Abstract
Lipopolysaccharide (LPS) has been implicated in sepsis-mediated heart failure and chronic cardiac myopathies. We determined that LPS directly and reversibly affects cardiac myocyte function by altering regulation of intracellular Ca2+ concentration ([Ca2+]i) in immortalized cardiomyocytes, HL-1 cells. [Ca2+]i oscillated (<0.4 Hz), displaying slow and transient components. LPS (1 μg/ml), derived either from Escherichia coli or from Salmonella enteritidis, reversibly abolished Ca2+ oscillations and decreased basal [Ca2+]i by 30–40 nM. HL-1 cells expressed Toll-like receptors, i.e., TLR-2 and TLR-4. Thus, we differentiated effects of LPS on [Ca2+]i and Ca2+ oscillations by addition of utlrapure LPS, a TLR-4 ligand. Ultrapure LPS had no effect on basal [Ca2+]i, but it reduced the rate of Ca2+ oscillations. Interestingly, Pam3CSK4, a TLR-2 ligand, affected neither Ca2+ parameter, and the effect of ultrapure LPS and Pam3CSK4 combined was similar to that of utlrapure LPS alone. Thus, unpurified LPS directly inhibits HL-1 calcium metabolism via TLR-4 and non-TLR-4-dependent mechanisms. Since others have shown that endotoxin impairs the hyperpolarization-activated, nonselective cationic pacemaker current (If), which is expressed in HL-1 cells, we utilized whole cell voltage-clamp techniques to demonstrate that LPS (1 μg/ml) reduced If in HL-1 cells. This inhibition was marginal at physiologic membrane potentials and significant at very negative potentials (P < 0.05 at −140, −150, and −160 mV). So, we also evaluated effects of LPS on tail currents of fully activated If. LPS reduced the slope conductance of the tail currents from 498 ± 140 pS/pF to 223 ± 65 pS/pF (P < 0.05) without affecting reversal potential of −11 mV. Ultrapure LPS had similar effect on If, whereas Pam3CSK4 had no effect on If. We conclude that LPS inhibits activation of If, enhances its deactivation, and impairs regulation of [Ca2+]i in HL-1 cardiomyocytes via TLR-4 and other mechanisms.
Keywords: Toll-like receptors, patch-clamp electrophysiology, Fura-2/AM Ca2+ fluorescence, pacemaker current
it is well established that sepsis releases into the circulation lipopolysaccharides (LPS) that have deleterious effects on cardiac function and play a significant role in development of chronic and acute heart failure (6, 27). LPS are membrane components of Gram-negative bacteria that activate the innate immune system via germline-encoded pattern recognition receptors or Toll-like receptors (TLR) (1). TLRs are type 1 integral membrane glycoproteins with leucine-rich repeats in the extracellular domain and an intracellular signaling domain homologous to the interleukin 1 receptor (IL-1R) designated the Toll/IL-1R homology domain (10). TLR-2 and -4 are the most studied TLRs in the heart (5). Lipoproteins, peptidoglycans, and other constituents of Gram-positive bacteria are thought to activate TLR-2, whereas LPS from Gram-negative bacteria primarily activate TLR-4 (1).
TLRs play a significant role in sepsis-mediated damage to the myocardium (6, 10); however, our understanding of the mechanisms is far from complete. TLR-4 is the primary receptor for LPS activation of inflammatory cells, and mice deficient in TLR-4 display reduced myocardial ischemia-reperfusion injury (19). Nonetheless, preconditioning, either by ischemia (18) or parenteral administration of LPS (4), ameliorates ischemic-reperfusion injury. We have reported that LPS mediates cardiac preconditioning via a phosphoinositide 3-kinase/Akt-dependent signaling mechanism (12). Yet, we are unable to differentiate the mechanisms of the cardioprotective effects of LPS in preconditioning from the mechanisms by which LPS damages the myocardium.
Endotoxemia in humans reduces heart rate variability (11), which alone has a poor prognosis (23). LPS added to rat neonatal cardiomyocytes in vitro effects a similar reduction in heart rate variability, which led to the conclusion that this results not from modulation of central or peripheral neurons but rather from remodeling of cardiac pacemaker cells (24). In a similar manner, LPS added in vitro for 6 to 10 h reduced the hyperpolarization-activated nonselective cation pacemaker current (If) in human myocytes isolated from right atrial appendages (29).
In the present study, we have utilized HL-1 cells, which are proliferating atrial myocytes established from a subcutaneous tumor of AT-1 cells that, in turn, were derived from the atria of a mouse transgenic for the simian virus 40 large T antigen under control of the atrial natriuretic factor promoter (7). These cells display oscillations of intracellular free Ca2+, [Ca2+]i, and If is present in about 30% of the cells (22). We report that LPS from Escherichia coli and from Salmonella enteritidis rapidly and reversibly reduce Ca2+ oscillations and total [Ca2+]i in HL-1 cells, as well as inhibit If.
