Abstract
Objective
Multipotent germline stem (mGS) cells derived from neonatal mouse testis, similar to embryonic stem (ES) cells, differentiate into various types of somatic cells in vitro and produce teratomas after inoculation into mice. In the present work, we examined mGS cells for hematopoietic progenitor potential in vitro and in vivo.
Materials and Methods
mGS cells were differentiated on OP9 stromal cells and induced into Flk1+ cells. Flk1+ cells were sorted and replated on OP9 stromal cells with various cytokines and emerging hematopoietic cells were analyzed for lineage marker expression by fluorescein-activated cell sorting, progenitor activity by colony assay, and stem cell transplantation assay.
Results
mGS cells, like ES cells, produce hematopoietic progenitors, including both primitive and definitive erythromyeloid, megakaryocyte, and B- and T-cell lineages via Flk1+ progenitors. When transplanted into the bone marrow (BM) of nonobese diabetic/severe combined immunodeficient (NOD/SCID) γcnull mice directly, mGS-derived green fluorescent protein (GFP)-positive cells were detected 4 months later in the BM and spleen. GFP+ donor cells were also identified in the Hoechst33342 side population, a feature of hematopoietic stem cells. However, these mGS-derived hematopoietic cells did not proliferate in vivo, even after exposure to hematopoietic stressors, such as 5-fluorouracil (5FU) injection or serial transplantation.
Conclusion
mGS cells produced multipotent hematopoietic progenitor cells with myeloid and lymphoid lineage potential in vitro and localized in the BM after intra-BM injection but, like ES cells, failed to expand or show stem cell repopulating ability in vivo.
Hematopoietic stem cells (HSCs) are defined as blood cells displaying the potential for self-renewal and multilineage differentiation. HSC transplantation has been widely used for treating hematological malignancies and inherited disorders. Peripheral blood and cord blood stem cells, as well as bone marrow cells, have been intensively studied and shown to be effective for clinical use. Recently, embryonic stem (ES) cells have been proposed as an alternative candidate source of HSCs. Many approaches have been attempted to obtain HSCs from ES cells, but this is challenging unless using enforced expression of genes, such as Hoxb4 [1] or Cdx4 [2] in ES cells. Even if a robust method for HSC derivation from ES cells were discovered, one would still need to address donor–host differences in histocompatibility antigens to permit ES-derived HSC engraftment in patients.
Multipotent germline stem (mGS) cells have been established from neonatal mouse testis and have been proven to have similar potential to ES cells, including germline transmission [3]. If mGS cells could be isolated from human testis and were utilized to produce HSC, then the problem of major histocompatibility complex (MHC) incompatibility would be solved because it might be possible to establish the patient's own mGS cells. In that sense, mGS cells may have a big advantage over ES cells in human application for cell therapies.
The methods for inducing hematopoietic cells from ES cells have been well-developed [4]. Flk1 is a candidate marker for mesoderm [5] and hemangioblast [6,7] cells, and Flk1 progeny have been proven to differentiate into all hematopoietic cell lineages [8]. Using the OP9 stromal cell line as a feeder layer, hematopoietic cells are effectively induced from ES cells [9], and FLK1+-derived definitive hematopoietic cells are obtained [10].
In a previous report [3], mGS cells have been shown to differentiate into CD45+ hematopoietic cells, including Gr-1+Mac1+ myeloid cells and Ter119+ erythroid cells, but the potential for mGS cells to differentiate into hematopoietic stem/progenitor has not been reported. We have observed multipotent hematopoietic progenitor cells with myeloid and lymphoid potential emerging from mGS FLK1+ cells using the OP9 feeder cell system and describe the localization of mGS-derived hematopoietic cells in the bone marrow (BM) cavity when directly injected into the BM of immunodeficient mice. However, the mGS-derived hematopoietic cells present in the host, like differentiated wild-type ES-derived hematopoietic cells, do not proliferate or display multilineage repopulating ability in vivo.
Materials and methods
Cell culture
mGS cells were established from mouse neonatal testis of DBA/2 mice or a green fluorescent protein (GFP)–expressing transgenic mouse (C57BL6 × DBA/2 F1 background), as described previously [3]. The CCE ES cell line was kindly provided by Dr. S. Nishikawa (RIKEN, Kobe, Japan). The ES cell line D3 was transfected with GFP gene driven by the ubiquitous CAG promoter. These CCE and D3 cell lines are derived from the 129 mouse strain. GFP+ mGS and D3 were used for the transplantation assay. The mGS or ES lines were maintained as described previously [3].
Differentiation to hematopoietic progenitor cells was induced as described [8,11]. Briefly, 104 of undifferentiated mGS and ES cells were seeded onto T-25 flask with confluent OP9 stromal cells (a gift from Dr. Kodama) in α–minimum essential medium supplemented with 10% fetal bovine serum and 5 × 10–5M 2-mercaptoenthanol. After 4 days, cultured cells were harvested with cell dissociation buffer (Gibco, Grand Island, NY, USA) and Flk1+ cells were collected using a FACSVantage flow cytometer (Becton Dickinson, Mountain View, CA, USA). The 5–10 × 103 Flk1+ cells per well in six-well plate with confluent OP9 stromal cells were cocultured again with added cytokines, such as 100 ng/mL mouse stem cell factor (SCF), 10 ng/mL human thrombopoietin (TPO), 10 ng/mL mouse Flt-3 ligand (FL), 4 u/mL human erythropoietin (EPO), and 100 u/mL mouse interleukin (IL)-7. Mouse SCF, human TPO, and human EPO were kindly provided from Kirin Brewery (Tokyo, Japan). Mouse FL and IL-7 were purchased from R&D Systems (Minneapolis, MN, USA). For T-cell induction, Flk1+ cells were cocultured with OP9-DL1 [12] stromal cells (kindly provided by Dr. Zuniga-Pflucker, University of Toronto) with 50 ng/mL IL-7.
