Abstract
In a companion study (Edwards A and Layton AT. Am J Physiol Renal Physiol. doi:10.1152/ajprenal.00680.2009), we developed a mathematical model of nitric oxide (NO), superoxide (O2−), and total peroxynitrite (ONOO) transport in mid-outer stripe and mid-inner stripe cross sections of the rat outer medulla (OM). We examined how the three-dimensional architecture of the rat OM, together with low medullary oxygen tension (Po2), affects the distribution of NO, O2−, and ONOO in the rat OM. In the current study, we sought to determine generation rate and permeability values that are compatible with measurements of medullary NO concentrations and to assess the importance of tubulovascular cross talk and NO-O2− interactions under physiological conditions. Our results suggest that the main determinants of NO concentrations in the rat OM are the rate of vascular and tubular NO synthesis under hypoxic conditions, and the red blood cell (RBC) permeability to NO (PNORBC). The lower the PNORBC, the lower the amount of NO that is scavenged by hemoglobin species, and the higher the extra-erythrocyte NO concentrations. In addition, our results indicate that basal endothelial NO production acts to significantly limit NaCl reabsorption across medullary thick ascending limbs and to sustain medullary perfusion, whereas basal epithelial NO production has a smaller impact on NaCl transport and a negligible effect on vascular tone. Our model also predicts that O2− consumption by NO significantly reduces medullary O2− concentrations, but that O2− , when present at subnanomolar concentrations, has a small impact on medullary NO bioavailability.
Keywords: mathematical model, rat kidney, generation rates, peroxynitrite, thick ascending limb sodium transport
in a companion study (16), we formulated a mathematical model that simulates the distribution of nitric oxide (NO), superoxide (O2−), and total peroxynitrite (the sum of ONOO− and ONOOH, denoted ONOO) in cross sections of the rat outer medulla (OM). The model represents the complex structural organization of the OM by means of four concentric regions, centered about a vascular bundle, following the region-based approach of Layton and Layton (27). The radial positions of structures is incorporated by assigning appropriate tubules and vasa recta (or fractions thereof) to each concentric region. It differs from our previous model of cross-sectional NO transport, referred to as the ZE model (49), in that it represents one model tubule or vessel for each class of tubules or vessels, whereas the ZE model explicitly represented all tubules and vessels associated with a vascular bundle. On the other hand, the current model predicts O2− concentrations (CO2−) instead of assuming fixed values, it incorporates medullary Po2 profiles and their effect on NO (GNO) and O2− (GO2−) generation rates instead of assuming well-oxygenated conditions, and it accounts for the capillary plexus which perfuses the interbundle region.
The companion study (16) predicts that regionalization, together with low medullary Po2, has a significant impact on the radial distribution of NO, O2−, and ONOO in the rat OM. In the present study, we used the model to examine the impact of kinetic and transport rates on the concentration profiles of these three species and to assess the importance of tubulovascular cross talk and NO-O2− interactions under in vivo conditions. As described below, fundamental questions related to basal GNO and GO2− and NO and O2− transport and consumption in the renal medulla remain unresolved.
The first question concerns the in vivo rate of NO production in the OM. At least three NO synthase (NOS) isoforms have been identified in the medulla: neuronal NOS (nNOS, or NOS1), inducible NOS (iNOS, or NOS2), and endothelial NOS (eNOS, or NOS3). Predicted rates of NO release by the dominant isoforms cannot account for reported perivascular NO concentrations (CNO) (7, 50). In addition, the relative contribution of each isoform to the regulation of vascular tone remains unclear. Studies by Mattson and colleagues (25) suggest that while nNOS accounts for a significant fraction of NO production in the renal medulla, basal blood flow in the renal cortex and medulla is regulated by eNOS. However, in response to ANG II, NO produced by nNOS mediates an increase in medullary blood flow (32). These results are consistent with the observations by Cowley and colleagues (15) that ANG II stimulates NO release from medullary thick ascending limb (mTAL) epithelia, and the subsequent diffusion of NO toward nearby vasa recta pericytes in vitro.
ANG II also stimulates the production of O2− in mTAL and thereby limits the diffusion of NO from mTAL to pericytes in vitro (33). The relative amount of O2− generated in different renal tubular segments was measured by Li and Zou (28), but absolute O2− production rates have not yet been determined in the kidney. In the current study, we seek to determine plausible NO and O2− endothelial and epithelial production rates that are compatible with experimental observations.
Another question relates to the endocrine function of NO. Under hypoxic conditions, red blood cells (RBCs) can release NO and thereby dilate blood vessels, indicating that NO bioactivity can be preserved within erythrocytes, despite the rapid conversion of NO by Hb into inactive metabolic products. To explain how low Po2 induces the release of NO by RBCs, Stamler and colleagues (38, 43) have proposed the S-nitrosohemoglobin (SNOHb) hypothesis, according to which NO bound to deoxyhemoglobin [Fe(II)NO] can be transferred from the ferrous ion group to the cysteine residue at position 93 on the β chain to form SNOHb. SNOHb can in turn transfer the NO group to cysteines of other molecules, such as glutathione and the RBC membrane protein AE1, thereby delivering NO to vascular smooth muscle and maintaining NO bioactivity. The distribution of NO between [Fe(II)NO] and SNOHb, and thus its availability for subsequent release, is modulated by the degree of hypoxia. The mechanisms underlying the heme-to-thiol NO transfer remain to be elucidated (2).
The observation that nitrite is a hypoxia-sensitive vasodilator has given rise to an alternative hypothesis (12). Under hypoxic conditions, RBC nitrite is reduced by deoxyhemoglobin to form NO, which can then be released from the lumen and promote vasodilation (13). According to the nitrite hypothesis, RBC nitrite represents a significant bioavailable pool of NO. In this study, we assess the contribution of both pathways to renal medullary NO levels.
NO has both vasodilatory and natriuretic effects, whereas O2− promotes vasoconstriction and sodium retention. The product of the NO-O2− reaction, ONOO−, also affects tubular function (20). In vitro studies have shown the importance of NO tubulovascular cross talk, and of the interactions between NO and O2− (15, 33). The current model is also used to investigate whether in vivo, the generation of NO by mTAL epithelia is likely to affect vascular tone, whether the generation of NO by vasa recta endothelia contributes to inhibition of sodium reabsorption, and whether O2− limits the tubulovascular cross talk of NO.
MODEL DESCRIPTION
The mathematical model used in this study and in the companion study (16) simulates the transport and reactions of NO, O2−, and ONOO in a cross section of the rat OM. The radial organization of the tubules and vessels around the vascular bundles is represented by means of four concentric regions centered on a vascular bundle: an innermost region containing the central vascular bundle (R1); a peripheral region of the vascular bundle (R2); a region neighboring the vascular bundle (R3); and the region most distant from the vascular bundle (R4). The position of tubules and vasa recta with respect to vascular bundles is represented by specifying the fractions of the tubules and vasa recta assigned to each concentric region at each medullary level; see Fig. 1 in the companion study (16).
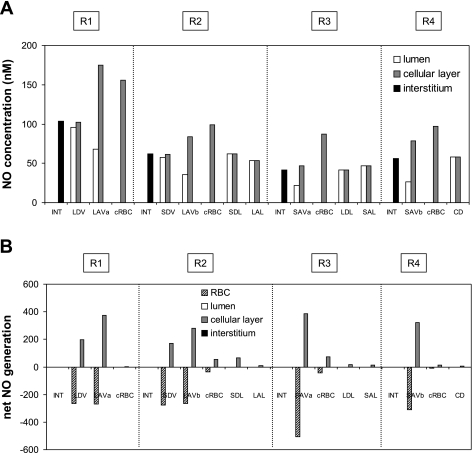
Fig. 1.
A: baseline nitric oxide (NO) concentrations (CNO) in the interstitium, vasa recta, capillaries, and tubules in each region (R1–R4), at the mid-inner stripe (IS). Results are shown for case C; CNO values in cases A, B, and C are nearly indistinguishable. Each tubule or vasa recta is assigned to the region with which it is in contact for 50% or more in the IS. The term “cellular layer” denotes the endothelium in vasa recta and capillaries, and the epithelium in tubules. CNO in red blood cells (RBC) are <1 nM and are not shown. LDV, long descending vasa recta; SDV, short descending vasa recta; LAVa and LVAb, 2 populations of long ascending vasa recta; SAVa and SAVb, 2 populations of short ascending vasa recta; LDL, long descending limb of Henle's loop; SDL, short descending limb; LAL; long ascending limb; SAL, short ascending limb; CD, collecting duct; cRBC, capillary red blood cells. B: baseline net generation (production minus consumption) rate of NO (×10−3 nmol·cm−1·s−1) in the interstitium, vasa recta, capillaries, and tubules at the mid-IS. The rates for a given type of tubule or vessel take into account the number of such tubules or vessels.
Solute conservation equations were formulated for separate compartments, which include the lumen and the surrounding epithelium in each tubule; the RBCs, plasma, and the surrounding endothelium in each vas rectum; the capillary red blood cell (cRBC) cytosol and the surrounding endothelium; and the interstitia of each of the four concentric regions. The conservation equations were solved to yield CNO and O2− (CO2−) and ONOO (CONOO) concentrations and fluxes for all compartments. Po2 and capillary flows were prescribed based on the results of a previous model of O2 transport (5).
