Abstract
Atheroprone regions of the arterial circulation are characterized by time-varying, reversing, and oscillatory wall shear stress. Several in vivo and in vitro studies have demonstrated that flow reversal (retrograde flow) is atherogenic and proinflammatory. The molecular and structural basis for the sensitivity of the endothelium to flow direction, however, has yet to be determined. It has been hypothesized that the ability to sense flow direction is dependent on the direction of inclination of the interendothelial junction. Immunostaining of the mouse aorta revealed an inclination of the cell-cell junction by 13° in direction of flow in the descending aorta where flow is unidirectional. In contrast, polygonal cells of the inner curvature where flow is disturbed did not have any preferential inclination. Using a membrane specific dye, the angle of inclination of the junction was dynamically monitored using live cell confocal microscopy in confluent human endothelial cell monolayers. Upon application of shear the junctions began inclining within minutes to a final angle of 10° in direction of flow. Retrograde flow led to a reversal of junctional inclination. Flow-induced junctional inclination was shown to be independent of the cytoskeleton or glycocalyx. Additionally, within seconds, retrograde flow led to significantly higher intracellular calcium responses than orthograde flow. Together, these results show for the first time that the endothelial intercellular junction inclination is dynamically responsive to flow direction and confers the ability to endothelial cells to rapidly sense and adapt to flow direction.
Keywords: shear stress, endothelium, mechanotransduction
the endothelium lining arteries plays a primary role in vascular health and disease. It is often seen as a continuum of neighboring endothelial cells (ECs) whose geometric shape and cellular responses quickly adapt to changes in external forces such as blood flow. Detailed analyses of fluid mechanics in atherosclerosis-prone regions of the vasculature reveal a strong correlation between EC morphology, proinflammatory activation, and areas of disturbed hemodynamic profiles (6, 24, 26, 34).
The physical properties of the endothelial plasma membrane itself change under flow (3, 4, 16–19, 38). Indeed, it was previously demonstrated that upon application of shear stress, endothelial membrane fluidity instantaneously increases. Changes in membrane fluidity have been shown to affect membrane enzyme activity through increases in membrane free volume (38). Similarly, increases in membrane fluidity using membrane fluidizing agents are able to simulate the shear-induced activation of G proteins (15), ERK1/2 (4), and the bradykinin receptor 2 in ECs (5).
Significant EC morphological adaptations to shear stress include elongation and orientation in the direction of flow [“alignment” (10, 11)], driven by cytoskeletal restructuring (20, 30), redistribution of focal adhesions and microtubule organization [planar cell polarity (27, 36)], and partial disassembly of the adherens junctions (30). The cell alignment process occurs over a 24-h time frame and is adaptive such that there is a reduction of spatial fluctuations in shear stress to which cells are exposed (2). However, the mechanisms by which ECs initially sense flow direction remain unclear.
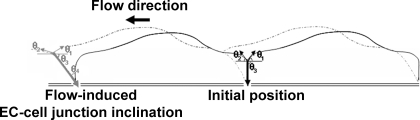
Fung and Liu demonstrated that ECs undergo another structural adaptation distinct from flow-induced alignment: they provided both a theoretical framework and supporting experimental evidence that fluid shear stress imposes tension on the EC membrane (13, 25). Their model showed that tension develops and propagates along an endothelial monolayer. The model predicts that there is increasing tension from the downstream side of a cell to the upstream junction, such that the membrane tension at the cell-cell junction is the highest. In fluctuating flows such as oscillatory flow and turbulent flow, the tension similarly oscillates, producing high rates of change of tension. In addition, it is shown that membrane tension can be propagated to an adjoining upstream cell by transmission of tension at the cell-cell junction. The degree of tension propagation is a function of the angle of inclination of the cell-cell junction relative to the underlying substrate on which the cells are attached.
Liu et al. (25) measured the angles of incidence of the membranes at the top of the cell-cell junction under flow using interference microscopy and observed that ECs adapt to steady flow within minutes by increasing the slope of the trailing-edge membrane (θ1) and decreasing the slope of the leading-edge membrane (θ2) at the top of the junction (Fig. 1). When flow was reversed, these angles reversed within minutes. However, the study was limited to surface geometry and did not measure the more important angle of inclination (θ3, hence the complementary angle θ4) and therefore their study could not conclude if the flow adaptation reduced or increased the propagation of tension from cell to cell.
Fig. 1.
Schematic of the flow-induced inclination hypothesis of the endothelial cell (EC)-cell junction in the direction of flow. Liu et al. (25) measured an increase in the slope of the trailing-edge membrane (θ1) and decrease in the slope of the leading-edge membrane (θ2) at the top of the junction after initiation of flow (gray vs. versus black: flow-unadapted condition). However, their study was limited to surface topology. Here, it was hypothesized that ECs also adapt to steady flow by inclination of their cell-cell junction (θ3, or the complementary angle θ4) to reduce the propagation of tension from cell to cell.
