3− absorption by 22%, from 15.8 ± 0.4 to 12.4 ± 0.3 pmol·min−1·mm−1 (Fig. 4B). The inhibition by both compounds is reversible and occurs with a time course similar to that observed with Pam3CSK4. These results support further the view that bacterial components recognized by TLR2 directly modify the transport function of the MTAL.
Fig. 4.
Lipoteichoic acid and peptidoglycan inhibit HCO3− absorption in the MTAL. MTALs from rats were perfused in vitro in control solution, and then lipoteichoic acid (1 μg/ml; A) or peptidoglycan (10 μg/ml; B) was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 2. Mean values are given in results.
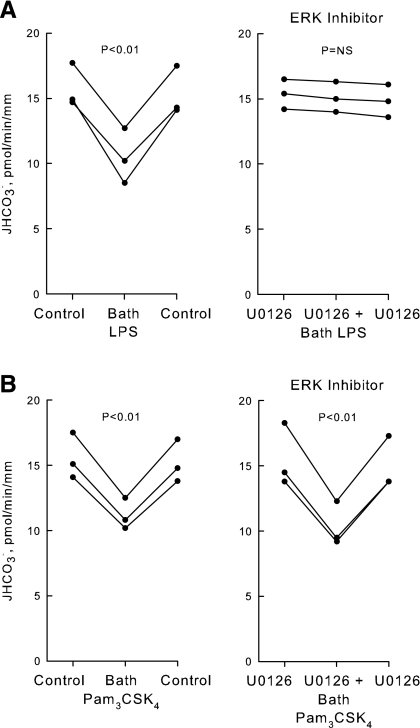
LPS and Pam3CSK4 inhibit HCO3− absorption through distinct signaling pathways.
Previously, we demonstrated that absorption of HCO3− by the MTAL is inhibited directly by LPS, the dominant cell wall molecule of gram-negative bacteria. The inhibition by LPS is mediated through activation of its cell-surface receptor TLR4 (34). Further studies were carried out to test whether the inhibition by bath LPS through TLR4 and the inhibition by Pam3CSK4 through TLR2 may be mediated through a common signaling pathway. The effects of LPS and Pam3CSK4 on HCO3− absorption were examined in the absence and presence of U0126, a MEK1/2 inhibitor that selectively blocks ERK activation and ERK-mediated inhibition of HCO3− absorption in the MTAL (33, 82, 83). Consistent with previous results (34), bath LPS decreased HCO3− absorption by 33 ± 3% under control conditions and this inhibition was eliminated completely by U0126 (Fig. 5A). In contrast, bath Pam3CSK4 decreased HCO3− absorption by 27 ± 1% in the absence and 31 ± 2% in the presence of U0126 (Fig. 5B). Thus, U0126 had no effect on the inhibition by bath Pam3CSK4. These results indicate that agonists of TLR4 and TLR2 in the basolateral membrane inhibit HCO3− absorption in the MTAL through different signal transduction pathways.
Fig. 5.
LPS and Pam3CSK4 inhibit HCO3− absorption through distinct signaling pathways. MTALs from rats were perfused in the absence (control) or presence of U0126 (15 μM) in the bath, and then LPS (500 ng/ml; A) or Pam3CSK4 (1 μg/ml; B) was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 2.
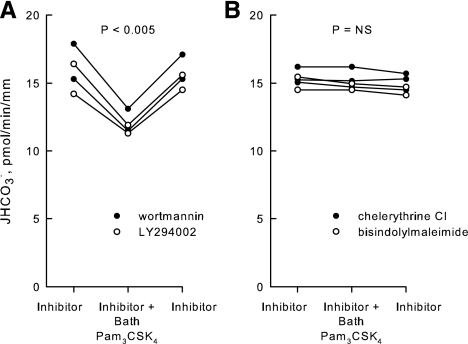
Inhibition by Pam3CSK4 is eliminated by inhibitors of PKC.