Hematopoietic colony-forming cell assay
In order to analyze emergence of immature hematopoietic progenitor cells from mGS cells, on each day after induction of Flk1+ cells on OP9, cells were harvested and plated in methylcellulose for colony-forming assay using a modification of the technique described previously [13,14]. All cultures were performed in triplicate and the number of colony-forming cells was scored at day 8 to 10. For megakaryocyte colonies, the cells were plated in Megacult (Stem Cell Technologies, Vancouver, Canada) according to manufacturer's instruction.
Antibodies and staining
The following primary antibodies were used: rat anti-mouse E-cadherin (ECCD2, from Calbiochem. San Diego, CA, USA), fluorescein isothiocyanate (FITC)–conjugated hamster anti-rat b1 integrin (Ha2/5), rat anti-mouse CD31 (MEC 13.3), allophycocyanin (APC)-conjugated rat anti-mouse c-kit (2B8), phycoerythrin (PE)-conjugated rat anti-Flk1 (AVAS12), PE-conjugated rat anti-mouse Ter119 (TER-119), APC-conjugated rat anti-mouse CD45 (30-F11), biotin-conjugated rat anti-mouse Gr-1 (RB6-8C5), biotin-conjugated rat anti-mouse Mac-1 (M1/70), purified rat CD41 antibody (MWReg30), and rabbit anti-embryonic hemoglobin antibody [15] (a gift from Dr. Takakura, Osaka University). PE-conjugated or alkaline phosphatase–conjugated anti-rat IgG, APC-conjugated streptavidin, or FITC–conjugated anti-rabbit immunoglobulin G were used as secondary antibodies. All antibodies except ECCD2 were purchased from Pharmingen (San Diego, CA, USA). For detection of side population (SP) cells, BM cells were stained with Hoechst 33324, as described previously [16–18]. Stained cells were analyzed using FACSCalibur or LSRII (Becton Dickinson).
Immunohistochemistry
Femurs of recipient mice were fixed with 4% paraformaldehyde, embedded in the optimal cutting temperature compound and frozen sections of 7-μm thickness were mounted on silan-coated glass slides and were stained with rabbit anti-GFP antibody (BD Bioscience Clontech, Palo Alto, CA, USA), as described previously [19]. Cytospin preparations and culture dishes were also stained with various antibodies, as described previously [20,21].
Mice and transplantation
Nonobese diabetic/severe combined immunodeficient (NOD/SCID) γcnull mice were kindly provided from the Central Institute of Experimental Animals (Kawasaki, Japan) and kept under specific pathogen-free conditions in accordance with the guidelines of the facility. Cultured mGS-derived hematopoietic cells and OP9 cells were collected and injected into the femoral BM of NOD/SCIDγcnull mice that were irradiated with 2.4 Gy before transplantation. The 2 × 105 BM mononuclear cells were injected as a positive control. After transplantation, mice were prophylactically provided sterile water with neomycin sulfate (Gibco BRL).
For serial transplantation, BM cells were collected from transplanted NOD/SCIDγcnull mice 4 months after primary transplantation and stained with anti-CD45.1+ (recipient) and CD45.2+ (donor) antibodies. CD45.2+ donor cells were sorted on FACS-Vantage (Becton Dickinson) and injected into the BM of 2.4-Gy irradiated NOD/SCIDαcnull mice. Peripheral blood (PB) and BM were analyzed 3 to 4 months after secondary transplantation.
RNA extraction and reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA was prepared using Trisol (Gibco BRL). Complementary DNA (cDNA) synthesis was performed using Superscript II and oligo (dT)12-18 primers (Invitrogen, Carlsbad, CA, USA) following manufacturer's instructions. The same cDNA sample was used for PCR amplification with different primer sets, using standard protocols using AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA). The primer sequences were as follows: βH1 forward: AGTCCCCATGGAGTCAAAGA, reverse: CTC AAG GAG ACC TTT GCT CA, β major forward: CTG ACA GAT GCT CTC TTG GG, reverse: CAC AAA CCC CAG AAA CAG ACA, GAPDH forward: TCC CAC TCT TCC ACC TTC, reverse: CTG TAG CCG TAT TCA TTG TC. For PCR to detect GFP, the primer sequence were as follows: GFP forward: CTG GTC GAG CTG GAC GGC GAC G, reverse: CAC GAA CTC CAG CAG GAC CAT G. Cycling parameters included: denaturation at 94° C for 30 seconds; annealing at various temperatures for 30 seconds; elongation at 72° C for 40 seconds. The number of cycle varied between 25 and 35 cycles.
Results
mGS cells can differentiate into Flk1+ cells on OP9 stromal cells
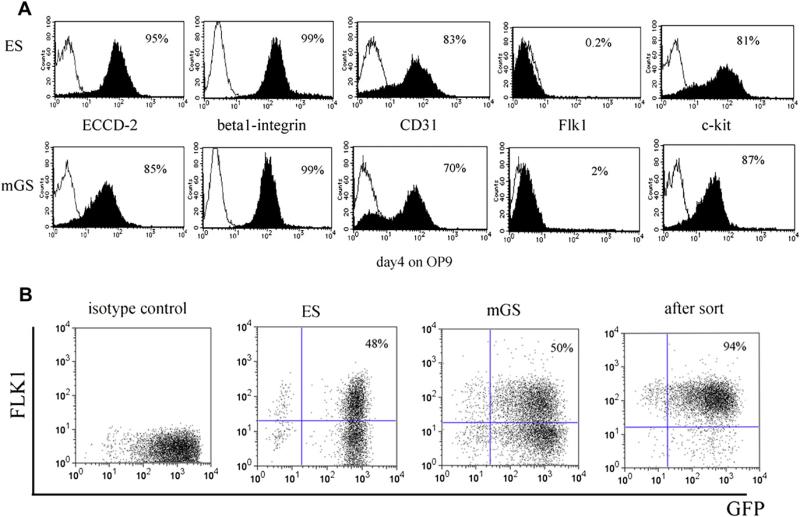
It has been reported that mGS cells are very similar to ES cells in their differentiation ability into multiple cell lineages in vitro and in vivo [3]. Undifferentiated mGS and ES cells express ECCD-2, β1-integrin, CD31 and c-kit, but are negative for Flk-1 expression (Fig. 1A). In order to examine the differentiation potential of these cells, we cocultured mGS or ES cells with OP9 stromal cells and assayed for emergence of Flk1+ cells 4 days after induction because Flk1 is thought to be a representative marker for mesodermal cells [5]. The percentage of Flk1+ cells derived from mGS cells (50%) was very similar to ES cells (48%) (Fig. 1B).
