Abstract
The acute administration of MDMA has been shown to promote glycogenolysis and increase the extracellular concentration of glucose in the striatum. In the present study the role of serotonergic and/or noradrenergic mechanisms in the MDMA-induced increase in extracellular glucose and glycogenolysis was assessed. The relationship of these responses to the hyperthermia produced by MDMA also was examined. The administration of MDMA (10 mg/kg, i.p.) resulted in a significant and sustained increase of 65-100% in the extracellular concentration of glucose in the striatum, as well as in the prefrontal cortex and hippocampus, and a 35% decrease in brain glycogen content. Peripheral blood glucose was modestly increased by 32% after MDMA treatment. Treatment of rats with fluoxetine (10 mg/kg, i.p.) significantly attenuated the MDMA-induced increase in extracellular glucose in the striatum but had no effect on MDMA-induced glycogenolysis or hyperthermia. Treatment with prazosin (1 mg/kg, i.p.) did not alter the glucose or glycogen responses to MDMA but completely suppressed MDMA-induced hyperthermia. Finally, propranolol (3 mg/kg, i.p.) significantly attenuated the MDMA-induced increase in extracellular glucose and glycogenolysis but did not alter MDMA-induced hyperthermia. The present results suggest that MDMA increases extracellular glucose in multiple brain regions, and that this response involves both serotonergic and noradrenergic mechanisms. Furthermore, β-adrenergic and α-adrenergic receptors appear to contribute to MDMA-induced glycogenolysis and hyperthermia, respectively. Finally, hyperthermia, glycogenolysis and elevated extracellular glucose appear to be independent, unrelated responses to acute MDMA administration.
Keywords: MDMA, glycogen, glucose, hyperthermia, microdialysis
1. Introduction
The recreational drug 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) is a synthetic analogue of amphetamine and methamphetamine. In general, the neuropharmacology of MDMA is similar to that of other amphetamines. In particular, it is well documented that MDMA promotes the release of dopamine, 5-HT and norepinephrine in multiple brain regions, presumably through an action on the respective monoamine transporters (c.f., Gudelsky and Yamamoto, 2008). More recently, a stimulatory effect of MDMA on cortical and hippocampal acetylcholine release also has been documented (Fischer et al., 2000; Acquas et al., 2001; Nair and Gudelsky, 2006). The pronounced stimulatory effect of MDMA on central 5-HT neurotransmission appears largely to mediate the psychoactive effects of the drug in humans (Leichti et al., 2000).
The acute pharmacological effects of MDMA in rodents consist of a variety of behavioral, neuroendocrinological and thermal responses. These include elicitation of a 5-HT behavioral syndrome (Spanos and Yamamoto, 1989), increased locomotor activity (Herin et al., 2005), increased serum concentrations of prolactin and corticosterone (Nash et al., 1988) and hyperthermia (Nash et al., 1988; Schmidt et al., 1990; Colado et al., 1993). In general, many of these responses are thought to be mediated, as noted above, by a MDMA-induced increase in the release of dopamine and/or 5-HT.
The neurochemical effects of MDMA also include an influence on brain cellular energetics. MDMA produces a complex pattern of alterations in local cerebral glucose utilization (Quate et al., 2004; Soto-Montenegro et al., 2007). MDMA also has been shown to reduce the brain concentration of glycogen, the main energy reserve in brain (Darvesh et al., 2002; Darvesh and Gudelsky, 2004). Under in vitro conditions, MDMA increases the activity of glycogen phosphorylase, an enzyme responsible for the breakdown of glycogen, in astro-glial rich primary cultures (Poblete and Azmitia, 1995). Furthermore, extracellular concentrations of glucose and lactate in the striatum are increased following MDMA administration (Darvesh et al., 2002; Gramsbergen and Cumming, 2007). In previous reports it has been suggested that activation of 5-HT2 receptors and/or hyperthermia contribute to the mechanism of substituted amphetamine-induced glycogenolysis and increased extracellular glucose in the brain (Huether et al., 1997; Darvesh et al., 2002). With the exception of these two reports, little is known about the neurotransmitter mechanisms involved in MDMA-induced glycogenolysis and elevated brain glucose. The goal of the present study was to assess the potential contribution of serotonergic and noradrenergic mechanisms in the effects of MDMA on brain glycogen and glucose. In addition, the inter-dependence of MDMA-induced glycogenolysis, increased extracellular glucose and hyperthermia was examined.