Here, it is hypothesized that shear stress rapidly induces EC-cell junction inclination in direction of flow and that junctional inclination has a profound role in modulating the mechanical stimulus in mechanotransduction, conferring the ability of the endothelial monolayer to sense flow reversal on a time scale of seconds. It is demonstrated for the first time the existence of a flow-oriented inclination in aortic ECs whose increased inclination is directly related to regions of increased unidirectional laminar flow in vivo. Moreover, ECs with junctions inclined in direction of flow had lower intracellular calcium responses than ECs with junctions inclined in the opposite direction, thereby showing that cellular responses are directly correlated to mechanoadaptation. Lastly, it is suggested that the remodeling of the EC-cell junctional geometry with flow-oriented inclination may represent the mechanism by which ECs adapt to flow with reduced mechanosensitivity.
MATERIALS AND METHODS
Animals.
All animal procedures were approved by the Institutional Animal Care and Use Committee of La Jolla Bioengineering Institute (assurance no. A3432), which is authorized by the Office of Laboratory Animal Welfare of the National Institutes of Health, as required by the Health Resource Extension Act of 1985. Six C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used for determination of the EC-cell junction inclination angle determination in the inner curvature and descending aorta. Mice were anesthetized with a cocktail of 90 mg/kg ketamine and 10 mg/kg xylazine administered intraperitoneally and were then perfused intracardially with 15 ml of 4% paraformaldehyde and 15% picric acid solution in 0.1 M phosphate buffer, pH 7.6. Aorta were then dissected under a stereoscopic binocular microscope from the aortic sinus to the renal artery bifurcation, cleaned of any fat residues, and cut longitudinally for further staining and en face microscopy.
Cell cultures and treatments.
Primary human umbilical vein EC (HUVEC) isolation was performed as previously described (12). Human umbilical cords were obtained from Sharp Memorial Hospital (San Diego, CA) under the auspices of Sharp Healthcare Institutional Review Board protocol no. 011081. Cells were seeded into 75-cm2 tissue culture-treated flasks and grown to confluence within a week in M199 media (Irvine Scientific, Santa Ana, CA). After detachment with trypsin, passage 1 cells were either seeded for at least 3 days on glass slides for flow experiments or on fibronectin-coated glass coverslips for live cell imaging. All cultures were studied as confluent monolayers of polygonal cells. Before all experimental procedures, the HUVECs were serum starved overnight in ATP-free media supplemented with 1% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO) to establish quiescence in the monolayer. In one study, HUVEC monolayers were pretreated with 50 μg/ml heparinase III (Hep, Sigma-Aldrich), 0.1 μM cytochalasin D (CD; Sigma-Aldrich), 50 μM blebbistatin (Bleb; Sigma-Aldrich), or 5 μM Taxol (Calbiochem, San Diego, CA) for 3 h, 2 min, 30 min, and 2 h, respectively. Removal of heparan sulfate by Hep was controlled by Western blot using an anti-heparan sulfate antibody (Seikagaku, Japan) using methods previously described (31). Cytoskeleton integrity was verified by immunocytochemistry as described below using antibodies against myosin IIA (Cell Signaling Technology, Danvers, MA), acetylated-tubulin (Sigma-Aldrich), and the F-actin probe phalloidin (Invitrogen, Carlsbad, CA).
For EC-cell junction inclination angle examination in live cells, HUVEC confluent monolayers were dyed with 10 μg/ml of the membrane stain Orange CellMask (Invitrogen) for 2 min on ice, followed by two quick rinses, and immediately processed for live cell flow imaging. Control experiments showed the CellMask stain remains in the membrane for at least 45 min before showing significant signs of endocytosis. For calcium studies, cells were incubated with 15 ng/ml Fluo-4 AM for 1 h supplemented with 2.5 mM probenicid, 20 mM HEPES, 0.042% pluronic F-127, and 0.1% BSA to facilitate the calcium indicator loading (all from Invitrogen unless specified). Cells were then rinsed and incubated at 33°C for 30 min for equilibration in ATP-free media before mounting slides onto the flow chamber.
Shear stress exposure.
HUVEC monolayers seeded on glass slides were used in a conventional parallel plate flow chamber where cells were subjected to a steady 16 dyn/cm2 fluid shear stress provided by a flow loop system maintained at 37°C and ventilated with 95% humidified air with 5% CO2 (12). Immediately after flow, monolayers were then fixed in 4% freshly made paraformaldehyde and processed for further immunostaining.
For live cell imaging and calcium studies, experiments were performed by using a parallel plate flow microchamber that allows exposure of HUVECs to variable values of shear stress in a flow channel (2 mm wide) that is optically accessible through a coverslip-based window. The temperature of the microchamber was maintained at 37°C, and a syringe pump delivered orthograde or retrograde flow rate via a computer-controlled PHD 2000 syringe pump (Harvard Apparatus, Holliston, MA) holding a 100-ml CO2 gas tight prewarmed syringe (SGE Analytical Science, Australia). The design of the microchamber ensured that there was no deformation of the glass coverslip (axial displacement was <0.05 μm) caused by flow-induced hydrostatic pressure (5). For calcium studies, the preconditioning orthograde flow consisted of a 5-min slowly ramped up flow to 5 dyn/cm2, followed by a 20-min steady flow period at 5 dyn/cm2, then a 5-min ramped down flow before cells were exposed to a 1-min step flow of similar magnitude. Sham controls were not exposed to the last minute step but let sit at 37°C instead. Static control slides were only removed from the incubator immediately before fixation and staining.
