Abstract
Nowadays, percutaneous pulmonary valve implantation is a successful alternative to surgery for patients requiring treatment of pulmonary valve dysfunction. However, owing to the wide variety of implantation site morphology, size and dynamics, only about 15 per cent of cases are suitable for current devices. In order to increase the number of patients who could benefit from minimally invasive procedures, a new valved stent graft for percutaneous implantation has been designed recently. In this study, patient-specific computational analyses have been applied to investigate the suitability of new device designs, using real data from 62 patients who had undergone surgical pulmonary valve replacement. Magnetic resonance images of these patients before surgery were elaborated using imaging post-processing software to reconstruct the three-dimensional volume of each patient's implantation site. Three stent designs were created and tested in these patient outflow tracts using finite-element simulations: stent graft SG1 resembles the first device tested in animals; stent graft SG2 is a custom device tailored for a specific patient morphology; and stent graft SG3 represents a hypothetical larger device. The three devices showed an implantation success rate of 37 per cent, 42 per cent and 63 per cent, respectively. Using patient-specific simulations, we have shown that a percutaneous approach with these new devices may be possible for many patients who are currently referred for surgery. Furthermore, when the new devices become available, the methodologies described may help clinicians in the decision-making process, by enabling virtual implantation prior to the actual procedure.
Keywords: patient specific, finite element, stent graft, percutaneous pulmonary valve implantation
1. Introduction
A decade ago, predictive medicine was highlighted as a ‘doorway to the future’ in the field of cardiovascular disease (Satava & Jones 1998). An important principle of predictive medicine is that it should be based on patient-specific data. This has recently become possible because: (i) development in three-dimensional imaging techniques, especially magnetic resonance (MR) and computed tomography (CT), has made quantification of mechanics in subject-specific anatomies (Schievano et al. 2007b) and physiological conditions (Lurz et al. 2009a) feasible and (ii) advances in computational techniques have reduced the gap between simplified models and the real clinical scenarios (Taylor & Figueroa 2009). Specific interest in this research field became concrete when the virtual physiological human initiative was launched to develop descriptive and predictive models of the human body that aim to improve diagnosis, treatment and prevention in healthcare (Fenner et al. 2008; Viceconti et al. 2008; Kohl & Noble 2009).
Patient-specific modelling of cardiovascular mechanics can play a key role in the development of medical devices. Over the last few years, finite-element (FE) methodologies have been applied to study various devices for the treatment of cardiovascular disease, such as stents and stent grafts (Taylor et al. 1999; Migliavacca et al. 2005; Capelli et al. 2008; De Beule et al. 2008; Gervaso et al. 2008; Kleinstreuer et al. 2008; Sadiq et al. 2008). These devices, which were originally designed to restore blood flow in obstructed vessels, have now been used as scaffolding structures for biological valves, thus opening up the possibility of treating valve disease with percutaneous, minimally invasive techniques rather than open-heart surgery. The first percutaneous heart valve was implanted in humans in 2000—percutaneous pulmonary valve implantation (PPVI) into a dysfunctional right ventricle (RV) to pulmonary artery conduit (Bonhoeffer et al. 2000). The device consists of a bovine jugular venous valve sutured into a balloon-expandable stent (Melody: Medtronic Inc., Minneapolis, MN, USA). The valved stent is mounted into a catheter and delivered via the femoral vein, into the right ventricular outflow tract (RVOT)/pulmonary trunk, where it is deployed by balloon inflation. Since the first implantation, more than 1100 patients have been treated worldwide with this technique (Khambadkone et al. 2005; Lurz et al. 2008; Momenah et al. 2009), thus establishing this procedure as a successful alternative to surgical valve replacement for dysfunctional conduits (Coats et al. 2006, 2007; Lurz et al. 2009b). The device has been commercially available in Europe since 2006, and recently approved by the FDA (Trombetti 2009).