METHODS
HL-1 cell culture.
HL-1 atrial cardiomyocytes were a gift of Dr. William Claycomb (Louisiana State University Medical Center, New Orleans, LA). They were grown in 5% CO2 at 37°C in Claycomb media (Sigma) supplemented with batch-specific 10% FBS (Sigma), 100 U/ml:100 ug/ml penicillin-streptomycin (Invitrogen), 0.1 mM norepinephrine (Sigma), and 2 mM l-glutamine (Invitrogen). Before culturing of cells, flasks were treated overnight with 0.02% Bacto gelatin (Fisher Scientific):0.5% Fibronectin (Invitrogen). For calcium measurements, cells were plated at a density of 3 × 105 cells/35-mm culture dish on glass coverslips (12 mm diameter) that had been briefly flamed to enhance coating and were then transferred to a 35-mm culture dish where they were treated with gelatin-fibronectin overnight.
Intracellular Ca2+ measurements.
Cells were loaded with Fura-2/AM (TefLabs, Austin, TX) by incubating them for 30 min at room temperature (22–23°C) with a standard external salt solution containing 2-μM Fura-2/AM. Cells were then washed with the external salt solution and incubated at 37°C with 5% CO2 for 30 min in the supplemented Claycomb media. The coverslip was transferred to an acrylic chamber (Warner Instruments, Hamden, CT) on the microscope stage and washed with the external salt solution for 5 min before measurements. Temperature throughout measurements was 37°C maintained by a stage/inline controller (Warner Instruments) Fluorescence was measured with an imaging system consisting of a filter wheel and a Basler A311F VGA camera connected to a AccuScope 3030 inverted fluorescence microscope. The filter wheel and data acquisition were controlled by the InCyte2 software (version 5.29; Intracellular Imaging, Cincinnati, OH). [Ca2+] was determined by interpolation from a standard curve generated by a Ca2+ calibration buffer kit #2 (Molecular Probes) and Fura-2/K5-salt. After correction for the individual background fluorescence, the ratio of the fluorescence at both excitation wavelengths (F340/F380) was monitored simultaneously in 30–40 cells. Once data collection began, each slide was perfused with the standard external solution for 2 min, followed by 5 min with 10 ng/ml LPS-binding protein (LBP; an adjuvant to enhance LPS binding to TLRs) in the standard external solution and then 37 min with the experimental solution plus LBP. At 37 min, the slide was washed with the standard external solution for 3 min, and at 47 min the data collection was stopped. Data were collected every 10 s, exported to Excel (Microsoft), and graphed using Origin 7.0 (OriginLab, Northampton, MA).
Whole cell voltage-clamp measurements.
Cells were grown for 1–2 days on 12-mm-diameter glass plastic coverslips, which were transferred to an acrylic chamber (Warner, New Haven, CT) on the stage of an inverted microscope (Olympus IMT-2) equipped with Hoffman modulation contrast optics. Cells were superfused at room temperature with a standard external salt solution. Patch pipettes (3–6 MΩ in the bath solution) were fabricated from glass capillaries [1.1–1.2 mm ID, 0.2-mm wall thickness, nonheparinized micro-hematocrit capillary tubes (Fisher)] with a Brown-Flaming horizontal micropipette puller (model P-97, Sutter Instruments, Novato, CA). A micromanipulator (model MO-202, Narishige, Tokyo) fixed to the microscope was used to position pipettes. The whole cell patch configurations were obtained by standard patch-clamp technique (13). Membrane currents were measured with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA) with the low-pass, Bessell filtering (−3 dB) set at 5 kHz. Whole cell currents from the patch-clamp amplifier were fed into a computer via a digital interface (Digidata 1322A) and processed by Clampex 8 software (Axon Instruments). Ag/AgCl half-cells constituted the electrodes, and an agar bridge (4% wt/vol in external solution) connected the reference electrode to the bath solution. Series resistances were compensated following whole cell access before recordings were obtained.
Toll-like receptor expression in HL-1 cells.
For flow cytometry analysis, HL-1 cells were lifted by scraping into lidocaine (21). Cells were blocked with 5% rabbit serum, 0.5% bovine serum albumin, and 5 mM EDTA before staining. Cells were stained with biotinylated anti-TLR-4 and anti-TLR-2 antibodies (eBioscience, San Diego, CA). Staining was performed according to conventional protocols at 4°C. Biotinylated antibodies were detected by streptavidin-phycoerythrin (Pharmingen). Cells were suspended in Pharmingen Stain Buffer and analyzed using a FACScalibur flow cytometer with CellQuest software (BD Biosciences, Mountain View, CA).
Solutions and chemicals.
