Abstract
Rhbg is a nonerythroid membrane glycoprotein belonging to the Rh antigen family. In the kidney, Rhbg is expressed at the basolateral membrane of intercalated cells of the distal nephron and is involved in NH4+ transport. We investigated the substrate specificity of Rhbg by comparing transport of NH3/NH4+ with that of methyl amine (hydrochloride) (MA/MA+), often used to replace NH3/NH4+, in oocytes expressing Rhbg. Methyl amine (HCl) in solution exists as neutral methyl amine (MA) in equilibrium with the protonated methyl ammonium (MA+). To assess transport, we used ion-selective microelectrodes and voltage-clamp experiments to measure NH3/NH4+- and MA/MA+-induced intracellular pH (pHi) changes and whole cell currents. Our data showed that in Rhbg oocytes, NH3/NH4+ caused an inward current and decrease in pHi consistent with electrogenic NH4+ transport. These changes were significantly larger than in H2O-injected oocytes. The NH3/NH4+-induced current was not inhibited in the presence of barium or in the absence of Na+. In Rhbg oocytes, MA/MA+ caused an inward current but an increase (rather than a decrease) in pHi. MA/MA+ did not cause any changes in H2O-injected oocytes. The MA/MA+-induced current and pHi increase were saturated at higher concentrations of MA/MA+. Amiloride inhibited MA/MA+-induced current and the increase in pHi in oocytes expressing Rhbg but had no effect on control oocytes. These results indicate that MA/MA+ is transported by Rhbg but differently than NH3/NH4+. The protonated MA+ is likely a direct substrate whose transport resembles that of NH4+. Transport of electroneutral MA is also enhanced by expression of Rhbg.
Keywords: methyl amine, ammonium ion, ammonia gas, Rh glycoproteins, acid-base transport, NH3
the mammalian Rhbg (human RhBG) and Rhcg (human RhCG) are two nonerythroid membrane proteins belonging to the Rh family of proteins in red blood cells originally recognized for its immunogenic properties and importance in pregnancy (2, 24). Rhbg and Rhcg are expressed in several tissues and mainly in liver, intestine, skin, and the kidney cortical collecting duct and connecting segment (15, 34). Rhbg and Rhcg are coexpressed in the same cells with Rhbg localized at the basolateral membrane and Rhcg mainly localized at the apical membrane (10, 29, 34). Both membrane proteins are presumably involved in NH3/NH4+ and possibly CO2 transport (4) (13, 16, 18, 21, 23, 27, 35, 36).
The suggestion that NH3/NH4+ transport is mediated by Rh glycoproteins was originally based on studies of yeast mutants rendered incapable of NH4+ transport by deletion of endogenous NH4+ transporter genes (18). These yeast transporters are known as Mep proteins, and their bacterial equivalents are Amt proteins. Both belong to the Rh family and are related to Rhbg and Rhcg. Recent investigations employing a variety of techniques further affirmed the relationship of Rh proteins to NH3/NH4+ transport.
However, the physiological role of Rhbg as a unique and essential NH4+ (or NH3) transporter is far from clear. For example, in a study on Sprague-Dawley rats, inducing chronic metabolic acidosis, which increases renal ammonia excretion, did not lead to any change in renal Rhbg protein expression by Western blot analysis or immunohistochemistry (30). On the other hand, studies on Rhbg showed that genetic deletion of pendrin, an apical Cl−-HCO3− exchanger, decreased Rhbg expression. Although earlier studies on Rhbg knockout (KO) mice (9) did not elicit abnormal acid-base balance or ammonium handling, later studies proved differently. Recently, Bishop et al. (5) generated intercalated cell-specific Rhbg KO mice and demonstrated that urinary ammonium excretion was significantly less in KO mice vs. controls. The same study showed that in control mice, HCl-induced acidosis increased Rhbg protein expression significantly in 3 days.
An important issue is to determine which substrate is transported by Rhbg and related glycoproteins. Although this question was investigated in several studies, the answer is not yet clear. Studies suggesting that Rhbg is an NH4+ transporter are the earliest and remain among the most convincing (14, 18). More recent studies confirmed that NH4+ is transported by Rhbg (16, 17, 36). Yet in several studies, methyl ammonium was used as a substitute of ammonium and was shown to be transported by Rhbg (16, 17). Other molecules have also been proposed to be the primary substrates of the Rh membrane proteins. In yeast colonies, NH3 is thought to mediate communications between neighboring colonies (26) and even to play a role in synchronizing their growth and development (25). Amt proteins have been suggested as bidirectional gas channels for NH3 (33). Two interesting studies argued that several Rh proteins, including Rh1 and Amt, actually mediate CO2 transport (13, 32).
Our study was undertaken to characterize substrate specificity of Rhbg transport. Using intracellular pH (pHi) measurements and voltage-clamp experiments, we investigated how NH3, NH4+, or methyl ammonium is transported by Rhbg. Our data clearly demonstrate that there are distinct differences between NH3/NH4+ and methyl amine/methyl ammonium (MA/MA+) transport by Rhbg. These differences are important in elucidating the mechanism by which NH4+ is transported.
METHODS
Solutions and Reagents
All chemicals and reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Standard “control” solution was ND96 medium containing (in mM) 100 NaCl, 2 KCl, and 1.8 CaCl2 and buffered with 5 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) to pH 7.5. Most NH3/NH4+ solutions typically contained 5 mM NH4Cl (replacing NaCl) at pH 7.5. Other NH3/NH4+ solutions contained 2, 5, 10, 15, and 20 mM NH4Cl at pH 7.5. Methylamine hydrochloride (Sigma) was used to prepare MA/MA+ solutions. Osmolarity of all solutions was ∼200 mosM. Oocytes were stored in OR3 medium (GIBCO BRL Leibovitz media) containing glutamate and 500 units each of penicillin-streptomycin, with pH adjusted to 7.5 and osmolarity adjusted to ∼200 mosM.
Isolation and Injection of Oocytes
All experiments were conducted on oocytes in stages 5 or 6. Isolation and injection of oocytes followed standard operating procedures as extensively described previously (21, 22). Briefly, the frog was anesthetized by mild hypothermia in water containing 0.2% tricaine. Following a 1-cm incision in the abdominal wall, a lobe of the ovary was externalized and its distal portion was cut. The wound was closed by a few stitches in the muscular plane of the peritoneum using 5-0 chromic gut (Ethicon) followed by two to three stitches in the abdominal skin using 6-0 silk. The excised piece of ovary containing oocytes was rinsed several times with Ca-free ND96 solution until the solution was clear. The tissue was then agitated in sterile-filtered Ca-free solution containing collagenase type I-A (2–3 mg/ml) for 30–40 min. Free oocytes were rinsed several times with sterile OR3 medium, sorted, and then stored at 18°C.
Preparation of cRNA
pGH19 plasmid containing Rhbg cDNA was purified by Wizard Plus Minipreps DNA Purification System (Promega, Madison, WI) according to protocol. The plasmid was then digested with Nhe1 (New England Biolabs, Ipswich, MA) to produce a linear template, followed by proteinase K (1 mg/ml) digestion. DNA was then extracted by phenol-chloroform twice followed by chloroform extraction and ethanol precipitation. cDNA was transcribed in vitro with T7 polymerase. The in vitro synthesis of capped RNA (cRNA) transcripts was then accomplished using mMessage mMachine T7 transcription kit from Ambion (Foster City, CA). The concentration of cRNA was determined by UV absorbance, and its quality was assessed by formaldehyde/MOPS/1% agarose gel electrophoresis.
