Abstract
Mammalian urine contains a range of macromolecule proteins that play critical roles in renal stone formation, among which Tamm-Horsfall protein (THP) is by far the most abundant. While THP is a potent inhibitor of crystal aggregation in vitro and its ablation in vivo predisposes one of the two existing mouse models to spontaneous intrarenal calcium crystallization, key controversies remain regarding the role of THP in nephrolithiasis. By carrying out a long-range follow-up of more than 250 THP-null mice and their wild-type controls, we demonstrate here that renal calcification is a highly consistent phenotype of the THP-null mice that is age and partially gene dosage dependent, but is gender and genetic background independent. Renal calcification in THP-null mice is progressive, and by 15 mo over 85% of all the THP-null mice develop spontaneous intrarenal crystals. The crystals consist primarily of calcium phosphate in the form of hydroxyapatite, are located more frequently in the interstitial space of the renal papillae than intratubularly, particularly in older animals, and lack accompanying inflammatory cell infiltration. The interstitial deposits of hydroxyapatite observed in THP-null mice bear strong resemblances to the renal crystals found in human kidneys bearing idiopathic calcium oxalate stones. Compared with 24-h urine from the wild-type mice, that of THP-null mice is supersaturated with brushite (calcium phosphate), a stone precursor, and has reduced urinary excretion of citrate, a stone inhibitor. While less frequent than renal calcinosis, renal pelvic and ureteral stones and hydronephrosis occur in the aged THP-null mice. These results provide direct in vivo evidence indicating that normal THP plays an important role in defending the urinary system against calcification and suggest that reduced expression and/or decreased function of THP could contribute to nephrolithiasis.
Keywords: knockout mice, nephrolithiasis, interstitial calcification, supersaturation, urolithiasis
renal stone formation or nephrolithiasis is a multistep process comprising nucleation, crystal growth and aggregation, and crystal adherence to the renal epithelial cells (24, 33, 38, 45). Each of these steps is believed to be influenced by a range of urinary factors, some acting to promote and others to inhibit nephrolithiasis (3, 28, 30, 35, 39). Among the critical forces that drive nucleation is the supersaturation of lithogenic components (4, 28). Indeed, patients with metabolic disorders that produce high urine concentrations of calcium (hypercalciuria), oxalate (hyperoxaluria), and phosphorus (hyperphosphaturia) are highly prone to developing kidney stones (1, 2, 6, 13, 36, 61). Even in idiopathic calcium stone formers without a history of metabolic disorders, their urine is often more supersaturated with the stone-forming mineral salts than non-stone formers (35). Notwithstanding this pivotal role in initiating renal stone formation, supersaturation per se cannot explain the full spectra of renal stone diseases or their pathogenesis, and factors that counter urinary supersaturation and/or inhibit crystal aggregation and epithelial attachment are also intimately involved in nephrolithiasis (5, 18, 35). Potential protectors against stones include inorganic ions such as magnesium, organic anions such as citrate, as well as macromolecules (29, 60).
A notable urinary macromolecule that has been implicated in modulating nephrolithiasis is Tamm-Horsfall protein (THP; also named uromodulin). THP is a kidney-specific, heavily glycosylated protein made by tubular cells in the thick ascending limb of the loop of Henle (TAL) (15, 32, 34, 53, 54). Mature THP is anchored to the luminal leaflet of the lipid bilayer of TAL's apical surface membrane via glycosylphosphatidyl inositol (GPI) linkage, and the protein backbone is released into the urine by the activity of proteases or phospholipases. THP is by far the most abundant urinary protein in most mammals, with a daily excretion amounting to 100 mg (34, 53).
Despite its abundance and frequent presence in the matrix of human kidney stones, the exact role of THP in stone formation remains controversial. First, while a reduced amount of THP was associated with one cohort of human stone formers (48), this association could not be verified in other patient cohorts (46). Aside from the disputed, quantitative defects, there is a renewed interest in THP's functional abnormalities, in particular its content of sialic acids. Thus THP from stone formers seems to bear a lower level of sialic acids than that from healthy individuals (21, 31, 54), suggesting a role for THP's complex-type carbohydrates in protection against nephrolithiasis. Second, in vitro analyses demonstrated that THP potently inhibits the aggregation of both calcium phosphate (CaP) and calcium oxalate (CaOx) crystals, and the protein is incorporated into CaP but not CaOx crystals (20, 22). In addition, whether THP inhibits or promotes or has no effect on CaOx crystallization in vitro depends largely on the pH and ionic strengths of the solution and THP concentration (23). Third, although gene inactivation of THP in mice has been accomplished, the two existing knockout models have yielded divergent results (8, 42). The model generated by Raffi and colleagues (47) had no renal crystals or stones after a 3-yr follow-up. In contrast, the model generated by our group exhibits spontaneous intrarenal calcification since an average of 16% of our THP-null male mice at 2–3 mo of age from two separate cohorts had spontaneous development of renal calcium crystals (42, 43). Chemical induction of hyperoxaluria in mice via the administration of ethylene glycol and vitamin D3 increased the frequency of CaOx crystallization in THP-null mice to ∼70%, whereas the same degree of hyperoxaluric condition elicited no CaOx crystal in the wild-type controls (42, 43).
While data from our THP-null mice provided in vivo evidence supporting the defensive role of normal THP against renal calcinosis, many key questions remain. For instance, it was unclear whether the appearance and frequency of renal calcification in our THP-null mice depended on a particular genetic background; whether the severity of crystal deposition was age and gender dependent; and whether a reduced gene dosage, not just a complete knockout, of THP could lead to renal calcinosis. It was also unknown whether the spontaneous renal crystals in our THP-null mice would eventually evolve to renal stones if we were to follow them for an extended period of time. The location of crystals (intratubular or interstitial) was also unclear as well as whether the crystals in mice resembled ultrastructurally the earliest stages of human nephrolithiasis. Finally, we could not rule out the possibility that the different targeting strategies were responsible for the divergent results between the two existing THP knockout models (8, 42, 44). The current study was therefore designed to address some of these fundamentally important issues and to shed light on the in vivo role of THP in nephrolithiasis.