En face immunohisto- and cytochemistry and image processing.
Longitudinally opened aortas were washed in phosphate-buffered saline, permeabilized in 0.3% Triton X-100 for 20 min, blocked for at least 1 h with 5% normal goat serum and 1% BSA, and incubated overnight with a rat anti-mouse platelet endothelial cell adhesion molecule 1 (PECAM-1) antibody (Biolegend, San Diego, CA). For HUVEC monolayers, after fixation, the cells were blocked and permeabilized as described above and incubated with a rabbit anti-human PECAM-1 antibody generated in our institute. Primary antibody incubations were followed by a final incubation step for 1 h with an anti-rat or -rabbit Alexa Fluor 488 antibody (Invitrogen). During mounting procedure in Vectashield (Vector, Burlingame, CA), tissues and cell monolayers were arranged on slides circled with a thin silicon gasket to avoid pressure from the coverslip and geometrical distortion. In some cases, Alexa Fluor 488 phalloidin (Invitrogen) staining was used at 66 nM for 15 min before mounting to visualize actin filament alterations.
Slides were examined under a confocal fluorescent microscope (Zeiss Pascal LSM5, Zeiss, Germany) equipped with a Plan-Apochromatic 63/1.4 objective and a Plan-NEOFLUAR 100/1.3 objective, and Z-stacks were examined using the Zeiss LSM Image Browser. XZ or YZ sections (parallel or perpendicular to the flow direction) were then analyzed and cropped using the three-dimensional Volume Viewer 1.31 plugin (Kai Uwe Barthel, Internationale Medieninformatik, Germany) for ImageJ software (National Institutes of Health, Bethesda, MD), and junctional inclination angles were measured using the ImageJ angle tool. While basal membrane was considered flat in culture cells, the inclination angle was measured between the EC-cell junction and an imaginary basal membrane drawn from the bottom of two EC-cell junctions in in vivo studies.
For calcium studies, picture sequences were acquired every 5 s and the average intensity in each selected cells was calculated using Volocity 5 software (Improvision/Perkin Elmer, Waltham, MA). To control for cell-to-cell variation in dye loading, all fluorescence measurements were expressed as a ratio (F/F0) of dynamic fluorescence intensity (F) to the basal fluorescence intensity measured before each experiment (F0) (21). Internal controls showed there was no photobleaching of the Fluo-4 AM during the time course of our experiments.
Statistical analysis.
Data are expressed as means ± SE, unless otherwise stated, from at least three independent experiments. Statistical comparisons between groups were performed using Student's t-test. A difference of P < 0.05 was judged as significant.
RESULTS
EC-cell junction inclinations in the mouse aorta.
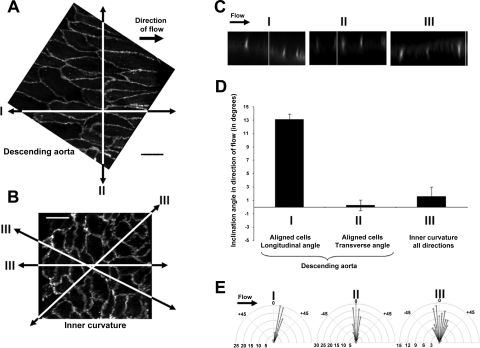
En face PECAM-1 immunostaining was used to observe and confirm previously published differences in three-dimensional morphology between ECs in the descending aorta and the inner curvature. ECs of descending aorta subjected to unidirectional laminar flow were long, fusiform, and aligned in the direction of flow, stretching on average to 57.1 ± 1.1 μm in length (n = 60, Fig. 2A). In contrast, ECs of the inner curvature, a location of oscillatory and reversing hemodynamic forces, displayed a polygonal shape whose average diameter was 22.0 ± 0.5 μm (n = 93, Fig. 2B) at the widest. XZ cross sections of the descending aorta were used to detect the existence of an inclination angle (referred as θ4 in Fig. 1) specifically at the extremities of the fusiform ECs with an average inclination value of 13.1 ± 0.8° in the direction of flow (n = 94, cross sections I, Fig. 2, C and D). YZ cross sections (perpendicular to the flow direction) cutting through side membranes (also referred to as sidewalls) did not show any significant inclination, and the vast majority of the EC-cell junction remained upright with an average of 0.3 ± 0.8° (n = 142, cross sections II, Fig. 2, C and D). In contrast, Z sections of ECs from the inner curvature had no preferred but heterogeneous orientations and angle values of inclination the EC-cell junction averaging 1.6 ± 1.4° of inclination in all directions (n = 105, cross sections III, Fig. 2, C and D). To illustrate this heterogeneity, a distribution of the frequency of the inclination angles is shown on the radial plots in Fig. 2E.