2. Materials and Methods
2.1 Animal Procedures
Adult male rats (200-275 g) of the Sprague-Dawley strain (Charles River Breeding Laboratories, Portage, Mich.) were used in the studies and were housed in a temperature (21-22°C) and humidity controlled (50%±10%) room with a 12 h light/dark cycle (lights on 0600 h). Food and water were available ad libitum. Animals undergoing surgery were housed one per cage post-operatively. All procedures were in strict adherence to National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committee.
2.2 Chemicals, Drugs and Drug Treatment
MDMA hydrochloride was provided by the National Institute on Drug Abuse. Fluoxetine hydrochloride was obtained from Eli Lilly and Company (Indianapolis, IN). Prazosin hydrochloride and propranolol hydrochloride were purchased from Tocris (Ellisville, MO) and Sigma-Aldrich (St. Louis, MO) respectively. All drugs were dissolved in 0.15 M NaCl. Hexokinase, glucose-6-phosphate dehydrogenase and amyloglycosidase were purchased from Roche Diagnostics Corp. (Indianapolis, IN), and NADP disodium salt was obtained from Sigma-Aldrich (St.Louis, MO). All reagents were prepared as described previously (Nahorski and Rogers, 1972).
Brain glycogen was determined 1 h following the administration of MDMA. Fluoxetine (10 mg/kg, i.p.) was administered 60 min prior to MDMA, whereas propranolol (3 mg/kg, i.p.) and prazosin (1 mg/kg, i.p.) were administered 30 min prior to the administration of MDMA. Doses of fluoxetine, propranolol and prazosin employed in the present study are based on doses used in previous reports (Shankaran et al., 1999; Sprague et al., 2003; Sprague et al., 2005).
2.3 Assay of tissue glycogen
Rats were killed by decapitation, and the brains were removed from the skull and immersed in liquid nitrogen within 10 s. The cerebellum was removed and the brains were stored at -80° C until analysis. Procedures for analysis of glycogen were similar to those described by Nahorski and Rogers (1972). Approximately 120 mg of brain tissue were weighed and homogenized in 3 ml of 0.03 N HCL at 0° C. The homogenate was placed in boiling water for 5 min. Assay tubes contained 300 μl of acetate buffer (pH 4.6), 100 μl of the homogenate or glycogen standard and 10 μl of amyloglycosidase or water. The tubes were vortexed and incubated at room temperature for 30 min. After incubation, 1.25 ml of Tris buffer (pH 7.8), 0.75 ml of MgCl2 (10 mM), 100 μl of ATP (2 mg/ml) and 10 μl of NADP (10 mg/ml) were added to each tube. The tubes were vortexed and subjected to centrifugation at 10,000 g for 5 min. The supernatants were transferred to other tubes. Ten μl of hexokinase (1 mg/ml) and 10 μl of glucose-6-phosphate dehydrogenase (0.2 mg/ml) were then added, and the tubes were vortexed and incubated at room temperature for 30 min. The fluorescence (excitation 350 nm/emission 460 nm) of reduced NADP was measured in a fluorescent spectrophotometer. The difference in the fluorescence values were corrected for sample, reagent, and enzyme blank. Glycogen values are reported as glucose equivalents (micromoles per gram of tissue).
2.4 Glucose assay
Glucose in the dialysis samples was assayed by using a modified method of the glycogen assay (Nahorski and Rogers, 1972). Assay tubes contained 1.25 ml of Tris buffer (pH 7.8), 0.75 ml of MgCl2 (10 mM), 100 μl of ATP (2 mg/ml), 10 μl of NADP (10 mg/ml), 20 μl of dialysate sample or glucose standard. Ten μl of hexokinase (1 mg/ml) and 10 μl of glucose-6-phosphate dehydrogenase (0.2 mg/ml) were added, and the tubes were vortexed and incubated at room temperature for 30 min. The fluorescence of the samples was measured as described above. Glucose values were reported as millimolar concentrations.