METHODS
Tamm-Horsfall protein (uromodulin) knockout.
A gene replacement strategy was employed to inactivate the THP gene in mice (44). Homologous recombination of the targeting vector with the endogenous THP gene allele in W4 embryonic stem cells (derived from mouse strain 129/SvEv) led to the deletion of a 650-bp proximal promoter and the first four exons of the THP gene. The resultant THP−/− mice were devoid of THP expression at the mRNA and protein levels, as evidenced by Northern blotting, RT-PCR, in situ hybridization, Western blotting, and polyclonal antibody staining (44). THP+/− mice that retained a wild-type THP allele expressed THP mRNA and protein, albeit at a lower level than that of the THP+/+ mice. The chimeric founder mice that were capable of transmitting the THP knockout allele to the offspring were crossed and back-crossed separately into two independent genetic backgrounds: 129/SvEv or C57BL/6. After more than 10 generations of backcrossing within each genetic background, the THP+/− siblings were intercrossed to produce the THP−/− mice. For the 129/SvEv background, only male THP−/− mice were analyzed, whereas for C57BL/6, both male and female THP+/− and THP−/− mice were analyzed. Wild-type mice were maintained in parallel, also in two separate, 129/SvEv or C57BL/6 backgrounds. All animal experiments were carried out in accordance with the regulations and policies set forth by Federal and local authorities and under an active protocol approved by the Institutional Animal Care and Use Committee.
Histochemical detection of renal calcification.
To visualize renal crystal deposits and ureteral stones, kidneys and ureters from the THP knockout mice as well as the wild-type controls were dissected from the animals after euthanasia and immediately fixed in PBS-buffered 10% formalin. The fixed tissues were routinely processed for paraffin embedding. Close attention was paid during paraffin block sectioning to preserve the renal papilla, which is of a very small size in mice. Five-micrometer-thick coronal sections were stained with a von Kossa solution that contained silver nitrate, which specifically reacts with calcium deposits (42). The sections were then counterstained in 1% neutral red solution and examined by light microscopy.
Determination of chemical composition of renal crystals.
Fourier transform infrared microspectroscopy (μ-FTIR) was employed to analyze the chemical components of histochemically identified renal papillary crystals using a method and instrument specifications described previously (43). Normal kidney tissues from the wild-type mice were used as a negative control. Spectra generated by μ-FTIR were compared with standard spectra of calcium oxalate, calcite, and calcium phosphate (hydroxyapatite).
Transmission electron microscopy.
Mouse kidneys were sliced along the coronal axis into two halves to expose the renal papillae, which were carefully dissected and immediately fixed. Two different methods of specimen processing were subsequently used. In method 1, the papillae were fixed in 2.5% glutaraldehyde prepared in 0.1 M sodium cacodylate buffer (pH 7.4) and postfixed in 2% (wt/vol) osmium tetroxide for a total of 4 h before dehydration and embedding. In method 2, the papillae were fixed in Trump's solution (1% glutaraldehyde and 3.7% formaldehyde in PBS) and stored at 4°C for 1–2 wk before further processing. The stored tissues were then processed with the aid of a laboratory microwave (Pelco; BioWave), consecutively in 0.1 M sodium cacodylate (pH 7.24) and 2% OsO4. The aforementioned differently fixed specimens with the two methods were then washed, dehydrated, and embedded in Epon 812. Ultrathin sections were poststained with 2% uranyl acetate and Reynold's lead citrate and examined with a Philips CM-12 electron microscope (FEI; Eindhoven, The Netherlands) and photographed with a Gatan (4 × 2.7 k) digital camera (Gatan, Pleasanton, CA).
Immunohistochemical staining.
Four-micrometer-thick sections of mouse kidneys were deparaffinized and hydrated in distilled water and then in phosphate-buffered saline. Antigen retrieval was performed by microwaving the deparaffinized sections in a citrate buffer (pH 6.0) with the maximum power output for 15 min. Nonspecific binding was blocked by incubating the sections with 3% bovine serum albumin in PBS, and the endogenous peroxidase was quenched with 3% hydrogen peroxide. The sections were then incubated with a rabbit anti-osteopontin antibody (1:500 dilution; Assay Designs, Ann Arbor, MI), followed by a goat anti-rabbit antibody conjugated with horseradish peroxidase. As a negative control, a nonimmune rabbit IgG was used as the primary antibody, with all subsequent procedures performed identically. Alternatively, the kidney sections were immunolabeled with a rabbit anti-mouse type IV collagen antibody (1:200 dilution, Abcam, Cambridge, MA) or antibodies specific for mouse resident macrophages (ER-mp20; 1:100 dilution, Abcam), as well as mouse activated macrophages [F4/80(CI:A3); 1:50 dilution, Novus, Littleton, CO]. Spleen sections from wild-type mice were used as positive controls for the macrophage antibodies. All reactions were developed in a 50 mM Tris·HCl buffer (pH 7.4) containing DAB and H2O2, and the sections were lightly counterstained with hemotoxylin.
Urine chemistry.
Wild-type and THP−/− mice (male, 5–8 mo old) were placed into autoclaved metabolic cages (VWR; NALGENE Single-Mouse Metabolic Cage), with one mouse per cage, in the morning of the day of urine collection. Food and water were provided ad libitum. Urine samples were collected from the collection cup the next morning at the same time. After centrifugation of the 24-h urine samples at 5,000 g at 4°C for 5 min, the cell debris was discarded and the supernatant was stored at −80°C until use. The concentrations of urinary constituents were determined at the Mayo Clinic Renal Function Laboratory or in the laboratory of Dr. Yasushi Nakagawa (University of Chicago). Supersaturation of key urine indices was calculated using the EQUIL2 program (57).