Fig. 2.
EC-cell junctions inclined uniformly in direction of flow in regions of laminar steady flow. A: en face tissue sections of the mouse aorta stained with platelet endothelial cell adhesion molecule 1 (PECAM-1) antibody show alignment of ECs with a fusiform shape in the descending aorta. B: a more disordered morphology (polygonal cells) was observed at the inner curvature of the aorta. Scale bars are 20 μm. C: XZ cross sections of the aortic endothelial layer cut longitudinally along the alignment trajectory showed an inclination of the tip of the EC-cell junction in direction of flow (sections I) as compared with transversal sections (perpendicular to flow, sections II) and arbitrary sections of the inner curvature (sections III). D: bar graph depicts EC-cell junction inclination angle mean values for each group. E: polar plots illustrate the distribution of the frequency of the inclination angles (by increment of 5° angles) for each group.
Flow-induced EC-cell junction inclination in vitro.
PECAM-1 immunostaining was used on confluent HUVECs to clearly delineate the interendothelial cell-cell junction outlining the cells. Static condition cells were of polygonal shape with an average diameter of 23.6 ± 0.6 μm (n = 82) at the widest. Compared with XY scans, confocal microscopy has a lower resolution on the Z scale due to limited optical slice overlaps. Hence an estimation of the height of the EC-cell junction between 3 and 4 μm was approximated. Three-dimensional reconstruction of the PECAM-1 EC-cell junction staining showed that static condition cells display a twisted ribbon-structure surrounding the cell (see Supplemental Material, Supplemental Video S1; Supplemental Material for this article is available online at the Journal website). EC-cell junctions of unsheared cells were not inclined in any preferred direction and the average angle value was 2.2 ± 1.1° (n = 32). Conversely, analysis of XZ sections from cells aligned with the direction of flow indicated an inclination of the EC-cell junction by 9.9 ± 1.3° (n = 32) after being subjected to 16 dyn/cm2 shear stress for 30 min (Fig. 3). The equilibrium angle of inclination was reached within 30 min of flow and did not vary as a function of imposed shear stress (Fig. 3, B and C: 8 dyn/cm2: 10.1 ± 0.9°, n = 73; 32 dyn/cm2: 9.5 ± 1.1°, n = 38). The inclination occurred in the absence of any discernible alignment of the cells in the direction of flow (Fig. 3A).
Fig. 3.
EC-cell junctions inclined after onset of flow in confluent human umbilical vein endothelial cells (HUVECs). A: PECAM-1 staining of HUVECs in unsheared condition (static, left) and after 30 min of a 16 dyn/cm2 steady shear stress (flow, right). EC-cell junction inclinations are shown on XZ cross sections (bottom). Scale bar is 20 μm. B: bar graph depicts EC-cell junction inclination angle mean values for various magnitudes of shear stress in the range of 8–32 dyn/cm2, which did not alter the equilibrium angle of inclination. NS, no significant difference. *P < 0.05. C: polar plots illustrate the distribution of the inclination angles as explained in Fig. 2.
EC-cell junction inclination was not changed following Hep treatment or cytoskeletal disruption.
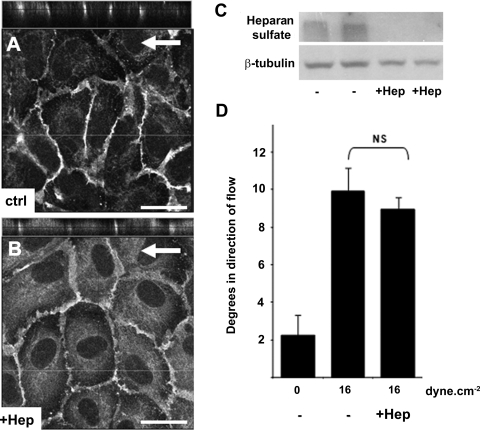
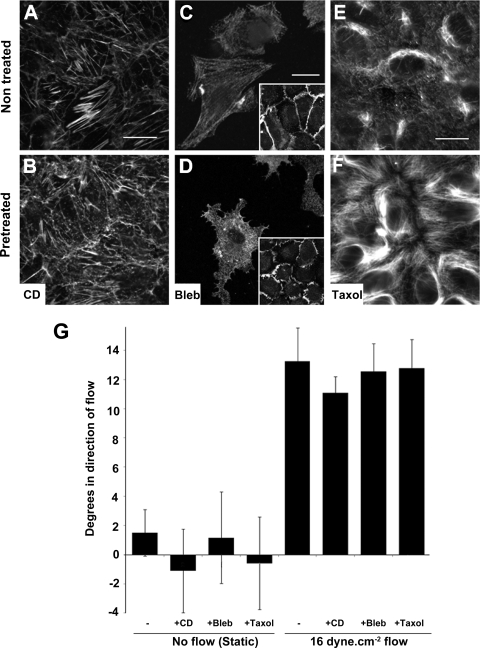
Both the glycocalyx and elements of the cytoskeleton are often described as primary mechanosensors (9, 22, 35). To investigate whether flow-induced junctional inclination requires a functional glycocalyx, HUVECs were pretreated with 50 μg/ml Hep for 3 h to remove glycocalyx-associated heparan sulfate. Hep-pretreated cells subjected to a steady 16 dyn/cm2 flow for 30 min displayed similar EC-cell junctional inclination to untreated control [Fig. 4; angles in degrees in direction of flow: control, 9.9 ± 1.3°, n = 32; Hep, 9.0 ± 0.7°, n = 61; CD, 11.1 ± 1.1°, n = 98]. Similarly, EC-cell junction inclination was investigated following cytoskeletal disruption. HUVECs were