Previous reports showed that stimulation of TLR2 leads to the induction of innate immune responses through the activation of PI3K- and PKC-dependent signaling pathways (8, 18, 27, 39, 64, 72, 77, 86). To examine the role of PI3K in the inhibition of HCO3− absorption by Pam3CSK4, MTALs were bathed with LY294002 or wortmannin, inhibitors that selectively block PI3K-mediated regulation of HCO3− absorption in the MTAL (33). In the presence of LY294002 or wortmannin, Pam3CSK4 decreased HCO3− absorption by 25%, from 15.8 ± 0.6 to 11.9 ± 0.4 pmol·min−1·mm−1 (Fig. 6A). Thus, the inhibition by Pam3CSK4 is not mediated through PI3K.
Fig. 6.
Inhibition of HCO3− absorption by Pam3CSK4 is blocked by inhibitors of PKC. MTALs were bathed with the PI3K inhibitors LY294002 (20 μM) or wortmannin (100 nM; A), or with the PKC inhibitors chelerythrine Cl (100 nM) or bisindolylmaleimide (100 nM; B), and then Pam3CSK4 (1 μg/ml) was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 2. Mean values are given in results.
TLR2 signaling in several cell systems leads to activation of PKC. The role of PKC in mediating Pam3CSK4-induced inhibition of HCO3− absorption in the MTAL was examined using the selective PKC inhibitors chelerythrine Cl and bisindolylmaleimide (32, 38, 78). As shown in Fig. 6B, the effect of Pam3CSK4 to inhibit HCO3− absorption was eliminated completely by the PKC inhibitors (15.1 ± 0.3 pmol·min−1·mm−1, inhibitors vs. 15.0 ± 0.3 pmol·min−1·mm−1, inhibitors + Pam3CSK4). These results support an essential role for PKC in mediating the inhibition of HCO3− absorption by Pam3CSK4 through TLR2.
Inhibition by TLR2 agonists is additive to inhibition by LPS.
Based on the preceding results, further experiments were carried out to determine whether inhibition of HCO3− absorption by TLR2 agonists was additive to inhibition by LPS. In tubules bathed with LPS, adding Pam3CSK4, lipoteichoic acid, or peptidoglycan to the bath decreased HCO3− absorption by 25%, from 11.8 ± 0.3 to 8.8 ± 0.4 pmol·min−1·mm−1 (Fig. 7, A and B). Similarly, in tubules bathed with Pam3CSK4, adding LPS to the bath decreased HCO3− absorption by 33%, from 11.0 ± 0.4 to 7.4 ± 0.3 pmol·min−1·mm−1 (Fig. 7C), an inhibition similar to that observed with bath LPS in the absence of Pam3CSK4 (Fig. 5A) (34). In the combined presence of a TLR2 and TLR4 agonist, the HCO3− absorption rate is reduced by ∼50% compared with the rate measured in tubules not exposed to bacterial stimuli.1 These results demonstrate that agonists of TLR2 and TLR4 function independently through the activation of distinct pathways to inhibit HCO3− absorption in the MTAL.
DISCUSSION
The development of kidney dysfunction during sepsis predicts a poor outcome and the risk for mortality doubles when renal insufficiency accompanies sepsis (43, 48, 56, 62, 67). Sepsis and endotoxemia induce a variety of functional defects within the nephron in association with alterations in fluid and electrolyte balance, including impaired urinary concentrating ability, increased fractional excretion of sodium, hypotension, and metabolic acidosis (10, 12, 22, 24, 36, 59, 65–67, 80). The mechanisms that underlie the development of kidney dysfunction during sepsis are incompletely understood. We examined the possibility that bacterial molecules act directly through TLRs to alter renal tubule function. In recent studies, we demonstrated that gram-negative bacterial LPS decreases HCO3− absorption in the MTAL through activation of TLR4 (34). In the present study, we demonstrate that MTAL HCO3− absorption is inhibited by bacterial lipoproteins and gram-positive bacterial cell wall molecules that activate TLR2. These studies establish that both TLR2 and TLR4 play a role in modulating renal tubule ion transport and that bacterial components can impair renal tubule function directly through interaction with these cell surface receptors. We show further that the inhibition of HCO3− absorption by bacterial components acting through TLR2 is additive to inhibition by LPS acting through TLR4 and that TLR2 and TLR4 agonists impair MTAL transport through the activation of different signal transduction pathways. Thus, gram-positive and gram-negative bacteria, which account for most cases of clinical sepsis, can function directly and independently to impair renal tubule function as a result of their specific molecular patterns activating different intracellular signaling pathways through distinct TLRs.