Statistical methods.
Student's t-test was used to compare the differences in urine indices of the wild-type mice vs. the THP knockout mice using Web-based SPSS software. A P value < 0.05 was considered statistically significant.
RESULTS
Progressive renal papillary calcification in THP−/− mice.
Although we previously noted spontaneous calcium crystal deposits in the renal parenchyma of our THP−/− mice, this phenotype occurred in only 16 and 14% of the young (2–3 mo old) male animals from two small cohorts consisting of 25 and 21 mice, respectively (42, 43). The crystals were small in size, and their exact locations (cortex, medulla or papilla; intratubular vs. interstitial) were not well defined. Additionally, it was unclear whether the extent of such renal calcification was dependent on age, gender, genetic background, and THP gene dosage. To address these important issues, we generated and systematically characterized, over a 15-mo period, larger cohorts of mice that represented different genetic backgrounds, genotypes, and age and gender groups (Table 1). We found that THP−/− mice in both 129/SvEv and C57BL/6 backgrounds were highly prone to spontaneous renal calcification, as evidenced by von Kossa histochemical staining (Table 1; Fig. 1). By 5–8 mo of age, ∼50% of the THP−/− mice in both genetic backgrounds developed renal calcium deposits. In stark contrast, renal crystals were never observed in the wild-type mice that were maintained in the same genetic backgrounds and with the same lengths of observation (Table 1; Fig. 1A). These results provide compelling evidence that intrarenal calcium crystallization does not occur in normal mice, but is a specific consequence of THP deficiency in our mouse cohorts and is independent of genetic background. By 15 mo of age, the frequency of renal crystal deposition increased dramatically, with 87.5% of the male THP knockout mice in the 129/SvEv background and 100% in the C57BL/6 background developing crystals (Table 1). While only male THP−/− mice in the 129/SvEv background were analyzed, female THP−/− mice in the C57BL/6 background were studied and also exhibited intrarenal crystals to a degree comparable to their male counterparts among all age groups. Interestingly, heterozygous deletion of the THP gene (as in THP+/− mice), which merely reduced the THP level (44), also rendered the animals susceptible to renal calcification (Table 1), suggesting that even reduced THP expression could predispose mice to renal calcium crystallization, albeit to a lesser extent than the total absence of THP.
Table 1.
Spontaneous kidney lesions in different genetic background, genotype, gender, and age groups of mice deficient for Tamm-Horsfall protein
| 129/SvEv |
C57BL/6 |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mo |
5–8 mo |
15 mo |
5–8 mo |
15 mo |
||||||||||||||
| WT |
THP−/− |
WT |
THP−/− |
WT |
THP−/− |
WT |
THP+/− |
THP−/− |
WT |
THP+/− |
THP−/− |
|||||||
| M | M | F | M | F | M | F | M | F | M | F | M | F | ||||||
| Total n | 46 | 47 | 22 | 20 | 5 | 16 | 12 | 9 | 18 | 8 | 9 | 11 | 6 | 4 | 4 | 3 | 11 | 2 |
| Papillary calcinosis | ||||||||||||||||||
| n | 0 | 8 | 0 | 10 | 0 | 14 | 0 | 0 | 6 | 2 | 5 | 5 | 0 | 0 | 3 | 1 | 11 | 2 |
| % | 0 | 17 | 0 | 50 | 0 | 87.5 | 0 | 0 | 33.3 | 25 | 55.6 | 45.4 | 0 | 0 | 75 | 33.3 | 100 | 100 |
| Ureteral stones/hydronephrosis | 0 | 0 | 0 | 3 | 0 | 5 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 3 | 1 | 7 | 2 |
WT, wild-type; THP, Tamm-Horsfall protein; M, male; F, female.
Fig. 1.
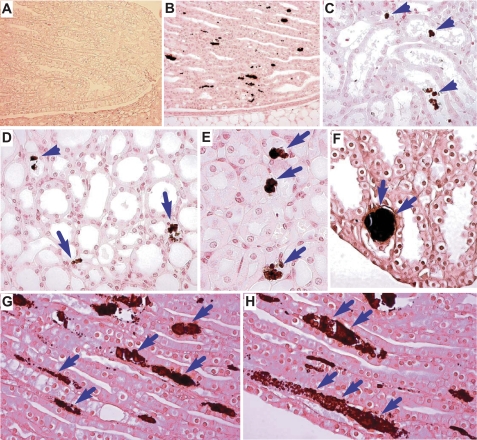
Existence of intratubular and interstitial calcium deposits in THP−/− mice. Kidney sections from a wild-type control (A) and THP−/− mice (B–H) were stained with the von Kossa method that specifically visualizes calcium deposits in tissue. Note the absence of any crystal in the papilla of the wild-type mouse (A) and the presence of dark-colored crystals in the renal papilla (B, D–H) and deep medulla of the THP−/− mice (C). Both intratubular (C and D, arrowheads) and interstitial deposits (D, E, G, H, arrows) could be seen. Large crystals were occasionally found plugging the terminal portion of the collecting tubules; adjacent tubules were markedly dilated (F).
Existence of both intratubular and interstitial calcium crystals in THP−/− mice.
Light microscopy of von Kossa-stained kidney sections demonstrated that calcium crystals in the THP−/− mice were restricted to the renal papillary region (Fig. 1B) and were completely absent in the cortex and outer medulla (data not shown). Both intratubular (Fig. 1C) and interstitial crystals (Fig. 1, C–H) were present, with the former representing 26.1% and the latter representing 73.9% of all the crystal locations in 5- to 8 mo-old THP−/− mice. In 15 mo-old THP−/− mice (Fig. 1, D–H), interstitial crystals were even more common, accounting for 93.2% of all the crystal locations. Interstitial crystals were localized primarily beneath the tubular cells of the collecting ducts (Fig. 1, G and H, arrows). The extent of crystal formation varied from tubule to tubule, from microcrystals spanning the length of 2–3 tubular cells to large crystal aggregates spanning up to 10–15 cells, possibly representing different stages of crystal accumulation. The basement membrane zone containing the crystals was markedly widened (Fig. 1, G and H). Occasionally, large plugging crystals could be seen within the ducts of Bellini, which were surrounded by enlarged collecting tubules (Fig. 1F).