Figure 1.
Surface markers of mGS and ES cells. Surface markers of undifferentiated mGS cells (A, lower panel) are similar to those of ES cells (A, upper panel). mGS cells were induced on OP9 stromal cells for 4 days and were confirmed to express Flk1 (B).
Primitive and definitive erythropoiesis can be derived in vitro in mGS culture
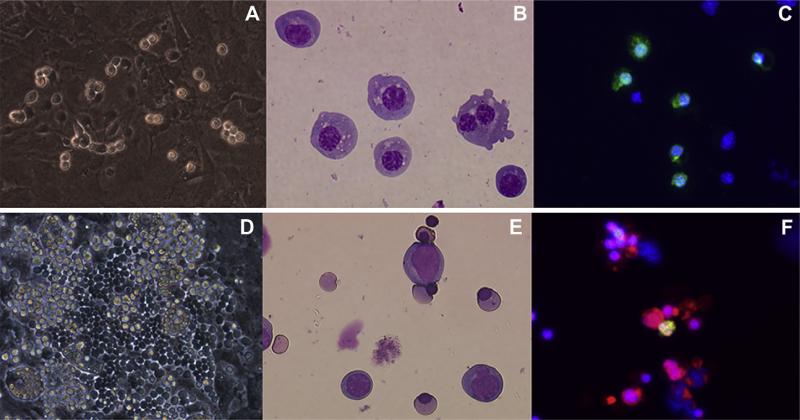
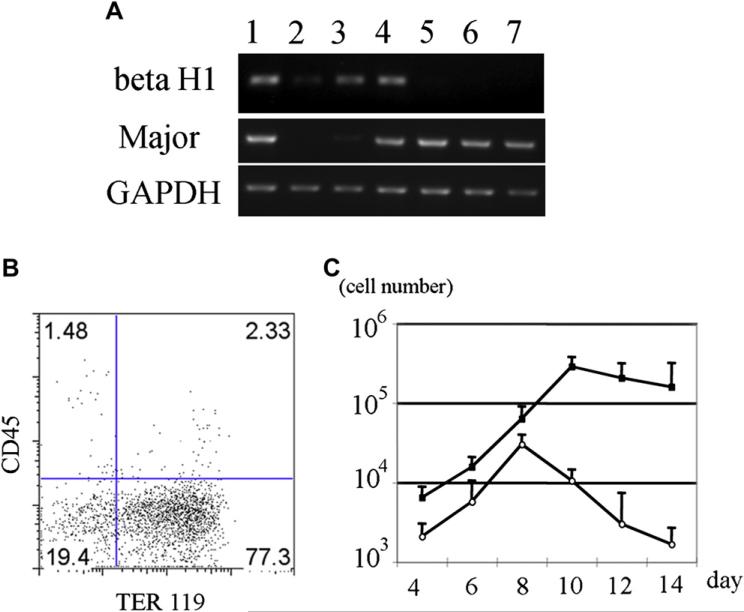
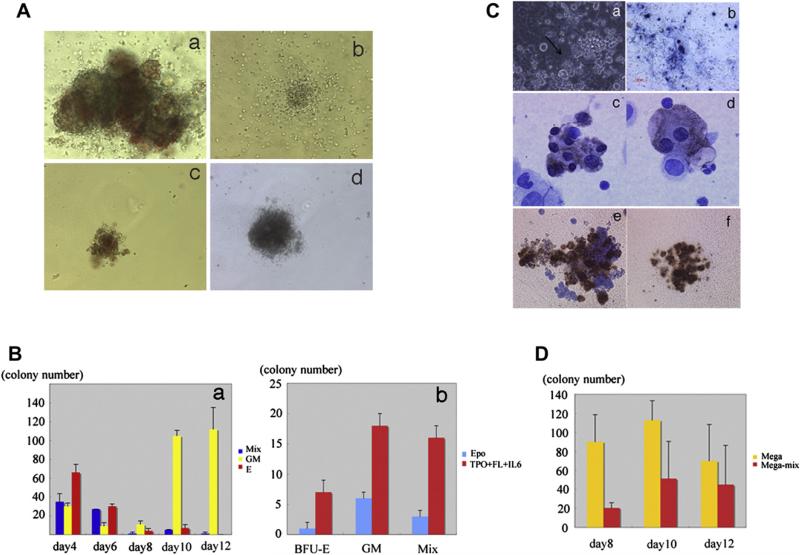
In order to examine red blood cell emergence from mGS-derived Flk1+ cells, Flk1+ cells were sorted and cocultured on OP9 stromal cells with added EPO. Three days later, small round hematopoietic cells were found (Fig. 2A). The cytospin preparation of these cells and May-Giemsa staining revealed the round, nucleated cell morphology of primitive erythrocytes (Fig. 2B). Immunostaining with an antiembryonic hemoglobin antibody confirmed that these cells were primitive erythrocytes (Fig. 2C). After 9 days of coculture, cobblestone-forming areas were observed in the cultures (Fig. 2D). Morphologic analysis of nonadherent cells revealed enucleated red blood cells (Fig. 2E). Immunostaining confirmed the cells to be definitive erythrocytes that express Ter119, but not embryonic hemoglobin molecules (Fig. 2F). Next, mGS Flk1+ cells were cocultured on OP9 cells in the presence of EPO or EPO and Ack45 (a blocking antibody of the c-kit signaling pathway). In this culture system, most of the emerging blood cells were Ter119+ erythroid cells (Fig. 3B), but the growth factor requirements of the cultured cells in each condition showed different patterns: an initial c-kit–independent period (days 4–8; Fig. 3C) and a second c-kit–dependent period during which erythropoiesis was blocked by the presence of the Ack45 antibody (days 8–14, Fig. 3C). Erythrocytes in the initial wave expressed βH1 hemoglobin mRNA, while only β-major hemoglobin mRNA was expressed in the second wave of erythropoiesis (Fig. 3A). These results indicated that cells in the initial wave represented primitive erythrocytes, while definitive erythrocytes comprised the second wave [22]. Thus, mGS cells can give rise to primitive and definitive erythropoiesis in vitro in the same manner as differentiated ES cells.