Glucose concentrations in peripheral blood were determined in blood obtained from a tail snip with the use of a hand-held glucometer.
2.5 In vivo microdialysis procedures
Rats were implanted with a stainless steel guide cannula under ketamine/xylazine (70:6 mg/kg, i.m.) induced anesthesia 48 to 72 h prior to the insertion of dialysis probe. On the day of the dialysis experiment, a concentric style dialysis probe was inserted through the guide cannula into the brain region of interest. The coordinates for the tip of the probe were A, 1.2 mm; L, 3.1 mm; and V, -7 mm from bregma for the striatum; A, -3.6 mm; L, 2.0 mm; and V, -4.4 mm from bregma for the dorsal hippocampus; A, 3.5 mm; L, 0.7 mm; and V, -4.0 mm from bregma for the prefrontal cortex according to the stereotaxic atlas of Paxinos and Watson (1986). The microdialysis probes were constructed as described previously (Yamamoto and Pehek, 1990). The dialysis surface of the membrane was 4.5 mm, 2.0 mm and 3.0 mm in length for the striatum, hippocampus and prefrontal cortex, respectively. On the day of the dialysis experiment, the probe was connected to an infusion pump set to deliver glucose-free Dulbecco's phosphate-buffered saline containing 1.2 mM CaCl2 at a rate of 2.0 μl/min. After a 1.5 h equilibration period, dialysis samples were collected every 30 min. At least 3 baseline samples were obtained prior to drug treatment.
2.6 Body temperature measurements
On the day of the experiment, the rats were allowed to acclimatize at 24° C for 2 h before body temperature were measured. Measurements of rectal temperature were made using a telethermometer and a thermister probe. The probe was lubricated with a small amount of petroleum jelly and inserted 5 cm into the rectum of each rat for at least 30 s until a stable temperature was obtained. Measurements were taken every 30 min for a 1-h period prior to the administration of fluoxetine, prazosin, propranolol or vehicle at time 0 and for a 2-h period following the injection of MDMA. Fluoxetine (10 mg/kg, i.p.) was administered 60 min prior to administration of MDMA (10 mg/kg, i.p.), whereas propranolol (3 mg/kg, i.p.) and prazosin (1 mg/kg, i.p.) were administered 30 min prior to administration of MDMA or vehicle. The change in body temperature was determined by subtracting the body temperature at time 0 from the maximal body temperature recorded after MDMA or vehicle administration.
2.7 Statistical analysis
The effects of MDMA, fluoxetine, propranolol, and prazosin on brain glycogen and body temperature were analyzed with two-way ANOVA. Glucose data from dialysis experiments were analyzed with two-way repeated measures ANOVA. Multiple pairwise comparisons were performed using the Student-Newman-Keuls test. Treatment differences for all the data were considered statistically significant at P<0.05.
3. Results
The effect of MDMA on the extracellular concentration of glucose in the striatum, prefrontal cortex and hippocampus is depicted in Fig. 1. Analysis of the effect of MDMA (10 mg/kg, i.p.) on the extracellular concentration of glucose revealed a significant effect of time in the striatum, prefrontal cortex and hippocampus [F (8, 229) = 23.66, P<0.001], whereas the main factor of brain region and the time × brain region interaction were not significant. MDMA increased extracellular glucose by 75-100%, and extracellular glucose remained elevated for at least 3 h following drug administration. Administration of the vehicle (0.15M NaCl) had no significant effect on extracellular glucose in any of the three brain regions (data not shown). The mean baseline value for glucose (mM) in the dialysis samples (uncorrected for probe recovery) was 0.1-0.3 mM.
Fig. 1. Effect of MDMA on the extracellular concentration of glucose in multiple brain regions.