The model represents the synthesis of NO and O2− in the vascular endothelium and tubular epithelium. The volumetric generation rates of these two solutes, respectively denoted GNOi and GO2−i in compartment i, depend on oxygen availability. Low Po2 inhibits NO synthesis, so that GNOi is determined using a Michaelis-Menten relationship (3)
| (1) |
where GNO, maxi is the maximum rate of NO production in compartment i. The Michaelis-Menten constant KO2NO, which corresponds to the Po2 at which the generation rate of NO synthesis is inhibited by half, is taken as 38 mmHg (47).
As described in the companion study (16), the effects of medullary hypoxia on O2− synthesis remain poorly understood. Some studies suggest that low Po2 stimulates O2− production, whereas others report an inhibitory effect. We thus consider three different scenarios. Case A assumes that low Po2 inhibits O2− synthesis, and the O2 dependence of the O2− generation rate is then modeled using a Michaelis-Menten relationship
| (2a) |
where GO2−i, high PO2 is fixed, and KO2O2− is taken as 15.4 mmHg (10). Case B assumes that the rate of O2− synthesis remains independent of Po2, that is
| (2b) |
Case C assumes that low Po2 increases O2− production by 50% (relative to well-oxygenated conditions), based upon the experimental data of Li et al. (28)
| (2c) |
In the simulations below, case C is chosen as the reference case: since it yields the highest CO2− under physiological Po2, this case generally exhibits the largest variations when parameter values are changed. Unless otherwise specified, concentrations are calculated at the mid-inner stripe.
RESULTS
A major objective of this study was to assess the importance of tubulovascular cross talk and NO-O2− interactions under physiological conditions. As a prerequisite, we first sought to determine NO and O2− kinetic and transport parameter values that yield model predictions consistent with experimental measurements of CNO in the rat OM, which are on the order of 100 nM (see below). We began with a simple analysis to gauge the relative importance of NO and O2− transport and kinetic rates. Consider for instance NO transport in a vas rectum. NO is generated within the endothelium (denoted “endo” in the equations below), and then diffuses to the surrounding interstitium (“int”) on one side and to the plasma on the other side. From the plasma, it diffuses into the RBC, where it is quickly consumed by hemoglobin species (Hb and HbO2, which we collectively write as Hb). Thus, neglecting the rates of NO consumption by oxygen and superoxide, both of which are >10+3 times slower than the Hb scavenging rate, the conservation of NO in the endothelium (“endo”) and plasma (“pl”) is given by
| (3) |
| (4) |
where Aendo is the cross-sectional area of the endothelial layer, and JNOi, j is the NO flux from compartment i to compartment j. Combining Eqs. 3 and 4, we obtain
| (5) |
The NO flux into the RBC can be expressed in terms of the plasma-to-RBC NO concentration difference (CNOpl − CNORBC), the RBC permeability to NO (PNORBC), and the RBC compartment radius (RRBC). Thus Eq. 5 can be written as
| (6) |
Neglecting the rates of NO consumption by O2 and O2− in the conservation equation for NO in RBCs as well, the NO flux into the RBC is also approximately equal to the rate of NO scavenging by hemoglobin, i.e.
| (7) |
where ARBC is the cross-sectional area of the RBC compartment, kHb is the Hb-NO reaction rate, and CHbRBC is an effective Hb concentration (which includes both Hb and HbO2). Since the endothelium-to-interstitium CNO gradient is not very significant (compare endothelial CNO and surrounding interstitial CNO in Fig. 1A), the NO flux into the interstitium, JNOendo, int, is small relative to the other terms in Eq. 6. Together, Eqs. 6 and 7 indicate that CNO values are mostly determined by GNO, membrane permeabilities to NO, and the rate of the Hb-NO reaction. The impact of these parameters is assessed below.
Similarly, consider the transport of O2− in a vas rectum. O2− is generated within the endothelium, from which it diffuses into the adjoining interstitium and plasma. In contrast to NO, the main O2− scavengers (i.e., SOD and to a lesser extent NO) are present everywhere. Thus CO2− are mostly determined by GO2−, SOD concentration, CNO, as well as the SOD-O2− and NO-O2− reaction rates.
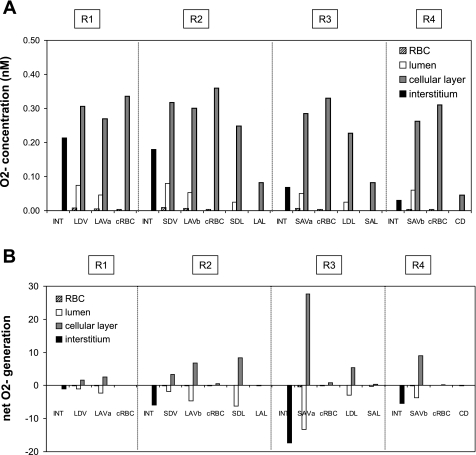
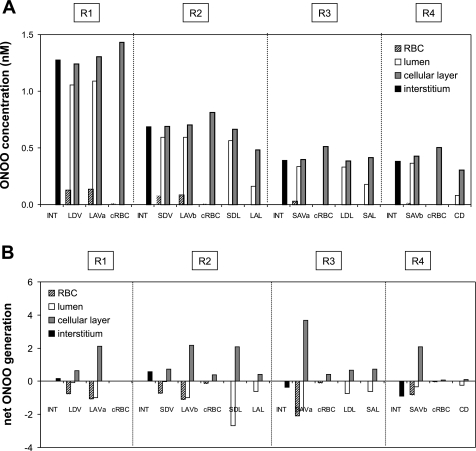
Under baseline conditions, KO2NO was taken as 38 mmHg and PNORBC as 0.1 cm/s, both of which represent midrange values. The maximal NO generation rate (GNO, max) in vasa recta endothelia was then chosen so as to yield OM interstitial CNO on the order of 100 nM. The GO2− under well-oxygenated conditions (GO2−highPO2) in vasa recta endothelia was taken to be similar to that in other endothelial cells (40). The baseline concentration profiles of NO, O2−, and ONOO are described in detail in the companion study (16). In the current study, Fig. 1, Fig. 2, and Fig. 3 display those concentration profiles as well as net generation rates at the mid-inner stripe for case C (baseline profiles for cases A and B can be found in the companion study). Transmembrane fluxes can be inferred from concentration differences between adjacent compartments. Note that even though the volumetric production rate of NO and that of O2− are taken to be the same in ascending (AVR) and descending (DVR) vasa recta, the generation rate is higher in AVR endothelia than in DVR endothelia because the former occupies a substantially greater surface area.
Fig. 2.
A: baseline O2− concentrations (CO2−) in the interstitium, vasa recta, capillaries, and tubules in each region, at the mid-IS. Results are shown for case C. In case B, CO2− is 33% lower everywhere. In case A, CO2− is ∼50% lower in R1, and 70–85% lower in R2–R4. B: base-case O2− net generation rates (×10−3 nmol·cm−1·s−1) in the interstitium, vasa recta, capillaries, and tubules at the mid-IS (case C).
Fig. 3.
A: baseline peroxynitrite (ONOO) concentrations (CONOO) in the interstitium, vasa recta, capillaries, and tubules in each region, at the mid-IS. Results are shown for case C. In case B, CONOO is 33% lower everywhere. In case A, CONOO is ∼50% lower in R1, and 70–85% lower in R2–R4. B: base-case ONOO net generation rates (×10−3 nmol·cm−1·s−1) in the interstitium, vasa recta, capillaries, and tubules at the mid-IS (case C).
Our first objective herein was to estimate plausible values for the NO and O2− transport parameters whose value remains uncertain, given a target CNO. To accomplish that goal, we conducted a series of parametric studies to assess the sensitivity of model predictions to those parameters. Specifically, we considered GNO and GO2−, the Hb-NO reaction rate, PNORBC, and SOD concentration. The effects of variations in KO2NO on CNO, CO2−, and CONOO profiles were examined in the companion study (16).
Estimates of GNO and GO2−
In vitro estimates of NOS activity in the kidney suggest that GNO is on the order of 0.3–3 fmol·mm−1·h−1 (48), or ∼0.1–10 nM/s depending on the kidney segment. However, using those estimates, medullary interstitial CNO are predicted to be subnanomolar (50). As reviewed by Chen and Popel (9), measured GNO in other tissues vary over a wide range, from ∼0.01 to 10 μM/s. In this study, the maximal value of GNO in vasa recta endothelia was set to 76.6 μM/s under baseline conditions, and we used the tubular-to-vascular GNO ratios obtained by Wu et al. (48) to determine GNO in tubular epithelia (16). The baseline vascular NO synthesis rate is comparable to theoretical estimates of GNO used in other NO transport models, which are in the 50–100 μM/s range (3, 26, 46). The ZE model assumed lower GNO values (e.g., 22.1 μM/s for vasa recta endothelia) but did not account for the rate-limiting effect of low Po2. It therefore predicted that the average interstitial CNO in R1–R4 ranges from 40 to 60 nM at the mid-inner stripe, vs. 42–104 nM in the current study; however, the ZE model considered local CNO differences within each region, and interstitial CNO could be as low as 30 nM in some areas and as high as 80 nM in others (49).