Standard external salt solution contained (in mM) 150 NaCl, 6 KCl, 1 MgCl2, 1.5 CaCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and 10 glucose (pH was adjusted to 7.41 with NaOH). External salt solution was modified from the preceding to measure If and contained (in mM) 25 KCl (isosmotically substituted for NaCl to maximize If) and added supplements, 2 NiCl2, 2 BaCl2, and 0.4 4-aminopyridine (pH was adjusted to 7.41 with NaOH). To measure voltage-activated Ca2+ currents, the external salt solution contained (in mM) 156 N-methyl-d-glucamine, 1 MgCl2, 5 CaCl2, 10 HEPES, and glucose 10 (pH was adjusted to 7.41 with HCl).
Pipette solution contained (in mM) 120 potassium aspartate, 10 TEA Cl, 0.4 Na2GTP, 5 Na2ATP, 2 MgCl2, 11 EGTA, 5 CaCl2, and 10 HEPES (pH was adjusted to 7.2 with KOH).
Lipopolysaccharides from S. enteritidis and E. coli strain 055:B5 were obtained from Sigma Chemical (St. Louis, MO); ultrapure LPS from K12 strain and Pam3CSK4, a synthetic diacylated lipopeptide, were obtained from Invivogen (San Diego, CA); and recombinant human LBP was obtained from R and D Systems (Minneapolis, MN).
Data analysis.
Results are expressed as means ± SE, and differences between means were computed by Student's paired t-test. Slope conductances were determined by linear regression analysis of current-voltage plots at the reversal potentials (−20 to 0 mV). Electrophysiological data were analyzed using WinASCD software (Guy Droogmans, Katholieke Universiteit, Leuven, Belgium; ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/winascd.zip).
RESULTS
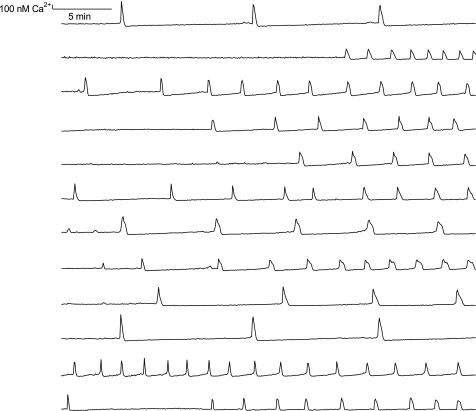
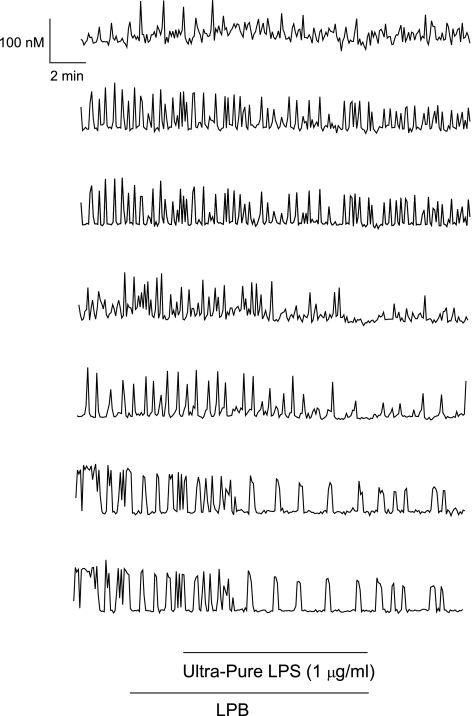
Intracellular Ca2+ concentrations, [Ca2+]i, in nonconfluent HL-1 cells oscillated asynchronously with a period of minutes (Fig. 1). Thorough examination showed that these oscillations consisted of two components: 1) slow increases in [Ca2+]i whose durations varied from seconds to minutes, followed by 2) transient increases in [Ca2+]i (Fig. 2). The latter presumably was elicited by action potentials involving voltage-dependent Ca2+ channels (28). The highest frequency for action potentials in nonconfluent HL-1 cells was 0.4 Hz as determined by on-cell measurements of outward delayed rectifier K+ current (Supplemental Fig. S1; Supplemental Material for this article is available online at the Journal website), which was considerably slower than 1.3 to 5 Hz reported for confluent HL-1 cells (22). However, only 39 ± 4.5% (n = 14 experiments; 434 cells total) of nonconfluent HL-1 cells displayed oscillations of [Ca2+]i despite the fact that all cells readily loaded Fura-2. These oscillations disappeared reversibly on reducing nominal external [Ca2+] to 0 mM (Supplemental Fig. S2), and their frequency increased as did total [Ca2+]i on addition of isoproterenol (10 μM) (Supplemental Fig. S3).
Fig. 1.