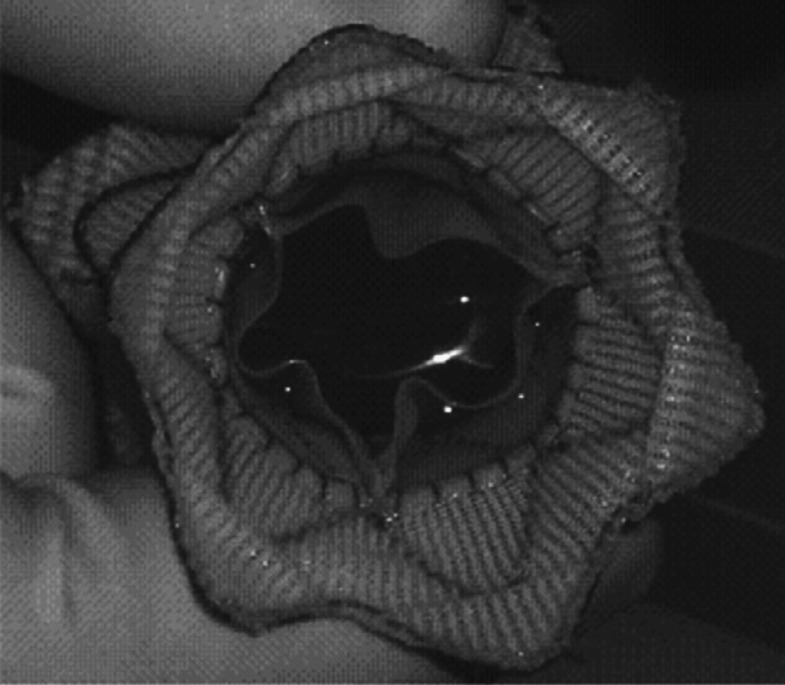
Despite the success of the Melody device, the implantation site of patients with congenital heart disease who require pulmonary valve replacement is highly variable in size, geometry and dynamics. This limits the suitability of the device to approximately 15 per cent of patients who require pulmonary valve replacement (Schievano et al. 2007a), thus committing 85 per cent of patients to surgical treatment options. A new valved stent graft, which would potentially increase the number of patients who could benefit from this minimally invasive procedure, has recently been designed in collaboration with Medtronic. The main novelties of this device are an hourglass geometry—larger diameters at the proximal and distal end, and smaller diameters in the central portion holding the valve—and the use of self-expanding Nitinol wires interwoven with a polymeric graft that should guarantee a greater adaptability of the device to the various RVOT morphologies. The device has undergone successful animal testing (Bonhoeffer et al. 2008). However, animal models do not always represent the wide variation of size and morphology that are seen in the human population. In January 2009, a patient was implanted with the next-generation PPVI device (figure 1) under compassionate use (Bonhoeffer 2009; Schievano et al. 2010). The design tested in animals had to be modified in order to fit the anatomy of this patient and guarantee a safe anchoring.
Figure 1.
Picture of the new percutaneous pulmonary valve device for implantation in humans.
To establish whether potential new device designs would safely fit the wide implantation site morphologies, thus increasing the number of patients who might benefit from percutaneous pulmonary treatments, we used patient-specific FE simulations to assess the prospective applicability of new devices in a group of patients who require treatment. The FE analyses mimicked virtual PPVI to provide morphological and structural information useful both for the development of the device design and for the clinicians in the decision-making process.
2. Material and methods
Data from 62 consecutive patients who underwent surgical pulmonary valve replacement at our centre between June 2006 and June 2008 were included in this study. For all patients, pre-operative three-dimensional MR images of the RV, RVOT and pulmonary arteries were available. This group of patients represents those patients who were referred to our centre for PPVI, but who were not morphologically or dimensionally suitable for implantation of the Melody device. From these three-dimensional datasets, patient-specific FE analysis of device implantation was performed. Institutional ethical approval for the study was obtained, and all patients gave informed consent for retrospective data analysis of the images.
(a). Image acquisition and processing
Cardiovascular MR was performed with a 1.5 T scanner (Symphony-Maestro class or Avanto: Siemens Medical Systems, Erlangen, Germany) using a four- or six-element body-phased array coil, conventional contrast-enhanced, gradient echo, three-dimensional MR angiogram sequence and isotropic image resolution (1.3 × 1.3 × 1.3 mm). MR images were acquired without ECG gating. The raw DICOM data of the patients' MR were imported into post-processing imaging software Mimics 12.1 (Materialise Inc., Leuven, Belgium) where the blood volume of RV, RVOT, pulmonary trunk and pulmonary bifurcation was reconstructed (Schievano et al. 2007b).