Injection of Oocytes
Oocytes in OR3 medium were visualized with a dissecting microscope and injected with 50 nl of cRNA for Rhbg (0.2 μg/μl, for a total of 10 ng of RNA). Control oocytes were injected with 50 nl of sterile H2O. The sterile pipettes have tip diameters of 20–30 μm. They were backfilled with paraffin oil and connected to a Nanoject motor-driven pipette (Drummond Scientific). Injected oocytes were used on the third day after injection with cRNA and for the following 4–5 days.
Electrophysiological Measurements in Frog Oocytes
pHi measurements and voltage-clamp experiments were previously reported (21, 23) and are briefly described here.
Measurements of pHi
The pH microelectrodes were of the liquid ion exchanger type, and the resin (H+ ionophore 1, cocktail B) was obtained from Fluka (Buchs, Switzerland). Briefly, single-barreled alumino-silicate glass tubings (1.5 mm OD × 0.86 mm ID; Frederick Haer, Brunswick, MD) were pulled to a tip < 0.2 μm and dried in an oven at 200°C for 2 h. The electrodes were silanized with bis(dimethylamino)-dimethyl silane vapor in a closed vessel (300 ml) as described by Siebens and Boron (31). The exchanger was then introduced into the tip of the electrodes by means of a very fine glass capillary. pH electrodes were backfilled with a buffer solution containing 0.04 M KH2PO4, 0.023 M NaOH, and 0.015 M NaC1, pH 7.0 (1). The electrodes were fitted with a holder with an Ag-AgC1 wire attached to a high impedance probe of a WPI FD-223 electrometer and calibrated. The pH electrodes were calibrated in standard solutions of pH 6 and 8. All the electrodes used in this study had a slope of >58 mV/pH. The pH electrodes were not selective to methyl ammonium over a concentration range from 1 to 20 mM.
Ling-Gerard (voltage) microelectrodes were pulled from 1.5 mm (OD) borosilicate fiber capillaries and filled with 3 M KCl. Their resistances were in the range of 8–16 MΩ and their tip potential was <4 mV.
Initial rates of change of pHi (dpHi/dt) were determined by fitting pHi vs. time to a linear regression line. Statistical significance was judged from Student's t-tests. Whenever possible, measurements were determined under control and test conditions in the same oocyte.
Two-Electrode Voltage Clamp
Two-electrode voltage clamp (OC-725, Warner Instruments, Hamden, CT) was used to measure whole cell currents. For these experiments, electrodes were pulled from borosilicate glass capillaries (1.5 mm OD, Warner Instruments) using a horizontal puller (model P-97, Sutter Instruments, Novato, CA). Electrodes were filled with 3 M KCl solution and had resistances of 1–4 MΩ. Bath electrodes were also filled with 3 M KCl and were directly immersed in the chamber. Oocytes were usually clamped at −60 mV, and long-term readings of current were sampled at a rate of once per second. Inward flow of cations is defined by convention as inward current (negative current).
RESULTS
NH3/NH4+ Transport by Rhbg: Basic Effect
All experiments in this study were performed on Xenopus oocytes either expressing Rhbg or injected with H2O as a control. Transport of NH3/NH4+ was assessed from measurements of NH3/NH4+-induced changes in voltage-clamped current or pHi as described in methods. In native oocytes, NH3/NH4+ transport is uniquely characterized by significant NH4+ transport and an apparent minimal NH3 transport. This is manifested, upon exposing the oocyte to a solution containing NH4Cl, by a significant pHi decrease, a depolarization of the cell, and an inward current (see Fig. 1 in Ref. 21). As described in earlier studies (8, 21, 22), the NH3/NH4+-induced changes are consistent with net NH4+ influx that is faster than NH3 diffusion, thus effectively masking any significant NH3-induced pHi changes. In oocytes expressing Rhbg, the pattern of NH3/NH4+-induced changes is similar. However, pHi acidification and the NH4+-induced current are significantly larger than in H2O-injected oocytes (21). In an earlier study, we titrated the effect of NH4+ in H2O-injected and Rhbg-expressing oocytes and demonstrated that, at 5 mM NH4Cl, the NH4+-induced changes in pHi, membrane potential (Vm), and inward currents in oocytes expressing Rhbg were significantly larger than those induced by 20 mM NH4Cl in H2O-injected oocytes. Those studies demonstrated that Rhbg transports NH4+ in an electrogenic manner and that NH3 transport is not significantly altered by expression of Rhbg.
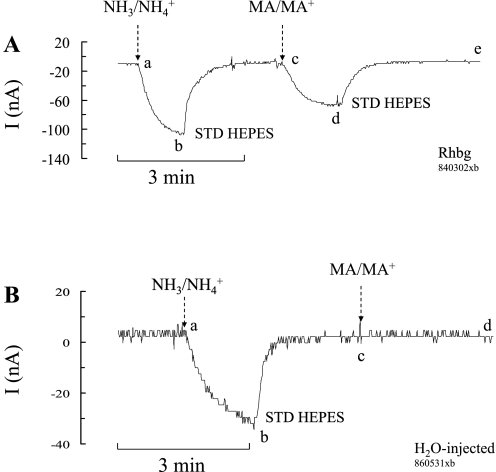
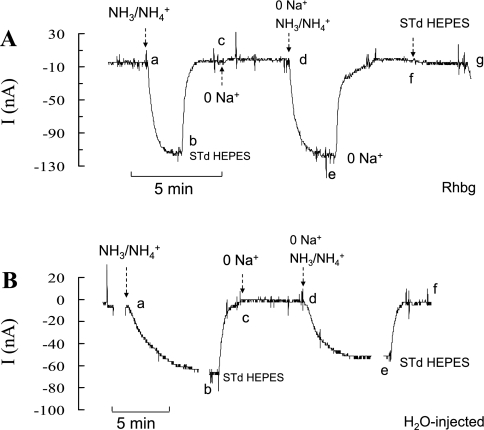
Fig. 1.
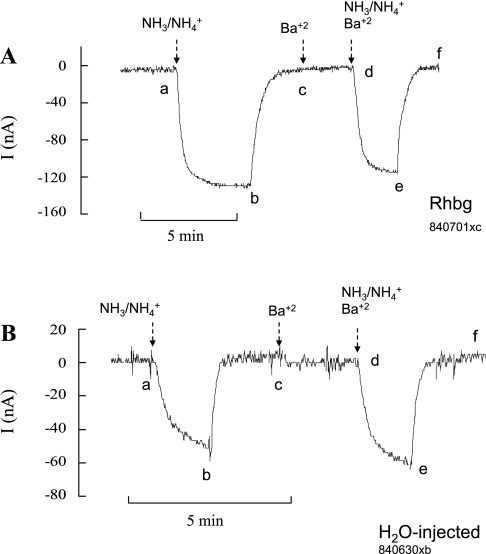
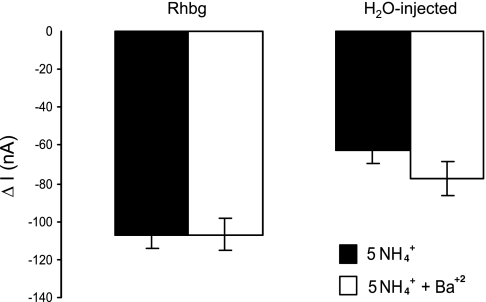
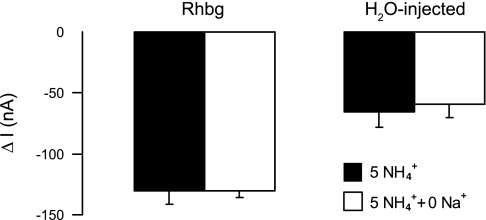
NH4+ and methyl ammonium (MA+) current (I) in Rhbg-expressing and H2O-injected oocytes. A: in oocytes expressing Rhbg (clamped at −60 mV), exposure to NH3/NH4+ (5 mM) induced an inward current (segment ab) of −83 ± 9.6 nA (n = 8). Exposure to methyl amine/methyl ammonium (MA/MA+; 5 mM) also caused an inward current (cd) of −63 ± 7.6 nA (n = 8) that was significantly smaller than the NH3/NH4+-induced current (P < 0.001). STD, standard. B: in H2O-injected (control) oocytes, exposure to NH3/NH4+ (5 mM) caused an inward current (segment ab) that averaged −52 ± 7.6 nA. Exposure to MA/MA+ (5 mM) did not cause any significant current (cd).