Chemical composition of papillary interstitial crystals.
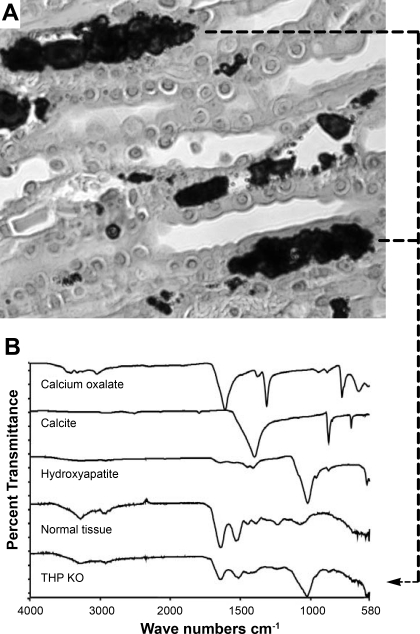
To determine their chemical composition, we performed μFTIR of histochemically identified crystals in the renal papillae (Fig. 2A). The spectrum of the interstitial crystals matched exactly that of the hydroxyapatite standard (Fig. 2B). The crystals also contained a small amount of calcium carbonate. The chemical composition of the intratubular crystals which were of much smaller sizes could not be determined with the current level of detection by μFTIR.
Fig. 2.
Chemical composition of the interstitial mineral deposits in THP−/− mice. A: interstitial mineral deposits shown by von Kossa staining. B: spectra of Fourier transform infrared microspectroscopy (FTIR) of chemical standards (calcium oxalate, calcite, and hydroxyapatite), crystal-free normal kidney tissue (normal tissue), and renal papillary crystals with clear interstitial localization, as illustrated in A (dashed lines), from THP knockout mice (THP KO). The spectrum of interstitial crystals in the THP KO mice matched the hydroxyapatite standard.
Stromal responses to hydroxyapatite crystal deposition.
To assess whether the pathological accumulation of hydroxyapatite crystals in the renal papillary interstitia could elicit any stromal responses, we studied the association of interstitial crystals with the status of osteopontin, type IV collagen, and resident and activated macrophages (Fig. 3). Since the same von Kossa-stained sections could not be used again for antibody staining and vice versa, we employed consecutive kidney sections, one for von Kossa staining and an adjacent one for antibody staining. We found intense osteopontin staining primarily in the center of the crystal aggregates, with surrounding collecting tubular cells being completely negative (Fig. 3B). Compared with the wild-type mice (Fig. 3F), THP−/− mice had significantly increased accumulation of type IV collagen in the basement zone (Fig. 3E) that contained hydroxyapatite crystals (Fig. 3D). Surprisingly, while antibodies against mouse resident and activated macrophages specifically labeled cells in the positive control tissues (spleens from the wild-type mice; Fig. 3, I and L), the antibodies did not recognize any cells adjacent to the interstitial crystals (Fig. 3, H and K).
Fig. 3.
Stromal responses to interstitial hydroxyapatite deposition. Potential association of osteopontin, type IV collagen, and resident and activated macrophages was studied using immunohistochemical staining. A–C: consecutive sections from a THP−/− mouse were stained with von Kossa method (A) or a rabbit anti-osteopontin antibody followed by a secondary, goat-anti-rabbit antibody conjugated with horseradish peroxidase (B), or a nonimmune rabbit IgG followed by a secondary goat-anti-rabbit antibody conjugated with horseradish peroxidase as a negative control (C). Note that osteopontin (dark brown), which is normally absent from the renal papillary parenchyma, was intermixed with the interstitial hydroxyapatite crystals (B). D and E: consecutive sections from a THP−/− mouse were stained with the von Kossa method (D) or immunohistochemically stained with an anti-type IV collagen antibody (E). Note that, compared with the wild-type mouse (F), type IV collagen was significantly elevated in the widened basement membrane zone underlying the collecting tubules in the THP KO mouse (E). G and H: consecutive sections from a THP−/− mouse stained with the von Kossa method (G) or an antibody specific for resident macrophages (H). Note that, while the antibody stained strongly resident macrophages in the spleen used as a positive control (I), it did not stain any cells surrounding the hydroxyapatite crystals (compare G and H). J and K: consecutive sections from a THP−/− mouse stained with the von Kossa method (J) or an antibody specific for activated macrophages (K). Note that, while the antibody stained strongly activated macrophages in the spleen used as a positive control (L), it did not stain any cells surrounding the hydroxyapatite crystals (compare J and K).
Ultrastructure of renal papillary crystals.
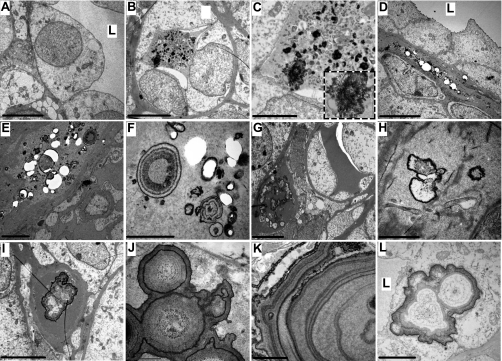
In support of the light microscopic data, transmission electron microscopy (TEM) detected intratubular and interstitial crystals in the THP−/− mice (Fig. 4). Intratubular crystals varied in size and shape and were mixed with noncrystal, amorphous materials and cell debris (Fig. 4, B and C). The intratubular crystals seemed to contain primarily electron-dense mineral-type materials (Fig. 4C, inset). No crystals were found within the collecting tubular epithelial cells (Fig. 4, B–D). A considerably widened basement membrane zone that contained numerous crystals was consistently observed, in some cases partially dislodged during sectioning, leaving many hollow circles (Fig. 4, D–F). In well-preserved areas and under higher magnification, high-density crystals exhibited ring- or tree-trunk-like structures, interspersed with less-dense areas, presumably consisting of proteinaceous materials (Fig. 4, E, F, J, and K). Other crystals contained fewer rings and more low-density areas (Fig. 4, G–I and L). In general, the core of both crystal types contained less density than their outer rings. In some instances, large crystals appeared attached to the apical surface of the tubular epithelial cells with portions of the epithelial cell migrating over to cover the crystals (Fig. 4L).