Asynchronous oscillations of intracellular Ca2+ concentration ([Ca2+]i) in nonconfluent, immortalized mouse cardiomyocytes, HL-1 cells. Traces shown are from simultaneous recordings of Fura-2 fluorescence ratios obtained from 12 cells in a single microscopic field.
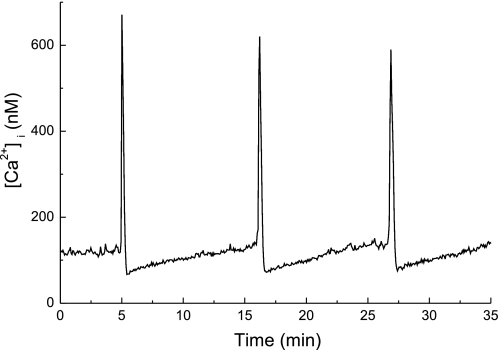
Fig. 2.
[Ca2+]i oscillations in HL-1 cells comprise two components: a slow increase in [Ca2+]i preceding a larger, transient increase in [Ca2+]i. The duration of the slow increase was 10 min but varied from seconds to minutes depending on the frequency of the transient increases in [Ca2+]i (not shown).
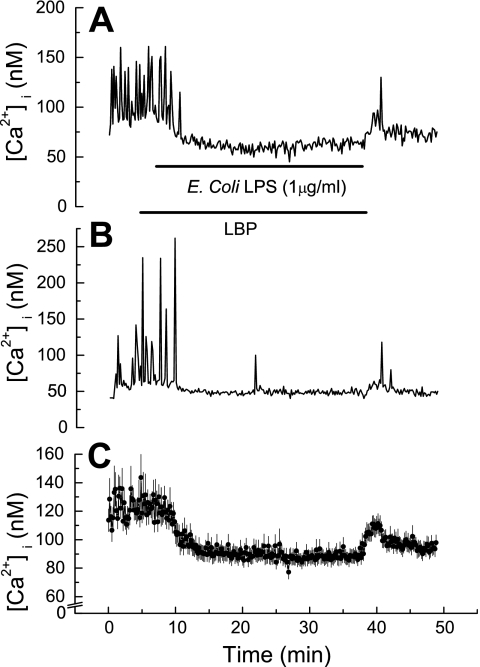
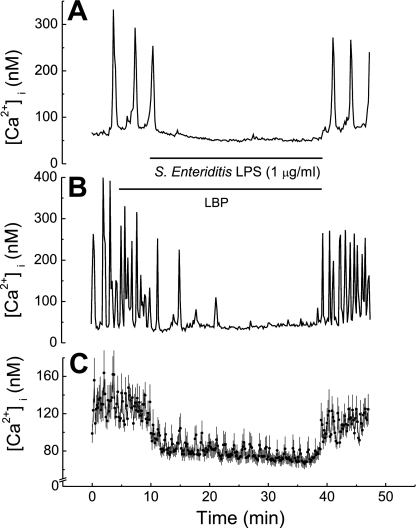
Considering that lipopolysaccharides have been implicated in endotoxin-mediated heart failure and chronic cardiac myopathies, we set out to determine whether lipopolysaccharides directly affect cardiac myocyte function by altering regulation of HL-1 cell [Ca2+]i. Superfusing HL-1 cells with LPS (1 μg/ml) derived from either E. coli (Fig. 3) or S. enteritidis (Fig. 4) resulted in rapid cessation of [Ca2+]i oscillations as well as a decrease in basal [Ca2+]i by 30–40 nM. The latter reversed quickly on washout of lipopolysaccharides in both instances (Figs. 3 and 4). However, washout of S. enteritidis LPS resulted in complete restoration of [Ca2+]i oscillations (Fig. 4), whereas washout of E. coli LPS resulted in modest restoration of [Ca2+]i oscillations (Fig. 3).
Fig. 3.
Effect of lipopolysaccharide (LPS) derived from Escherichia coli on basal [Ca2+]i and [Ca2+]i oscillations in HL-1 cells. A: [Ca2+]i versus time in a single HL-1 cell. Trace shows both inhibition of [Ca2+]i oscillations plus reduction in basal [Ca2+]i. B: [Ca2+]i versus time in a single HL-1 cell. Trace shows only inhibition of [Ca2+]i oscillations. C: [Ca2+]i versus time. Values are means ± SE (n = 30 cells). Horizontal bars show application time of LPS-binding protein (LBP; 10 ng/ml) and of LPS for all traces.
Fig. 4.
Effect of LPS derived from Salmonella enteritidis on basal [Ca2+]i and [Ca2+]i oscillations in HL-1 cells. A: [Ca2+]i versus time in a single HL-1 cell. Trace shows both inhibition of [Ca2+]i oscillations plus reduction in basal [Ca2+]i. B: [Ca2+]i versus time in a single HL-1 cell. Trace shows only inhibition of [Ca2+]i oscillations. C: [Ca2+]i versus time. Values are means ± SE (n = 16 cells). Horizontal bars show application time of LBP (10 ng/ml) and of LPS for all traces.
