Abstract
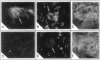
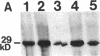
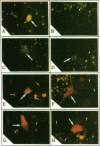
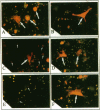
The bone resorbing cells, osteoclasts, express high levels of carbonic anhydrase II (CA II) and vacuolar H(+)-ATPase (V-ATPase) during bone resorption. We have used antisense RNA and DNA molecules targeted against CA II, and against 16- and 60-kD subunits of vacuolar H(+)-ATPase (V-ATPase), to block the expression of these proteins in vitro. Osteoclastic bone resorption was studied in two in vitro culture systems: release of 45Calcium from prelabeled newborn mouse calvaria cultures, and resorption pit assays performed with rat osteoclasts cultured on bovine bone slices. Both antisense RNA and DNA against CA II and the V-ATPase were used to compare their specificities as regards inhibiting bone resorption in vitro. The antisense molecules inhibited the synthesis of these proteins by decreasing the amounts of mRNA in the cells in a highly specific manner. In osteoclast cultures treated with the 16-kD V-ATPase antisense RNA, acidification of an unknown population of intracellular vesicles was highly stimulated. The acidification of these vesicles was not sensitive to amiloride or bafilomycin A1. This suggests the existence of a back-up system for acidification of intracellular vesicles, when the expression of the V-ATPase is blocked. Our results further indicate that blocking the expression of CA II and V-ATPase with antisense RNA or DNA leads to decreased bone resorption.
Full text
PDF

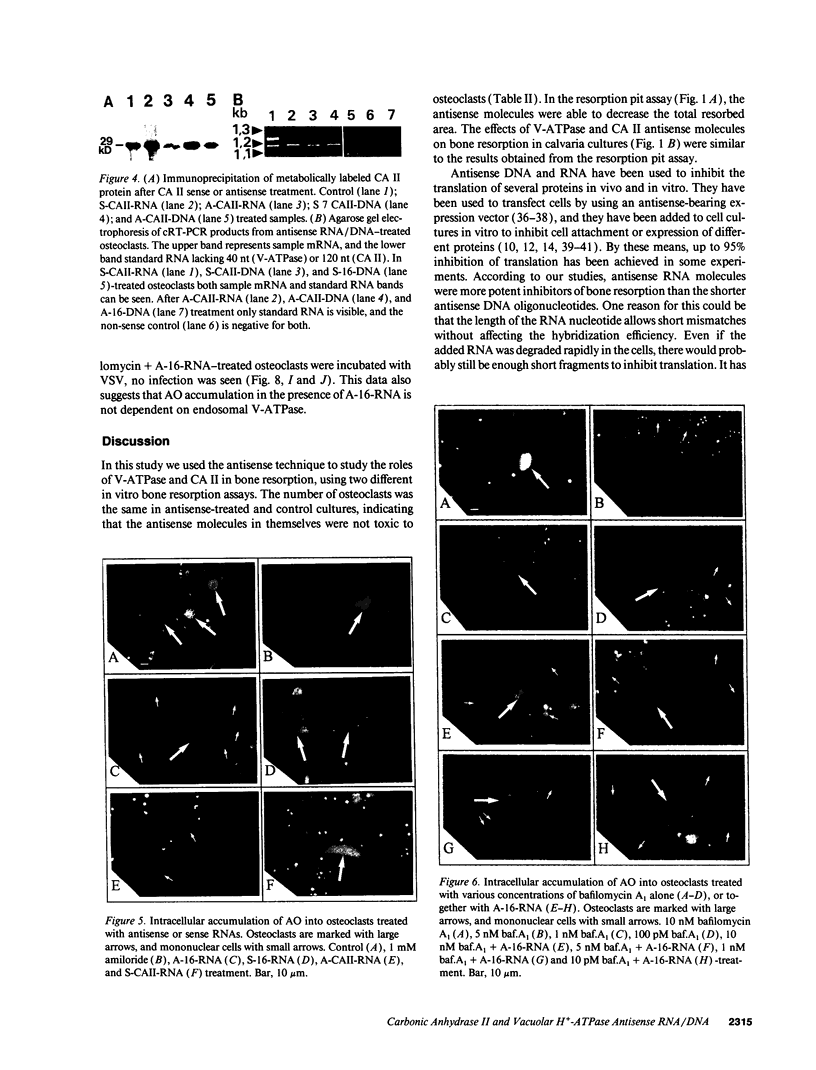
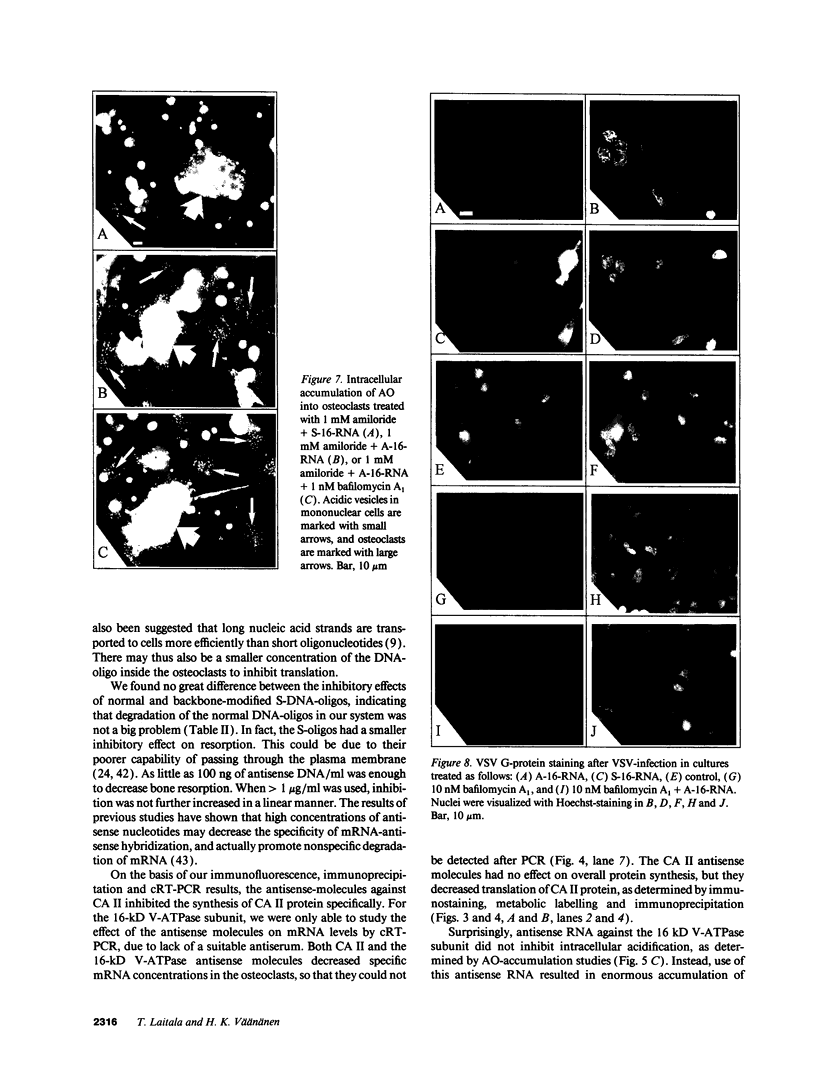


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabino S. M., Sproat B. S., Lamond A. I. Antisense probes targeted to an internal domain in U2 snRNP specifically inhibit the second step of pre-mRNA splicing. Nucleic Acids Res. 1992 Sep 11;20(17):4457–4464. doi: 10.1093/nar/20.17.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro S., Fu Y. M., Yu Z. X., Epstein S. E. Inhibitory effects of antisense oligodeoxynucleotides targeting c-myc mRNA on smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):654–658. doi: 10.1073/pnas.90.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989 Aug 25;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Murphy R. F. A chloroquine-resistant Swiss 3T3 cell line with a defect in late endocytic acidification. J Cell Biol. 1988 Feb;106(2):269–277. doi: 10.1083/jcb.106.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. J., Withers E., Demuth D., Watt R., Venta P. J., Tashian R. E. The nucleotide sequence and derived amino acid sequence of cDNA coding for mouse carbonic anhydrase II. Gene. 1983 Nov;25(2-3):325–332. doi: 10.1016/0378-1119(83)90237-8. [DOI] [PubMed] [Google Scholar]