Effects of varying GNO.
To assess the degree to which such a high GNO, max value is justified, we first varied that parameter and assessed its effects on model CNO, CO2− and CONOO profiles. A 10-fold decrease in endothelial and epithelial GNO is predicted to reduce CNO by a factor of 10 in every compartment. As summarized in Table 1, interstitial CNO then range from 4.2 nM (in R3) to 10.4 nM (in R1) at the mid-inner stripe; these values are significantly lower than reported CNO measurements (100–800 nM).
Table 1.
Effect of variations in generation rates on NO and O2− concentrations (in nM) at the mid-inner stripe
| CNO in R1 | CNO in R2 | CNO in R3 | CNO in R4 | CO2− in R1 | CO2− in R2 | CO2− in R3 | CO2− in R4 | |
|---|---|---|---|---|---|---|---|---|
| Case A | 104 | 63 | 43 | 57 | 0.109 | 0.049 | 0.010 | 0.009 |
| Case B | 104 | 63 | 42 | 56 | 0.142 | 0.119 | 0.045 | 0.020 |
| Case C | 104 | 62 | 42 | 56 | 0.213 | 0.179 | 0.067 | 0.030 |
| 10-Fold decrease in GNO | 10.4 | 6.3 | 4.3 | 5.7 | 0.159 | 0.062 | 0.012 | 0.012 |
| 10.4 | 6.3 | 4.3 | 5.6 | 0.207 | 0.150 | 0.055 | 0.027 | |
| 10.3 | 6.2 | 4.2 | 5.6 | 0.311 | 0.224 | 0.082 | 0.040 | |
| No capillary NO synthesis | 104 | 59 | 39 | 55 | 0.109 | 0.050 | 0.010 | 0.009 |
| 103 | 59 | 38 | 54 | 0.142 | 0.121 | 0.046 | 0.020 | |
| 103 | 58 | 38 | 53 | 0.213 | 0.182 | 0.069 | 0.030 | |
| 10-Fold increase in GO2− | 100 | 60 | 41 | 54 | 1.100 | 0.493 | 0.100 | 0.087 |
| 98 | 54 | 34 | 48 | 1.445 | 1.223 | 0.465 | 0.206 | |
| 95 | 51 | 31 | 44 | 2.190 | 1.859 | 0.709 | 0.314 | |
| 10-Fold decrease in GO2− | 105 | 64 | 43 | 57 | 0.011 | 0.005 | 0.0009 | 0.0008 |
| 105 | 64 | 43 | 57 | 0.014 | 0.012 | 0.0045 | 0.0020 | |
| 105 | 64 | 43 | 57 | 0.021 | 0.018 | 0.0067 | 0.0029 | |
| No capillary O2− synthesis | 104 | 63 | 43 | 57 | 0.108 | 0.045 | 0.009 | 0.008 |
| 104 | 63 | 42 | 56 | 0.142 | 0.113 | 0.043 | 0.019 | |
| 104 | 62 | 42 | 56 | 0.213 | 0.170 | 0.065 | 0.029 | |
| SOD concentration =10 μM in cells | 105 | 64 | 43 | 57 | 0.009 | 0.003 | 0.0003 | 0.0003 |
| 105 | 64 | 43 | 57 | 0.011 | 0.006 | 0.0013 | 0.0006 | |
| 105 | 64 | 43 | 57 | 0.017 | 0.010 | 0.0020 | 0.0009 |
In each set of simulations, results are shown for cases A, B, and C. Baseline values are in bold. NO, nitric oxide; O2−, superoxide; CNO and CO2−, NO and O2− concentration, respectively; GNO and GO2−, NO and O2− generation, respectively. The rate of O2− synthesis is taken to either decrease with decreasing Po2 (case A), to remain independent of Po2 (case B), or to increase with decreasing Po2 (case C).
As CNO decreases, so does the rate of reaction between NO and O2−. As a result, CO2− values increase everywhere. The CO2− increase, however, is considerably smaller than the 10-fold decrease in CNO: interstitial CO2− increases by 45% in R1 and 20–35% in R2–R4 (Table 1). Recall that NO is neither the only, nor the predominant, O2− scavenger: the consumption of O2− by SOD is significantly more rapid than that by NO (∼10–100 times, depending on the compartment). Hence, a 10-fold decrease in CNO does not raise CO2− by the same factor. Our model predicts that CO2− increases more in R1, where CNO values are higher and the rate of O2− scavenging is therefore also higher, than in the surrounding regions.
The GNO decrease also affects the CONOO, which is formed by the reaction between NO and O2−. The net effect of the 10-fold decrease in CNO and the lesser increase in CO2− is to reduce CONOO by a factor of 7–9 (i.e., not 10) in all compartments.
In the above results, we did not account for the SNOHb- or nitrite-mediated delivery of NO to plasma. As described at the beginning of this paper, the release of NO by RBCs under hypoxic conditions has led investigators to postulate that SNOHb or RBC nitrite constitutes a pool of bioavailable NO (13, 38). To account for the generation of NO via the SNOHb and nitrite pathways, we used the same approach, and the same kinetic parameters, as in the modeling studies of Chen et al. (6, 8). Both the approach and the parameters are described in Model Description in the companion study (16). Our model predicts that the SNOHb and nitrite pathways have a negligible effect (<0.1%) on medullary CNO, because their NO release rate is 10+3-10+4 times lower relative to that of endothelial and epithelial cells. Thus even an increase in the rate of NO release by SNOHb and nitrite by a factor of 10 or 100 would not significantly affect predicted concentration profiles.
In contrast to the ZE model, the current model accounts for the presence of the capillary plexus that irrigates the OM. In the absence of specific data, we assumed that the volumetric rates of NO and O2− synthesis were equal in the endothelium of capillaries and that of vasa recta. Whether these capillaries express NOS to the same extent as vasa recta, if at all, remains unknown. We therefore examined the limiting case in which the capillary endothelium does not produce NO. As summarized in Table 1, CNO would be 0–10% lower at the mid-inner stripe, depending on the region, in the absence of NO synthesis by the capillary endothelium. Indeed, at that medullary level, the capillary RBC cytosol and endothelium (collectively referred to as cRBC) occupy a significant surface area (see Table 2 in Ref. 16). The capillary-to-interstitium surface area ratio is 0.04 in R1, 0.92 in R2, 0.14 in R3, and 0.04 in R4 at the mid-inner stripe. At the mid-outer stripe, the cRBC-to-interstitium surface area ratios are lower (0.003, 0.04, 0.02, and 0.005, respectively), and CNO would be reduced by <1% everywhere in the absence of capillary NO synthesis (results not shown).
Effects of varying GO2−.
The absolute GO2− in the renal medulla has not been measured, to the best of our knowledge. Under well-oxygenated conditions, endothelial GO2− is taken as 0.7 μM/s, based upon experimental measurements in other cells (40). A 10-fold increase in endothelial and epithelial GO2− is predicted to raise CO2− by a factor of 10 in every compartment. The resulting increase in the reaction rate between NO and O2− lowers CNO everywhere (Table 1). Since the rate of the reaction between NO and O2− is ∼103 times lower than GNO and the rate of NO scavenging by hemoglobin, the reduction in CNO is comparatively small: it is <8% in case A (in which GO2− is significantly rate limited by low medullary Po2 and therefore CO2− is the lowest), <24% in case B, and <32% in case C (in which CO2− values are highest). These results are similar to the predictions of the ZE model, which suggested that below a concentration of 1 nM, superoxide has negligible effects on NO consumption and that CO2− has to rise above 10 nM to reduce the baseline CNO by a factor of 2 (49).
Since the rate of ONOO formation is proportional to the product of CNO and CO2−, the 10-fold increase in GO2−, which yields an ∼10-fold increase in CO2− and a 10–30% decrease in CNO, raises CONOO by a factor of ∼9.
Conversely, a 10-fold decrease in GO2− reduces CO2− and CONOO by a factor of 10 everywhere, but has a small effect on CNO (<1, 3 and 5% in cases A, B, and C, respectively) since the NO-O2− reaction rate is then even smaller than under baseline conditions, given the lower CO2−.
We also examined the hypothesis that the capillary endothelium does not produce O2−. In that case, CO2− would be 0–12% lower at the mid-inner stripe (Table 1), relative to baseline values. The variations would be largest in R2 and R3, the regions where the capillary-to-interstitium surface area ratio is highest (see above). The CNO would not be significantly affected, and CONOO would vary to the same extent as CO2−.
Effects of varying SOD concentration.
The baseline concentration of SOD was taken to be 1 μM everywhere, as in other modeling studies (3). A recent review (19) suggests that SOD may be >10 μM in some cells. We therefore performed simulations in which the concentration of SOD within RBC, endothelia, epithelia, and interstitium was equal to 10 μM (and 1 μM in the vascular and tubular lumen). The results are qualitatively similar to those corresponding to a decrease in GO2−. Given that SOD is the dominant O2− scavenger, a 10-fold increase in cellular SOD concentrations drastically lowers CO2− in all compartments. Predicted CO2− values decrease by 78–98% in all regions (Table 1). At the mid-inner stripe, interstitial CO2− ranges from 0.3 pM in R4 to 9 pM in R1 (vs. 9 -109 pM assuming a uniform SOD concentration of 1 μM) in case A, and from 0.9 pM in R4 to 17 pM in R1 (vs. 30–213 pM) in case C. As the reaction rate between NO and O2− is subsequently reduced, CONOO decreases by 80–90% in all compartments, but the effect on CNO is small (<5%), since O2− is not a strong scavenger of NO under basal conditions.