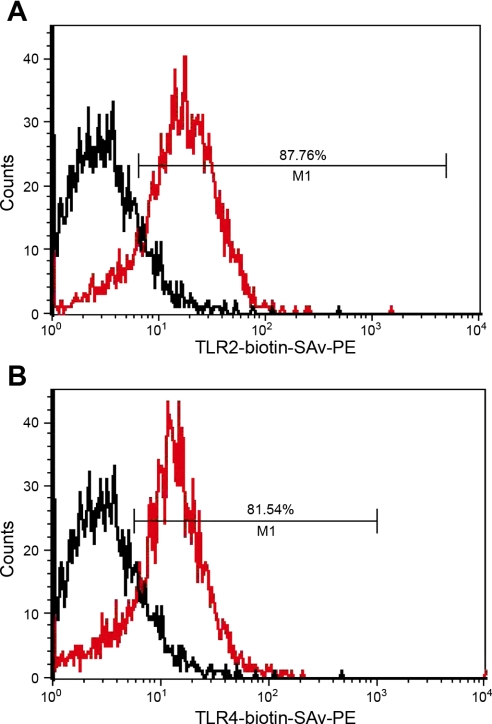
Toll-like receptors play a central role in innate immune response to lipopolysaccharides of Gram-negative pathogens, and TLR-4 has been specifically implicated in the innate response in the heart (19). HL-1 cells express TLR-4 as well as TLR-2 as determined by flow cytometry (Fig. 5, A and B). Thus we applied utlrapure LPS to HL-1 cells, since it has been proposed to be a primary TLR-4 agonist (14). Utlrapure LPS (1 μg/ml) had no effect on basal [Ca2+]i, but it decreased the rate of [Ca2+]i oscillations (Fig. 6 and Table 1). In an effort to differentiate TLR-4 from TLR-2 effects, we also assessed effects of Pam3CSK4, which is thought to be a pure agonist for TLR-2 (2, 20). It had no effect on either basal [Ca2+]i or [Ca2+]i oscillations (Table 1). The combination of Pam3CSK4 plus ultrapure LPS reduced the rate of [Ca2+]i oscillations with no effect on basal [Ca2+]i, which was identical to effects of ultrapure LPS alone (Table 1).
Fig. 5.
Flow cytometry analysis of Toll-like receptors (TLR) in live intact HL-1 cells. Black, unstained HL-1 cells; red, antibody-stained HL-1 cells. A: TLR-4. B: TLR-2. Horizontal lines in each trace indicate the percentage of cells staining positive for TLR. SAv-PE, streptavidin-phycoerythrin.
Fig. 6.
Effect of ultrapure LPS on [Ca2+]i oscillations in HL-1 cells. Results are shown from seven cells. LBP, 10 ng/ml.
Table 1.
Effect of inhibitors of TLR-4 (ultrapure LPS), TLR-2 (Pam3CSK4), and If (CsCl) on the rate of [Ca2+]i oscillations in HL-1 cells
| Agent | Control, Hz | Inhibitor, Hz | Washout, Hz |
|---|---|---|---|
| Ultrapure LPS, 1 μg/ml | 0.045 ± 0.003 (13) | 0.016 ± 0.003* | NA |
| Pam3CSK4, 10 μg/ml | 0.043 ± 0.004 (10) | 0.048 ± 0.005 | NA |
| Ultrapure LPS + Pam3CSK4 | 0.037 ± 0.006 (12) | 0.015 ± 0.003* | NA |
| CsCl, 4 mM | 0.037 ± 0.004 (3) | 0.013 ± 0.002† | 0.043 ± 0.008 |
Values are means ± SE; no. of paired measurements is shown in parentheses. TLR, Toll-like receptor; If, pacemaker current; [Ca2+]i, intracellular Ca2+ concentration; NA, not applicable.
Significantly different from control, P < 0.001;
significantly different from control and washout, P < 0.05.
Characterization of electrical properties of HL-1 demonstrated that action potentials could be elicited readily by current stimulation (Supplemental Fig. S4A). In addition, voltage activation elicited both inward and outward currents (Supplemental Fig. S4B), of which the inward current also was voltage inactivated (Supplemental Fig. S4C). These occurred readily in all HL-1 cells on stimulation.