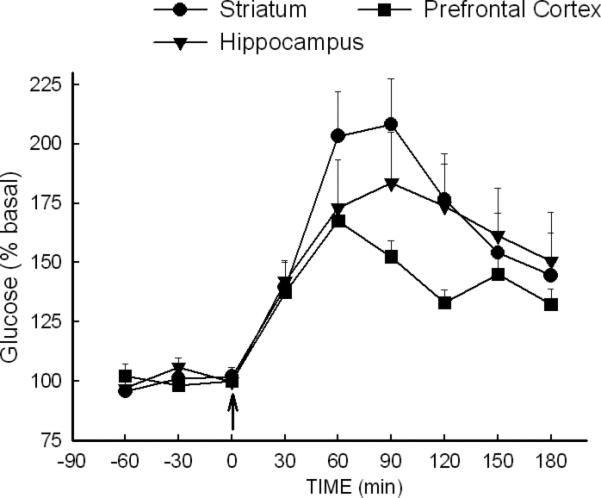
Dialysis samples were collected from the striatum, prefrontal cortex and hippocampus of rats treated with MDMA (10 mg/kg, i.p.) at time 0. N=8-13 rats/group.
In a separate group of non-fasted rats, it was determined that MDMA produced a modest (30%), but significant (P<0.05), increase in peripheral blood glucose at 30 and 60 min following drug administration (Fig. 2). The ANOVA indicated a significant effect of treatment [F (1,24) = 6.27, P<0.05], significant effect of time [F (2,48) = 17.04, P<0.001] and a significant interaction between time and treatment [F (2, 48)=8.13, P<0.001].
Fig. 2. Effect of MDMA on blood glucose concentrations.
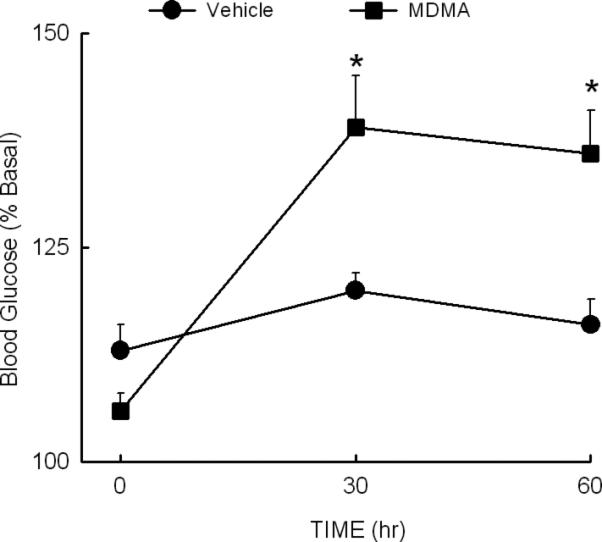
Rats were injected with vehicle or MDMA (10 mg/kg, i.p.) at time 0. Blood samples were obtained from a tail snip at the indicated times. *Indicates P<0.05 compared to the value at time 0. N=6 rats/group.
Serotonergic contributions to the increase in the extracellular concentration of glucose in the striatum and brain glycogenolysis elicited by MDMA was determined subsequently by assessing the effects in fluoxetine-treated rats. Treatment with fluoxetine markedly suppressed (P<0.05) the MDMA-induced increase in extracellular glucose in the striatum (Fig. 3). Extracellular glucose concentrations in rats treated with fluoxetine and MDMA were no greater than those for animals treated with fluoxetine alone. However, fluoxetine alone produced a modest, but significant (P<0.05), increase in extracellular glucose concentrations (Fig. 3). Analysis of the data revealed a significant main effect of drug [F (3, 329) = 6.96, P<0.001], time [F (10, 329) = 13.50, P<0.01] and drug × time interaction [F (30, 329) = 4.13, P<0.001]. In separate groups of rats, MDMA (10 mg/kg, i.p.) produced a significant (P<0.05) 25-30% depletion of brain glycogen, and there was no significant effect of fluoxetine on MDMA-induced glycogenolysis (Fig. 4).