Transport of MA/MA+ by Rhbg
MA/MA+ current.
Methyl amine hydrochloride (MA/MA+) has been used extensively in transport studies as a substitute for NH4Cl (NH3/NH4+) to measure NH4+ uptake. Methyl amine (HCl) has the advantage that it can be easily radiolabeled as compared with NH4+. Methyl amine (HCl) in solution exists as neutral CH3NH2 (MA) in equilibrium with the positively charged methyl ammonium CH3NH3+ (MA+). To investigate the substrate specificity of Rhbg, we compared MA/MA+- and NH3/NH4+-induced cellular changes in Rhbg and H2O-injected oocytes. As shown in Fig. 1A, in oocytes expressing Rhbg, exposure to 5 mM NH4Cl caused an inward current (segment ab) that was readily reversed upon removal of NH4Cl from the bath (bc). Exposing the oocyte to a bath solution containing 5 mM MA/MA+ also resulted in an inward current (cd) that was slightly less than that caused by 5 mM NH4Cl. This current was readily reversed upon removal of external MA/MA+ (segment de).
In H2O-injected oocytes (Fig. 1B), 5 mM NH4Cl in the bath caused an inward current (segment ab) that readily reversed upon removal of NH4Cl (bc). In contrast, addition of 5 mM MA/MA+ to the bath did not cause any change in the whole cell current (cd). These observations were reported in our earlier study (21), and similar experiments are included here to show the basic effect of NH3/NH4+ and MA/MA+. These results indicate that electrogenic transport of MA/MA+ is absent in H2O-injected oocytes but evident in oocytes expressing Rhbg. It is to be noted that subtracting the endogenous NH4+ current (measured in H2O-injected oocytes) from the NH4+-induced current in oocytes expressing Rhbg yields a net current whose magnitude (−64 nA) is comparable to that of MA+-induced current in Rhbg oocytes (−63 nA). However, simple comparison of the subtracted net NH4+ current with the MA+-induced current must be interpreted with caution because the exact nature of the endogenous NH4+-current in H2O-injected oocytes is not clear.
MA/MA+-induced pHi changes.
To further characterize MA/MA+ transport, we compared MA/MA+- and NH3/NH4+-induced pHi changes in H2O-injected oocytes and those expressing Rhbg. In H2O-injected oocytes (Fig. 2), 5 mM NH4Cl in the bath caused a pHi decrease and significant depolarization (segment ab) as reported previously (21, 23). These changes were reversed upon removal of bath NH4Cl (bc). When MA/MA+ (5 mM) was then added to the bath, a slow and very small increase in pHi occurred (cd) and there was no effect on Vm. Removal of bath MA/MA+ resulted in pHi recovery toward its steady-state value (de).
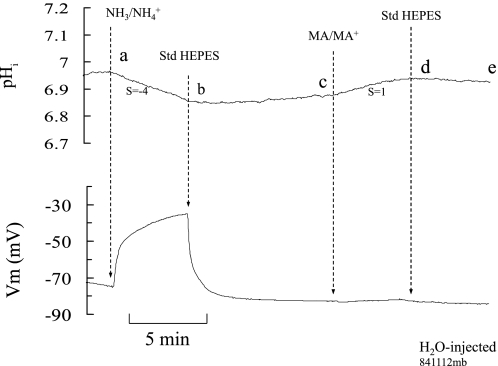
Fig. 2.
NH3/NH4+ and MA/MA+ effects on intracellular pH (pHi) and membrane potential (Vm) in H2O-injected oocytes. Exposing oocytes to 5 mM NH3/NH4+ caused a slow and small decrease in pHi but a large depolarization of the cell (segment ab). On the other hand, exposing the oocyte to 5 mM MA/MA+ caused a small increase in pHi and no depolarization (cd). S, rate of pHi change (dpHi/dt) in pH/s × 10−4.
In oocytes expressing Rhbg (Fig. 3), addition of 5 mM NH4Cl to the bath caused a rapid and fast decrease in pHi (ab) and depolarization of the cell. These changes were reversed upon removal of bath NH4Cl (bc). On the other hand, addition of MA/MA+ (5 mM) to the bath caused a rapid increase in pHi (cd), but the cell still depolarized significantly. These changes were also reversed when MA/MA+ was removed from the bath (de). It is highly significant that, whereas the MA/MA+ effect on Vm resembled that of NH3/NH4+ (depolarization), its effect on pHi was in the opposite direction, causing alkalinization rather than acidification. Figure 4A summarizes the Vm and pHi effects of MA/MA+ and NH4Cl in oocytes expressing Rhbg. Figure 4B is a summary graph comparing the effects of MA/MA+ in oocytes expressing Rhbg to those in H2O-injected oocytes.
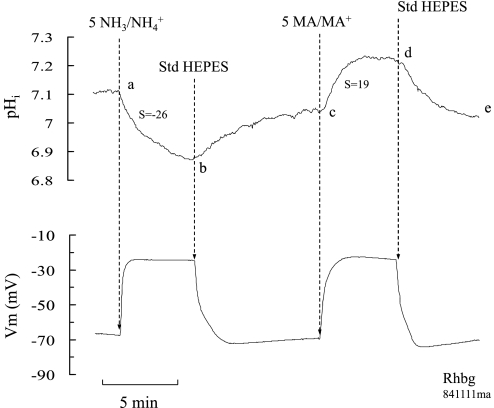
Fig. 3.
NH3/NH4+ and MA/MA+ effects on pHi and Vm in oocytes expressing Rhbg. NH3/NH4+ (5 mM) caused a fast and significant decrease in pHi and depolarized the oocyte (segment ab). In contrast, MA/MA+ (5 mM) caused an increase (not a decrease) in pHi but also depolarized the cell (segment cd).
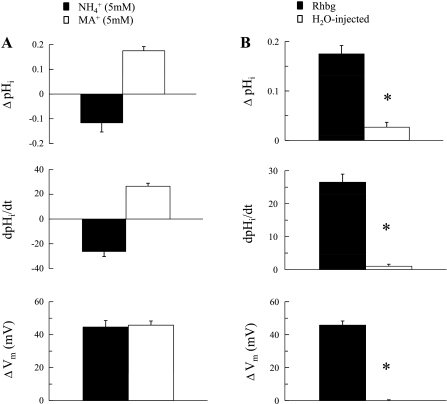
Fig. 4.