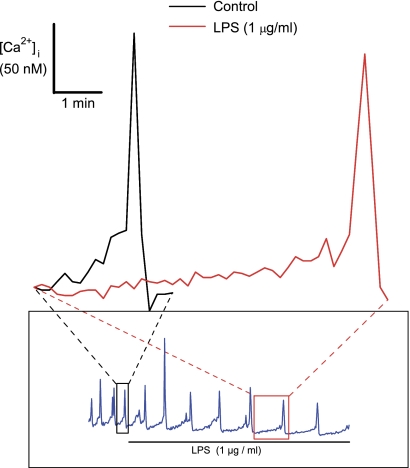
Added E. coli LPS reduced the rate of increase of the slow component that preceded the transient increase in [Ca2+]i attributable to action potentials from 607 ± 80 pM/s to 123 ± 45 pM/s (P < 0.002; n = 5; Fig. 7). Moreover, endotoxin impairs the human pacemaker current, If, (29), and this current is expressed in HL-1 cells, along with corresponding hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channels (22). Thus, we sought to determine whether LPS might alter If in HL-1 cells. In particular, we aimed to determined whether rapid effects on If might account for the speed by which endotoxins reduce [Ca2+]i oscillations in HL-1 cells. As an initial step toward this end, we employed two standard voltage-clamp protocols to measure 1) voltage dependence of hyperpolarization-activated If, and 2) current-voltage relationships of fully activated If in HL-1 cells to determine reversal potentials (Supplemental Fig. S5, A and B). We opted not to correct for time-independent or leakage components of our recordings, thinking that this might mask currents of biologic importance.
Fig. 7.
Traces showing expanded time courses of the effect of E. coli LPS on the increases of slow and transient components of [Ca2+]i oscillations in an HL-1 cell. Inset: original traces from which the expanded traces were obtained. Red and black boxes and dashed lines indicate the original position of the expanded traces. LBP (10 ng/ml) was present but is not indicated.
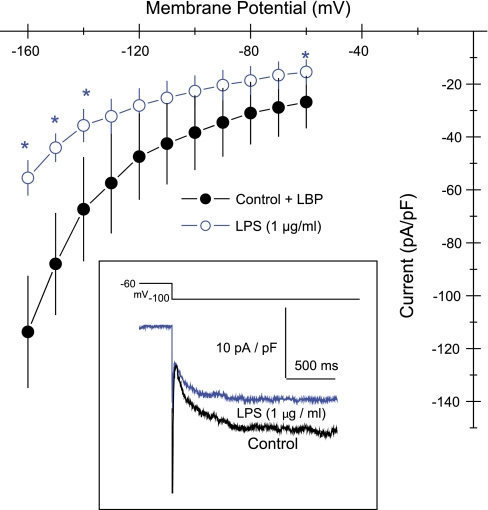
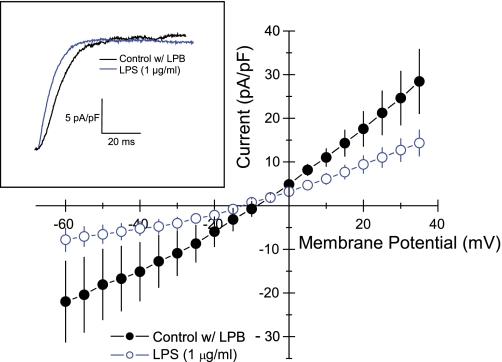
LPS (1 μg/ml) reduced the hyperpolarization-activated nonselective cationic current in HL-1 cells (Fig. 8, inset). This inhibition was significant only at very negative potentials (P < 0.05 at −140, −150, and −160 mV and at the holding potential of −60 mV; Fig. 8). Thus, we further evaluated effects of LPS on tail currents of If fully activated at −120 mV. Here, LPS (1 μg/ml) reduced the nonselective cationic current over a range of voltages ranging from −60 to 35 mV (Fig. 9). Slope conductance of control cells measured at the reversal potential was 498 ± 140 pS/pF (n = 5). Added LPS (1 μg/ml) reduced this to 223 ± 65 pS/pF (n = 5; P < 0.03). However, reversal potentials of −11 ± 2 mV (n = 5) for control cells were unaffected by added LPS (−13 ± 3 mV; n = 5). The disparity between the inhibitions of LPS on If activation at very negative membrane potentials compared with those on tail currents, which occurred over a range of physiologic membrane potentials, indicated that LPS more readily enhances deactivation of If compared with blocking its activation. As an initial step to examine this possibility, we compared the time courses of If decreases during step changes of the voltage clamp from −160 mV to −30 mV in the same cell before and after added LPS (E. coli, 1 μg/ml). The current deactivated considerably faster after LPS treatment (Fig. 9, inset). In addition, Cs+ (4 mM), which inhibits If and spontaneous beating in HL-1 cells (22), markedly slowed the rate of [Ca2+]i oscillations (Table 1).
Fig. 8.
Current-voltage plots of hyperpolarization-activated inward current, If, in HL-1 cells. ●, Control cells; ○, cells treated with E. coli LPS. Values are means ± SE (n = 6). *Significantly different from control (P < 0.05). Inset: representative traces from the same cell before (black) and after (blue) added LPS. Holding potential (HP) = −60 mV.