The TLRs are a family of closely related transmembrane receptors that participate in innate immunity by recognizing distinct microbial structures (2, 40). To date, at least 13 members of the TLR family have been identified in mammalian cells (40). In response to pathogen recognition, TLRs activate intracellular signal transduction pathways that lead to activation of specific kinases and transcription factors and the production of proinflammatory mediators (2, 22, 40). TLR2 plays a major role in the recognition of gram-positive bacterial components, including lipoprotein, lipoteichoic acid, and peptidoglycan, and is important in host defense against gram-positive bacterial infection (2, 15, 40, 73, 87). Surprisingly, however, despite the fact that gram-positive bacteria account for at least half of sepsis cases (3, 14, 22, 50), the role of TLR2 in sepsis-induced kidney injury is undefined.
Our results indicate that TLR2 plays a direct role in modulating the transport function of renal tubules. The ability of the MTAL to absorb HCO3− is decreased by the synthetic bacterial lipopeptide Pam3CSK4 and by a purified preparation of the gram-positive bacterial cell wall glycolipid lipoteichoic acid, two TLR2-specific ligands (2, 5, 17, 20, 54, 57, 69, 74, 75). The inhibition by Pam3CSK4 is eliminated in MTALs from TLR2−/− mice, confirming that TLR2 is the receptor responsible for the transport inhibition. MTAL HCO3− absorption is also inhibited by peptidoglycan, an additional gram-positive cell wall molecule reported to be recognized by TLR2 (2, 4, 57, 69, 73, 87). The direct action of bacterial molecules to impair luminal acidification in renal tubules may accentuate the pathogenicity of sepsis through several mechanisms. The development of systemic metabolic acidosis contributes to multiple organ dysfunction (particularly instability of the cardiovascular system) during sepsis (12, 22, 44, 59, 67) and is independently associated with increased mortality in septic patients (30, 45). The inhibition of renal tubule acid secretion by bacterial components mediated through TLR2 would exacerbate and impair the correction of sepsis-induced acidemia. Within the kidney, the relative alkalinization of the luminal fluid would promote a variety of pathogenic processes, including increased attachment of bacteria to renal tubule cells that is critical for bacterial colonization (68), increased bacterial cell growth (29, 41, 68), the formation of bacteria-associated phosphate and calcium stones (61), and resistance to certain antibiotics (88). Thus, the ability of bacterial molecules to directly inhibit MTAL HCO3− absorption through TLR2 can adversely affect the severity and progression of sepsis at both the systemic and kidney levels.