Figure 2.
Primitive and definitive erythroid cells are differentiated from mGS–derived Flk1+ cells. mGS-derived Flk1+ cells were cultured on OP9 cells and small round cells and cobblestone-forming areas were found [(A) day 3, (D) day 9). May-Giemsa staining of a cytospin preparation [(B); day 3, (E); day 9)]. Immunostaining of E1 antigen and Ter119 (C,F). (C) E1 antigen; green. (F) E1 antigen; green Ter119; red. Nuclear staining with Hoechst 33324; blue. Magnification (A,D): ×100; (B,C,E,F): ×200.
Figure 3.
Erythropoiesis from mGS Flk1+ cells shows two different waves. Flk1+ cells were cultured on OP9 with erythropoietin (EPO) and with or without ACK45. The nonadherent cells in the culture were collected every other day, counted the number (C), examined the surface markers (B) and hemoglobin gene expression (A). Sequential RT-PCR analysis shows expression of β H1 hemoglobin mRNA, followed by b-major hemoglobin mRNA expression (A). E12.5 fetal liver (lane 1), E8.0 embryo (lane 2), mGS- Flk1+ cells on OP9 for 4 days (lane 3), for 6 days (lane 4), for 8 days (lane 5), for 10 days (lane 6), for 12 days (lane 7). Flk1+ cells were cultured on OP9 cells with EPO and with (white circle) or without ACK45 (black square) (C). Most cells in the culture were Ter119+ erythrocyte (B). Definitive erythropoiesis was blocked by ACK45 (C). GAPDH = glyceraldehydes phosphate dehydrogenase.
Flk1+ cells derived from mGS cell can differentiate into multiple lineages including B and T cells
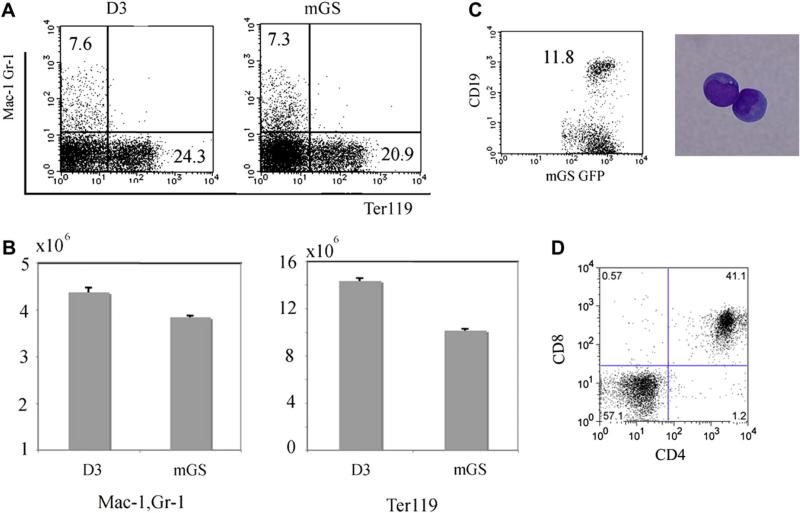
When mGS-derived Flk1+ cells were cocultured on OP9 cells with added SCF, granulocyte colony-stimulating factor (G-CSF), IL-3, and EPO, Flk1+ cells can also differentiate into Mac1+Gr1+ cells and Ter119+ cells (7.3% and 20.9%, respectively) similar to ES cells (7.6% and 24.3%, respectively) (Fig. 4A). Furthermore, mGS-derived Flk1+ cells also gave rise to CD19+ B cells and CD4+CD8+ T cells when cultured on OP9 cells or OP9-DL1 cells with added IL7 (Fig. 4C and D).
Figure 4.
Myelolymphoid potential of mGS cells. Mac1+Gr1+, Ter119+, CD19+, and CD4+CD8+ cells were differentiated from mGS-derived Flk-1+ cells within OP9 culture (A,B,C) or OP9-DL1 culture (D). For Mac1+Gr1+, Ter119+ cells, cells were collected from 8–10 days culture (A,B). The numbers of myeloid and erythroid cells differentiated from mGS and ES cells were similar (B). For CD19+ (C), and CD4+CD8+ cells (D), cells were collected from 14–21 days culture. These FACS data are representative among three independent experiments.