A: summary graph comparing the effects of NH3/NH4+ and MA/MA+ on pHi and Vm in oocytes expressing Rhbg. In paired experiments (n = 8), NH3/NH4+ (5 mM) caused pHi to decrease by 0.12 units at a rate of −26.5 ± 0.4 × 10−4 pH/s and depolarized the cell by 45 ± 3.9 mV. MA/MA+ (5 mM) caused pHi to increase by 0.18 ± 0.02 at a rate of 26.5 ± 2.5 × 10−4 pH/s and depolarized the cell by 46 ± 2.6 mV. The depolarizations by NH3/NH4+ and MA/MA+ were not statistically different (P > 0.05). B: effects of MA/MA+ on pHi and Vm in oocytes expressing Rhbg compared with the effects on H2O-injected oocytes. In H2O-injected oocytes, MA/MA+ (5 mM) caused a small and very slow increase in pHi (0.03 ± 0.01 units at a rate of 1.0 ± 0.06 × 10−4 pH/s) and caused no significant depolarization of the cell. These changes were much smaller than those in oocytes expressing Rhbg. *Statistical significance.
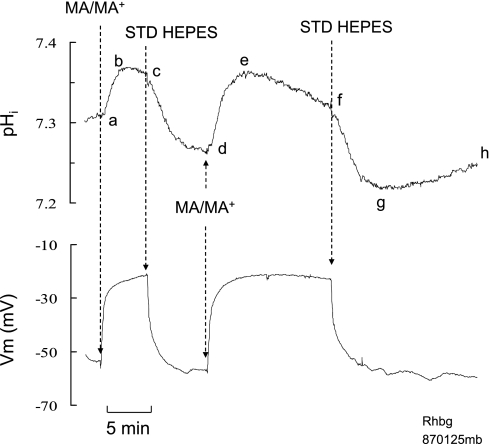
In oocytes expressing Rhbg, the pHi and current changes induced by exposure to MA/MA+ suggest that transport of MA as well as MA+ is enhanced. If this is the case, then transport of MA/MA+ should resemble that of NH3/NH4+ prepulse in mammalian cells, an initial pHi increase followed by a slower decrease in pHi. To test for this, we exposed the oocytes expressing Rhbg to MA/MA+ for a longer period of time. As shown in Fig. 5, exposing the oocyte to 5 mM MA/MA+ for a short period of time (3–5 min) caused an increase in pHi (segment abc) of 0.10 ± 0.01 that was reversed upon removal of MA/MA+ from the bath (segment cd). Reexposing the oocyte to MA/MA+ for a longer period time (>10 min) caused an initial increase in pHi of 0.11 ± 0.02 (segment de) followed by a slow acidification (ef). Removal of bath MA/MA+ caused a rapid decrease in pHi (fg) with a significant undershoot (compare points g and d). This was followed by spontaneous pHi recovery toward initial steady-state value (segment gh). In both cases the cell depolarized by 39 ± 3.7 mV (n = 8).
Fig. 5.
Effect of prolonged exposure to MA/MA+ on pHi in oocytes expressing Rhbg. Exposing oocytes to MA/MA+ (5 mM) for a short period of time (3–5 min) caused the usual reversible increase in pHi (ab–cd). Reexposing the oocyte to MA/MA+ for a longer time (>10 min) caused an initial increase in pHi (de) followed by a slow acidification (ef). Removal of bath MA/MA+ caused a rapid decrease in pHi (fg) with a significant undershoot (compare points g and d). This was followed by spontaneous pHi recovery toward initial steady-state value (segment gh). Eight similar experiments were performed.
Concentration Dependence of MA/MA+-Induced Changes
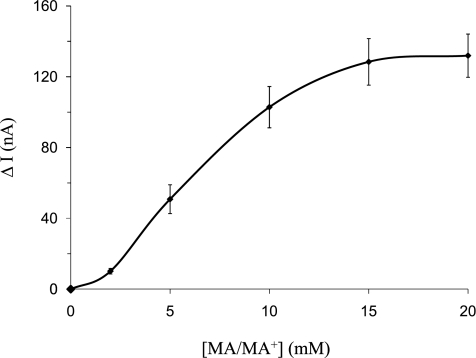
The experiments described above indicate that, in oocytes expressing Rhbg, the following features characterize transport of MA/MA+: a significant MA/MA+-induced inward current, depolarization of the cell, and rapid pHi increase, none of which are present in H2O-injected oocytes. To further characterize the MA/MA+ effect in oocytes expressing Rhbg, we measured the MA/MA+-induced current and pHi changes in response to varying concentrations of MA/MA+ in the bath. Fig. 6 is a plot of net MA/MA+ current as a function of total MA/MA+ concentration ([MA/MA+]) in the bath. These data indicate that net MA/MA+-induced inward current due to Rhbg is saturated at higher concentrations of MA/MA+.
Fig. 6.
MA+-induced currents as a function of total MA/MA+ concentration ([MA/MA+]) in oocytes expressing Rhbg. The MA+-induced current is saturated at higher concentrations of bath MA/MA+. The concentration of bath MA/MA+ was varied from 2 to 20 mM.
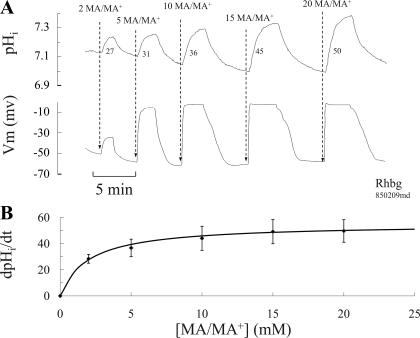
The effect of different concentrations of MA/MA+ on pHi was also investigated in the experiments depicted in Fig. 7. In those experiments, oocytes expressing Rhbg were exposed to bath solutions containing different concentrations of MA/MA+ ranging from 2 to 20 mM. As shown in Fig. 7A, increasing the concentration of MA/MA+ in the bath resulted in progressive increase in pHi and Vm changes. It was clearly evident that MA/MA+-induced Vm changes quickly saturated at higher MA/MA+ concentrations, as previously observed with the MA/MA+-induced current changes. Moreover, ΔpHi and the rate of pHi change (dpHi/dt) also increased with exposure to higher concentrations of MA/MA+. Figure 7B is a plot of the rate of pHi increase as a function of total [MA/MA+] in the bath and clearly shows saturation. The solid line is a Michaelis-Menten best fit of the data indicating a Km of 2 mM. Measurement of the rate of pHi increase is of significance because it reflects measurements of MA/MA+-induced H+ consumption presumably due to influx of electroneutral methyl amine and not the charged methyl ammonium. The latter is expected to cause a decrease rather than an increase in pHi.
Fig. 7.
Concentration dependence of pHi and Vm caused by MA/MA+ in oocytes expressing Rhbg. A: exposing oocytes to increasing concentrations of MA/MA+ in the bath caused proportionately faster and bigger changes in pHi. Changes in Vm quickly saturated at concentration of 10 mM MA/MA+. The numbers next to the pHi tracing are rates of pHi increase in pH/s × 10−4. B: plot of MA-induced pHi increase as a function of total MA/MA+ concentration in the bath. The rate of pHi increases owing to MA saturated at higher concentration of MA/MA+ in the bath. The solid line is a Michaelis-Menten best fit of the data with a Vmax of 50 and a Km of 2 mM (R2 = 0.997).
Inhibition by Amiloride
Effect on MA/MA+ current.