Effects of varying NO permeability.
Given that the synthesis of NO and O2−, as well as the scavenging of NO and ONOO by hemoglobin, are restricted to certain compartments, the concentration of these species is likely to depend significantly on intercompartmental permeabilities (see Eqs. 6 and 7). As described in the companion study (16), estimates of PNORBC vary from ∼0.05 to 5 cm/s; we chose a baseline value of 0.1 cm/s. Since the NO permeability (PNO) of vessels and tubules is scaled by that of RBC (see Model Description in Ref. 16), all PNO values depend on the selected PNORBC value. To assess the extent to which PNO affects CNO and CO2−, we conducted simulations in which we decreased or increased PNORBC, and therefore vascular and tubular PNO, by a factor of 5.
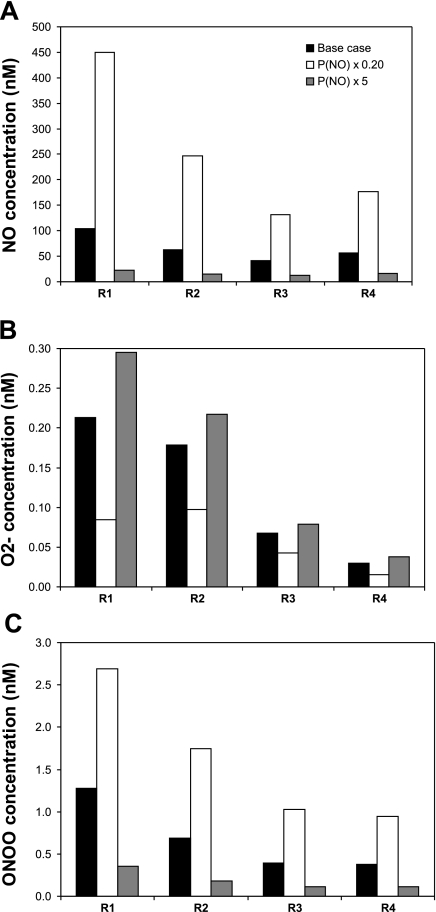
A fivefold decrease in PNO significantly raises CNO in all compartments except in RBCs, and accentuates CNO gradients within each region. As the resistance to NO diffusion from plasma to RBC is increased, the fraction of NO that enters RBCs, and that is subsequently consumed by Hb, is greatly reduced; hence the overall increase in medullary CNO, as displayed in Fig. 4A. At the mid-inner stripe, predicted interstitial concentrations then range from 131 nM in R3 (vs. 42 nM under baseline conditions) to 450 nM in R1 (vs. 104 nM under baseline conditions), which is more than a threefold increase. These values correspond to case C; in case A, CNO is 0.9–11.4% higher, and in case B 0.6–4.3% higher, and the trends are nearly identical.
Fig. 4.
Effect of varying NO permeability (PNO) on interstitial CNO (A), CO2− (B), and CONOO (C) in each region. RBC, vascular, and tubular PNO are taken to vary together. PNO is set to its base-case value, decreased by a factor of 5, and increased by a factor of 5. Results are shown for case C. Relative concentration increases or decreases are almost identical in cases A, B, and C.
The fivefold decrease in PNO enhances the differences between interstitial, endothelial, epithelial, and luminal CNO within each region. The peak CNO (853 nM) is found in the endothelia of the AVR located in R1, as it is in the baseline, since the volumetric rate of NO synthesis is largest in vasa recta. Moreover, in contrast with DVR, AVR do not express aquaporin-1 (AQP1) water channels; their PNO is therefore comparatively small, so that more NO is preserved within the endothelium where it is generated. A fivefold reduction in PNO leads to the redistribution of O2− and ONOO as well. The permeability-induced CNO increase in turn raises O2− consumption (and ONOO− production). Thus, as illustrated in Fig. 4, B and C, interstitial CO2− is predicted to decrease, and interstitial CONOO to increase, by a factor of ∼2–3 in all regions following a fivefold reduction in PNO. Note that relative concentration increases (CONOO) or decreases (CO2−) are nearly identical in cases A, B, and C.
Conversely, a fivefold increase in PNO reduces CNO everywhere except in RBCs, and homogenizes NO concentrations within a given region. Since more NO is carried to the RBCs where it is rapidly scavenged, elsewhere CNO are ∼80% lower than under baseline conditions. Interstitial CNO is then predicted to vary between 12 and 23 nM (vs. 42 and 104 nM in the base case) at the mid-inner stripe (Fig. 4A). The subsequent reduction in O2− consumption, therefore in ONOO production, is predicted to raise CO2− by 10–60% and to decrease CONOO by ∼70% (Fig. 4, B and C).
Effects of varying consumption rate of NO in RBCs.
Experimental studies have suggested that the consumption rate of NO by Hb in RBCs is several orders of magnitude lower than that of an equivalent solution of free Hb (44, 45). Whether the rate-limiting step resides in the unstirred plasma layer that surrounds each RBC, in the RBC membrane and the underlying cytoskeleton, or in the RBC cytosol, remains uncertain. The magnitude of the difference in NO uptake by RBCs and free Hb is also controversial (44). In a combined experimental and modeling study, Vaughn et al. (45) measured the production rate of metHb, which is the product of the reaction between NO and HbO2 (the rate constant of which is denoted as koxy). To fit their predictions to the experimental data, they either fixed PNORBC and varied koxy, or varied PNORBC and fixed koxy: in both cases, the best fit was obtained when the product of PNORBC and koxy was 1 × 10+6 cm·M−1·s−2. In the present model, baseline estimates of koxy and kdeoxy (the Hb-NO rate constant) come from kinetic studies of NO with free hemoglobin (4, 17); both are taken as 2.5 × 107 M−1·s−1. The baseline value of PNORBC is 0.1 cm/s, so that the product of PNORBC and koxy equals 2.5 × 10+6 cm·M−1·s−2 in our model, or 2.5 times higher than the value suggested by the study by Vaughn et al. (45).
To examine the effect of decreasing the rates of NO consumption by Hb, we obtained model results using rate constants (koxy and kdeoxy) that are reduced by factor of 2.5 relative to baseline conditions. Our model predicts that CNO then increase by approximately the same factor in RBC. At the mid-inner stripe, CNO values range from 30 pM in the RBC of short ascending vasa recta (vs. 12 in the base case) to 241 pM in the RBC of long descending vasa recta (vs. 97 in the base case). In contrast, the effects on CNO in the surrounding layers (plasma, endothelium, and interstitium) are negligible (<0.3%). The finding that a 2.5-fold reduction in the rate of NO scavenging in RBC has only minimal effect on NO outside of the RBC is attributable to the order-of-magnitude difference between CNO in plasma (∼50 nM in the base case) and CNO in RBC (0.01–0.1 nM in the base case). This means that the CNO difference across the RBC membrane is approximately equal to plasma CNO; thus a 2.5-fold increase in RBC CNO does not significantly affect that gradient.
Implication for GNO.
The results described above, taken together, suggest that 1) the consumption of NO by O2− is small relative to that by Hb in RBCs; 2) the diffusion of NO into the RBC cytosol is the bottleneck in the scavenging of NO by hemoglobin. Thus, at steady state, NO diffusion into RBC is roughly balanced by NO generation in the vascular endothelium; in other words, the product of PNORBC and the plasma NO concentration (CNOpl) scales with GNOendo. Since plasma, endothelial, and interstitial CNO are all of same order of magnitude, we have
| (8) |
The baseline results suggest that the target interstitial CNO (∼100 nM) can be reached with GNOmaxendo ∼ 100 μM/s and PNORBC = 0.1 cm/s, assuming that the Po2 at which NO synthesis is inhibited by half is ∼40 mmHg. According to Eq. 8, and the results shown in Fig. 4, the same target can be attained with GNOmaxendo ∼ 300 μM/s and PNORBC = 0.5 cm/s, or with GNOmaxendo ∼ 30 μM/s and PNORBC = 0.05 cm/s. Note that according to this analysis, the lowest possible GNOmaxendo that generates CNO predictions consistent with measured values in the OM, in conjunction with PNO within the experimental range (i.e., ≥0.05 cm/s), is ∼30 μM/s, which is three orders of magnitude higher than the values of NOS activity measured in dissected kidney segments (48).
Effects of varying O2− diffusivity.