Flk1+ cells derived from mGS cell can differentiate into multipotent progenitors
To determine whether mGS cells can give rise to clonogenic hematopoietic progenitors, Flk1+ cells derived from mGS cells were cocultured on OP9 cells and all the cells after 4 to 10 days culture were plated in methylcellulose with added growth factors. Burst-forming unit-erythroid (BFU-E), colony-forming unit granulocyte-macrophage (CFU-GM), and CFU-Mix were observed (Fig. 5Aa–d). Interestingly, the number of CFU-mix was decreased after 8 days’ coculture (Fig. 5Ba), and CFU-GM was the main population detectable after 10 day's culture of mGS cells on OP9 cells. To examine whether production of these clonogenic progenitors from Flk1+ cells was influenced by cytokines, Flk1+ cells were cocultured on OP9 cells with only EPO or with SCF, TPO, and FL. The numbers of all kind of colonies were increased when cocultured with SCF, TPO, and FL (Fig. 5Bb). Thus, mGS-derived Flk1+ cells generate multipotent hematopoietic progenitors, and this effect was promoted by addition of multiple cytokines, similar to differentiated ES cells.
Figure 5.
Colony-forming ability and megakaryocyte potential of mGS Flk1+-derived cells. mGS-derived Flk-1+ cells produce hematopoietic progenitor cells that can form various kinds of colonies [(Aa) mixed colony, (Ab) granulocyte-macrophage (GM) colony, (Ac) burst-forming unit erythroid (BFU-E), (Ad) mast cell colony]. Mixed colonies were predominant when mGS-derived Flk1+ cells were cultured on OP9 cells for 4 to 6 days (Ba). Blue bar: mixed colony, yellow bar: GM colony, red bar: Erythroid colony. The number of all kinds of colonies was increased when mGS Flk1+ cells were cultured on OP9 with thrombopoietin (TPO) and interleukin (IL)-6 (Bb). Blue bar: with erythropoietin (EPO), red bar: with TPO and IL6. Megakaryocyte or proplatelet mGS-derived Flk1+ cells were observed within OP9 culture for 8–12 days. Proplatelet like cells (arrow Ca), Immunostaining of cultured cells with CD41 antibody secondary detected by alkaline phosphatase–conjugated antibody (Cb blue). Immunostaining of a cytospin preparation with CD41 antibody secondary detected by peroxidase conjugated antibody (Cc, Cd brown). After transferring cultured cells into Megacult conditions, megakaryocyte colonies (Cf) include mega-mix colonies (Ce) were found. These cells expressed acethylcholinestrase as evidenced by brown staining. The CFU-Mega was maintained during culturing day 8 to 12 in the presence of TPO and stem cell factor (D). Yellow bar: Megakaryo-colony, red bar: Mega-Mix colony. Magnification: (Ca, Cb) ×100; (Cc, Cd) ×200; (Ce, Cf) ×100.
Megakaryopoiesis through Flk1+ cells derived from mGS cells
We also evaluated megakaryocyte production from mGS-derived Flk1+ cells. Eight days after Flk1+ cells were co-cultured on OP9 cells in the presence of TPO and SCF, large round cells with proplatelets were observed (Fig. 5Ca). These cells expressed the cell surface protein CD41, a well-recognized marker of the platelet lineage (Fig. 5Cb). Cytospin preparations of culture fluid from these culture dishes showed large multinucleated cells that were CD41-positive (Fig. 5Cc and Cd). These cells contained both CFU-megakaryocyte and CFU-mega-mix confirmed by specific megakaryocyte progenitor cultures (Fig. 5Ce and Cf). CFU-megakaryocyte progenitors were maintained during culturing between day 8 and day 12 of mGS-derived Flk1+ cells in the presence of TPO and SCF (Fig. 5D). Differentiated ES cells also showed similar trends of megakaryocyte colony production (data not shown). Thus, Flk1+ cells derived from mGS cells were shown to produce megakaryocytes as well as megakaryocyte progenitor cells.
Flk1-derived hematopoietic cells can engraft in the BM of NOD/SCIDγcnull mice by intra-BM injection
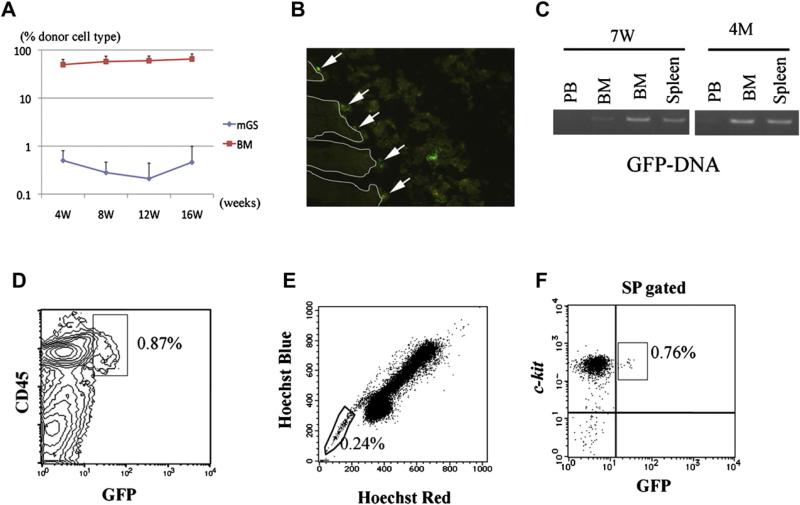
Finally, day-6 cocultured mGS-derived Flk1+ cells and OP9 cells were recovered and injected directly into the BM of NOD/SCIDγcnull mice (n = 4). As a positive control, 2 × 105 BM mononuclear cells were injected into the BM of NOD/SCIDγcnull mice. Every 4 weeks after transplantation, PB was analyzed for evidence of donor-cell engraftment. While the BM cells engrafted, mGS-derived cells were barely detected (<0.1%, Fig. 6A). After 7 weeks, fluorescein-activated cell sorting (FACS) analysis revealed donor mGS-derived GFP+ cells in the BM, but at low chimerism of <0.1% (data not shown). PCR analysis confirmed the presence of GFP-DNA in the BM and the spleen (Fig. 6C). Four months after transplantation, a very small number of donor CD45+GFP+ cells were detected in the BM and the spleen by FACS analysis and confirmed by PCR (Fig. 6A, C, and D). Furthermore, when BM cells of transplanted mice were stained with Hoechst33324, GFP+ cells were detected in the SP region (Fig. 6E and F). Immunostaining of the femur revealed that GFP+ cells were attached to the endosteal region, where HSCs are considered to reside (Fig. 6B). Three of four transplanted mice were confirmed to display this type of “stem cell-like” engraftment (0.03% ± 0.03%). Thus, mGS-derived hematopoietic cells are found in the BM 4 months after transplantation. It is of note that no teratoma formation was observed in any of the transplanted animals.