(b). FE analysis
Large deformation analyses were performed using the FE commercial code Abaqus/Explicit 6.8 (Simulia, Providence, RI, USA).
(i). FE model
The 62 reconstructed three-dimensional anatomies of RV, RVOT and pulmonary arteries were imported into the FE software and meshed using rigid three-dimensional shell elements. The number of elements varied between 7172 and 13 104 according to the complexity of the geometry.
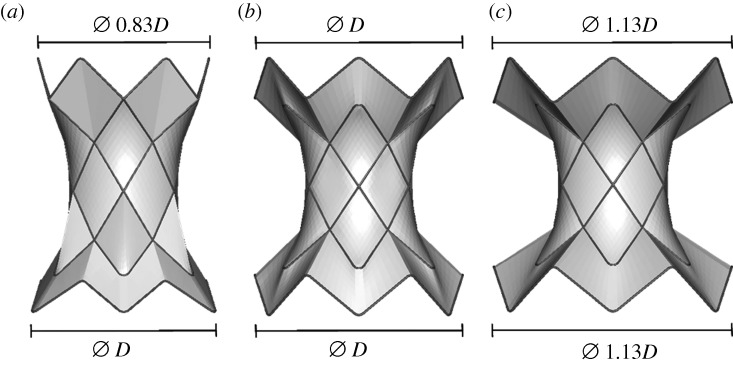
Three models of stent grafts were created using CAD software (Rhinoceros 4.0, McNeel & Associates, Seattle, WA, USA). The models had equivalent central ring diameters (22 mm), but differed in the proximal (ring 1) and distal (ring 6) struts’ dimensions in order to evaluate the suitability of the different designs in the selected patient population.
— Stent graft 1 (SG1): asymmetrical shape with the inflow diameter (D = 40 mm) approximately 1.2 times the outflow diameter (figure 2a). This resembles the animal experiment device.
— Stent graft 2 (SG2): symmetrical device specifically customized for the first human implant with equivalent inflow and outflow diameters (D = 40 mm; figure 2b).
— Stent graft 3 (SG3): symmetrical but larger device proposed for human implants with equivalent inflow (=1.13D) and outflow diameters (figure 2c).
Figure 2.
Models of the three stent grafts: (a) SG1, (b) SG2 and (c) SG3.
Surfaces were created in between the struts to model the polyester fabric. The valve was neglected in the FE analyses.
One-dimensional beam elements were chosen to mesh the stent wires. A characteristic element length of 0.7 mm was preferred after performing a sensitivity analysis. SG1, SG2 and SG3 were meshed with 696, 696 and 912 beam elements, respectively. The graft fabric was meshed by membrane elements that offer strength in the plane of the element, but have no bending stiffness. To discretize SG1, SG2 and SG3, 3850, 3408 and 3909 membrane elements were used, respectively. A tight, rigid contact was assumed to simulate the suture between the stent and the graft.
The shape memory alloy model implemented in Abaqus was used to describe the Nitinol material behaviour of the stent wires. A hyperelastic, isotropic constitutive model based on a reduced polynomial strain energy density function (C10 = 0.38, C20 = 4.36, C30 = 80.56, C40 = −134.72, C50 = 86.24, C60 = −19.74) was used for the fabric graft material. These material models were validated with experimental tests carried out for the Nitinol wires and fabric samples.
(ii). Analyses
The device was placed inside each patient's outflow tract model, as close as possible to the bifurcation, without obstructing the pulmonary arteries, according to the judgement of an experienced interventional cardiologist. The implantation of each device (SG1, SG2 and SG3) into the 62 patients' outflow tract models was divided in two steps. First, the stent was crimped down to 7 mm diameter using displacement control conditions. Second, the displacement constraints were removed under quasi-static conditions and the stent graft tended to recover its original shape. In this step, a general contact algorithm (Abaqus 2007) was defined to allow interaction between the device and the RV, RVOT and pulmonary trunk internal wall.