TLR2 is expressed constitutively in renal tubule segments of rat, mouse, and human kidney, including segments of the proximal tubule and thick ascending limb (1, 23, 25, 42, 46, 49, 70, 84). Our results show that TLR2 is expressed in the basolateral membrane domain of the rat and mouse MTAL. This finding is consistent with the study of Shigeoka et al. (70), which reported that TLR2 was localized to the basolateral membrane of renal tubule cells in mouse outer medulla. The selective expression of TLR2 in the basolateral membrane differs from the expression of TLR4, which is localized in both basolateral and apical membrane domains of the MTAL (34). The luminal location of TLR4 is thought to be important for its crucial role in defense against ascending urinary tract infections, which are due predominantly to gram-negative bacteria (19, 25, 37, 58, 60). The coexpression of TLR2 and TLR4 in the basolateral membrane indicates that these two receptors are situated to monitor the composition of the interstitial fluid. This would enable the MTAL to recognize and respond rapidly to gram-positive and gram-negative bacteria that spread from the intravascular compartment to the interstitial space in the renal outer medulla during hematogenous infection. This would initiate innate immune responses that may aid in eliminating the invading bacteria. However, bacterial recognition also triggers intracellular signals that impair renal tubule function (Figs. 2 and 4) (34) and that may lead to inflammatory responses that contribute to sepsis-induced acute kidney injury. Activation of TLR2 by bacterial lipopeptide stimulated the production of proinflammatory cytokines and chemokines in cultured renal tubule epithelial cells (20, 79), consistent with a potential role for TLR2 in mediating kidney inflammation during sepsis. TLR2 on renal cells has been shown to play an important role in mediating inflammatory kidney injury in a variety of noninfectious conditions, including acute ischemia-reperfusion injury (42, 47, 52, 70, 84), nephrotoxic antibody-induced glomerulonephritis (16), obstructive nephropathy (46), and possibly exposure to nephrotoxic drugs (1, 49). Inflammatory injury in these conditions is associated with increased levels of TLR2 protein in the renal tubules, including increased expression of TLR2 in the thick ascending limb during ischemia-reperfusion and cyclosporine-induced renal injury (42, 46, 49, 84). TLR2-mediated inflammation and injury in these conditions are thought to involve receptor activation by endogenous “danger” molecules that are released by damaged cells (6, 42, 46, 49, 70, 71). These molecules, which include heat shock proteins, extracellular matrix components such as hyaluronan and biglycan, and the nuclear protein HMBG1, accumulate in the interstitial space during cellular injury and are recognized by TLR2 to result in the initiation of inflammatory responses (13, 46, 51, 52, 71). Our results raise the possibility that activation of TLR2 on the basolateral membrane of the MTAL by endogenous ligands may also contribute directly to alterations in renal tubule transport during these pathological conditions. Whether TLR2-induced inflammatory responses may play a role in kidney injury during sepsis remains to be determined.
A unique feature of TLR2 is its ability to interact functionally and physically with other TLRs (TLR1 and TLR6) to recognize different bacterial lipoproteins (2, 57). In particular, TLR2 cooperates with TLR1 to recognize Pam3CSK4 and other triacylated lipopeptides (17, 40, 75). Studies using cells from TLR2-deficient mice and TLR reconstitution experiments showed that expression of TLR2 is essential for responses to Pam3CSK4 and that coexpression of TLR1 significantly enhances Pam3CSK4-induced TLR2 activation (17, 75). Alternatively, TLR2 cooperates with TLR6 to discriminate diacylated lipopeptides (17, 40, 54, 57, 74) and recent evidence suggests that the responses of TLR2 to peptidoglycan and lipoteichoic acid are enhanced by interaction with TLR6 (54, 57, 74). The mechanisms involved in these cooperative interactions are incompletely understood but are thought to involve the heterodimerization of TLR2 with TLR1 or TLR6 (17, 40, 54, 57, 74, 75). TLR1 and TLR6 were detected in mouse renal tubule epithelial cells in culture (79), but to our knowledge neither receptor has been localized in native renal tubules. Whether TLR1 and/or TLR6 is coexpressed with TLR2 in the basolateral membrane of the MTAL and functions to enhance TLR2 signaling in response to bacterial structures, or to confer specificity of TLR2 ligand recognition, will be important areas for future investigation.