Fig. 4.
Ultrastructural studies of renal calcium deposits by transmission electron microscopy (TEM). The renal papillary regions of kidneys from wild-type (A) and THP−/− mice (B–L) were subjected to TEM using 2 different processing methods (A, D–F, H, and J from method 1; B, C, G, I, K, and L from method 2; see methods for details). In general, method 2 showed better crystal preservation than method 1. Whereas the wild-type mouse was devoid of renal crystals (A), THP−/− mice harbored various forms of intratubular (B and C) and interstitial crystals (D–K). The majority of the crystals were located in the widened basement membrane zone (D–I). The presence of onion-ring or tree-trunk crystals was the most often observed (E, F, J, and K). Occasional epithelial migration over the luminal crystals was also present (L). L, tubular lumen. Bars in A–L represent 10, 25, 10, 5, 2, 1, 30, 1, 25, 1, 5.5, and 20 μm, respectively.
Ureteral stone formation and hydronephrosis in aged THP−/− mice.
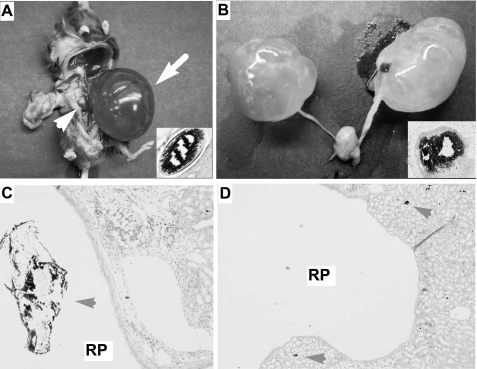
In addition to the microscopic renal calcification described above, a significant portion (10–60% depending on age) of our THP+/− and THP−/− mice exhibited varying degrees of hydronephrosis upon necropsy (Table 1; Fig. 5). Both unilateral (Fig. 5, A and B) and bilateral (Fig. 5, B and D) hydronephrosis were observed. More severe hydronephrosis and a higher frequency of this abnormality were invariably associated with aged mice (Table 1). Serial sections of the ureters and kidneys from these hydronephrotic mice revealed stone-like structures in the ureter (Fig. 5, A and B, inset) and renal pelvis (Fig. 5C) in a great majority of these mice, suggesting stone-mediated blockage of urine flow as the cause of hydronephrosis.
Fig. 5.
Ureteral stone formation and hydronephrosis in THP−/− mice. A: severe unilateral hydronephrosis and obstructive ureteral stone in a 15-mo-old male THP−/− mouse. B: severe bilateral hydronephrosis and ureteral stone in a 10-mo-old male THP−/− mouse. C: von Kossa staining showing a dislodged stone (with loss of mineral materials during specimen processing/sectioning/staining) in the renal pelvic cavity of a THP−/− mouse. D: von Kossa staining showing highly expanded renal pelvic cavity with small deposits in the papilla (arrowheads). RP, renal pelvic cavity. Magnification: C and D, ×50.
Supersaturation of calcium phosphate, but not calcium oxalate, in THP−/− mice.
To begin to understand the pathophysiological basis of the intrarenal calcification in THP−/− mice, we compared key urine indices of the knockout mice with those of wild-type controls. Twenty-four-hour urine samples were collected from 5- to 8-mo-old mice in metabolic cages. Compared with the wild-type controls, the urine of THP knockout mice had a significantly lower 24-h volume (Table. 2). Most urine constituents in the THP−/− mice were also more concentrated than those in the wild-type mice. All solutes were affected, with the concentrations of Cl, K, Ca, Mg, P, uric acid, creatinine, and ammonium nearly doubled in the THP−/− mice. Perhaps not surprisingly, urine pH was also significantly lower in the THP−/− mice. Against the general trend toward increased concentration, the concentrations of organic anions such as citrate and oxalate were actually slightly lower in the THP−/− mice compared with the wild-type mice (Table 2). Interestingly, brushite, a common component in the nucleus of human idiopathic kidney stones, was significantly supersaturated in the THP−/− mouse urine. However, another common human stone component, calcium oxalate, was not significantly supersaturated in the THP−/− mice. Finally, THP−/− mice were significantly supersaturated for uric acid and sodium urate. While the underlying physiologic reasons for these alterations in the THP−/− mice are not readily apparent and need further investigation, the selective supersaturation for brushite but not calcium oxalate likely influenced the type of crystallization that the THP−/− mice develop.
Table 2.