Figure 6.
Transplanted hematopoietic cells from GFP+ mGS cells can be detected in bone marrow (BM) 4 months after transplantation and displays stem cell phenotype. After transplantation, peripheral blood (PB) was analyzed every 4 weeks (A). BM cells from recipient mice 4 months after transplantation were analyzed by LSR II (D, E, F). GFP+CD45+ cells were detected (D). When the BM cells of the recipient mice were stained with Hoechst 33324, GFP+ cells were detected in the SP region (E, F). In the section of recipient BM, GFP+ cells were found attached to the endosteal region (B, arrow). RT-PCR shows donor-derived DNA in the BM and spleen at 7 weeks and 4 months after transplantation (C).
mGS-derived hematopoietic cells did not show stem cell potential by serial transplantation
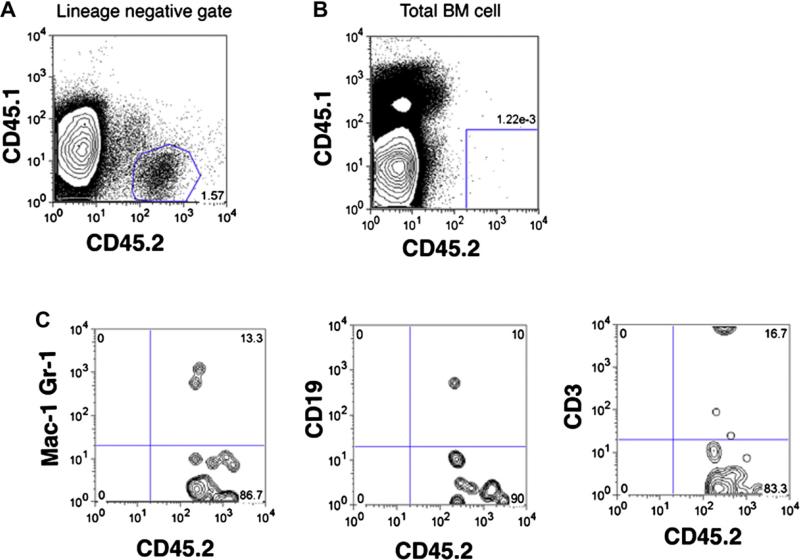
Because HSCs are enriched in the SP fraction and normally reside in the endosteal region of the BM [23–25], we speculated that hematopoietic progeny of mGS-derived Flk1+ cells engrafted in the BM and remained in the stem cell fraction. In order to prove that these GFP+ cells in the BM were HSCs, we hypothesized that hematopoietic stress may induce further expansion of the donor HSCs and thus, 5FU injection and serial transplantation were performed. 5FU was injected intraperitoneally at a standard dose of 100 ug/g into transplanted mice that received mGS-derived hematopoietic cells and the PB was analyzed with CD45.1 and CD45.2 antibodies every week after 5FU injection. When the blood cell count recovered, we expected donor-derived cells would increase in number in PB, however, no donor mGS-derived cells were observed (data not shown). For secondary transplantation analysis, we collected BM cells of primary recipient mice. Interestingly, more donor cells were detected in the lineage-negative population of the recipient BM cells (Fig. 7A) than in the whole BM (Fig. 7B). There were only 0.001% donor cells that displayed myelolymphoid lineage markers (Fig. 7B and C). Then we sorted CD45.2+ mGS-derived cells from the BM of primary recipient mice (Fig. 7A) and injected 104 mGS-derived donor cells into the BM of irradiated secondary recipient NOD/SCIDγcnull mice. However, none of the host mice displayed donor-derived cells in the PB or BM after 4 months of the secondary transplantation. Thus, mGS-derived multipotent hematopoietic cells en-grafted in the BM, but could not show stem cell potential by expanding and repopulating all the hematopoietic lineages posttransplantation for > 16 weeks.
Figure 7.
Analysis of primary recipient mice transplanted with mGS–derived hematopoietic cells. There are small percentages of mGS-derived cells in lineage negative fraction in the recipient bone marrow (BM) cells (A). When analyzing total recipient BM cells, mGS-derived cell can be detectable in very small percentage (B), but showed multi-lineage cell types (C).