Our results demonstrate further that the inhibition of HCO3− absorption by bacterial molecules acting through TLR2 is additive to inhibition by gram-negative LPS acting through TLR4. This finding has important implications for the pathogenesis of renal tubule dysfunction during septic conditions in which a mixture of gram-positive and gram-negative bacteria is responsible for the sepsis response. These include sites of infection involving the gastrointestinal tract (peritonitis), lung (pneumonia), and skin (burn wounds). Our results indicate that gram-positive and gram-negative bacterial components can act independently through different TLRs to impair renal tubule function during polymicrobial sepsis. The ability of TLR2 and TLR4 agonists to inhibit HCO3− absorption additively in the MTAL is a result of their activating different intracellular signaling pathways. Consistent with previous results (34), the inhibition of HCO3− absorption by basolateral LPS is eliminated by U0126, which selectively blocks ERK activation and ERK-mediated inhibition of HCO3− absorption in the MTAL (33, 82, 83). In contrast, U0126 had no effect on the inhibition by Pam3CSK4. Thus, the recognition of specific bacterial pathogens by TLR4 and TLR2 results in inhibition of HCO3− absorption through the activation of distinct downstream mediators.
Our results indicate that PKC is an essential component of the TLR2 signaling pathway that leads to inhibition of HCO3− absorption in the MTAL. The inhibition by Pam3CSK4 was eliminated by chelerythrine Cl and bisindolylmaleimide, two selective and chemically unrelated PKC inhibitors with different mechanisms of action (38, 78). PKC has been shown to play a role in mediating innate immune responses induced by TLR2 in a variety of systems, including macrophages, neutrophils, and intestinal epithelial cell lines (18, 27, 64, 76, 77, 86). These responses are associated with TLR2-mediated activation of specific PKC isoforms, including PKC-α, -δ, -ε, and -ζ (18, 27, 64, 77, 86). We showed that the MTAL constitutively expresses members of the conventional (α, βII), novel (δ, ε), and atypical (ζ) PKC subfamilies and that PKC-dependent transport regulation in the MTAL may involve the activation of specific PKC isoforms (9). It will be important in future studies to determine the role of distinct PKC isoforms in TLR2 signaling in the MTAL, the mechanisms involved in TLR2-induced PKC activation (phosphorylation, subcellular relocalization), and the upstream activators and downstream substrates leading to PKC-dependent inhibition of HCO3− absorption. Our results provide new evidence of a role for PKC in TLR2-induced signaling in renal epithelial cells. These findings raise the possibility that the PKC pathway may be a therapeutic target for modulating TLR2 signaling in renal tubules and, potentially, for suppressing TLR2-dependent signals that lead to inflammatory kidney injury (16, 25, 46, 47, 49, 70).
TLR2 contains a consensus binding site for the p85 subunit of PI3K and activation of PI3K through TLR2 stimulation plays a role in the induction of inflammatory responses in immune cells (8, 39, 72). TLR2 signaling also involves ERK activation in other cell systems, including renal tubule epithelial cells (11, 17, 52). Activation of the PI3K and ERK pathways has been shown to inhibit HCO3− absorption in the MTAL (33, 82, 83). However, the results of experiments using highly selective inhibitors indicate that the PI3K and ERK pathways are not involved in mediating TLR2-dependent inhibition of HCO3− absorption in the MTAL. Thus, either activation of PI3K and ERK is not a component of Pam3CSK4-induced TLR2 signaling in the MTAL or activation of these pathways through TLR2 is not coupled to the inhibition of HCO3− absorption.