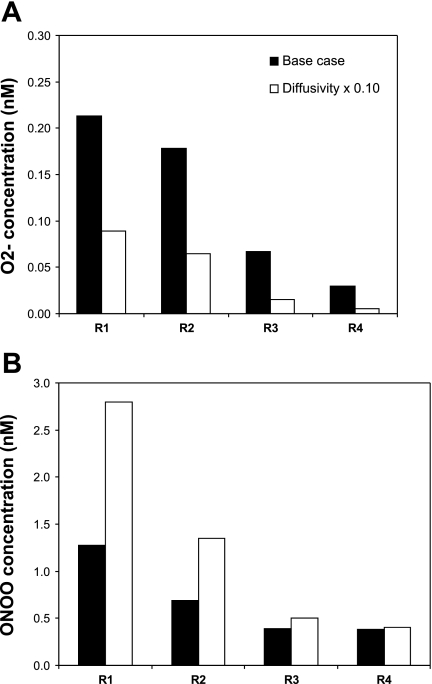
In the next set of simulations, we examined the sensitivity of model predictions to vascular and tubular permeability to O2− (PO2−), which are determined based on the diffusivity of O2− (16). As a baseline, we assumed that the diffusivity of O2− (DO2−) in membranes is five times lower than that in dilute solution, as is the case for NO (14). However, as discussed by Ferrer-Sueta and Radi (19), it is possible that the electric charge of O2− restricts its diffusion more significantly. We thus examined the impact of a 10-fold decrease in DO2−, i.e., in vascular and tubular PO2− (Eq. 14 in the companion study). As expected, the permeability reduction preserves more superoxide in the epithelia and endothelia where it is produced. In particular, interstitial CO2− decrease, as shown in Fig. 5A. Maximal CO2−, which is found in DVR endothelia, reaches 0.15 (case A) and 0.47 nM (case C) when DO2− is reduced 10-fold, vs. 0.10 (case A) and 0.32 nM (case C) with the baseline DO2− value. Minimal CO2−, in the collecting duct lumen, is as low as 0.01 (case A) and 0.04 pM (case C), vs. 0.1 (case A) and 0.4 pM (case C) under baseline conditions.
Fig. 5.
Effect of varying the diffusivity of O2− (DO2−) and ONOO (DONOO) on interstitial CO2− (A) and CONOO (B). DO2− and DONOO are set to their base-case values and decreased by a factor of 10. Results are shown for case C. Relative concentration increases or decreases are almost identical in cases A, B, and C. O2− permeability reduction preserves more O2− in the epithelia and endothelia where it is produced; hence the reduction in interstitial CO2−. ONOO permeability reduction reduces ONOO transport to RBC and its subsequent rapid scavenging by HbO2 and peroxiredoxin 2 (Prx2), thereby raising CONOO elsewhere.
By significantly raising CO2− in vascular and tubular walls, where CNO is also the highest, the 10-fold DO2− decrease leads to a significant increase in ONOO− production. Medullary CONOO subsequently increases everywhere, by ∼10%. If the membrane diffusivity of ONOO− is concomitantly decreased 10-fold, CONOO profiles change more significantly. The ONOO permeability reduction favors ONOO preservation in tubular epithelia and vascular endothelia. As less ONOO diffuses into RBCs, the amount of ONOO that is promptly scavenged by HbO2 and peroxiredoxin 2 (Prx2) is significantly reduced, leading to an overall increase in CONOO elsewhere (Fig. 5B). CONOO values decrease by 80–90% in RBC, whereas they increase by a factor of 2–3 in vascular endothelia. In the interstitium of R1, the region where vasa recta are most tightly packed, CONOO reaches 1.4 and 2.8 nM in cases A and C, respectively, vs. 0.65 and 1.3 nM assuming baseline diffusivity values.
Effect of AQP1 permeability to NO.
Recent studies have shown that AQP1 transports NO and thereby regulates endothelium-dependent vasorelaxation (21, 22). In the renal medulla, AQP1 is expressed by DVR and descending limbs in addition to erythrocytes. To examine the impact of AQP1 permeability to NO in the OM, we performed simulations in which PNORBC and PNO in DVR endothelia and descending limb epithelia was reduced by a factor of 10, to simulate the condition under which NO transport through AQP1 is inhibited. The predicted effects are qualitatively similar to those obtained assuming a factor of 5 decrease in PNO (shown in Fig. 4). As the resistance to NO diffusion from the DVR endothelium toward the plasma is increased, the fraction of NO that enters the plasma and the RBCs decreases significantly. As a result, the amount of NO that is consumed by Hb is reduced, which preserves more NO in the DVR endothelium and the surrounding interstitium. The volumetric rate of NO synthesis is six times greater in DVR than in descending limbs. Moreover, the fractional surface area occupied by DVR is four to eight times greater in R1 than in R2. Thus inhibition of NO transport through AQP1 as simulated herein is predicted to raise interstitial CNO to a greater extent in R1 (by a factor of 7–8) than in R2-R4 (by a factor of 5–6).
Tubulovascular Cross Talk
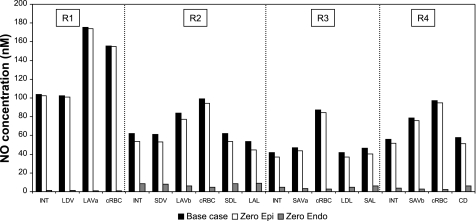
Tubular synthesis of NO is thought to affect vascular function; vice-versa, vascular synthesis of NO may affect tubular function (37). To examine these tubular-vascular interactions, we simulated the inhibition of either epithelial or endothelial production of NO. As shown in Fig. 6, complete inhibition of NO synthesis by vasa recta endothelia reduces CNO drastically, by a factor of 70–80 in R1 (which does not contain any tubules) and ∼10 in the outer regions, even as NO synthesis by tubular epithelia is maintained. The second-largest source of NO in the medulla after the vasa recta are the descending limbs of Henle. In the inner stripe, two-thirds of the descending limbs (all the short ones) are in R2, and one-third (all the long ones) are in R3. Thus, in the absence of vascular NO synthesis, concentrations are highest in R2 (Fig. 6). In the outer stripe, the descending limbs are more evenly distributed among R2, R3, and R4, so that concentrations are very similar in these three regions (results not shown). Nevertheless, at both levels, predicted CNO remain below 12 nM everywhere, because the GNO, max of long descending limbs (12.3 μM/s) remains small relative to that of endothelial cells (76.6 μM/s). Inhibition of endothelial NO production (i.e., the main source of NO in the OM) reduces luminal CNO in long ascending limbs from 54 to 9 nM at the mid-inner stripe (case C); at the mid-outer stripe, the decrease is even sharper, as luminal CNO falls from 69 to 12 nM in mTAL (case C). As discussed below, these reductions in mTAL CNO should have a large impact on sodium reabsorption across mTAL.
Fig. 6.
Effect of NO synthesis inhibition on CNO in the interstitium, vasa recta endothelium, capillary endothelium, and tubular epithelium in each region. Results are displayed for case C; CNO values in cases A, B, and C are nearly indistinguishable. Shown are concentrations under baseline conditions (black bars), assuming inhibition of tubular epithelial NO synthesis only (white bars, “zero epi”), and assuming inhibition of vascular endothelial NO synthesis only (grey bars, “zero endo”). Results suggest that vascular NO synthesis acts to limit Na+ reabsorption across mTAL, whereas tubular NO synthesis does not significantly affect vascular tone.
Complete inhibition of NO synthesis by the epithelia of the loops of Henle and the collecting duct (while that of endothelia remains as in the baseline) has a smaller effect on medullary NO levels, since tubular NO production is one to two orders of magnitude lower than vascular NO production (see Table 4 in Ref. 16). As illustrated in Fig. 6, in that case, CNO is reduced by 1–20% everywhere, and the largest differences are found in R2 and R3, which have the highest density of descending limbs (i.e., the largest tubular suppliers of NO). Maximal NO levels are still found in R1, where interstitial CNO is 111 nM (vs. 112 nM under baseline conditions) at the mid-outer stripe, and 102 nM (vs. 104 nM under baseline conditions) at the mid-inner stripe (case C). Luminal CNO in long ascending limbs is predicted to be 57 nM at the mid-outer stripe, and 45 nM at the mid-inner stripe (case C).
Similarly, the ZE model had predicted that a 104-fold decrease in endothelial NO synthesis lowers CNO by one to three orders of magnitude in R1 and R2, whereas a 104-fold decrease in epithelial NO synthesis has a relatively small effect on CNO in these regions (49).
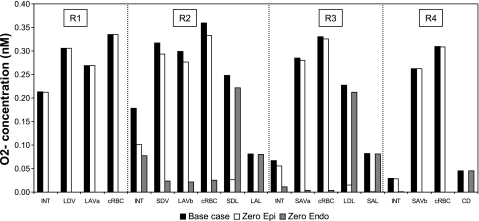
The relative importance of epithelial vs. endothelial O2− synthesis was also investigated with the model. Complete inhibition of endothelial (but not epithelial) O2− synthesis is predicted to lower CO2− significantly everywhere, since medullary vascular GO2− (0.7 μM/s at high Po2) is taken to be significantly higher than tubular GO2− (which ranges from 0.061 μM/s in collecting ducts to 0.508 μM/s in descending limbs at high Po2). In the vascular bundle core, a region with no tubules, CO2− drop below 1 pM (i.e., by >99%) in all three cases. At the mid-outer stripe, where the descending limbs are relatively well dispersed among R2, R3, and R4, interstitial CNO drops by 70–85% in the outer regions. At the mid-inner stripe, where the descending limbs are localized in R2 and R3 only, interstitial CO2− decreases more in R4 (98% in case A, 97% in cases B and C), a region where collecting ducts produce relative little O2−, than in R2 (62% in case A, 57% in cases B and C) and R3 (84% in case A, 83% in cases B and C), as displayed in Fig. 7.