- Eidelman O., Schlegel R., Tralka T. S., Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984 Apr 10;259(7):4622–4628. [PubMed] [Google Scholar]
- Gay C. V., Mueller W. J. Carbonic anhydrase and osteoclasts: localization by labeled inhibitor autoradiography. Science. 1974 Feb 1;183(4123):432–434. doi: 10.1126/science.183.4123.432. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Fichmann J., Braun Y., Morgan L., Styles P., Taiz S. L., DeLapp K., Taiz L. The use of antisense mRNA to inhibit the tonoplast H+ ATPase in carrot. Plant Cell. 1992 Jul;4(7):851–864. doi: 10.1105/tpc.4.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. E., Kenny A. D. Role of carbonic anhydrase in bone resorption: effect of acetazolamide on basal and parathyroid hormone-induced bone metabolism. Calcif Tissue Int. 1987 Apr;40(4):212–218. doi: 10.1007/BF02556624. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Laitala T., Vänänen K. Proton channel part of vacuolar H(+)-ATPase and carbonic anhydrase II expression is stimulated in resorbing osteoclasts. J Bone Miner Res. 1993 Jan;8(1):119–126. doi: 10.1002/jbmr.5650080115. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P. T., Vänänen H. K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J Bone Miner Res. 1991 Aug;6(8):817–826. doi: 10.1002/jbmr.5650060806. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P., Tuukkanen J., Hentunen T., Järvelin K., Vänänen K. Organization of osteoclast microfilaments during the attachment to bone surface in vitro. J Bone Miner Res. 1989 Dec;4(6):817–825. doi: 10.1002/jbmr.5650040605. [DOI] [PubMed] [Google Scholar]
- Lallier T., Bronner-Fraser M. Inhibition of neural crest cell attachment by integrin antisense oligonucleotides. Science. 1993 Jan 29;259(5095):692–695. doi: 10.1126/science.8430321. [DOI] [PubMed] [Google Scholar]
- Larrouy B., Blonski C., Boiziau C., Stuer M., Moreau S., Shire D., Toulmé J. J. RNase H-mediated inhibition of translation by antisense oligodeoxyribonucleotides: use of backbone modification to improve specificity. Gene. 1992 Nov 16;121(2):189–194. doi: 10.1016/0378-1119(92)90121-5. [DOI] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Moriyama Y., Hulmes J. D., Pan Y. C., Nelson H., Nelson N. cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5521–5524. doi: 10.1073/pnas.85.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987 Sep;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Hentunen T., Vänänen K. Local expression and exocytosis of viral glycoproteins in multinucleated muscle cells. J Cell Biol. 1992 Jun;117(5):987–995. doi: 10.1083/jcb.117.