In summary, our data provide new evidence of a role for TLR2 in the regulation of renal tubule ion transport. TLR2, which plays a major role in recognition of gram-positive bacteria (2), is expressed in the basolateral membrane of the MTAL. Absorption of HCO3− by the MTAL is inhibited by bacterial components recognized by TLR2, including the bacterial lipopeptide Pam3CSK4 and the gram-positive bacterial cell wall molecules lipoteichoic acid and peptidoglycan. Inhibition of HCO3− absorption by gram-positive bacterial components acting through TLR2 is additive to inhibition by LPS acting through TLR4. This is a result of the TLR2 and TLR4 agonists activating different intracellular signal transduction pathways: inhibition of HCO3− absorption through TLR2 is blocked by inhibitors of PKC, whereas inhibition through TLR4 is blocked by inhibitors of ERK. Thus, gram-positive and gram-negative bacterial molecules can act independently during sepsis to impair renal tubule function. Understanding the distinct molecular components of the TLR2 and TLR4 pathways that are triggered by different bacterial molecules to inhibit MTAL transport, and the ability to manipulate these pathways, will be important for therapeutic strategies aimed at treating and preventing renal tubule dysfunction during sepsis.
GRANTS
This work was supported by American Heart Association, South Central Affiliate, Grant-in-Aid no. 0855057F and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-038217.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
The initial control rate of HCO3− absorption was measured in 6 of 10 experiments in Fig. 7 before the addition of bacterial compounds. For those tubules, the initial HCO3− absorption rate was 15.9 ± 0.4 pmol·min−1·mm−1 compared with a rate of 8.1 ± 0.5 pmol·min−1·mm−1 measured in the combined presence of a TLR2 agonist plus LPS (49% inhibition). This inhibition is in good agreement with that predicted by adding the individual inhibitory effects of TLR2 agonists (25%) and LPS (33%) when these agents are studied in separate tubules.
REFERENCES
- 1.Ahn KO, Lim SW, Yang HJ, Ghee JY, Kim JY, Kim SH, Kim J, Yang CW. Influence of angiotensin II on expression of Toll-like receptor 2 and maturation of dendritic cells in chronic cyclosporine nephropathy. Transplantation 83: 938–947, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulme R, Lepage E, Le Gall JR. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med 28: 108–121, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med 8: 878–884, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor 2. Science 285: 736–739, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Anders HJ, Banas B, Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288: 862–871, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Aristimuno PC, Good DW. PKC isoforms in rat medullary thick ascending limb: selective activation of the δ isoform by PGE2. Am J Physiol Renal Physiol 272: F624–F631, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Austgen TR, Chen MK, Moore W, Souba WW. Endotoxin and renal glutamine metabolism. Arch Surg 126: 23–27, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA 103: 3274–3279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgart K, Radermacher P, Calzia E, Hauser B. Pathophysiology of tissue acidosis in septic shock: blocked microcirculation or impaired cellular respiration. Crit Care Med 36: 640–642, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol 23: 509–512, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandia F, Tamayo L, Collado J, Garcia-Labattut A, Carriedo D, Valledor M, De Frutos M, Lopez MJ, Caballero A, Guerra J, Alvarez B, Mayo A, Villar J. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 12: R158–R171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285: 732–736, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Brown HJ, Lock HR, Sacks SH, Robson MG. TLR2 stimulation of intrinsic renal cells in the induction of immune-mediated glomerulonephritis. J Immunol 177: 1925–1931, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 281: 9049–9057, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Cario E, Guido G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127: 224–238, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury P, Sacks SH, Sheerin NS. Minireview: functions of the renal tract epithelium in coordinating the innate immune response to infection. Kidney Int 66: 1334–1344, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury P, Sacks SH, Sheerin NS. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin Exp Immunol 145: 346–356, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 19: 403–434, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 23.DeGroot K, Kuklik K, Brocker V, Schwarz A, Gwinner W, Kreipe H, Haller H, Fliser D, Mengel M. Toll-like receptor 2 and renal allograft function. Am J Nephrol 28: 583–588, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Dollins M, Kraus MA, Molitoris BA. Intensive care nephropathy. Sepsis. In: Brenner and Rector's The Kidney, vol. 2, edited by Brenner BM. Philadelphia, PA: Saunders, 2008, p. 2045–2050 [Google Scholar]