mGS-derived hematopoietic cells did not express CXCR4 or CDX4, but expressed HOXB4
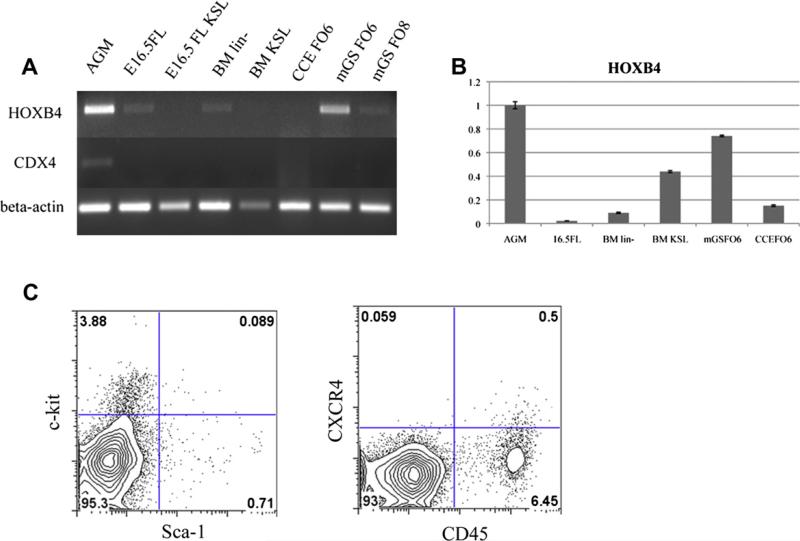
Because mGS-derived hematopoietic cells did not engraft in the BM efficiently, we examined these cells for evidence of expression of a well-known and important homing receptor, CXCR4 (Fig. 8C). Indeed, very few mGS-derived CD45+ cells expressed CXCR4. Also, c-kit expression was only 3% and the c-kit+Sca-1+ population represented only 0.09% of the culture elements. Because CDX4 and HOXB4 overexpression have enabled ES cells to engraft in vivo [2], the expression level of these genes was examined in mGS-derived cells using both RT-PCR and quantitative PCR. mGS-derived cells, as well as fetal liver and BM stem/progenitor cells, did not express mRNA for CDX4 (Fig. 8A). However, using the embryonic aorta-gonadomesonephros (AGM) tissue as a reference (positive control tissue), the relative expression of HOXB4 in mGS-derived cells (0.76) was higher than embryonic day 16.5 fetal liver cells (0.02), BM lin– cells (0.09), BM Sca-1+c-kit+lin– cells (0.43), and CCE-derived cells (0.15) (Fig. 8B). From these results, it appears that the lack of CXCR4 expression of mGS-derived cells may be a primary reason that the transplanted cells could not expand in the recipient BM compartment.
Figure 8.
mGS–derived cells express HOXB4, but not CDX4 or CXCR4. RT-PCR for CDX4 and HOXB4 among various hematopoitic cells (A). KSL = c-kit+Sca-1+lineage– cells, BM lin– = bone marrow lineage-negative cells, FO6 = Flk1 culture on OP9 day 6, FO8 = Flk1 culture on OP9 day 8. Relative HOXB4 expression in the various hematopoitic cells (B). With AGM as a reference, the relative expressions of HOXB4 in each sample were follows; 16.5FL 0.02, BM lin– 0.09, BM KSL 0.43, mGS FO6 0.74, CCE FO6 0.15. c-kit, Sca-1, and CXCR4 expression on mGS-derived cells [(C) 6 days culture].
Discussion
We report that multipotent hematopoietic progenitor cells are derived from mGS cells. mGS cells differentiate into Flk1+ cells similar to differentiated ES cells. From mGS-derived Flk1+ cells, erythroid, myeloid, lymphoid, and megakaryocyte hematopoietic progenitors were induced efficiently in vitro in coculture with OP9 stromal cells. Furthermore, mGS-derived hematopoietic cells engrafted in the BM of NOD/SCIDγcnull mice, although their chimeric rate was very low. We have also transplanted hematopoietic cells differentiated from mGS-derived Flk1+ cells into lethally irradiated mice via tail vein, but found no significant number of donor cells in the PB or BM after transplantation (more than 30 transplanted mice, data not shown). mGS-derived cells were found in the recipient BM only when directly transplanted into the femoral BM of NOD/SCIDγcnull mice.
There may be several reasons why the mGS-derived Flk1+ progeny only engraft upon direct injection into the marrow. It is known that emergence of hematopoiesis from ES cells in vitro mirrors the emergence of hematopoiesis in the murine embryos [26]. Considering this observation, hematopoietic cells derived from mGS and ES cells should have similar characteristics to hematopoietic cells in embryos during early–mid gestation. Yolk sac cells and para-aortic splanchnopleura cells as early as E9.0 engraft in sublethally conditioned neonatal mice, but not in adult BM [27,28]. These facts lead us to speculate that mGS-derived hematopoietic cells may be too immature to express homing receptors and thus failed to find the HSC niche in the adult BM when circulating. Indeed, very few mGS-derived cells expressed CXCR4, c-kit, or Sca-1 (Fig. 8C). Furthermore, the mGS-derived Flk1+ progeny may fail to express high levels of human leukocyte antigen (HLA) and thus are trapped by host natural killer (NK) cells. However, NOD/SCIDγcnull mice have no NK cell activity [29,30] and are thus suitable for transplantation with embryonic HSCs that have just emerged from the aorta-gonado-mesonephros region at E10.5 [31]. Finally, we may have observed low chimerism due to the limited number of cells infused (1 × 105). Transplanting a larger number of cells might improve the chimerism. This limited proliferative ability of transplanted cells is similar to the behavior of human ES-derived cells transplanted in NOD/SCID mice [32].
It is interesting that donor mGS–derived hematopoietic cells that engrafted remained at the endosteal region, reported to be a niche for HSC, and that they were resident in the SP fraction 4 months after transplantation. However, these mGS-derived hematopoietic cells could not proliferate even after 5FU injection; a hematopoietic stress that normally recruits quiescent HSC into cycle and replenishment of the 5FU–depleted progenitor cell pool. In the secondary transplantation studies, as many as 30,000 to 40,000 cells were sorted from the BM of the primary recipients and injected into secondary recipient BM, and no donor cells were observed. While the most quiescent dormant HSCs may divide every 145 days, even dormant HSC can proliferate after 5FU injection [33]. Therefore, it is unlikely that our mGS-derived multipotent cells represent dormant HSCs.
We have also shown that mGS-derived Flk-1+ cells could produce B- and T-lymphoid cells, as well as erythromyeloid cells, using OP9 and OP9-DL1 stromal cell line cocultures. These results were very similar to ES cell differentiation studies reported previously [12,22]. Thus, mGS cells have at least the same hematopoietic potential as ES cells in vitro and in vivo.