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989 Oct;21(5):553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- Pallen M. J., Puckey L. H., Wren B. W. A rapid, simple method for detecting PCR failure. PCR Methods Appl. 1992 Aug;2(1):91–92. doi: 10.1101/gr.2.1.91. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G. Acridine orange as a probe for measuring pH gradients across membranes: mechanism and limitations. Anal Biochem. 1991 Feb 1;192(2):316–321. doi: 10.1016/0003-2697(91)90542-2. [DOI] [PubMed] [Google Scholar]
- Pepin M. C., Pothier F., Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992 Feb 20;355(6362):725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Sizeland A. M., Burgess A. W. Anti-sense transforming growth factor alpha oligonucleotides inhibit autocrine stimulated proliferation of a colon carcinoma cell line. Mol Biol Cell. 1992 Nov;3(11):1235–1243. doi: 10.1091/mbc.3.11.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C., Sacks P. G., Adler-Storthz K., Shillitoe E. J. Effect on cancer cells of plasmids that express antisense RNA of human papillomavirus type 18. Cancer Res. 1992 Sep 1;52(17):4706–4711. [PubMed] [Google Scholar]
- Sundquist K. T., Leppilampi M., Järvelin K., Kumpulainen T., Vänänen H. K. Carbonic anhydrase isoenzymes in isolated rat peripheral monocytes, tissue macrophages, and osteoclasts. Bone. 1987;8(1):33–38. doi: 10.1016/8756-3282(87)90129-3. [DOI] [PubMed] [Google Scholar]
- Sundquist K., Lakkakorpi P., Wallmark B., Vänänen K. Inhibition of osteoclast proton transport by bafilomycin A1 abolishes bone resorption. Biochem Biophys Res Commun. 1990 Apr 16;168(1):309–313. doi: 10.1016/0006-291x(90)91709-2. [DOI] [PubMed] [Google Scholar]
- Vänänen H. K., Karhukorpi E. K., Sundquist K., Wallmark B., Roininen I., Hentunen T., Tuukkanen J., Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol. 1990 Sep;111(3):1305–1311. doi: 10.1083/jcb.111.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen H. K., Parvinen E. K. High active isoenzyme of carbonic anhydrase in rat calvaria osteoclasts. Immunohistochemical study. Histochemistry. 1983;78(4):481–485. doi: 10.1007/BF00496199. [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Watkins D. C., Malbon C. C. Antisense oligodeoxynucleotides to GS protein alpha-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992 Jul 23;358(6384):334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Specific inhibition of hepatitis B viral gene expression in vitro by targeted antisense oligonucleotides. J Biol Chem. 1992 Jun 25;267(18):12436–12439. [PubMed] [Google Scholar]