Major urinary indices in THP−/− mice
| WT | KO | |
|---|---|---|
| Volume, μl | 1,840 ± 619 | 657 ± 266* |
| pH | 6.4 ± 0.2 | 6.0 ± 0.2* |
| Na, meq/l | 91.7 ± 20.1 | 153.3 ± 56.4* |
| Cl, meq/l | 126.2 ± 40.6 | 239.9 ± 92.9* |
| K, meq/l | 148.8 ± 52.2 | 330.1 ± 121.5* |
| Ca, mg/dl | 5.4 ± 3.5 | 10.0 ± 5.3* |
| Mg, mg/dl | 18.1 ± 10.0 | 58.0 ± 20.9* |
| P, mg/dl | 155.9 ± 54.3 | 354.9 ± 140.8* |
| Citrate, mg/dl | 46.4 ± 32.4 | 35.8 ± 24.5 |
| Ox, mg/dl | 3.9 ± 1.2 | 3.6 ± 0.9 |
| UA, mg/dl | 10.2 ± 7.3 | 17.0 ± 6.5* |
| Creatinine, mg/dl | 25.5 ± 4.9 | 49.8 ± 17.6* |
| Ammonium, mmol/l | 30.0 ± 11.8 | 82.2 ± 105.3 |
| CaOx SS (DG) | 0.41 ± 1.09 | 0.63 ± 0.99 |
| BR SS (DG) | −0.24 ± 1.09 | 1.00 ± 1.15* |
| UA SS (DG) | −7.24 ± 6.01 | −1.29 ± 1.45* |
| NaUr SS (DG) | −1.60 ± 2.73 | 0.89 ± 1.01* |
Values are means ± SD. KO, knockout; CaOx SS, calcium oxalate supersaturation; BR, brushite; UA, uric acid. Urinalysis was performed on 24-h urine samples collected using metabolic cages from 5-to 8-mo-old WT mice (n = 19) and THP KO mice (n = 19) both in 129/SvEv background.
P < 0.01.
DISCUSSION
Intrarenal calcification: a consistent phenotype of THP-null mice.
Through the systematic dissection of a cohort of >250 mice, we provide strong evidence indicating that intrarenal calcification is a reproducible phenotype in mice deficient for THP. Compared with the previous two small cohorts where ∼16% of the 2- to 3-mo-old male mice harbored spontaneous renal crystals (42, 43), the present cohort shows a clear increase in frequency and severity of renal calcification with increasing age, to the extent that by 15 mo >85% of our THP-null mice harbor spontaneous renal crystals (Table 1). Genetic background, on the other hand, does not seem to play a principal role, because THP-null mice maintained in both 129/SvEv and C57BL/6 backgrounds are equally susceptible to spontaneous crystallization. The same holds true for different genders, as the rate of renal crystal formation in male and female mice is comparable in most age groups (Table 1). Finally, mice heterozygous for the THP gene also develop spontaneous renal crystals, albeit at a lower frequency, suggesting that even a reduced amount of THP predisposes mice to renal calcification. The reduced expression of THP is particularly relevant to human conditions such as type I diabetes, acute and chronic renal insufficiency, kidney transplantation, hyperprostaglandin E syndrome, and active lupus nephritis (41, 50, 52, 55). Some of these conditions are known to be associated with an increased risk of renal stones. Taken together, the data presented here provide new information that extends well beyond what we described before, and they reveal a hitherto unrecognized progressive feature of renal calcinosis during THP deficiency.
Intratubular vs. interstitial crystals.
Another interesting finding with implications regarding the pathogenesis of nephrolithiasis is the location of the renal crystals that were restricted to the renal papillae, with the cortex and outer medulla being devoid of crystals (Fig. 1). Light and electron microscopy further showed the existence of intratubular as well as interstitial crystals (Figs. 1 and 4), two different locations that may have entirely different pathological consequences. On the one hand, intratubular microcrystals can readily pass through the renal tubules and be eliminated from the urinary system, thus posing minimal long-term risk to stone formation. In fact, the formation of intratubular crystals is often regarded as a host defense to reduce the mineral salt concentration and supersaturation of the urine (33, 35). Interstitial microcrystals (Figs. 1 and 4), on the other hand, are conceivably much more difficult to eradicate, and their clearance may have to rely on inflammatory cells such as macrophages (27). However, in this model, we demonstrated a lack of macrophage infiltration in the vicinity of the interstitial crystals (Fig. 3). This could explain, at least in part, why the interstitial crystals in our THP-null mice not only persist but also worsen with the increasing age.
Although intratubular crystal formation in our THP-null mice may be caused by the supersaturation of the urine with mineral salts, particularly CaP (Table 2), the origin and mechanism of interstitial crystal formation remain unclear. One scenario is that interstitial crystals are derived from intratubular ones that have been internalized into the cytoplasm of the tubular cells and later transported out of the cells to the interstitium (35). Cells that have internalized crystals could also undergo apoptosis, exposing the basement membrane to urinary constituents and leading to crystal deposition. However, this scenario is not supported by our TEM studies, which failed to detect any “intermediate-stage,” intracellular crystals (Fig. 4). Another scenario is that the intratubular microcrystals could be directly transported to the interstitium via the intercellular route. Finally, one cannot exclude the possibility that urinary supersaturation of CaP leads to interstitial supersaturation and direct nucleation there. Clearly, additional investigation is needed to pinpoint how crystals form in the interstitial space, as these studies may help shed light on the earliest stages of human nephrolithiasis.
It should be noted that the interstitial crystals in our THP-null mice bear a close resemblance to features observed in human kidneys harboring idiopathic calcium oxalate stones. The interstitial crystals in humans are also located at the papilla and deposited primarily in the widened basement membrane zone enriched with collagen (11, 13, 40). They also are composed of CaP, are mixed with osteopontin, and are not accompanied by inflammatory cell infiltration. These CaP crystals are thought to initially develop in the basement membrane of the thin limb and then slowly extend through the interstitium to the urothelial surface of the renal pelvis, forming so-called Randall's plaque over which CaOx crystals grow into stones (19). Aged THP-null mice do sometimes exhibit stones in the renal pelvis and proximal ureter (Fig. 5), the later of which might have been dislodged from the renal pelvis. Due to the extremely small size of the mouse renal pelvis, we currently do not know whether THP-null mice develop pathology equivalent to human Randall's plaques. More careful dissection studies are needed to clarify this issue, and human-relevant mouse models can provide an excellent in vivo platform to facilitate this process.
THP mutation vs. THP knockout.