(c). Quantities of interest
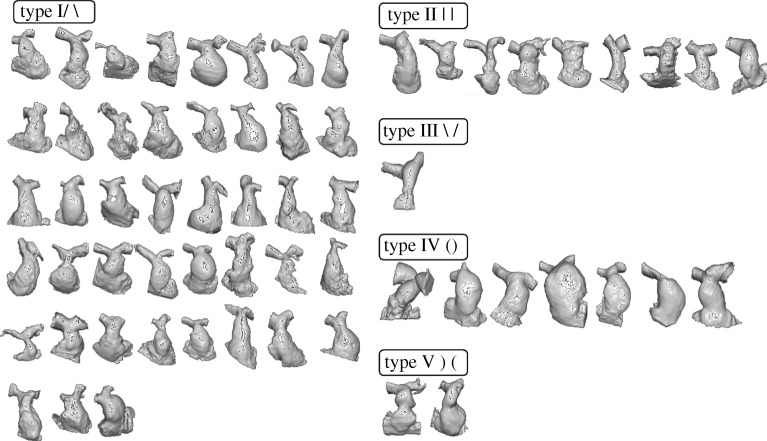
Patients' RVOT/PA morphologies were visually classified into five types as defined by Schievano et al. (2007a): type I—convergent, type II—straight, type III—divergent, type IV—barrel and type V—hourglass.
Anchoring of the device inside the artery was considered optimal if the proximal and distal diameters measured less than 80 per cent of the original diameters, according to manufacturer's specifications. Furthermore, the central portion of the anatomy should not restrict the device valve diameter, so that the valve sewn inside can be fully deployed (greater than 18 mm). These criteria were used to define successful implantation. Diameters after deployment were quantified in the proximal, central and distal sections of the stents. Standardized diameters were calculated by normalizing to a circumference the perimeter of the irregular hexagon that connected all the vertices of the zig-zag rings at each section. Resultant diameters were compared with the limits defined for each stent graft for successful implantation.
3. Results
Forty-three patients were classified as type I (69.3%), nine patients as type II (14.5%), one patient as type III (1.6%), seven patients as type IV (11.3%) and two patients as type V (3.2%) (figure 3).
Figure 3.
Sixty-two patients' RVOTs grouped by morphological type.
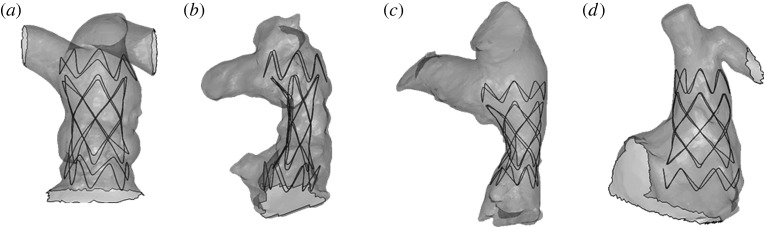
The different phases of virtual deployment of SG2 into a type II anatomy are shown in figure 4. Figure 4a illustrates the initial crimped positioning of the device inside the RVOT. The stent graft, once the displacement conditions were released, adapted its shape to the arterial wall of each specific patient (figure 4b–d). Figure 4e shows the area of contact between the device and the implantation site at the end of the simulation.
Figure 4.
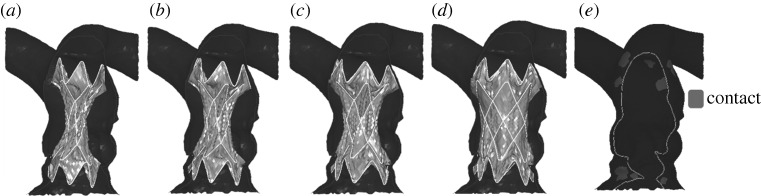
Deployment phases of SG2 inside a patient's implantation site model: (a) crimped SG2 positioning; (b) beginning of self-expansion; (c) contact with the arterial wall model; (d) final configuration of the implant; and (e) implantation site areas with contact between the device and the arterial wall are highlighted in lighter grey.