Fig. 9.
Current-voltage plots of fully activated If (tail currents) in HL-1 cells. ●, Control cells; ○, cells treated with E. coli LPS. Values are means ± SE (n = 5). HP = −60 mV; activation potential = −120 mV. Inset: superimposed time courses of deactivation current before (black) and after (blue) added LPS. HP = 60 mV; activation potential = −160 mV; tail potential = −30 mV.
Voltage-activated Ca2+ currents also contribute to cardiac pacemaker potentials, particularly the long-lasting or L-type Ca2+ currents (16), which are expressed in HL-1 cells (28). LPS had no effect on L-type Ca2+ current in HL-1 cells, at least not in the time course in which LPS inhibited Ca2+ oscillations in these cells (Supplemental Fig. S6, A and B).
DISCUSSION
These results show a direct inhibitory effect of E. coli- and S. enteritidis-derived LPS on mouse cardiomyocyte [Ca2+]i, both on rate of oscillations and on total ion concentration. [Ca2+]i controls the inotropic state of the myocardium. Since LPS diminishes [Ca2+]i, we conclude that, as such, it impairs contractility and thus may account in part for chronic and acute failure of the heart resulting from infection and endotoxin release into the circulation. This implies that harmful effects of endotoxins on cardiac function also result from their direct actions on cardiac myocytes in addition to deleterious effects mediated by secondary agents liberated from their activation of systemic inflammatory responses (6).
These findings also indicate that effects of LPS on cardiomyocyte [Ca2+]i are mediated in part by Toll-like receptors, particularly TLR-4. Ultrapure LPS activates only the TLR-4 pathway (14, 15), so we conclude that LPS slows Ca2+ oscillations in HL-1 cells through activation of TLR-4. However, both E. coli and S. enteritidis LPS had greater inhibitory effects on the Ca2+ variables in HL-1 cells than those observed with ultrapure LPS. Impurities such as lipopeptides found in cruder extractions of LPS are known to activate other TLRs, specifically, TLR-2. However, we ruled out TLR-2 activation, because Pam3CSK4, a TLR-2 agonist, had no effect on either Ca2+ oscillations or basal [Ca2+]i, either alone or in combination with ultrapure LPS. These findings suggest that LPS in its crude form interacts with TLR-4 and other, yet unknown HL-1 receptors to directly and rapidly inhibit Ca2+ metabolism.
Our findings confirm the existence of If in HL-1 cells (22). Our computation of its maximal slope conductance is greater than reported by these investigators (22), which may result because we did not correct for time-independent components of the tail currents. Yet, it agrees well with that reported for mouse sinoatrial cells (17, 26). Added LPS significantly decreases this slope conductance, which indicates that LPS diminishes the rate of Ca2+ oscillations in HL-1 cells in part by inhibiting the If. LPS has been reported to impair If measured 6 to 10 h following administration to human myocytes obtained from right atrial appendages (29). Our results expand this finding by showing that this direct effect of LPS on cardiac myocytes occurs within minutes of its application to HL-1 cells. This observation strongly suggests a direct effect of LPS on HL-1 Ca2+ metabolism and it argues against the release of second messengers, such as cytokines, feeding back on HL-1 cells in an autocrine/paracrine fashion as mediators of the effect.
Inhibition of If activation by LPS was marginal in the physiologic range of transmembrane voltage and significant at transmembrane voltages more negative than the diastolic membrane potential of cardiac myocytes. It is noteworthy that the predominant expression of HCN ion channels by HL-1 cells comprises HCN-1 and HCN-2 compared with the predominance of HCN-4 by mouse sinoatrial node (3, 22). Nonetheless, we do not know whether these isoforms have differential sensitivity to endotoxins. A more likely explanation of our findings is that LPS enhances If deactivation with greater propensity than it inhibits its activation. We conclude, therefore, that LPS diminishes If in HL-1 cells by a novel mechanism, which may inhibit oscillations of [Ca2+]i. This latter conclusion, however, is not unequivocal until If and [Ca2+]i are measured isochronously during spontaneous [Ca2+]i oscillations.
A thorough analysis of the effects of LPS on kinetic parameters for activation and deactivation of If also is needed to delineate mechanisms by which LPS reduces If and [Ca2+]i oscillations in cardiac myocytes. However, this may require an expression system for the various HCN channels that constitute cardiac If, because the low percentage of HL-1 cells that express HCN channels places a technical limit on using HL-1 cells for this purpose. We have ruled out possible inhibition by LPS of voltage-dependent L-type Ca2+ currents. However, we cannot rule out other nonselective cation currents that may affect cardiac rhythm (16). The nonselective cation channel transient receptor potential melastatin 4 (TRPM4) is functionally expressed in mouse sinoatrial node cells and is purported to play a role in generating cardiac rhythm (9). It displays both Ca2+- and voltage-dependent activation, but its role as contributing to pacemaker current remains provisional (25). Its close homologue, TRPM5, plays a significant role in Ca2+ oscillations and glucose-induced insulin release from mouse pancreatic islets (8).