It was recently discovered that germline mutations of the THP gene can cause familial, juvenile-onset kidney diseases such as hyperuricemic nephropathy, medullary cystic disease, and glomerulocystic disease (10, 15, 51, 59). However, these conditions are not known to be associated with an increased risk of CaP stone formation. Conversely, we have not observed hyperuricemia or medullary cystic changes found in patients with THP mutations in our THP-null mice (data not shown). The absence of hyperuricemia is perhaps not surprising as mouse liver expresses the uric acid-degrading enzyme uricase, which lost its activity during human evolution (63). Therefore, serum uric acid would not be expected to rise in the THP-null mice even if renal defects exist that increase uric acid reabsorption and/or prevent renal uric acid excretion (12). An alternative explanation for the lack of hyperuricemia in the THP-null mice is that THP mutations may exert somewhat different effects on the TAL cells than the loss of THP. As has been shown both in vitro and in vivo, THP mutations cause THP misfolding and entrapment in the endoplasmic reticulum (ER) (9, 58). Like other ER-stress/overload disorders, the retention of a mutated protein can adversely affect the synthesis of other cellular proteins in the affected cells, thus causing broad functional impairment (49, 62). It is conceivable that loss of THP would not lead to ER stress or have the same cellular effects as THP mutations. The total lack of THP in the TAL could potentially mediate the apparent differences in urinary solute concentrations in the knockout mice, although more experiments will need to be performed to rigorously test this hypothesis.
Divergence of the two existing THP knockout models.
Given the reproducibility of our THP-null mice in spontaneous renal crystallization, it is puzzling why another THP knockout model developed by Bates and colleagues (8, 47) is crystal free even in aged animals. We demonstrated in the present study that THP ablation in both 129/SvEv and C57BL/6 backgrounds resulted in renal calcification (Table 1). Nevertheless, we cannot rule out the possibility that, due to the complicated cross-breeding scheme, the Bates model is dissimilar to either of the aforementioned backgrounds. As has been documented in numerous knockout studies, including those involving genes affecting nephrolithiasis, genetic background can play a major role in phenotypic variation (16, 25, 37, 56). Difference in targeting strategies could be a key contributing factor as well. The Bates knockout model was generated using the so-called “insertion strategy,” where a neo gene was inserted into exon 3 of the THP gene to disrupt THP expression (8). This strategy is sometimes associated with partial expression of a gene fragment preceding the neo-bearing exon, and/or exon (neo gene) skipping, leading to leakage expression (14, 17, 26). Residual THP expression was indeed noted by in situ hybridization in a previous publication using the Bates model (7). In comparison, our model was generated using a “replacement strategy,” where a THP gene fragment encompassing a 650-bp proximal promoter and exons 1–4 were deleted (44). Due to the deletion of the THP promoter, no residual THP expression was present in our null mice, as evidenced by Northern blotting, in situ hybridization, RT-PCR, real-time quantitative PCR, Western blotting, and polyclonal antibody staining (42–44). The divergent phenotypes of the two existing THP knockout models are intriguing and present a unique opportunity for comparative studies, which may offer insights into the bases of the phenotypic divergence and yield useful clues on the molecular mechanisms of renal calcification.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK56903 (to X.-R. Wu) and the Mayo Clinic O'Brien Urology Research Center (NIDDK Grant P50 DK083007 to J. C. Lieske).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Alpay H, Ozen A, Gokce I, Biyikli N. Clinical and metabolic features of urolithiasis and microlithiasis in children. Pediatr Nephrol 24: 2203–2209, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Asplin JR. Obesity and urolithiasis. Adv Chronic Kidney Dis 16: 11–20, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Asplin JR, Arsenault D, Parks JH, Coe FL, Hoyer JR. Contribution of human uropontin to inhibition of calcium oxalate crystallization. Kidney Int 53: 194–199, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Asplin JR, Parks JH, Coe FL. Dependence of upper limit of metastability on supersaturation in nephrolithiasis. Kidney Int 52: 1602–1608, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Asplin JR, Parks JH, Nakagawa Y, Coe FL. Reduced crystallization inhibition by urine from women with nephrolithiasis. Kidney Int 61: 1821–1829, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Assimos DG, Holmes RP. Role of diet in the therapy of urolithiasis. Urol Clin North Am 27: 255–268, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65: 791–797, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bernascone I, Vavassori S, Di Pentima A, Santambrogio S, Lamorte G, Amoroso A, Scolari F, Ghiggeri GM, Casari G, Polishchuk R, Rampoldi L. Defective intracellular trafficking of uromodulin mutant isoforms. Traffic 7: 1567–1579, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Bleyer AJ, Trachtman H, Sandhu J, Gorry MC, Hart TC. Renal manifestations of a mutation in the uromodulin (Tamm Horsfall protein) gene. Am J Kidney Dis 42: E20–E26, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bushinsky DA. Nephrolithiasis: site of the initial solid phase. J Clin Invest 111: 602–605, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron MA, Sakhaee K. Uric acid nephrolithiasis. Urol Clin North Am 34: 335–346, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 115: 2598–2608, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet 17: 346–349, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Devuyst O, Dahan K, Pirson Y. Tamm-Horsfall protein or uromodulin: new ideas about an old molecule. Nephrol Dial Transplant 20: 1290–1294, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Doetschman T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol 530: 423–433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorin JR, Stevenson BJ, Fleming S, Alton EW, Dickinson P, Porteous DJ. Long-term survival of the exon 10 insertional cystic fibrosis mutant mouse is a consequence of low level residual wild-type Cftr gene expression. Mamm Genome 5: 465–472, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Dussol B, Berland Y. Urinary kidney stone inhibitors. What is the news? Urol Int 60: 69–73, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Evan A, Lingeman J, Coe FL, Worcester E. Randall's plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int 69: 1313–1318, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Grover PK, Ryall RL, Marshall VR. Does Tamm-Horsfall mucoprotein inhibit or promote calcium oxalate crystallization in human urine? Clin Chim Acta 190: 223–238, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Hallson PC, Choong SK, Kasidas GP, Samuell CT. Effects of Tamm-Horsfall protein with normal and reduced sialic acid content upon the crystallization of calcium phosphate and calcium oxalate in human urine. Br J Urol 80: 533–538, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Hess B. The role of Tamm-Horsfall glycoprotein and nephrocalcin in calcium oxalate monohydrate crystallization processes. Scanning Microsc 5: 689–695, 1991 [PubMed] [Google Scholar]