pretreated with 0.1 μM CD, 50 μM Bleb, or 5 μM Taxol for 2 min, 30 min, and 2 h, respectively. Disruption of actin, tubulin, and myosin filaments was confirmed by immunostaining (Fig. 5, A–F). None of the cytoskeleton disruptive agents significantly changed the EC-cell junction inclination after 30 min of a steady 16 dyn/cm2 flow as compared with their pretreated counterparts in static condition (Fig. 5G; nontreated static, 1.5 ± 1.6°, n = 42; nontreated flow, 13.3 ± 2.3°, n = 15; CD static, −1.1 ± 2.9°, n = 74; CD flow, 11.1 ± 2.3°, n = 98; Bleb static, 1.2 ± 3.1°, n = 43; Bleb flow, 12.6 ± 1.9°, n = 49; Taxol static, −0.6 ± 3.2°, n = 42; Taxol flow, 12.8 ± 2°, n = 76).
Fig. 4.
Glycocalyx degradation did not alter flow-induced EC-cell junction inclination. HUVECs were subjected to a 16 dyn/cm2 steady shear stress for 30 min after 2-h pretreatment with 50 μg/ml heparinase III (Hep). A and B: PECAM-1 immunostaining with XZ cross sections showing inclination of the EC-cell junction in Hep-pretreated cells and control (Ctrl)-untreated experiment. Scale bars are 20 μm. C: removal of heparan sulfate by Hep treatment was confirmed by Western blot. D: EC-cell junction inclination angles for each condition described above.
Fig. 5.
Flow-induced EC-cell junction inclination was independent of cytoskeletal structure. The cytoskeletal structure was disrupted by pretreatment of HUVEC confluent monolayers with 0.1 μM cytochalasin D (CD), 50 μM blebbistatin (Bleb), or 5 μM Taxol for 2 min, 30 min, and 2 h, respectively. Cytoskeleton disruption was verified by staining for phalloidin (A and B) and myosin IIA (C and D) and Taxol-induced inhibition of depolymerization of microtubules by acetylated-tubulin (E and F). Scale bars are 20 μm. Differences in myosin IIA staining were more detectable when using a lower cell seeding density where cytoplasmic retraction was observed (D). However, no cell retraction was observed on confluent monolayers. A wider PECAM-1 membrane staining including increased number of protusions after blebbistatin pretreatment was noticed (insets). G: steady shear stress of 30 min at 16 dyn/cm2 did not show any significant difference in EC-cell inclination after cytoskeletal disruption.
Retrograde flow induced a rapid reversal of the EC wall inclination.
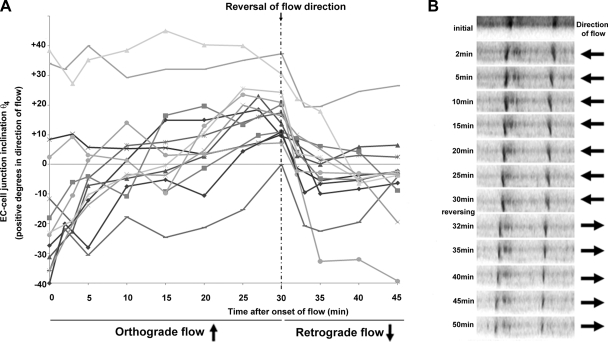
A cell membrane stain was used to observe adaptive changes of EC-cell inclination in live confluent HUVECs subjected to a 16 dyn/cm2 shear stress. Supplemental Fig. S1 illustrates an EC-cell junction, initially in an upright position, subjected to 60 min of steady flow with Z stacks acquired every 2 to 5 min. To investigate the effects of retrograde flow on EC-junctional inclination, naïve HUVEC cultures were preconditioned with 30 min of flow, and then flow was reversed (retrograde, Fig. 6 and Supplemental Video S2). For clarity, a range of 12 different EC-cell junctions is shown with initial inclination from various directions, i.e., in opposite direction to the flow (negative angle values), upright (close to zero values), and already in direction of flow (positive angle values). XZ sections were acquired every 2 to 5 min over a 45-min period. Immediately after onset of flow, cell walls leaning against the flow direction started straightening up and gradually inclined with shear flow. Depending on the value of the initial angle, lateral cell walls required between 5 and 25 min to reach an equilibrium angle of inclination in the direction of flow. EC-cell junctions already inclined in direction of flow did not further incline. After 30 min, cells were subjected to retrograde steady flow of similar shear stress. All cell walls rapidly responded uniformly to the change of shear stress profile by inclining in the opposite direction with an average angular velocity of 4.0 ± 0.4° per minute over the first 5 min of retrograde flow. Most of the EC-cell junctions inclined to a final angle of 10° to 15° within the 15 min of retrograde flow.