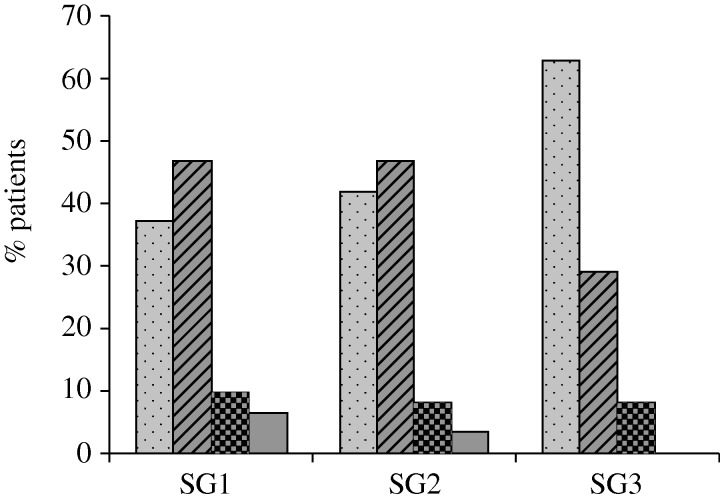
Measures of diameters showed that SG1 would be suitable for 37 per cent of the patients. Ten per cent were excluded because the central section of the stent that holds the valve was not fully deployed; 6 per cent had a distal diameter greater than 80 per cent of the original device diameter; 47 per cent had a proximal diameter greater than 80 per cent of the original device diameter.
Forty-two per cent of the patients would be suitable for the SG2 device (figure 5a); 8 per cent were excluded because of an inadequate deployment of the central section (figure 5b); 3 per cent of the patients had distal diameters greater than 80 per cent the original device diameter (figure 5c) and the remaining 47 per cent had proximal diameter greater than 80 per cent of the original device diameter (figure 5d).
Figure 5.
Four examples of patients in which device SG2 was virtually implanted: (a) successful implant; (b) central section of the device not fully opened; (c) distal ring not in safe contact; (d) proximal crown not in safe contact.
Sixty-three per cent of the patients would be suitable for the SG3 device; 8 per cent were excluded because of an inadequate deployment of the central section; and the remaining 29 per cent had a proximal diameter greater than 80 per cent of the original device diameter (figure 6).
Figure 6.
Percentage of suitable and not suitable patients for the implant of SG1, SG2 and SG3. Unsuitable patients are subdivided according to the regions of the stent that did not respect the criteria of safe implant (NO proximal, NO central and NO distal). Dotted bars, suitable; striped bars, NO proximal; checked bars, NO central; shaded bars, NO distal.
Table 1 summarizes the potential suitable candidates for the three different devices divided according to the morphological type.
Table 1.
Number of patients potentially suitable for the new stents grouped according to the morphological type.
| type I | type II | type III | type IV | type V | total | |
|---|---|---|---|---|---|---|
| patients | 43 | 9 | 1 | 7 | 2 | 62 |
| suitable for SG1 | 15 | 3 | 0 | 5 | 0 | 23 |
| suitable for SG2 | 15 | 5 | 0 | 5 | 1 | 26 |
| suitable for SG3 | 24 | 7 | 1 | 6 | 1 | 39 |
4. Discussion
In this study, we have used realistic patient-specific data to demonstrate the utility of a new PPVI device. We have shown that by varying the dimensions of the device, the number of patients that could potentially benefit from this new treatment can be increased. Furthermore, once devices are developed, the methodologies presented could offer tools to help clinicians in the decision-making process for planning treatment with the correctly sized device.
The percutaneous implantation of a new valved stent is a procedure that has the right characteristics, and potential difficulties, to be approached using patient-specific methodologies. Firstly, the procedure itself is relatively new, but of proven benefit in a small subset of patients; secondly, there is an identified patient group who would benefit from a wider variety of devices; thirdly, each patient's anatomy is completely individual in terms of size, shape and dynamics; fourthly, it represents an area where integration between device technology, imaging science, computer analysis, computed-aided design and clinical cardiology is essential to take the field forward; fifthly, developments in the small but well-defined field of congenital heart disease can influence developments in larger markets for devices in adults, for example, percutaneous aortic valve implantation; and finally, it represents an area where the use of clinical data from patients can influence device design and hopefully reduce the number of animal experiments necessary to bring new devices into the clinical arena.