- 23.Hess B. Tamm-Horsfall glycoprotein and calcium nephrolithiasis. Miner Electrolyte Metab 20: 393–398, 1994 [PubMed] [Google Scholar]
- 24.Holmes RP, Assimos DG, Goodman HO. Molecular basis of inherited renal lithiasis. Curr Opin Urol 8: 315–319, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Joyner AL. Gene Targeting: A Practical Approach. Oxford, UK: Oxford Univ. Press, 1993 [Google Scholar]
- 27.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol 8: 75–88, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Khan SR, Canales BK. Genetic basis of renal cellular dysfunction and the formation of kidney stones. Urol Res 37: 169–180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci 9: 1450–1482, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kleinman JG, Wesson JA, Hughes J. Osteopontin and calcium stone formation. Nephron Physiol 98: 43–47, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Knorle R, Schnierle P, Koch A, Buchholz NP, Hering F, Seiler H, Ackermann T, Rutishauser G. Tamm-Horsfall glycoprotein: role in inhibition and promotion of renal calcium oxalate stone formation studied with Fourier-transform infrared spectroscopy. Clin Chem 40: 1739–1743, 1994 [PubMed] [Google Scholar]
- 32.Kokot F, Dulawa J. Tamm-Horsfall protein updated. Nephron 85: 97–102, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Koul HK, Koul S, Fu S, Santosham V, Seikhon A, Menon M. Oxalate: from crystal formation to crystal retention. J Am Soc Nephrol 10, Suppl 14: S417–S421, 1999 [PubMed] [Google Scholar]
- 34.Kumar S, Muchmore A. Tamm-Horsfall protein—uromodulin (1950–1990). Kidney Int 37: 1395–1401, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens 15: 374–380, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Liebman SE, Taylor JG, Bushinsky DA. Idiopathic hypercalciuria. Curr Rheumatol Rep 8: 70–75, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Linder CC. Genetic variables that influence phenotype. Ilar J 47: 132–140, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Mandel N. Mechanism of stone formation. Semin Nephrol 16: 364–374, 1996 [PubMed] [Google Scholar]
- 39.Marangella M, Vitale C, Petrarulo M, Bagnis C, Bruno M, Ramello A. Renal stones: from metabolic to physicochemical abnormalities. How useful are inhibitors? J Nephrol 13, Suppl 3: S51–S60, 2000 [PubMed] [Google Scholar]
- 40.Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall's plaques in the pathogenesis of calcium stones. J Urol 177: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin PJ, Aikawa A, Davies HM, Ward RG, Bakran A, Sells RA, Johnson PM. Uromodulin levels are decreased in urine during acute tubular necrosis but not during immune rejection after renal transplantation. Clin Sci (Lond) 84: 243–246, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66: 1159–1166, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Mo L, Liaw L, Evan AP, Sommer AJ, Lieske JC, Wu XR. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol Renal Physiol 293: F1935–F1943, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Moe OW. Kidney stones: pathophysiology and medical management. Lancet 367: 333–344, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Pourmand G, Nasseh H, Sarrafnejad A, Mojtahedi A, Mehrsai A, Alamdari DH, Nourijelyani K. Comparison of urinary proteins in calcium stone formers and healthy individuals: a case-control study. Urol Int 76: 163–168, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Raffi H, Bates JM, Laszik Z, Kumar S. Tamm-Horsfall protein knockout mice do not develop medullary cystic kidney disease. Kidney Int 69: 1914–1915, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Romero MC, Nocera S, Nesse AB. Decreased Tamm-Horsfall protein in lithiasic patients. Clin Biochem 30: 63–67, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Schroter J, Timmermans G, Seyberth HW, Greven J, Bachmann S. Marked reduction of Tamm-Horsfall protein synthesis in hyperprostaglandin E-syndrome. Kidney Int 44: 401–410, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Scolari F, Caridi G, Rampoldi L, Tardanico R, Izzi C, Pirulli D, Amoroso A, Casari G, Ghiggeri GM. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis 44: 987–999, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Sejdiu I, Torffvit O. Decreased urinary concentration of Tamm-Horsfall protein is associated with development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetic patients. Scand J Urol Nephrol 42: 168–174, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Serafini-Cessi F, Monti A, Cavallone D. N-glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J 22: 383–394, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron 85: 207–214, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Werness PG, Brown CM, Smith LH, Finlayson B. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 58.Williams SE, Reed AA, Galvanovskis J, Antignac C, Goodship T, Karet FE, Kotanko P, Lhotta K, Moriniere V, Williams P, Wong W, Rorsman P, Thakker RV. Uromodulin mutations causing familial juvenile hyperuricaemic nephropathy lead to protein maturation defects and retention in the endoplasmic reticulum. Hum Mol Genet 18: 2963–2974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf MT, Hoskins BE, Beck BB, Hoppe B, Tasic V, Otto EA, Hildebrandt F. Mutation analysis of the uromodulin gene in 96 individuals with urinary tract anomalies (CAKUT). Pediatr Nephrol 24: 55–60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Worcester EM. Inhibitors of stone formation. Semin Nephrol 16: 474–486, 1996 [PubMed] [Google Scholar]
- 61.Worcester EM, Coe FL. Nephrolithiasis. Prim Care 35: 369–391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Worcester EM, Parks JH, Evan AP, Coe FL. Renal function in patients with nephrolithiasis. J Urol 176: 600–603, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C, Jr, Jones P, Bradley A, Caskey CT. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci USA 91: 742–746, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]