Fig. 6.
Dynamic monitoring of the EC-cell junction inclination. A: representation of the inclination angle θ4 of a range of 12 EC-cell junctions initially inclined in opposite direction of flow (θ4 < 0), in upright position, or in direction of flow (θ4 > 0) followed over a time course of 30 min of a 16 dyn/cm2 orthograde flow. After 25 min of shear stress, all junctions were considered as flow adapted because they all inclined in the direction of flow. Step retrograde flow immediately after the 30-min time point led to a uniformly rapid reversal of the junctional inclination. XZ sections were acquired every 2 to 5 min over the 45-min period. B: XZ scans illustrating two EC-cell junctions gradually inclining in direction of flow in the first 30 min then suddenly readapting their position to the new flow direction. A representative video of dynamic inclination is provided in the Supplemental Material (Video S2).
Abrupt changes in flow direction were associated with higher intracellular calcium responses.
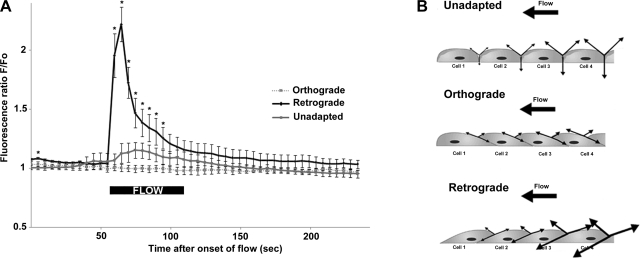
Intracellular calcium (Ca2+i) release is one of the earliest cellular responses to shear stress (38). Therefore, Ca2+i levels were monitored to assess the relative effects of orthograde and retrograde flow on mechanotransduction. Cells were inclined in the direction of flow by subjecting them to a 30-min ramped flow at 5 dyn/cm2. This mild and ramped flow preconditioning did not affect resting Ca2+i levels. A step increase in orthograde flow similarly produced minimal increases in Ca2+i in flow-adapted (orthograde) and unsheared cells (unadapted) (Fig. 7A and Supplemental Video S3). In sharp contrast, retrograde flow induced a dramatic increase in Ca2+i.
Fig. 7.
Step changes of flow direction were associated with higher intracellular calcium (Ca2+i) responses. A: after an initial 30-min period of slowly ramped-up and -down flow, flow-adapted HUVECs were subjected to a 1-min step flow in either forward or reverse direction. Unadapted cells were not subjected to the initial slow pre-shear but were left mounted on the chamber for 30 min with a reduced flow (0.5 dyn/cm2 for 1 min) every 10 min to avoid hypoxia. Sudden step shear of 5 dyn/cm2 minimally stimulated Ca2+i in both unadapted (gray line, n = 24 cells) and preinclined cells (“orthograde,” dashed line, n = 20 cells). In contrast, dramatic Ca2+i increases were observed when flow was reversed (“retrograde,” solid line, n = 24 cells). Values are means ± SD. *Significant differences of Ca2+i responses in retrograde flow condition as compared with both orthograde flow and unadapted (P < 0.05). For all conditions, experiments were replicated five times with different HUVECs. Data were acquired every 5 s over a 4-min period. B: junctional tension in static (unadapted), orthograde, and retrograde flow. A potential explanation for the sudden increase in Ca2+i responses in retrograde flow can be given by the theory of tension force propagation at the membrane first proposed by Fung and Liu (13). Inclination of the cell-cell junction in flow-adapted cells reduces transmission of tension forces, while a sudden retrograde flow dramatically increases membrane tension from cell to cell. This is likely the basis for the increased permeability of the endothelium in regions of oscillatory flow in the vasculature.
DISCUSSION
Using a fluorescent marker for the plasma membrane together with laser-scanning confocal microscopy, we have developed the ability to monitor adaptive changes in the angle of inclination at the EC-cell junction in response to flow. This approach allowed for the first time the observation in confluent endothelial cells of an inclination of the EC-cell junction within seconds reaching a maximal angle of inclination of 10° in the direction of flow within minutes. These measurements correlated with in vivo observation. A 13° EC-cell junction inclination was found in aortic regions subjected to unidirectional flow but not in regions with disturbed and reversing hemodynamic profiles.
The minor difference in inclination angle values between both in vivo and in vitro measurements maybe accounted for the fact that the bottom reference for the basal surface is flat on glass slides while sometimes corrugated in fixed vessels, making measurements more challenging. Alternatively, ECs in vivo have an apical surface topography which is smoother than naïve EC cultures, illustrating their chronic flow-adapted morphology (2, 28).