Wild-type ES cells fail to reconstitute mouse BM without prior genetic modification [1,2]. Considering that only CDX4- and HOX B4-expressing ES cells can engraft and proliferate in vivo, it might be possible that ES or mGS-derived hematopoietic cells originally lack the proliferation potential required for in vivo expansion following engraftment. Actually, our mGS-derived cells, as well as fetal liver and BM stem/progenitor cells, did not express CDX4 transcripts. On the other hand, mGS-derived cells did express HOXB4. Because mGS-derived cells could not expand in vivo in spite of higher HOXB4 expression than fetal liver and BM progenitors, lack of CXCR4 expression may be a primary reason for the low level of donor cell chimerism rather than lack of CDX4 expression, although the question of whether upregulating CDX4 expression may enhance repopulating ability of the transplanted mGS cells needs further study. Nevertheless, this is the first report that transplanted hematopoietic cells derived from ES-like cells (mGS or ES cells) may reside in a long-lived multipotent hematopoietic cell fraction in vivo.
Recently, multipotent germline stem cells have been established from adult mouse testis [34]. Multipotent adult germline stem cells display similar characteristics to ES cells, including contributions to various organs (even germ-line transmission) when injected into an early blastocyst. The multipotent adult GS cells possess an advantage over ES or embryonic germ cells because they can be stably established from the postnatal mouse. Establishing multipo-tent adult GS cells from human subjects should be feasible both technically and ethically. Indeed, pluripotent stem cells from adult human testis have been generated [35,36]. Additionally, induced pluripotent stem cells have been established from mouse and human somatic cells and upon differentiation induced pluripotent stem cell progeny appear to have the same potential as ES-derived cells [37–39]. Which cell source will ultimately prove most efficacious for human therapy remains fertile ground for future translational research.
Acknowledgments
This study was supported by grants for Scientific Research (S) (19109006) and Scientific Research (B) (18390298,20390296) from the Ministry of Education, Science, Technology, Sports and Culture of Japan (Tokyo, Japan), Uehara Memorial Foundation (Tokyo, Japan) and by the Riley Children's Foundation (Indianapolis, IN, USA).
Footnotes
Conflict of Interest Disclosure
No financial interests/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka H, Takakura N, Nishikawa S, et al. Expressions of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev Growth Differ. 1997;39:729–740. doi: 10.1046/j.1440-169x.1997.t01-5-00009.x. [DOI] [PubMed] [Google Scholar]
- 6.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 7.Shalaby F, Ho J, Stanford WL, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 9.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa SI, Nishikawa S, Kawamoto H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 11.Iida M, Heike T, Yoshimoto M, Baba S, Doi H, Nakahata T. Identification of cardiac stem cells with FLK1, CD31, and VE-cadherin expression during embryonic stem cell differentiation. FASEB J. 2005;19:371–378. doi: 10.1096/fj.04-1998com. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 13.Nakahata T, Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982;79:3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakahata T, Ogawa M. Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. J Clin Invest. 1982;70:1324–1328. doi: 10.1172/JCI110734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miwa Y, Atsumi T, Imai N, Ikawa Y. Primitive erythropoiesis of mouse teratocarcinoma stem cells PCC3/A/1 in serum-free medium. Development. 1991;111:543–549. doi: 10.1242/dev.111.2.543. [DOI] [PubMed] [Google Scholar]
- 16.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya A, Heike T, Fujino H, et al. Long-term extensive expansion of mouse hepatic stem/progenitor cells in a novel serum-free culture system. Gastroenterology. 2005;128:2089–2104. doi: 10.1053/j.gastro.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Nagato M, Heike T, Kato T, et al. Prospective characterization of neural stem cells by flow cytometry analysis using a combination of surface markers. J Neurosci Res. 2005;80:456–466. doi: 10.1002/jnr.20442. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimoto M, Chang H, Shiota M, et al. Two different roles of purified CD45+c-Kit+Sca-1+Lin- cells after transplantation in muscles. Stem Cells. 2005;23:610–618. doi: 10.1634/stemcells.2004-0220. [DOI] [PubMed] [Google Scholar]
- 20.Umeda K, Heike T, Yoshimoto M, et al. Development of primitive and definitive hematopoiesis from nonhuman primate embryonic stem cells in vitro. Development. 2004;131:1869–1879. doi: 10.1242/dev.01065. [DOI] [PubMed] [Google Scholar]
- 21.Umeda K, Heike T, Yoshimoto M, et al. Identification and characterization of hemoangiogenic progenitors during cynomolgus monkey embryonic stem cell differentiation. Stem Cells. 2006;24:1348–1358. doi: 10.1634/stemcells.2005-0165. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto T, Ogawa M, Minegishi N, et al. Step-wise divergence of primitive and definitive haematopoietic and endothelial cell lineages during embryonic stem cell differentiation. Genes Cells. 2001;6:1113–1127. doi: 10.1046/j.1365-2443.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto M, Shinohara T, Heike T, Shiota M, Kanatsu-Shinohara M, Nakahata T. Direct visualization of transplanted hematopoietic cell reconstitution in intact mouse organs indicates the presence of a niche. Exp Hematol. 2003;31:733–740. doi: 10.1016/s0301-472x(03)00108-5. [DOI] [PubMed] [Google Scholar]
- 24.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 26.Choi K. The hemangioblast: a common progenitor of hematopoietic and endothelial cells. J Hematother Stem Cell Res. 2002;11:91–101. doi: 10.1089/152581602753448568. [DOI] [PubMed] [Google Scholar]
- 27.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 28.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 30.Hiramatsu H, Nishikomori R, Heike T, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 31.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 35.Conrad S, Renninger M, Hennenlotter J, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 36.Kossack N, Meneses J, Shefi S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]