Over the last 2 years, a new PPVI device has been designed to potentially increase the number of patients who might benefit from PPVI. The device has been developed using information from those patients who were not suitable for the current Melody device—dilated and dynamic RVOT/pulmonary trunk—and had undergone safety testing in animals. Using FE analysis, we have shown that 37 per cent of those patients who are currently still treated with surgery would potentially be suitable for PPVI with a new device that has already provided good results in animal models (Bonhoeffer et al. 2008). Furthermore, we have shown that if the dimensions of this new device are theoretically increased at the distal end (SG2) or both at proximal and distal ends (SG3), the number of PPVI patients would increase by a further 5 per cent and 36 per cent, respectively. Importantly, these dimensions would be difficult to test in animal experiments because of the lack of relevant sizes in these settings. Although animal testing remains important, FE modelling could be integrated into preclinical testing to predict how such devices behave when implanted into the human situation.
Though it would be possible to custom-design a device specific to each individual patient's anatomy, such a manufacturing process would be prohibitively expensive. Furthermore, while it might be tempting to use only the largest device, larger devices require bigger delivery systems that would be difficult to bend through the vascular pathway, to the right atrium, through the tricuspid valve and into the pulmonary position of young patients. An excessively large stent may also exert extremely high radial forces against the arterial wall, whose role is still debated as a potential cause of erosions (Carrol 2009). The balance would, therefore, be to have a family of a small number of devices that could be selected for each individual case to provide easy deliverability and the best-fit solution. With the introduction in clinical practice of the new device in three sizes, the total number of patients requiring pulmonary valve treatment who could benefit from a percutaneous approach (Melody device + three sizes of the new stent graft) would be approximately 70 per cent. This would potentially have a big impact on the cost benefit for healthcare, by reducing hospital stay and improving the speed at which patients can get back to normal daily activities.
Using our described models, approximately 30 per cent of patients would still not be suitable for PPVI, and among these the large majority have a pyramidal-shaped RVOT/pulmonary trunk morphology (table 1). All patients selected for this study underwent surgery of the RVOT and, when visually assessed, nearly 70 per cent had type I morphology. This is because the Melody device is felt unsuitable (high risk of embolization) for PPVI in these patients that consequently undergo surgery. The new stent graft would allow the treatment of some of these type I patients, who are currently excluded with Melody. However, in some, the risk of device embolization in the RV would still be too high and would still require surgery. Therefore, a further device with an even larger proximal ring is worth designing and testing.
The methodologies described in this study not only provide information that may influence device design, but, once such devices enter clinical practice, may also offer a tool to help select the most appropriate prosthesis for any individual patient taking into account their specific anatomy. This can be crucial in the ‘first-in-human’ phase of the introduction of new devices into clinical practice, where realistic implantation site morphologies and FE analyses, in combination with conventional assessment tests can help enhance the safety and success of the procedure.
5. Limitations
Despite the applicability of patient-specific computer models for personalized and predictive healthcare and for modelling human anatomy, many aspects still limit the possibility to mimic the human physiology.
— The current MR images provide an average of RVOT/pulmonary artery shape and size over the cardiac cycle. Data regarding dynamics effects were not available in our study, but are essential to investigate the long-term behaviour of the device. Despite these limitations, we have previously demonstrated with phantoms that the MR reconstructions are very accurate, and hence provide validation data for the MR technique (Schievano et al. 2007b).
— The FE model of the implantation site was simplified using rigid elements to discretize the structure. This can be improved if the material properties of the arterial wall are available, allowing the model to become mechanically characterized.
— The FE model of the stent (modelled with beams) is simplified to guarantee fast results. This did not affect the results of our study that aimed to study the stent-deployed configuration. However, to perform a structural analysis on the long-term mechanical behaviour of the stent itself, the struts will need to be modelled using solid elements.
— The mechanics of device deployment was simplified and the presence of the retractable sheath was not modelled. However, the diameters of the stent grafts were measured at the end of the device recovery phase when the effects of the transient expansion process were negligible.
— The presence of the valve was neglected in this work. Future fluid-dynamics analyses that will include the valve will be performed to evaluate the effects of the device positioning on the resulting pulmonary blood flow, valve regurgitation and, therefore, potential clinical outcomes.