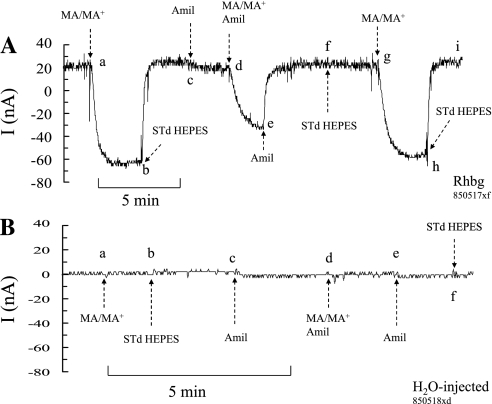
Amiloride is an inhibitor of several transport mechanisms including epithelial Na+ channel, Na-H exchange, and T-type calcium channels. We investigated whether amiloride affected MA/MA+ transport by Rhbg. In paired experiments on the same oocyte, we measured the MA/MA+-induced currents in the presence and absence of amiloride. In oocytes expressing Rhbg (Fig. 8A), perfusing the bath with a solution containing 5 mM MA/MA+ induced an inward current (segment ab) that readily recovered upon removal of MA/MA+ from the bath (segment bc). At point c, amiloride (1 mM) was added to the bath, causing a very small change in current (segment cd). In the continued presence of amiloride, exposing the oocyte to 5 mM MA/MA+ caused an inward current that was significantly smaller than that in the absence of amiloride (segment de). Removal of MA/MA+ from the bath reversed the MA/MA+-induced current (segment ef). At point f, amiloride was removed from the bath. When the cell was exposed again to MA/MA+ after washout of amiloride, the MA/MA+-induced inward current (segment gh) was larger than in the presence of amiloride and very comparable to the first pulse (ab) before amiloride was added. In 19 similar experiments, the MA/MA+-induced current in the absence of amiloride averaged −83 ± 8.4 nA but only −62 ± 7.7 nA in the presence of amiloride (P < 0.005).
Fig. 8.
Effect of amiloride (Amil) on MA+-induced current. A: in oocytes expressing Rhbg, MA/MA+ current (segments ab and gh) averaged −83 ± 8.4 nA (n = 19), which was significantly inhibited to −68 ± 7.7 nA (P < 0.005) in the presence of 1 mM amiloride (segment de). Addition of amiloride per se did not cause any effect on whole cell current (segment cd). B: in H2O-injected oocytes, there was no effect of MA+ or amiloride on current (clamped at −60 mV).
Figure 8B shows a similar experiment conducted with an H2O-injected oocyte. As shown in this experiment, exposing the oocyte to 5 mM MA/MA+ did not cause a change in current (segment ab). Similarly, no significant change in current was evident upon addition of amiloride to the bath (segment cd) or when MA/MA+ was added in the presence of amiloride (segment de). These experiments indicate that amiloride inhibits MA/MA+ current in oocytes expressing Rhbg.
Effect on MA/MA+-induced pHi changes.
To further investigate the effect of amiloride, we monitored the pHi changes caused by MA/MA+ in the presence and absence of amiloride. As shown in Fig. 9, in oocytes expressing Rhbg, adding MA/MA+ (5 mM) to the bath caused a significant increase in pHi (segment ab) and depolarization of the cell as shown previously (see Fig. 3). Removal of bath MA/MA+ reversed these effects (segment bc). Amiloride (1 mM) added to the bath at point c did not affect pHi and caused a small depolarization (segment cd). Exposing the oocyte to MA/MA+ in the presence of amiloride still caused an increase in pHi and depolarization of the cell (segment de). Subsequent removal of MA/MA+ from the bath in the continuing presence of amiloride resulted in recovery of pHi and Vm (segment ef). At point (f), amiloride was removed from the bath causing a small hyperpolarization and no effect on pHi (segment fg). Exposing the oocyte to MA/MA+ again in the absence of amiloride resulted in rapid increase in pHi and depolarization of the cell (segment gh). In seven paired experiments, the pHi increase induced by MA/MA+ in the absence of amiloride averaged 0.13 ± 0.01 pH units, which was not significantly different from 0.11 ± 0.04 in the presence of amiloride (P > 0.1). However, the rate of pHi increase of 22 ± 4.9 × 10−4 pH/s in the absence of amiloride was significantly higher than 15 ± 3.3 × 10−4 pH/s in the presence of amiloride (P < 0.01). Similarly, MA/MA+-induced depolarization in the absence of amiloride (39 ± 1.9 mV) was significantly bigger than 32 ± 2.4 mV in the presence of amiloride (P < 0.005).
Fig. 9.
Amiloride inhibition of MA/MA+-induced pHi and Vm changes in oocytes expressing Rhbg. Exposing oocytes to MA/MA+ (5 mM) caused an increase in pHi (segments ab and gh) that was significantly faster than in the presence of amiloride (segment de). Similarly, the MA/MA+-induced depolarization was inhibited in the presence of amiloride. The numbers next to the pHi tracing indicate rate of pHi increase (dpHi/dt) in pH/s × 10−4. Amiloride alone did not cause any significant effect on pHi or Vm (segment cd).
We conducted similar experiments on H2O-injected oocytes. As shown in Fig. 10, there was no significant change in pHi or Vm when MA/MA+ was added to the bath (segment ab) or when removed (segment bc). Addition of amiloride to the bath at point c caused a small depolarization as observed in oocytes expressing Rhbg (see Fig. 9). In the presence of amiloride, addition of MA/MA+ to the bath (segment de) or its removal (segment ef) did not cause significant changes in pHi or Vm.
Fig. 10.
Effect of amiloride on MA/MA+-induced pHi and Vm changes in H2O-injected oocytes. MA/MA+ had no significant effect on pHi or Vm in the absence of amiloride (segment ab) or in its presence (segment de).
Effect of Barium
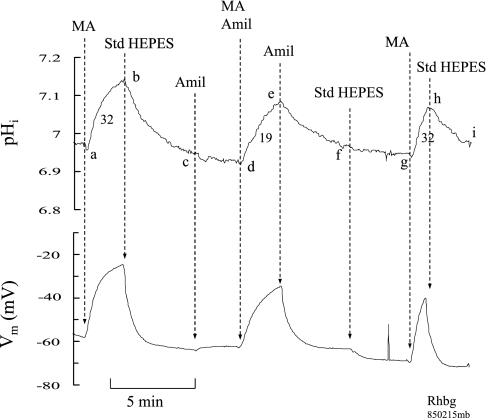
In an earlier study, we demonstrated that NH3/NH4+-induced inward current was slightly but significantly enhanced in the absence of bath K+ (21). To further investigate whether K+ channels may be involved in NH3/NH4+ transport, we used Ba+2 (2 mM) to block K+ conductance. As shown in Fig. 11A, in oocytes expressing Rhbg, exposure to 5 mM NH4Cl in the absence of Ba+2 caused the usual inward current (ab), which readily recovered upon removal of bath NH4Cl (bc). Addition of Ba+2 to the bath did not cause any change in current (cd). Exposing the oocyte to 5 mM NH4Cl in the presence of Ba+2 also caused an inward current (de), which fully recovered upon removal of NH4Cl from the bath (ef). The NH4+-induced inward current in the absence of Ba+2 (−107 ± 9.1 nA) was not different from that in the presence of Ba+2 (−106 ± 8.8 nA; P > 0.05, n = 8).
Fig. 11.
Effect of barium (2 mM) on NH3/NH4+-induced current. A: in oocytes expressing Rhbg, 5 mM NH3/NH4+ induced an inward current in the absence of Ba+2 (segment ab) that was not different from that in absence of Ba+2 (segment de). B: in H2O-injected oocytes, the NH3/NH4+-induced current in the absence of Ba+2 (segment ab) was also not inhibited by Ba+2 (segment de).
Similar experiments were conducted on H2O-injected oocytes. As shown in Fig. 11B, the magnitude of the NH4+-induced current in the absence of Ba+2 (−62 ± 11.3 nA; segment ab) was not statistically different from that in the presence of Ba+2(−78 ± 8.8 nA; P > 0.05, n = 8). The results of these experiments are summarized in Fig. 12.
Fig. 12.