The kinetics of the flow-induced inclination of the junctional membrane appeared to be similar to that of the incident slopes as previously observed (25), suggesting that these phenomena occur simultaneously. Strong support for the concept that part of the forces induced by flow are propagated as tension in the membrane was provided by Butler and colleagues (4), who measured membrane diffusivity using fluorescence recovery after photobleaching along the length of ECs subjected to step increases in flow. Instantaneous increases in membrane diffusivity on the upstream end of cells relative to the downstream end were noted. Because membrane diffusivity is strongly correlated with membrane fluidity (3, 16), this study showed that shear can induce upstream junctional membrane fluidization and mechanotransduction. Likewise, subcellular differences of shear stress have been detected within minutes after the exposure to flow by observing displacement of intermediate filaments (20). Similarly, microtubule remodeling occurs preferentially in the upstream side of the cell (14).
Decreasing Z-stack scanning resolution and thereby decreasing the time necessary for image collection enabled the detection of adaptive changes in membrane inclination as early as 20 s after onset of flow. Similarly, upon sudden retrograde flow, a reversal of the angular inclination of 4° per minute was observed. Consequently, a cell with a 3- to 4-μm high cell-cell junction would displace its luminal membrane by a range of 100 to 200 nm/min. This time scale and length are in the same range as the early vesicle movements in response to flow observed by Dangaria and Butler (8) using particle-tracking microrheology on subconfluent bovine aortic ECs. Using similar cells, Mott and Helmke (29) observed increased actin polymerization into lamellipodia at onset of shear stress. Similarly, using confocal microscopy in labeled cells, Ueki and colleagues (37) observed increased displacement of the cytoplasm in the apical region of the cell upon onset of shear. However, recent investigations have suggested that the initial signal transduction in mechanochemical sensation preferentially occurs at locations deeper within the cells such as the cytoskeleton and focal adhesion sites (9, 22, 33). Plasma membrane and cytoskeleton networks are intrinsically bound together and thus alteration of the stiffness of the cytoplasm is expected to modulate EC-cell junction inclination properties. Nevertheless, disruption of the cytoskeleton did not alter EC-cell junction inclination in our study, demonstrating that junctional inclination may occur independently of cytoskeletal remodeling.
Similarly, our data showed that EC-junctional inclination does not require an intact glycocalyx. The glycocalyx has previously been demonstrated to be a major mechanosensing element in ECs (35). Although the in vitro presence of a fully developed glycocalyx has been controversial, removal of heparan sulfate was clearly demonstrated in our fresh primary endothelial cells. Our results indicated that the heparan sulfate component of the glycocalyx is not involved in the mechanism by which the EC-cell junctions incline. Fung and Liu (13) also noted that the membrane tension model is qualitatively valid even if the internal contents are more solid-like, such as with a significant cytoskeletal network. Interestingly, the presence of a glycocalyx does not alter the model, because the membrane with the attached glycocalyx will still transmit tension. By experimentally observing that the outward curvature of the endothelial apical membranes and applying Laplace's law, Liu and colleagues (25) verified that tension does in fact develop in the membrane when ECs are subjected to fluid shear stress. Taken together, these studies and ours support the concept that the plasma membrane tension is involved in mechanotransduction.
Cells subjected to retrograde flow were dramatically more mechanosensitive than cells subjected to orthograde flow. While Fung and Liu's (13) theoretical model appears oversimplified by assuming that the intracellular contents are fluid-like and therefore the entire stress is imposed on the cell membrane (including any associated glycocalyx), it is likely that retrograde flow dramatically increases tension forces from cell to cell in a continuum layer of ECs (Fig. 7B). However, if the cell contents are more solid-like because of the presence of the cytoskeletal network, then the stress applied to the membrane can be expressed as a fraction ϵ of the applied fluid shear stress. This is supported by the observation that flow-induced nitric oxide stimulation in HUVECs is enhanced in the presence of cytoskeletal disrupting agents (23). Nonetheless, the expressions developed by Fung and Liu (13) remain qualitatively correct.
An important consequence of the membrane tension model and our finding of EC-cell junction inclination is the demonstration of the existence of large tension amplification at the junction when flow is reversed on ECs that were previously adapted to orthograde flow. It is shown here a dramatic increase in Ca2+i but increased tension also likely leads to other amplified atherogenic signals such as increased cellular responses, cell-cell junction permeability, and leukocyte adhesiveness. Indeed, Adamson and colleagues (1) recently observed increased permeability in rat microvessels subjected to retrograde shear stress. This hypothesis is also strongly supported by the increased atherogenic gene expression such as endothelin-1 and platelet-derived growth factor B expression levels when flow is reversed on flow adapted ECs (32). Hence, because of its short time scale, compared with cell realignment and cytoskeleton reorganization, EC-cell junction inclination should be considered as a potential preconditioning treatment to vessels intended to be used in retrograde flow such as the saphenous vein used in bypass surgery. More importantly, this hypothesis provides a molecular mechanism for the enhanced atherogenic signaling which correlates with reverse and oscillatory flow [where there is a significant reverse flow component (7)].