6. Conclusions
This study uses patient-specific data to demonstrate the feasibility of a new PPVI device, and shows how the device can be altered to deal with various human conditions. Our analyses demonstrate that by using patient-specific data we can guide device design. Importantly, device design characteristics developed from animal models may not always be suitable for patients, in particular where animal models do not exist that represent the clinical condition, as is the case for congenital heart disease where there are such a wide range of patient anatomies. The methodologies presented in this study may help overcome some of these restrictions.
Acknowledgements
This work was supported by the British Heart Foundation, the Royal Academy of Engineering/EPSRC, Medtronic and the UK National Institute of Health Research. P.B. is a consultant for Medtronic and receives funding from them. The other authors declare that they have no conflict of interest.
Footnotes
One contribution of 13 to a Theme Issue ‘The virtual physiological human: computer simulation for integrative biomedicine II’.
References
- Abaqus. Abaqus analysis user's manual, v. 6.7-EF. vol. V. Vélizy-Villacoublay, France: Dassault Systèmes; 2007. 31.4. [Google Scholar]
- Bonhoeffer P., et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0. ( ) [DOI] [PubMed] [Google Scholar]
- Bonhoeffer P.2009RVOT dysfunction: future perspectives Workshop IPC and ISHAC Joint Meeting, Milan, Italy. See http://www.abmedica.it/workshopipc/7th_workshop/video/0051.wmv [Google Scholar]
- Bonhoeffer P., Huynh R., House M., Douk N., Kopcak M., Hill A., Rafiee N. Transcatheter pulmonic valve replacement in sheep using a grafted self-expanding stent with tissue valve. Circulation. 2008;118:S_812. [Google Scholar]
- Capelli C., Gervaso F., Petrini L., Dubini G., Migliavacca F. Assessment of tissue prolapse after balloon-expandable stenting: influence of stent cell geometry. Med. Eng. Phys. 2008;31:441–447. doi: 10.1016/j.medengphy.2008.11.002. ( ) [DOI] [PubMed] [Google Scholar]
- Carrol J. D. Device erosion. Catheter. Cardiovasc. Interv. 2009;73:931–932. doi: 10.1002/ccd.22103. ( ) [DOI] [PubMed] [Google Scholar]
- Coats L., et al. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113:2037–2044. doi: 10.1161/CIRCULATIONAHA.105.591438. ( ) [DOI] [PubMed] [Google Scholar]
- Coats L., et al. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. Eur. Heart J. 2007;28:1886–1893. doi: 10.1093/eurheartj/ehm181. ( ) [DOI] [PubMed] [Google Scholar]
- De Beule M., Mortier P., Carlier S. G., Verhegghe B., Van Impe R., Verdonck P. Realistic finite element-based stent design: the impact of balloon folding. J. Biomech. 2008;41:383–389. doi: 10.1016/j.jbiomech.2007.08.014. ( ) [DOI] [PubMed] [Google Scholar]
- Fenner J. W., et al. The EuroPhysiome, STEP and a roadmap for the virtual physiological human. Phil. Trans. R. Soc. A. 2008;366:2979–2999. doi: 10.1098/rsta.2008.0089. ( ) [DOI] [PubMed] [Google Scholar]
- Gervaso F., Capelli C., Petrini L., Lattanzio S., Di Virgilio L., Migliavacca F. On the effects of different strategies in modelling balloon-expandable stenting by means of finite element method. J. Biomech. 2008;41:1206–1212. doi: 10.1016/j.jbiomech.2008.01.027. ( ) [DOI] [PubMed] [Google Scholar]
- Khambadkone S., et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112:1189–1197. doi: 10.1161/CIRCULATIONAHA.104.523266. ( ) [DOI] [PubMed] [Google Scholar]
- Kleinstreuer C., Li Z., Basciano C. A., Seelecke S., Farber M. A. Computational mechanics of Nitinol stent grafts. J. Biomech. 2008;41:2370–2378. doi: 10.1016/j.jbiomech.2008.05.032. ( ) [DOI] [PubMed] [Google Scholar]