Only 39% of HL-1 cells exhibit oscillations of [Ca2+]i, this occuring despite the fact that all voltage-clamped cells display voltage-activated inward and outward current. This percentage of HL-1 cells showing oscillations of [Ca2+]i agrees quite well with the report that If is present in only 30% of HL-1 cells and is absent from cells lacking a spontaneous diastolic depolarization preceding the action potential (22). We conclude, therefore, that limited expression of If by HL-1 cells accounts for the limited number of cells that display oscillations of [Ca2+]i. This suggests that the HL-1 cell line comprises mixed phenotypes. It also is possible that HL-1 cells express If and Ca2+ oscillations in limited phases of the cell cycle; however, expression of If does not vary with the cell density (22).
In summary, we have utilized HL-1 immortalized cardiomyocytes to examine effects of endotoxin on [Ca2+]i oscillations by these cells. LPS from both E. coli and S. enteritidis directly and reversibly decreased [Ca2+]i oscillations and basal [Ca2+]i oscillations in these cells. We conclude that LPS primarily mediates effects on cardiomyocyte [Ca2+]i oscillations via TLR-4, whereas the effect of LPS on basal Ca2+ concentration is mediated via an non-TLR-4-dependent mechanism. Moreover, added LPS decreased If in HL-1 cells by inhibiting its activation and enhancing its deactivation.
GRANTS
This work was supported by National Institutes of Health Grants GM-083016 (to C. Li and D. L. Williams), HL-0718337 (to C. Li), and GM-53522 to (D. L. Williams).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Authors' contributions—RW: designed experiments, oversaw and conducted Ca2+ measurements, performed all electrophysiological measurements, wrote and edited the manuscript, and submitted the manuscript; BMG: maintained HL-1 cells in culture, performed Ca2+ measurements, and wrote and edited the manuscript; TRO-S: performed all flow cytometry measurements of Toll-like receptors and wrote and edited the manuscript; CL: designed experiments, contributed all reagents for TLR measurements by flow cytometry, and wrote and edited the manuscript; DLW: contributed extensively to the experimental design, routinely conferred with all authors and regularly convened the group for discussions on experimental design and on progress, and wrote and edited the manuscript.
Supplementary Material
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285: 736–739, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA 86: 2516–2520, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charalambous BM, Stephens RC, Feavers IM, Montgomery HE. Role of bacterial endotoxin in chronic heart failure: the gut of the matter. Shock 28: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci USA 107: 5208–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73: 531–538, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med 4: 444–454, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med 24: 1117–1124, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res 78: 546–553, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 165: 618–622, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest 105: 497–504, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev 88: 919–982, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mangoni ME, Nargeot J. Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res 52: 51–64, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA 97: 13766–13771, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinovitch M, DeStefano MJ. Use of the local anesthetic lidocaine for cell harvesting and subcultivation. In Vitro 11: 379–381, 1975 [DOI] [PubMed] [Google Scholar]
- 22.Sartiani L, Bochet P, Cerbai E, Mugelli A, Fischmeister R. Functional expression of the hyperpolarization-activated, non-selective cation current I(f) in immortalized HL-1 cardiomyocytes. J Physiol 545: 81–92, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt H, Muller-Werdan U, Hoffmann T, Francis DP, Piepoli MF, Rauchhaus M, Prondzinsky R, Loppnow H, Buerke M, Hoyer D, Werdan K. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med 33: 1994–2002, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt H, Saworski J, Werdan K, Muller-Werdan U. Decreased beating rate variability of spontaneously contracting cardiomyocytes after co-incubation with endotoxin. J Endotoxin Res 13: 339–342, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handbk Exper Pharmacol: 269–285, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Verkerk AO, van Ginneken AC, Wilders R. Pacemaker activity of the human sinoatrial node: role of the hyperpolarization-activated current, I(f). Int J Cardiol 132: 318–336, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Werdan K, Schmidt H, Ebelt H, Zorn-Pauly K, Koidl B, Hoke RS, Heinroth K, Muller-Werdan U. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol 87: 266–274, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Xia M, Salata JJ, Figueroa DJ, Lawlor AM, Liang HA, Liu Y, Connolly TM. Functional expression of L- and T-type Ca2+ channels in murine HL-1 cells. J Mol Cell Cardiol 36: 111–119, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Zorn-Pauly K, Pelzmann B, Lang P, Machler H, Schmidt H, Ebelt H, Werdan K, Koidl B, Muller-Werdan U. Endotoxin impairs the human pacemaker current If. Shock 28: 655–661, 2007 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.