NH3/NH4+-induced current in the presence and absence of Ba+2. In oocytes expressing Rhbg, NH3/NH4+-induced current averaged −107 ± 3.1 nA (n = 8) in the absence of Ba+2 and −106 ± 8.8 nA in the presence of Ba+2 (P > 0.5). Similar results were obtained in H2O-injected oocytes: −62 ± 11.3 nA in the presence of Ba+2 and −78 ± 8.8 nA in its absence (P > 0.05). The NH3/NH4+-induced current in H2O-injected oocytes is significantly smaller than that in Rhbg oocytes.
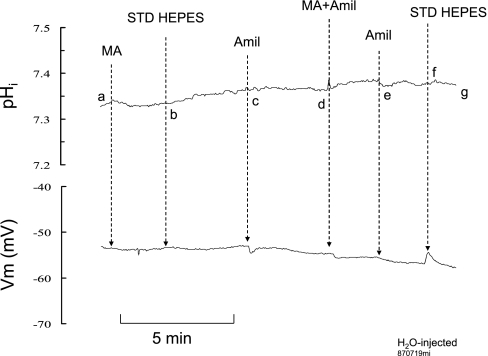
Dependence on Extracellular Na+
We also investigated whether Na+ is involved in NH3/NH4+ transport by Rhbg. To do so we measured NH3/NH4+-induced current in the presence and absence of extracellular Na+. In oocytes expressing Rhbg (Fig. 13A), 5 mM NH4Cl in the bath caused an inward current (ab) that readily recovered when NH4Cl was removed (bc). Removal of bath Na+ did not cause a significant change in current (cd). In the absence of Na+, reexposing the oocyte to 5 mM NH4Cl caused an inward current (de) that readily recovered upon removal of bath NH4Cl (ef). In five paired experiments, the NH4+-induced current in the presence of Na+ (−130 ± 11 nA) was not statistically different from that in the absence of Na+ (−130 ± 6 nA). At point f, bath Na+ was restored to normal, with no significant change in current (fg).
Fig. 13.
NH3/NH4+-induced current in the presence and absence of Na+. A: in oocytes expressing Rhbg, NH3/NH4+ induced an inward current in the presence of Na+ (segment ab) that was not different from that in the absence of Na+ (segment de). Removal of Na+ did not cause any change in current (segment cd). B: in H2O-injected oocytes, similar results were obtained. NH3/NH4+-induced current in the presence of Na+ (segment ab) was not different from that in its absence (segment de).
Similar experiments were conducted on H2O-injected oocytes. Figure 13B shows similar results, whereby the NH4+-induced current in the presence of Na+ (segment ab; −65 ± 13 nA) was not different from NH4+-current in the absence of Na+ (segment ef, −60 ± 11 nA; n = 5; P > 0.05). The results of these experiments are summarized in Fig. 14.
Fig. 14.
Summary graph comparing the effects of Na+ on NH3/NH4+-induced current. In Rhbg oocytes, NH3/NH4+-induced current was −130 ± 11 nA (n = 5) in the presence of Na+ and −130 ± 6 nA in the absence of Na+ (P > 0.05). In H2O-injected oocytes, NH3/NH4+-induced current was −65 ± 13 nA (n = 5) in the presence of Na+ and −60 ± 11 nA in its absence (P > 0.05).
DISCUSSION
In this study, we investigated transport of MA/MA+ in oocytes expressing Rhbg. We made the novel observation that MA/MA+, often used as a substitute of NH3/NH4+ in uptake studies, causes different pHi changes than NH3/NH4+. The distinct differences between MA/MA+ and NH3/NH4+ transport allow for probing the substrate specificity of Rhbg. We used these data to further characterize NH3/NH4+ transport and to discuss some of the proposed models of NH3/NH4+ transport by Rhbg.
The present experiments also confirmed our previous findings with respect to NH3/NH4+ transport in control oocytes and those expressing Rhbg (21, 23). In two electrode voltage-clamp experiments, bath NH4Cl caused an NH4+-induced inward current. In oocytes expressing Rhbg, this NH4+ current was significantly larger than in H2O-injected oocytes. In experiments where intracellular pH was monitored, bath NH4Cl induced a pHi decrease and a sustained depolarization of the cell. In oocytes expressing Rhbg, the NH4+-induced pHi decrease and ΔVm were respectively faster and bigger than in H2O-injected oocytes. These changes confirm that Rhbg transports net NH4+ in an electrogenic manner.
NH3/NH4+ Transport by Rhbg
Studying NH3/NH4+ transport in oocytes expressing Rhbg is complicated by the presence of background “endogenous” NH3/NH4+ transport in control Xenopus oocytes. The NH3/NH4+-induced cellular changes (pHi decrease, inward current, and depolarization) are unique in the oocyte and do not resemble NH3/NH4+-induced changes in other cells. The unusual intracellular acidification and its magnitude were referred to as “paradoxical” because 1) a pHi decrease, presumably due to NH4+ influx, precludes that NH4+ transport masks NH3 transport, which is usually more permeable than NH4+ in most membranes; 2) the magnitude of pHi decrease (ΔpHi), induced by NH3/NH4+, can only be reached if intracellular NH3 is sequestered or removed thus allowing more NH4+ to enter the cell; and 3) the pHi recovery upon removal of NH3/NH4+ is uncharacteristically slow. These changes were confirmed by several investigators (6, 8, 12), and several possibilities to explain these observations were recently discussed (19). It is unlikely that cellular utilization (e.g., metabolism) leading to generation of H+ is responsible for the NH4+-induced pHi decrease. The relatively fast pHi change and the reversibility of the pHi changes upon removal of NH3/NH4+ argue against this possibility. Nevertheless, NH3 must be sequestered or removed to allow for this decrease in pHi as discussed in our earlier study (22). Thus, the two hallmarks of NH3/NH4+ transport in oocytes are a pathway for NH4+ influx suggested to be a nonselective cationic channel (8) and the need to dissipate buildup of intracellular NH3 that may prevent NH4+ influx (19, 21).
Rhbg as a mechanism for NH4+ transport is supported by several findings. Xenopus oocytes expressing Rhbg exhibit a higher whole cell membrane conductance, particularly in the presence of NH4Cl (16, 17, 21). Even though in H2O-injected oocytes, bath NH4Cl induced a pHi decrease, depolarization of the cell, and an inward current, these changes were significantly less than in oocytes expressing Rhbg (3, 21, 23, 36). Moreover, NH4Cl, at various concentrations, induced an enhanced inward current and faster rates of pHi decrease, suggesting that Rhbg mediates NH4+ transport (3, 21, 23, 36).
In contrast, it was also proposed that Rhbg mediated electroneutral NH3/NH4+ transport. One study (16) reported an NH3/NH4+-induced alkalinization of intracellular pH, suggesting transport of NH3. However, in that study, pHi was monitored by fluorescence measurements, whereas all other studies employing microelectrode measurement detected only a pHi decrease induced by NH4Cl (4, 21, 28). Two other studies (11, 16), relying on measurements of labeled MA/MA+, suggested that Rhbg acts as NH4+-H+ exchanger, although one of those studies reported that there was an increase in whole cell conductance. One main difference between our studies and those suggesting that Rhbg is an electroneutral NH4+-H+ exchanger is that, in the latter studies, methyl amine (HCl) was mostly used to assess NH3/NH4+ transport. Our data clearly indicate that Rhbg transports MA/MA+ in a different mode than NH3/NH4+ as discussed below.
One question often raised in expression studies is how to rule out that Rhbg (or other Rh proteins) is indirectly responsible for the observed pHi, current, and Vm. Although unlikely, it is possible that expression of Rhbg in a heterologous system may activate an endogenous mechanism(s) that in turn mediates transport of NH3/NH4+ and MA/MA+. Another suggestion is that, in the oocyte, Rhbg affects activity of other cationic channels, which leads to the NH3/NH4+-induced changes in current or Vm.