Fig. 7.
Effect of O2− synthesis inhibition on CO2− in the interstitium, vasa recta endothelium, capillary endothelium, and tubular epithelium in each region. Results are displayed for case C. Relative CO2− changes are similar in cases A, B, and C. Shown are concentrations under baseline conditions (black bars), assuming inhibition of tubular epithelial O2− synthesis only (white bars, “zero epi”), and assuming inhibition of vascular endothelial O2− synthesis only (grey bars, “zero endo”).
Conversely, complete inhibition of epithelial (but not endothelial) O2− synthesis reduces CO2− significantly in R2 and R3, but not in R1. Since the GO2− is smaller in epithelia than in endothelia, the CO2− decreases are smaller in this case than in the previous one (Fig. 7). At the mid-inner stripe, interstitial CO2− is <1% lower in R1 (relative to baseline conditions), 38–44% lower in R2, 16–18% in R3, and 2–4% in R4, depending on the case. CO2− in short DVR endothelia is predicted to be 6–8% lower in the absence of tubular O2− production, which suggests that O2− diffusion from tubules to vessels is not significant under basal conditions.
Interactions Between NO and O2−
Experiments performed on rat OM microtissue strips showed that a tempol-induced decrease in medullary CO2− increases the diffusion of NO from mTAL to DVR pericytes, suggesting that NO cross talk between tubules and vessels may be inhibited by O2− (33). Vice versa, O2− cross talk between mTALs and vasa recta was observed to be inhibited by NO (33). To assess the importance of NO-O2− interactions, we performed simulations in which the reaction between these two radicals was completely inhibited. The reaction rate, which is taken as 6.7 × 109 M/s under baseline conditions, was set to zero in these simulations.
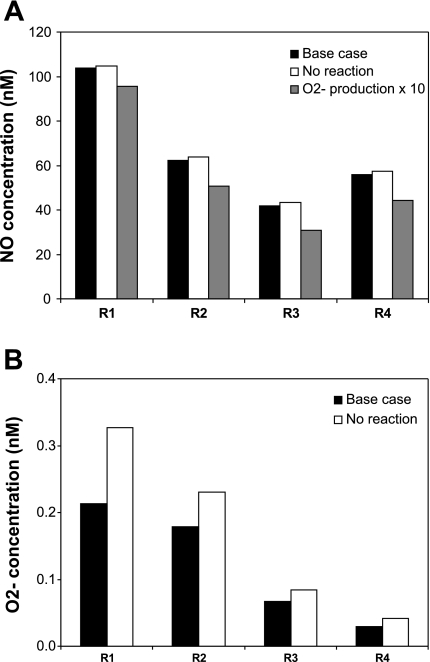
In agreement with in vitro observations, our model predicted that CO2− values were significantly higher relative to baseline conditions. In all three cases (A, B, and C), interstitial CO2− increased by ∼55% in the vascular bundle (where NO is more concentrated and the reaction therefore faster), and by 25–40% in the outermost regions, as shown in Fig. 8. In contrast to experimental findings, however, the model predicts that inhibition of the NO-O2− reaction has a small effect on CNO (<1, 4, and 5%, in cases A, B, and C, respectively). Indeed, the NO-O2− reaction rate is several orders of magnitude (∼10+3 times) lower than the rate of vascular NO synthesis and the rate of NO scavenging by hemoglobin. Thus eliminating NO scavenging by O2− does not raise CNO very significantly. The ZE model reported a similar finding (see Fig. 5 in Ref. 49).
Fig. 8.
Effect of NO-O2− interactions on interstitial CNO (A) and CO2− (B) in each region. Concentrations are given for baseline conditions (black bars), in the absence of reaction between NO and O2− (white bars, “no reaction”), or if O2− generation were 10 times higher (grey bars, “O2− production × 10”). In the latter case, CO2− would be 10 times greater than in the base case. Results correspond to case C; as described in the text, in cases A and B the relative changes would be smaller for CNO, and identical for CO2−. The model predicts that O2− consumption by NO significantly reduces medullary CO2−, but that O2− has a small impact on medullary NO bioavailability under physiological conditions.
As discussed below, it is possible that our baseline GO2− are too low. If epithelial and endothelial GO2− were increased 10-fold, relative to baseline values, CO2− would increase by a factor of 10 in all compartments, and basal NO levels would be 10–30% lower in case C (Fig. 8). The CNO reduction would be lower in cases A (3–8%) and B (5–25%), which assume smaller basal GO2−. If the concentration of SOD were then raised from 1 μM to 1 mM to mimic the addition of 1 mM tempol, CNO would subsequently rise by ∼10% in R1, 25% in R2, and 30–50% in R3 and R4 in case C. Note that the relative increase in CNO would be lower in cases A (at most 10%) and B (at most 35%). These results indicate overall that if GO2− were higher (∼10 μM/s), O2− would have a more significant impact on the tubulovascular cross talk of NO.
Another possible explanation for the discrepancy between experimental and theoretical findings lies in the experimental conditions themselves. In the microtissue strip studies that suggested that the cross talk of NO from the mTAL to the pericytes is inhibited by O2−, the vasa recta were obtained from kidneys perfused with microspheres to disrupt the endothelium (33); thus the vascular production of NO must have been significantly below its physiological value. Moreover, blood flow was absent, i.e., there was no scavenging of NO by RBCs. Under these conditions, the relative importance of the NO-O2− reaction would have been significantly enhanced.
Effects of ANG II
ANG II has been found to stimulate mTAL NO production and the subsequent diffusion of NO toward DVR pericytes in isolated microtissue strips (15). ANG II also increases mTAL production of O2−, and the cross talk of O2− from mTAL to vasa recta is significant in the presence of NO synthesis inhibitors (33). To determine whether ANG II-mediated increase in mTAL production rates may affect CNO and CO2− in vivo, we simultaneously varied the mTAL epithelial production rates of NO and O2−.
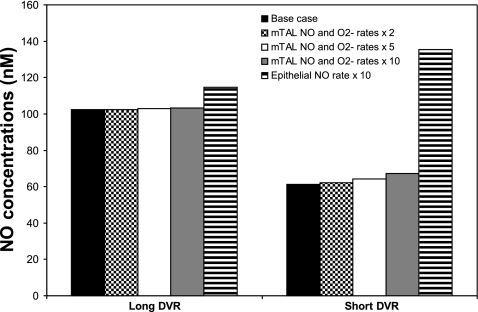
The predicted effects of increasing mTAL GNO and GO2− by a factor of 2, 5, and 10 on DVR endothelial CNO are displayed in Fig. 9. The vasoactive properties of DVR are imparted by pericytes, smooth muscle cells that surround DVR endothelia. Since pericytes are not represented explicitly in this model, endothelial CNO is taken to approximate pericyte CNO. Our model predicts that CNO is generally higher in long DVR, which reach into the inner medulla and are located in the core of the vascular bundle (R1), than in short DVR, which peel off from the immediate periphery of the bundle (R2); the reason is that the concentration of O2, a precursor in the synthesis of NO, is significantly higher in R1 than in R2, as described in the companion study (16). Increasing the rates of NO and O2− synthesis in mTALs, which are localized in the outer regions, raises CNO to a greater extent in short DVR than in long DVR (Fig. 9). However, the predicted CNO increases are relatively modest, since the major source of NO and O2− in the OM remains the vascular endothelium: even when mTAL GNO and GO2− are multiplied 10-fold, endothelial CNO rises by 1–2% in long DVR and 10–15% in short DVR, depending on the case.
Fig. 9.
Effect of increasing rates of NO and O2− synthesis on CNO in long and short DVR. Results are shown for baseline conditions, assuming that the NO and O2− production rates by ascending limb epithelia simultaneously increase by a factor of 2, 5, and 10 (so as to mimic the effects of ANG II), and assuming that the NO generation rate (GNO) by tubular (i.e., descending limb, ascending limb, and collecting duct) epithelia separately increases 10-fold. Concentration values correspond to case C, and are similar in cases A and B. Results suggest that a significant, isolated elevation in epithelial GNO affects the distribution of blood flow between the inner and outer medulla.
In agreement with experimental observations, ANG II-mediated increases in the mTAL rates of NO and O2− synthesis are not predicted to significantly enhance O2− tubulovascular cross talk. This is because the simultaneous increase in the mTAL rates of NO and O2− production means that the increase in GO2− is partly, or even completely, offset by the increase in NO-mediated O2− consumption. At the mid-inner stripe, a 10-fold increase in mTAL GNO and GO2− raises interstitial CO2− by <7% in R2, and actually reduces CO2− by 1% in R1. That is, in the vascular bundle core, the additional amount of O2− scavenged by NO (following the mTAL GNO increase) is greater than the additional amount of O2− diffusing from the tubules (following the mTAL GO2− increase). Within mTAL, CO2− changes are more significant: at the mid-inner stripe CO2− increases by a factor of ∼9 in the endothelia of mTAL, and by a factor of ∼8 in their lumen.