Here, it is demonstrated for the first time that fluid flow is directly responsible for three-dimensional geometrical changes in the plasma membrane topography. Indeed, it is shown both in vitro and in the mouse descending aorta that ECs adapt to steady shear by inclining the cell-cell junction in direction of flow. A reversal of flow is immediately accompanied by a repositioning of the EC-cell junction and a burst of Ca2+i response. Hence, it is proposed that the EC-cell junction inclines in response to unidirectional flow in a manner that reduces the propagation of tension, thereby reducing junctional tension and tension rates, leading to reduced mechanotransduction from the junction. The correlation between increased junctional inclinations and reduced cellular responses is an indicator of the effective mechanoadaptation of aligned cells in atheroprotective regions.
GRANTS
This work was supported by National Institutes of Health MERIT Award R37 HL040696.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sharp Memorial Hospital (San Diego, CA) for consistent availability of fresh umbilical cords, Alex Meilan for collecting and culturing primary HUVEC cultures, and Natalie Kardos for significant support in calcium dye loading experiments. Authors also acknowledge Nikhil C. Bhowmick for assistance in movie editing and Gallen Triana-Baltzer for reviewing and criticism of the manuscript.
REFERENCES
- 1.Adamson RH, Sarai RK, Weinbaum S, Curry FE. Retrograde shear stress modulates rat mesentery microvessel permeability and endothelial adhesion structures. FASEB J 23: 950–959, 2009. 19001055 [Google Scholar]
- 2.Barbee KA, Mundel T, Lal R, Davies PF. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am J Physiol Heart Circ Physiol 268: H1765–H1772, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol 280: C962–C969, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Butler PJ, Tsou TC, Li JY, Usami S, Chien S. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: a role for membrane perturbation in shear-induced MAPK activation. FASEB J 16: 216–218, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82: 532–539, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Chien S. Role of shear stress direction in endothelial mechanotransduction. Mol Cell Biomech 5: 1–8, 2008 [PubMed] [Google Scholar]
- 8.Dangaria JH, Butler PJ. Macrorheology and adaptive microrheology of endothelial cells subjected to fluid shear stress. Am J Physiol Cell Physiol 293: C1568–C1575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103: 177–185, 1981 [DOI] [PubMed] [Google Scholar]
- 11.Eskin SG, Ives CL, Frangos JA, McIntire LV. Cultured endothelium: the response to flow. ASAIO J 8: 109–112, 1985 [Google Scholar]
- 12.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng 32: 1053–1060, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Fung YC, Liu SQ. Elementary mechanics of the endothelium of blood vessels. J Biomech Eng 115: 1–12, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton 40: 317–330, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haidekker MA, L'Heureux N, Frangos JA. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am J Physiol Heart Circ Physiol 278: H1401–H1406, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Haidekker MA, Ling T, Anglo M, Stevens HY, Frangos JA, Theodorakis EA. New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol 8: 123–131, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Haidekker MA, White CR, Frangos JA. Analysis of temporal shear stress gradients during the onset phase of flow over a backward-facing step. J Biomech Eng 123: 455–463, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Haidekker MA, Stevens HY, Frangos JA. Cell membrane fluidity changes and membrane undulations observed using a laser scattering technique. Ann Biomed Eng 32: 531–536, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Helmke BP, Goldman RD, Davies PF. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res 86: 745–752, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Hong D, Jaron D, Buerk DG, Barbee KA. Heterogeneous response of microvascular endothelial cells to shear stress. Am J Physiol Heart Circ Physiol 290: H2498–H2508, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Katoh K, Kano Y, Ookawara S. Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cells in situ. Vasc Health Risk Manag 4: 1273–1282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen HL, Frangos JA. Role of cytoskeleton in shear stress-induced endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 273: H347–H355, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Liu SQ, Yen M, Fung YC. On measuring the third dimension of cultured endothelial cells in shear flow. Proc Natl Acad Sci USA 91: 8782–8786, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol 561: 575–582, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCue S, Dajnowiec D, Xu F, Zhang M, Jackson MR, Langille BL. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ Res 98: 939–946, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki H, Hayashi K. Atomic force microscopic measurement of the mechanical properties of intact endothelial cells in fresh arteries. Med Biol Eng Comput 37: 530–536, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol 293: C1616–C1626, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res 85: 504–514, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 587: 2365–2373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passerini AG, Milsted A, Rittgers SE. Shear stress magnitude and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. J Vasc Surg 37: 182–190, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Suzuki K, Ueki Y, Ohashi T. Microelastic mapping of living endothelial cells exposed to shear stress in relation to three-dimensional distribution of actin filaments. Acta Biomater 3: 311–319, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol 27: 346–351, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci Signal 1: pt8, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem 278: 31020–31023, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Ueki Y, Sakamoto N, Sato M. Direct measurement of shear strain in adherent vascular endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun 394: 94–99, 2010 [DOI] [PubMed] [Google Scholar]
- 38.White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci 362: 1459–1467, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.