It is difficult to rule out these possibilities, especially since a specific inhibitor of Rhbg, or of other Rh proteins, is not yet available. However, in our preparation, several observations render these possibilities unlikely. First, the pHi response to NH3/NH4+ and MA/MA+ in oocytes expressing Rhbg clearly indicates a solute-specific effect. Specifically, NH3/NH4+ in Rhbg-expressing oocytes induced different changes in pHi (acidification) than MA/MA+ (alkalinization). This is likely due to enhanced transport of the solute by Rhbg leading to the observed effect and not due to a nonspecific effect caused by Rhbg expression. Second, if Rhbg expression promotes NH3/NH4+ transport through induced cationic channels, then only two possibilities may be raised: either K+ channels are blocked or a native Na+ channel is activated to explain the inward current and the depolarization of the membrane. In an earlier study (21), we showed that NH4+-induced current was enhanced in the absence of external K+ rather than blocked and was not blocked by Ba+2 (Figs. 11 and 12) or in the absence of external Na+ (Figs. 13 and 14). These experiments suggest that Rhbg-mediated NH4+ transport is not caused by a change in conductance of either of these two cations. Finally, and most directly, we demonstrated that a mutation of Rhbg (W230F) did not affect expression in the oocyte membrane but caused complete loss of function (20). This makes it very unlikely that the effect of Rhbg on NH3/NH4+ (and MA/MA+) is indirect.
MA/MA+ Transport by Rhbg
Similar to NH4Cl, methyl amine (HCl) in solution exists as neutral CH3NH2 (MA) in equilibrium with its protonated acid form CH3NH3+ (MA+).
The main advantage of using methyl amine (HCl) instead of NH4Cl is the ability to measure uptake of [14C]methyl amine. However, transport of MA/MA+ by Rhbg in oocytes differs from that of NH3/NH4+. In voltage-clamp experiments (Fig. 1A), exposure to MA/MA+ did induce an inward current in oocytes expressing Rhbg. This is consistent with electrogenic transport of the protonated positively charged MA+ by Rhbg as observed with NH4+. On the other hand, MA/MA+ did not cause any inward current in H2O-injected oocytes (Fig. 1B). This suggests that MA+ is a good substrate for Rhbg and that there is minimal apparent endogenous transport of MA+ in oocytes.
If MA+ is the only species transported by Rhbg (as suggested by measurement of current), then the Vm and pHi changes should resemble those caused by NH4+. In H2O-injected oocytes, NH4Cl caused the expected depolarization and small pHi decrease (see Fig. 2). In contrast, MA/MA+ did not cause any change in Vm, and only a small and very slow increase in pHi (Fig. 2, segment cd). The absence of MA/MA+-induced change in Vm and pHi is consistent with minimal transport of MA/MA+ in control (H2O-injected) oocytes.
In oocytes expressing Rhbg, MA/MA+ induced a substantial and sustained depolarization, similar to that observed with NH3/NH4+ (Fig. 3). However, MA/MA+ also caused a fast and significant pHi increase (Fig. 3, segment cd). Whereas influx of MA+ can explain the MA/MA+-induced inward current and depolarization, it cannot explain the pHi increase. Instead, transport of the neutral MA by Rhbg could explain the pHi increase. This suggests two distinct pathways of MA and MA+ transport by Rhbg: 1) uptake of neutral MA leading to a pHi increase and 2) uptake of charged hydrogenated MA+ leading to depolarization and inward current.
Electrogenic MA+ Transport by Rhbg
Our model assumes that Rhbg expression in oocytes promotes two pathways of MA/MA+ transport. The first one mediates MA+ transport in an electrogenic manner much like NH4+ transport by Rhbg. The second allows for increased permeability (or transport) of electroneutral MA. To further characterize each pathway, we measured the MA+-induced current at different concentrations of MA+. The results of these experiments (Fig. 6) clearly indicate a saturable process suggesting that MA+ transport is carrier-mediated. This is similar to NH4+ transport by Rhbg as shown earlier (21). Uptake studies also agree with saturation kinetics of labeled MA/MA+ uptake as mentioned (16, 17). However, in those studies, uptake of labeled [C14]MA/MA+ is a reflection of total uptake of MA and MA+ and cannot distinguish between the two. In our study, measurement of current is likely a measure of MA+ transport and not the neutral MA.
Electroneutral MA Transport by Rhbg
To assess electroneutral transport by Rhbg, we relied on measurements of MA-induced pHi increase at different MA/MA+ concentrations. Our model predicts that 1) electroneutral MA transport is responsible for pHi increase and 2) Rhbg expression promotes increased transport of MA. Our results show that the MA-induced pHi change progressively increased with the MA/MA+ concentration gradient (Fig. 7A) and clearly saturated at higher concentrations of bath MA/MA+ (Km = 2 mM, Fig. 7B). Either overexpression of a membrane protein like Rhbg might have affected the intrinsic permeability of the cell membrane lipid bilayer to noncharged molecules like MA, or the Rhbg protein facilitates influx of MA.
Relative Fluxes of MA/MA+ and NH3/NH4+ in Oocytes Expressing Rhbg
On the basis of measurements of pHi and whole cell currents, our data support enhanced transport of NH3/NH4+ and MA/MA+ by Rhbg. In both cases, pHi changes are likely determined by the relative fluxes of NH3 to NH4+ (i.e., JNH3/JNH4+ or JMA/JMA+) when oocytes are exposed to solutions containing these salts. For pHi not to change upon exposure to NH3/NH4+, the influx of NH3 (JNH3) should be matched by an influx of NH4+ (JNH4+) equivalent to the ratio of 10(pHi−pKa). In oocytes expressing Rhbg, exposure to 5 mM NH4Cl caused a bigger decrease in pHi (ΔpHi) at a faster rate and a larger inward current compared with H2O-injected oocytes. Both changes are consistent with a net increase in the ratio of JNH4+ to JNH3, which is greater in Rhbg than in H2O-injected oocytes. Any increase in JNH4+ is relative and does not exclude an increase in JNH3 as well. Several models addressing the effect of NH3 and NH4+ fluxes on predicted pHi and surface pH changes have been recently described (19). In a previous study (22) we reported that NH4+-induced pHi decrease in H2O-injected oocytes has to be accompanied by sequestration (or removal) of intracellular NH3 (whether resulting from NH3 influx or intracellular generation) to allow for the observed magnitude of pHi decrease to occur. Thus, because influx of NH3 as well as NH4+ may occur simultaneously, it is very difficult to assess individual contribution of each to the observed pHi or current change.
As for MA/MA+, estimates of permeability based on pHi measurements are more difficult because of the evidence that fluxes of MA as well as MA+ are enhanced by expression of Rhbg. Following the same approach as above, and assuming the increase in pHi is caused by MA influx, expressing Rhbg causes a significant increase in JMA/JMA+. It is to be noted that accurate assessment of relative permeabilities is complex and needs additional measurements and modeling of transport of the different entities (MA, MA+, NH3, and NH4+) that are beyond the scope of this work.
Amiloride Inhibition of MA/MA+ Transport by Rhbg
We have previously shown that amiloride inhibited electrogenic NH4+ transport by Rhbg. In the present study, we showed in voltage-clamp experiments that amiloride also inhibited MA+-induced current (Fig. 8). This effect was evident in oocytes expressing Rhbg but not in H2O-injected oocytes. This suggests that the mechanism of electrogenic MA+ transport by Rhbg is similar to that of NH4+. The degree of inhibition of the MA+ current is moderate (25%) but consistent (P < 0.005). Amiloride also caused an inhibition of the rate of MA-induced pHi increase as shown in Fig. 9. This effect is unlikely if electroneutral MA transport is purely diffusive through the lipid bilayer and not mediated by Rhbg.