- 25.El-Achkar TM, Dagher PC. Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol 2: 568–581, 2006 [DOI] [PubMed] [Google Scholar]
- 26.El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gram-negative sepsis. Eur J Clin Invest 38: 39–44, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The scaffold MyD88 acts to couple protein kinase Cε to Toll-like receptors. J Biol Chem 283: 18591–18600, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Foxman B, Barlow R, D'Arcy H, Gillespi B, Sobel JD. Urinary tract infection. Self-reported incidence and associated costs. Ann Epidemiol 10: 509–515, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Geerlings SE, Brouwer EC, Gaastra W, Verhoef J, Hoepelman AI. Effect of glucose and pH on uropathogenic and nonuropathogenic Escherichia coli: studies with urine from diabetic and non-diabetic individuals. J Med Microbiol 48: 535–539, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Gomez J, Garcia-Vazquez E, Banos R, Canteras M, Ruiz J, Banos V, Herrero JA, Valdes M. Predictors of mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia: the role of empiric antibiotic therapy. Eur J Clin Microbiol Infect Dis 26: 239–245, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Good DW. Inhibition of bicarbonate absorption by peptide hormones and cyclic adenosine monophosphate in rat medullary thick ascending limb. J Clin Invest 85: 1006–1013, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Good DW. PGE2 reverses AVP inhibition of HCO3− absorption in rat MTAL by activation of protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 270: F978–F985, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Good DW, George T, Watts BA., III Nerve growth factor inhibits Na+/H+ exchange and HCO3− absorption through parallel phosphatidylinositol 3-kinase-mTOR and ERK pathways in thick ascending limb. J Biol Chem 283: 26602–26611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Good DW, George T, Watts BA., III Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol 297: F866–F874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Good DW, Watts BA, III, George T, Meyer J, Shull GE. Transepithelial HCO3− absorption is defective in renal thick ascending limbs from NHE1 Na+/H+ exchanger null mutant mice. Am J Physiol Renal Physiol 287: F1244–F1249, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Grinevich V, Knepper M, Verbalis J, Reyes I, Aguilera G. Acute endotoxemia in rats induces downregulation of V2 vasopressin receptors and aquaporin-2 content in the kidney medulla. Kidney Int 65: 54–62, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Eden CS. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46: 839–844, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 172: 993–999, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Jones BW, Heldwein KA, Means TK, Fenton MJ. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann Rheum Dis 60: iii6–iii12, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawai T, Akira S. TLR signaling. Cell Death Differ 13: 816–825, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kaye D. Antibacterial activity of human urine. J Clin Invest 47: 2374–2390, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Klenzak J, Himmelfarb J. Sepsis and the kidney. Crit Care Clin 21: 211–222, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Kraut JA, Kurtz I. Use of base in the treatment of severe acidemic states. Am J Kidney Dis 38: 703–727, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Lee SW, Hong YS, Park DW, Chio SH, Moon SW, Park JS, Kim JY, Baek KJ. Acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg Med J 25: 659–665, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Leemans JC, Butter LM, Pulskens PC, Teske GJD, Claessen N, van der Poll T, Florquin S. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS ONE 4: e5704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 49.Lim SW, Li C, Ahn KO, Moon IS, Ahn C, Lee JR, Yang CW. Cyclosporine-induced renal injury induces Toll-like receptor and maturation of dendritic cells. Transplantation 80: 691–699, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Marik PE, Varon J. Sepsis: state of the art. Dis Mon 47: 465–532, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 19: 3–10, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Mkaddem SB, Werts C, Goujon JM, Bens M, Pedruzzi E, Ogier-Denis E, Vandewalle A. Heat shock protein gp96 interacts with protein phosphatase 5 and controls Toll-like receptor 2-mediated activation of extracellular signal-regulated kinase 1/2 in posthypoxic kidney cells. J Biol Chem 284: 12541–12549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med 193: 393–397, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morr M, Takeuchi O, Akira S, Simon MM, Muhlradt PF. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol 32: 3337–3347, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Naresh G, Mansharamani MD, Koziel H. Chronic lung sepsis: lung abscess, bronchiectasis, and empyema. Curr Opin Pulm Med 9: 181–185, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, Frei U, Eckardt KU, Loeffler M, John S. Acute renal failure in patients with severe sepsis and septic shock–a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant 23: 904–909, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA 97: 13766–13771, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patole PS, Schubert S, Hildinger K, Khandoga S, Khandoga A, Segerer S, Henger A, Kretzler M, Werner M, Krombach F, Schlondorff D, Anders HJ. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int 68: 2582–2587, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Pittet JF, Wiener-Kronish JP, McElroy MC, Folkesson HG, Matthay MA. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest 94: 663–671, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr Mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Rahman N, Meng M, Stoller M. Infections and urinary stone disease. Curr Pharm Des 9: 975–981, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, Schein R, Summer W, Wright P, Walley KR. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med 28: 3405–3411, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Schwartz JS, Moore R, Johnson BL, Jr, Platt R. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 278: 234–240, 1997 [PubMed] [Google Scholar]
- 64.Scheibner KA, Litz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronin fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Schmidt C, Hocherl K, Bucher M. Regulation of renal glucose transporters during severe inflammation. Am J Physiol Renal Physiol 292: F804–F811, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Schwan W, Lee J, Lenard F, Mathews B, Beck M. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect Immun 70: 1391–1402, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem 274: 17406–17409, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WK, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178: 6252–6258, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Shirali AC, Goldstein DR. Tracking the Toll of kidney disease. J Am Soc Nephrol 19: 1444–1450, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Strassheim D, Asehnoune K, Park JS, Kim JY, Qianbin H, Richter D, Kuhn K, Mitra S, Abraham E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol 172: 5727–5733, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11: 443–451, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13: 933–940, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169: 10–14, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signaling. Biochem J 376: 545–552, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teusch N, Lombardo E, Eddleston J, Knaus UG. The low molecular weight GTPase RhoA and atypical protein kinase Cζ are required for TLR2-mediated gene transcription. J Immunol 173: 507–514, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991 [PubMed] [Google Scholar]
- 79.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami KI, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of Toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Li C, Summer SN, Falk S, Wang W, Ljubanovic D, Schrier RJ. Role of AQP1 in endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1473–F1480, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Watts BA, III, George T, Good DW. The basolateral NHE1 Na+/H+ exchanger regulates transepithelial HCO3− absorption through actin cytoskeleton remodeling in renal thick ascending limb. J Biol Chem 280: 11439–11447, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Watts BA, III, George T, Good DW. Aldosterone inhibits apical NHE3 and HCO3− absorption via a nongenomic, ERK-dependent pathway in medullary thick ascending limb. Am J Physiol Renal Physiol 291: F1005–F1013, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Watts BA, III, Good DW. Extracellular signal-regulated kinase mediates inhibition of Na+/H+ exchange and HCO3− absorption by nerve growth factor in MTAL. Am J Physiol Renal Physiol 282: F1056–F1063, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Wolfs TGAM, Buurman WA, van Schadewijk A, de Vries B, Daemen MARC, Hiemstra PS, van't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated upregulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Wooten RM, Ma Y, Yoder RA, Brown JP, Weiss JH, Zachary JF, Kirshning CJ, Weiss JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borellia burgdorferi. J Immunol 168: 348–355, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, Akira S, Paik TH, Jo EK. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol 9: 382–396, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol 163: 1–5, 1999 [PubMed] [Google Scholar]
- 88.Zhanel GG, Karlowsky JA, Davidson RJ, Hoban DJ. Influence of human urine on the in vitro activity and postantibiotic effect of ciprofloxacin against Escherichia coli. Chemotherapy 37: 218–223, 1991 [DOI] [PubMed] [Google Scholar]