DISCUSSION
The main goal of this study was to assess the importance of tubulovascular cross talk and NO-O2− interactions in the OM. In contrast to our previous models of medullary NO transport (49, 50), the current study explicitly includes O2− conservation equations, the O2 dependence of generation rates, solute transport by capillary flow, and NO transport by AQP1 water channels. As discussed below, these effects are found to significantly affect predicted concentrations. As a prerequisite, we sought to determine NO and O2− kinetic and transport parameter values that are compatible with a variety of experimental measurements in the rat OM. This task is complicated by several facts: 1) reported values of CNO in the OM vary between 100 and 800 nM as described below; 2) CO2− in the medulla have not been measured, to the best of our knowledge; 3) the permeability of RBC, vessel walls, and tubular epithelia to NO, O2−, and ONOO remains highly uncertain; 4) and measurements of KO2NO range from ∼5 to 60 mmHg.
GNO
Measurements of physiological CNO in the kidney vary over a large range. Fenoy and colleagues, using differential normal pulse voltammetry, obtained values on the order of 400 nM in the cortex (30) and 800 nM in the outer medulla (41, 42). In contrast, Zou and Cowley (52) reported that CNO, as determined by the microdialysis-Hb-trapping assay, averaged ∼60 and 100 nM, respectively, in the renal cortex and medulla. Using the same technique, Kakoki and colleagues (24, 25) found baseline NO levels of ∼120 nM in the medulla. In this study, we attempted to find sets of parameter values that yield baseline interstitial CNO predictions in agreement with the lower range CNO measurements. Our results suggest that the main determinants of interstitial CNO are the maximal rate of vascular NO synthesis (GNO, max), KO2NO, and PNORBC.
Given that Po2 is low in the medulla, oxygen is predicted to exert a substantial rate-limiting effect on the production of NO, as described in the companion study (16). However, hypoxia may induce compensatory mechanisms. Heyman et al. (23) observed that amplification of OM hypoxia increases medullary NO levels, perhaps through enhanced binding to oxyhemoglobin. Other investigators reported that hypoxia stimulates NO production in the proximal tubule (29). To explain these experimental findings, it has been postulated that low Po2 induces the release of NO by RBCs via SNOHb or RBC nitrite (13, 38). To test that hypothesis, in a set of simulations, we accounted for the generation of NO via the SNOHb and nitrite pathways using an approach similar to that developed by Chen et al. (6, 8). In their models, an unspecified mechanism is assumed to facilitate the export out of RBC of the NO thereby formed. Those models predict that the amount of NO delivered by SNOHb that reaches the vascular wall is ∼1–10 pM (8), and that delivered by nitrite is ∼100 pM (6). These concentrations are significantly below the CNO required for half-maximal activation of sGC (EC50 ∼ 10 nM; see Ref. 7), which suggests that these two mechanisms cannot, by themselves, induce hypoxic vasodilation. Not surprisingly, in our study, too, both pathways were predicted to have a negligible effect (<0.1%) on medullary CNO. Thus the means by which hypoxia raises CNO remain unclear.
In contrast, variations in PNORBC were found to significantly affect CNO (Fig. 4). Indeed, the rate of NO scavenging by hemoglobin in RBCs is sufficiently fast that restricting NO diffusion toward RBCs significantly enhances CNO elsewhere. Our results suggest that to predict interstitial CNO on the order of 100 nM, the rates of vascular NO production (which may include sources activated by hypoxia) should be ∼10–100 μM/s if PNORBC is taken as 0.05 or 0.1 cm/s. In the base case, (GNO, max = 76.6 μM/s in vasa recta, KO2NO = 38 mmHg, PNORBC = 0.1 cm/s) calculated interstitial NO values range from 63 to 112 nM at the mid-outer stripe, and from 42 to 104 nM at the mid-inner stripe.
Effect of AQP1 Permeability to NO
NO is transported by AQP1 (21, 22), which is expressed by erythrocytes, as well as DVR and descending limbs in the renal medulla. Our simulation results suggest that NO transport via AQP1 lowers pericyte concentrations of NO, presumably decreasing its vasodilatory effects; the higher the permeability of RBCs and endothelia to NO, the greater the diffusion of NO toward RBCs, where it is promptly consumed. In contrast, Herrera and Garvin (21) observed that aortic ring vasorelaxation induced by acetylcholine (which increases endogenous NO) was significantly reduced in vessels from AQP1 null mice relative to those from control mice. However, their in vitro studies were performed in the absence of blood flow; under these conditions, AQP1 facilitates NO diffusion out of endothelial cells, but it does not enhance NO consumption by Hb. Further experimental studies are needed to resolve this discrepancy.
Tubulovascular NO Cross Talk
NO has both vasodilatory and natriuretic effects, and several studies have demonstrated the importance of NO tubular-vascular interactions (15, 33). In microtissue strips, ANG II stimulates NO synthesis by mTAL epithelia and subsequent diffusion to adjacent pericytes (15). Thus the generation of NO by mTAL epithelia could affect vascular tone (37). Conversely, the generation of NO by vasa recta endothelia may increase CNO in mTAL and thereby inhibit sodium reabsorption.
The epithelial-to-endothelial GNO, max ratios used in our model were determined based upon experimental measurements (48). With these values, our model suggests that basal epithelial NO synthesis has a negligible effect on vascular tone (Fig. 6). At the mid-inner stripe, the simulated inhibition of epithelial NO production reduces endothelial CNO in long DVR only from 102 to 101 nM, and in short DVR only from 61 to 53 nM. However, a 10-fold increase in epithelial NO synthesis (by all tubules) is predicted to reverse the long-to-short DVR CNO difference at the mid-inner stripe, from +41 to −21 nM, as displayed in Fig. 9. Hence, a significant elevation in epithelial GNO could affect the distribution of blood flow between the inner medulla and OM.
The current study predicts that basal endothelial NO synthesis has a large impact on tubular NaCl transport. The simulated inhibition of endothelial NO production reduces luminal CNO in long ascending limbs from 54 to 9 nM, and in short ascending limbs from 47 to 7 nM at the mid-inner stripe (Fig. 6). At the mid-outer stripe, the reduction is even larger: luminal CNO is predicted to decrease from 69 to 12 nM in long ascending limbs, and from 67 to 10 nM in short ascending limbs. Ortiz et al. (36) observed that 10 μM spermine NONOate (or SPM, an NO donor) inhibits mTAL Cl− reabsorption by 46%. At a concentration of 10 μM, SPM is expected to result in a bath CNO of 50–60 nM (39). Thus a decrease in long ascending limb CNO levels from 60 to 10 nM should increase NaCl reabsorption across mTAL by ∼40%. In other words, endothelial NO production is predicted to strongly inhibit tubular NaCl transport under basal conditions.
When active NaCl transport across mTALs is inhibited by local NO, the consumption of O2 by thick limbs decreases. Since NO synthesis is rate limited by low Po2, an elevation in Po2 will raise local NO generation and concentration. Thus the blunting of mTAL transport by NO and the generation of NO together form a positive feedback loop, which may be an aspect of tubulovascular crosstalk not captured by the present model, because the model does not explicitly represent mTAL NaCl transport and assumes that Po2 values are known a priori. That positive feedback loop may increase CNO and Po2 locally through the processes described above, and, to a lesser extent, globally through diffusion. Nonetheless, those increases are bounded, because local Po2 is limited by O2 supply to the medulla and because for sufficiently high Po2, GNO approaches its maximal rate. Moreover, at some point, the reduction in NaCl reabsorption will activate the renin-angiotensin-aldosterone system, which will act to enhance NaCl transport and counteract the effects of NO.
Interactions Between NO and O2−
The relative GO2− of different medullary segments were measured by Li et al. (28), but absolute rates have not been determined, to the best of our knowledge. Our GO2− estimate for vasa recta under well-oxygenated conditions (0.7 μM/s) is comparable to that of other studies (3, 40). As described in the companion study (16), the effects of medullary hypoxia on GO2− remain controversial, so we considered three scenarios: cases A, B, and C, respectively, assume that low Po2 inhibits, does not affect, or stimulates O2− synthesis. The resulting CO2− in all three cases (on the order of 0.01–0.2 nM in tissue) are too small to significantly affect NO transport, since the rate of NO consumption by O2− is ∼10+3 times lower than the rate of NO scavenging by hemoglobin as well as the vascular rate of NO synthesis. Thus our model predicts that inhibiting the NO-O2− reaction raises CNO by <5% in case C, 4% in case B, and 1% in case A.
In our previous study of NO transport (49), the effect of NO scavenging by O2− was found to be more significant under certain conditions: the ZE model predicted that a simultaneous 10-fold increase in CO2− and epithelial NO synthesis would raise CNO in R2–R4 by 70–140%, whereas an isolated increase in epithelial NO synthesis would raise CNO in R2–R4 by 110–220% (49). The greater effect of O2− on NO levels predicted by the ZE model is likely due to the fact that CO2− was fixed in the ZE model, not calculated, and the basal CO2− values were significantly higher in the interstitium than the ones predicted by the current model (0.25 nM in the ZE model, vs. 0.01 to 0.22 nM herein). At lower CO2−, the ZE model also predicted that O2− has a small impact on NO levels.