Our model suggests that MA/MA+ transport by Rhbg should resemble NH3/NH4+ transport in mammalian cells as described by Boron and De Weer (7). In most mammalian cells there is an initial NH3-induced alkalinization followed by a plateau acidification caused by a slower NH4+ influx. If this is true for MA/MA+ transport by Rhbg, then in a large cell like the oocyte, prolonged exposure to MA/MA+ should also show an initial alkalinization followed by a slower acidification. To test this hypothesis, we exposed oocytes expressing Rhbg to MA/MA+ first for a short pulse followed by another pulse of longer exposure for more than 10 min. As shown in Fig. 5, exposing oocytes to MA/MA+ for a longer period of time clearly shows initial alkalinization (de) followed by a slower acidification (ef). Subsequent removal of bath MA/MA+ caused recovery of pHi (fgh) with an evident undershoot (fg) in pHi. These pHi transients resemble those caused by NH3/NH4+ in mammalian cells where permeability to NH3 and NH4+ occurs. These data indicate that transport of MA and MA+ is likely enhanced by expression of Rhbg.
Two interesting points are observed in these studies. One anomalous characteristic is that any MA effect in H2O-injected oocytes is small (no or little alkalinization, as shown in Fig. 2), suggesting low-permeability diffusion to MA. In contrast, in mammalian cells, transport of neutral species (e.g., NH3) by diffusion through the lipid bilayer is significant and fast. Another unique and unusual feature in this case is that Rhbg expression seems to enhance transport of both species, MA as well as MA+, further suggesting that MA transport is not simply by permeability diffusion.
The results of this study provide for possible reconsideration of the MA+-H+ exchanger hypothesis for Rhbg. Most studies supporting this mechanism relied on measurements of labeled MA/MA+ that were not blocked by manipulating the membrane potential. Our studies indicate two components of MA/MA+ transport by Rhbg: 1) electrogenic MA+ and 2) electroneutral MA. Both components seem to be enhanced by Rhbg expression. Consequently, it is very difficult to distinguish between the two from labeled uptake studies that measure a sum total of both. In one study (17), uptake of labeled MA/MA+ was enhanced in oocytes expressing Rhbg but was not affected by clamping the oocyte at different membrane potentials. Hence it was concluded that uptake of MA/MA+ by Rhbg was electroneutral. However, those data cannot rule out that enhanced uptake of labeled compounds by Rhbg was due to enhanced uptake of MA through the electroneutral component, which our studies show to be stimulated by Rhbg expression as well.
In conclusion, we have demonstrated that MA/MA+ is transported by Rhbg. An electroneutral component, namely, MA, is enhanced by Rhbg expression. An electrogenic component, MA+, is transported by Rhbg. The charged MA+ seems to be a direct substrate for Rhbg whose transport likely resembles that of NH4+. Our data do not support the model that Rhbg is an electroneutral NH4+-H+ exchanger. This study shows that a specific signature could be utilized to distinguish between the two components of MA/MA+ transport, pHi for MA and current for MA+. Thus teasing out an NH3 from NH4+ signal (or MA from MA+), for example, is very valuable. This is important because one of the unanswered questions in studies of Rhbg and related Rh membrane proteins is which physiologic substrate is carried by Rhbg. Is it NH4+, NH3, or maybe CO2, and how closely does transport of MA/MA+ resemble that of NH3/NH4+? Our data clearly indicate distinct differences between MA/MA+ and NH3/NH4+ transport by oocytes expressing Rhbg. These differences may well explain some of the controversies related to the mechanism of NH4+ transport by Rhbg.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-62295 and American Heart Association Southern Affiliate Grant 0255258B.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Ammann D, Lanter F, Steiner RA, Schulthess P, Shijo Y, Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem 53: 2267–2269, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Avent ND, Reid ME. The Rh blood group system: a review. Blood 95: 375–387, 2000 [PubMed] [Google Scholar]
- 3.Bakouh N, Benjelloun F, Cherif-Zahar B, Planelles G. The challenge of understanding ammonium homeostasis and the role of the Rh glycoproteins. Transfus Clin Biol 13: 139–146, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem 279: 15975–15983, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bishop J, Verlander JW, Lee HW, Nelson RD, Handlogten ME, Weiner AJ, Weiner D. Role of the ammonia transporter family member, RhB glycoprotein, in acidosis stimulated renal ammonia excretion. (Online Abstract) Renal Week 2009: American Society of Nephrology (ASN) 2009 Annual Meeting. http://www.abstracts2view.com/asn/index.php
- 6.Boldt M, Burckhardt G, Burckhardt BC. NH(4)(+) conductance in Xenopus laevis oocytes. III. Effect of NH(3). Pflügers Arch 446: 652–657, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burckhardt BC, Fromter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch 420: 83–86, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in the mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH(3)/NH(4)(+) transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Keicher E, Meech R. Endogenous Na(+)-K+ (or NH4+)-2Cl− cotransport in Rana oocytes; anomalous effect of external NH4+ on pHi. J Physiol (Lond) 475: 45–57, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kustu S, Inwood W. Biological gas channels for NH3 and CO2: evidence that Rh (rhesus) proteins are CO2 channels. Transfus Clin Biol 13: 103–110, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Chen Y, Mo R, Hui C, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J Physiol 559: 751–759, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4(+). J Membr Biol 228: 15–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakhoul N, Abdulnour-Nakhoul S, Le M, Rabon E, Doetjes R, Hamm L. Trp-230 in the hydrophobic pore of Rhbg is essential for transport of NH3/NH4+. FASEB J 23: 1012, 2009 [Google Scholar]
- 21.Nakhoul NL, Dejong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281: F255–F263, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Nakhoul NL, Schmidt E, Abdulnour-Nakhoul SM, Hamm LL. Electrogenic ammonium transport by renal Rhbg. Transfus Clin Biol 13: 147–153, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Nicolas V, Mouro-Chanteloup I, Lopez C, Gane P, Gimm A, Mohandas N, Cartron JP, Le Van Kim C, Colin Y. Functional interaction between Rh proteins and the spectrin-based skeleton in erythroid and epithelial cells. Transfus Clin Biol 13: 23–28, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Palkova Z, Forstova J. Yeast colonies synchronise their growth and development. J Cell Sci 113: 1923–1928, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Palkova Z, Janderova B, Gabriel J, Zikanova B, Pospisek M, Forstova J. Ammonia mediates communication between yeast colonies. Nature 390: 532–536, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Peng J, Huang CH. Rh proteins vs Amt proteins: an organismal and phylogenetic perspective on CO2 and NH3 gas channels. Transfus Clin Biol 13: 85–94, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Planelles G. Ammonium homeostasis and human Rhesus glycoproteins. Nephron Physiol 105: p11–p17, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Siebens AW, Boron WF. Effect of electroneutral luminal and basolateral lactate transport on intracellular pH in salamander proximal tubules. J Gen Physiol 90: 799–831, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soupene E, Chu T, Corbin RW, Hunt DF, Kustu S. Gas channels for NH(3): proteins from hyperthermophiles complement an Escherichia coli mutant. J Bacteriol 184: 3396–3400, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soupene E, Lee H, Kustu S. Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc Natl Acad Sci USA 99: 3926–3931, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Westhoff CM, Wylie DE. Transport characteristics of mammalian Rh and Rh glycoproteins expressed in heterologous systems. Transfus Clin Biol 13: 132–138, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391: 33–40, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]