- Kohl P., Noble D. Systems biology and the virtual physiological human. Mol. Syst. Biol. 2009;5:292. doi: 10.1038/msb.2009.51. ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurz P., et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. doi: 10.1161/CIRCULATIONAHA.107.735779. ( ) [DOI] [PubMed] [Google Scholar]
- Lurz P., Nordmeyer J., Muthurangu V., Khambadkone S., Derrick G., Yates R., Sury M., Bonhoeffer P., Taylor A. M.2009aComparison of bare metal stenting and percutaneous pulmonary valve implantation for treatment of right ventricular outflow tract obstruction: use of an x-ray/magnetic resonance hybrid laboratory for acute physiological assessment Circulation 1192995–3001.( 10.1161/CIRCULATIONAHA.108.836312) [DOI] [PubMed] [Google Scholar]
- Lurz P., Puranik R., Nordmeyer J., Muthurangu V., Hansen M. S., Schievano S., Marek J., Bonhoeffer P., Taylor A. M.2009bImprovement in left ventricular filling properties after relief of right ventricle to pulmonary artery conduit obstruction: contribution of septal motion and interventricular mechanical delay Eur. Heart J. 302266–2274.( 10.1093/eurheartj/ehp258) [DOI] [PubMed] [Google Scholar]
- Migliavacca F., Petrini L., Montanari V., Quagliana I., Auricchio F., Dubini G. A predictive study of the mechanical behaviour of coronary stents by computer modelling. Med. Eng. Phys. 2005;27:13–18. doi: 10.1016/j.medengphy.2004.08.012. ( ) [DOI] [PubMed] [Google Scholar]
- Momenah T. S., El Oakley R., Al Najashi K., Khoshhal S., Al Qethamy H., Bonhoeffer P. Extended application of percutaneous pulmonary valve implantation. J. Am. Coll. Cardiol. 2009;53:1859–1863. doi: 10.1016/j.jacc.2008.08.061. ( ) [DOI] [PubMed] [Google Scholar]
- Sadiq S. K., et al. Patient-specific simulation as a basis for clinical decision-making. Phil. Trans. R. Soc. A. 2008;366:3199–3219. doi: 10.1098/rsta.2008.0100. ( ) [DOI] [PubMed] [Google Scholar]
- Satava R. M., Jones S. B. Current and future applications of virtual reality for medicine. Proc. IEEE. 1998;86:484–489. doi: 10.1109/5.662873. ( ) [DOI] [Google Scholar]
- Schievano S., Coats L., Migliavacca F., Norman W., Frigiola A., Deanfield J., Bonhoeffer P., Taylor A. M.2007aVariations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation J. Cardiovasc. Magn. Reson. 9687–695.( 10.1080/10976640601187596) [DOI] [PubMed] [Google Scholar]
- Schievano S., Migliavacca F., Coats L., Khambadkone S., Carminati M., Wilson N., Deanfield J. E., Bonhoeffer P., Taylor A. M.2007bPercutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data Radiology 242490–497.( 10.1148/radiol.2422051994) [DOI] [PubMed] [Google Scholar]
- Schievano S., et al. A new approach to medical device development—first-in-man implantation of a novel percutaneous valve. EuroIntervention. 2010;5:745–750. doi: 10.4244/eijv5i6a122. [DOI] [PubMed] [Google Scholar]
- Taylor C. A., Draney M. T., Ku J. P., Parker D., Steele B. N., Wang K., Zarins C. K. Predictive medicine: computational techniques in therapeutic decision-making. Comput. Aided Surg. 1999;4:231–247. doi: 10.3109/10929089909148176. ( ) [DOI] [PubMed] [Google Scholar]
- Taylor C. A., Figueroa C. A. Patient-specific modeling of cardiovascular mechanics. Annu. Rev. Biomed. Eng. 2009;11:109–134. doi: 10.1146/annurev.bioeng.10.061807.160521. ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetti J.2009FDA approves with conditions Medtronic, Inc. Melody transcatheter pulmonary valve. See http://www.dotmed.com/news/story/9754/ [Google Scholar]
- Viceconti M., Clapworthy G., Van Sint Jan S. The virtual physiological human—a European initiative for in silico human modelling. J. Physiol. Sci. 2008;58:441–446. doi: 10.2170/physiolsci.RP009908. ( ) [DOI] [PubMed] [Google Scholar]