The baseline O2− production rates were perhaps underestimated in our study. The enzymatic production of O2− has been found to be significantly greater in the OM than in the inner medulla and the cortex (51), which suggests that GO2− in the OM may be comparatively high. If baseline GO2− were increased by a factor of 10, the rate of the reaction between O2− and NO would increase accordingly. A subsequent 103-fold increase in the rate of O2− scavenging by SOD would raise CNO everywhere by 10–55% in case C (and by <10% in case A, and <35% in case B). Thus, if we assume higher basal GO2−, the model predicts that the bioavailability of NO in the renal medulla is more significantly reduced by O2− under physiological conditions.
If, however, our basal O2− synthesis rate (on the order of 1 μM/s) is appropriate for the OM, the model prediction that NO scavenging by O2− is not very significant would appear to contradict a number of experimental results at first glance. Mori and Cowley (33) found that 1 mM tempol (a SOD mimetic) enhances the diffusion of NO from mTAL to DVR pericytes. Ortiz and Garvin (35) measured the production of NO in isolated, perfused TALs and found that the addition of tempol to the bath significantly increased NO release by TALs. More recently, Abe et al. (1) showed that increases in mTAL luminal Na+ concentration and/or flow rate can increase the generation of superoxide and decrease NO in mTAL. In all of these experiments, however, the vascular endothelium (i.e., the main source of NO in vivo) was either absent or disrupted; nor were there RBCs, which constitute a sink for NO due to Hb scavenging. Thus the rate of (epithelial) NO synthesis and that of the NO-O2− reaction were likely comparable in these experiments. Our model suggests that under physiological conditions, the relative contribution of the latter reaction to medullary NO levels is much smaller.
Under pathological conditions associated with oxidative stress, elevated medullary O2− levels should enhance NO consumption, thereby indirectly affecting vascular tone and NaCl transport. A 10-fold increase in vascular and tubular O2− synthesis rates is predicted to lower CNO by ∼25–30% in ascending limbs, and by 10–20% in descending vasa recta, in case C. Superoxide also exerts direct effects on vascular and tubular functions: it enhances NaCl reabsorption in the mTAL (20), and reduces medullary blood flow, although the underlying mechanisms remain uncertain (18). Thus a shift in the balance between the production of NO and O2− in favor of O2−, such as seen in diabetes, atherosclerosis, and renin-angiotensin system activation, promotes renal vasoconstriction and enhanced tubular sodium reabsorption, ultimately leading to the progression of renal disease and hypertension (18).
Comparison with a Previous Modeling Study
Both this study and the ZE model predict that basal NO synthesis by the tubular epithelium does not raise CNO significantly in the vasoactive pericytes surrounding DVR. Both models also suggest that below concentrations of 1 nM, superoxide is a minor scavenger of NO.
The most significant difference between this study and the ZE model lies in the fact that the latter did not include the rate-limiting effects of medullary hypoxia on NO generation, and therefore predicted that CNO is the highest in R2 (and specifically greater in short DVR than in long DVR), as discussed in the companion study (16). Among other substantial differences, the current model incorporates O2− conservation equations: basal CO2− is predicted to range from 0.1 pM (in the collecting duct lumen) to 300 pM (in DVR endothelium), i.e., a greater span than the fixed one we assumed in the ZE model (50–250 pM). NO transport via AQP1, neglected in the ZE model, is predicted to significantly increase NO diffusion into RBCs and its subsequent scavenging by hemoglobin, whereas NO synthesis by the capillary plexus (equally omitted in the ZE model) is predicted to enhance NO bioavailability in the peripheral regions by up to 10%.
Conclusions
NO exerts at least two distinct effects in the renal medulla: it modulates DVR vasoactivity and thereby helps to maintain medullary perfusion and O2 supply, and it inhibits NaCl reabsorption across mTALs, thus decreasing O2 consumption. As shown experimentally, acute and chronic inhibition of NO synthesis reduces medullary blood flow and leads to sodium retention and the development of hypertension (11, 31, 34). Our theoretical results suggest that under physiological conditions, endothelial (as opposed to epithelial) NO synthesis is paramount: our model predicts that basal endothelial NO production acts to significantly limit NaCl reabsorption across mTALs and to prevent vasoconstriction, whereas basal epithelial NO production has a much lesser effect on NaCl transport and a negligible impact on vascular tone. In addition, our model predicts that O2− consumption by NO significantly reduces medullary CO2−, but that O2−, when present at subnanomolar concentrations, has a small impact on medullary NO bioavailability. This study also emphasizes the need for additional experimental investigations; a full understanding of the effects of NO and O2− in the renal OM will require measuring the absolute rates of NO and O2− synthesis in the medulla (particularly under hypoxic conditions), the vascular and tubular PNO and PO2−, and tubulovascular cross talk in the presence of blood flow.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases DK053775 to A. Edwards.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Angelo M, Singel DJ, Stamler JS. An S-nitrosolthiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA 103: 8366–8371, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buerk D, Lamkin-Kennard K, Jaron D. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med 34: 1488–1503, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Cassoly R, Gibson QH. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol 91: 301–313, 1975. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Edwards A, Layton AT. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol, 298: F1369–F1383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide 18: 47–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 10: 1185–1198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K, Pittman RN, Popel AS. Vascular smooth muscle NO exposure from intraerythrocytic SNOHb: a mathematical model. Antioxid Redox Signal 9: 1097–1110, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chen K, Popel AS. Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radic Biol Med 41: 668–680, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol 289: F749–F753, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chin SY, Wang CT, Majid DSA, Navar LG. Renoprotective effects of nitric oxide in angiotensin II-induced hypertension in the rat. Am J Physiol Renal Physiol 274: F876–F882, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denicola A, Souza JM, Radi R, Lissi E. Nitric oxide diffusion in membranes determined by fluorescence quenching. Arch Biochem Biophys 328: 208–212, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Edwards A, Layton AT. Nitric oxide and superoxide transport in a cross section of the rat outer medulla. I. Effects of low medullary oxygen tension. Am J Physiol Renal Physiol (doi:10/1152/ajprenal.00680.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS. Mechanism of NO-Induced oxidation of myoglobin and hemoglobin. Biochemistry 35: 6976–6983, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Evans R, Fitzgerald S. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 14: 9–15, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol 4: 161–177, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Garvin J, Ortiz P. The role of reactive oxygen species in the regulation of tubular function. Acta Physiol Scand 179: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol 292: F1443–F1451, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension 48: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Heyman SN, Goldfard M, Darmon D, Brezis M. Tissue oxygenation modified nitric oxide bioavailability. Microcirculation 6: 199–203, 1999 [PubMed] [Google Scholar]
- 24.Kakoki M, Kim HS, Arendshorst WJ, Mattson DL. l-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol 287: R1478–R1485, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kakoki M, Zou AP, Mattson DL. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am J Physiol Regul Integr Comp Physiol 281: R91–R97, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol 97: 293–301, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Liang M, Knox FG. Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol Regul Integr Comp Physiol 278: R1117–R1124, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol Renal Fluid Electrolyte Physiol 264: F79–F87, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Mattson DL, Meister CJ. Renal cortical and medullary blood flow responses to l-NAME and ANG II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am J Physiol Regul Integr Comp Physiol 289: R991–R997, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi K, Mattson DL, Cowley AW., Jr Role of renal medullary blood flow in the development of l-NAME hypertension in rats. Am J Physiol Regul Integr Comp Physiol 268: R317–R323, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Ortiz PA, Garvin JL. Interaction of O2− and NO in the thick ascending limb. Hypertension 39: 591–596, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature 409: 622–626, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Quijano C, Romero N, Radi R. Tyrosine nitration by superoxide and nitric oxide fluxes in biological systems: Modeling the impact of superoxide dismutase and nitric oxide diffusion. Free Radic Biol Med 39: 728–741, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Salom MG, Arregui B, Carbonell LF, Ruiz F, Gonzalez-Mora JL, Fenoy FJ. Renal ischemia induces an increase in nitric oxide levels from tissue stores. Am J Physiol Regul Integr Comp Physiol 289: R1459–R1466, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Salom MG, Nieto Ceron S, Rodriguez F, Lopez B, Hernandez I, Gil Martinez J, Martinez Losa A, Fenoy FJ. Heme oxygenase-1 induction improves ischemic renal failure: role of nitric oxide and peroxynitrite. Am J Physiol Heart Circ Physiol 293: H3542–H3549, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA 89: 7674–7677, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsoukias NM, Popel AS. Erythrocyte consumption of nitric oxide in presence and absence of plasma-based hemoglobin. Am J Physiol Heart Circ Physiol 282: H2265–H2277, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocyte consumption of nitric oxide: competition experiment and model analysis. Nitric Oxide 5: 18–31, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates. Am J Physiol Heart Circ Physiol 274: H2163–H2176, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Whorton AR, Simonds DB, Piantadosi CA. Regulation of nitric oxide synthesis by oxygen in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 272: L1161–L1166, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Edwards A. A model of nitric oxide tubulovascular cross talk in a renal outer medullary cross section. Am J Physiol Renal Physiol 292: F711–F722, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Pibulsonggram T, Edwards A. Determinants of basal nitric oxide concentration in the renal medullary microcirculation. Am J Physiol Renal Physiol 287: F1189–F1203, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Zou AP, Wu F, Cowley AW., Jr Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31: 271–276, 1998 [DOI] [PubMed] [Google Scholar]