Fig. 3. Effect of fluoxetine on the MDMA-induced increase in extracellular glucose in the striatum.

Rats were treated with vehicle (Veh) or fluoxetine (FLX) (10 mg/kg, i.p.) 60 min prior to the administration of MDMA (10 mg/kg i.p.) at time 0. *P<0.05 compared to values at the corresponding time for Veh+Veh rats. #P<0.05 for values of the FLX+MDMA animals compared to those of the Veh+MDMA rats. N=7-13 rats/group
Fig. 4. Effect of fluoxetine on MDMA-induced glycogenolysis.

Rats received an i.p. injection of vehicle (Veh) or fluoxetine (FLX) (10 mg/kg) 60 min prior to treatment with MDMA. Rats were sacrificed 1 h after treatment with MDMA (10mg/kg, i.p.). *P<0.05 compared to the values for the appropriate vehicle treated control animals. #P<0.05 compared to the values for Veh+VEH rats. N=9-12 rats/group
Noradrenergic contributions to the MDMA-induced increases in extracellular glucose and brain glycogenolysis were evaluated using the β- and α-adrenergic antagonists, propranolol and prazosin, respectively. The MDMA-induced increase in the extracellular concentration of glucose in the striatum was markedly suppressed (P<0.05) in propranolol-treated rats (Fig. 5). The ANOVA revealed a significant effect of treatment [F (3, 176) = 13.16, P<0.001], and time [F (7,176) = 12.97, P<0.001]. Moreover, the interaction of treatment × time also was significant [F (21,176) = 4.05, P<0.001]. In a similar manner, MDMA-induced glycogenolysis was absent in propranolol treated rats (Fig. 6). Whereas MDMA produced a 28% (P<0.05) decrease in brain glycogen in control animals, there was no significant decrease in brain glycogen in propranolol-treated rats.
Fig. 5. Effect of propranolol on the MDMA-induced increase in extracellular glucose in the striatum.
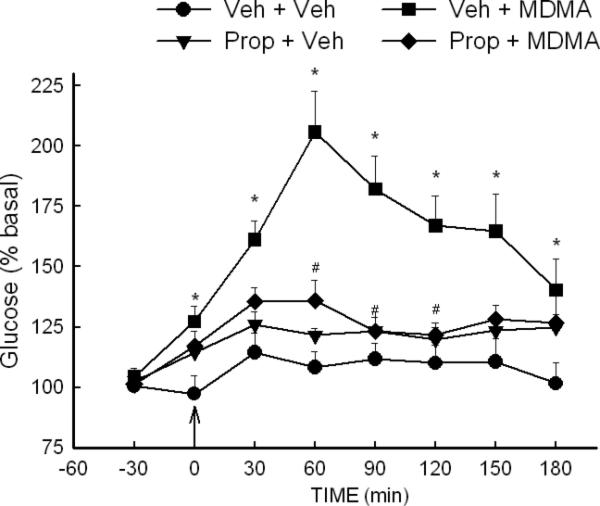
Rats were treated with vehicle (Veh) or propranolol (Prop) (3 mg/kg, i.p.) 30 min prior to the administration of MDMA (10 mg/kg, i.p.) at time 0. *P<0.05 compared to values at corresponding time for Veh+Veh rats. #P<0.05 compared to values at corresponding time for Veh+MDMA rats. N=7-9 rats/group.
Fig. 6. Effect of propranolol on MDMA-induced glycogenolysis.

Rats received Vehicle (Veh) or propranolol (Prop) 30 min prior to treatment with MDMA. Rats were sacrificed 1 h after treatment with MDMA (10mg/kg, i.p.). *P<0.05 compared to the values for the appropriate vehicle treated control animals N=5-6 rats/group.
Treatment or rats with the α-adrenergic antagonist prazosin resulted in a pattern of antagonism of MDMA-induced increases in brain glucose and glycogenolysis that differed from that of both fluoxetine and propranolol. With regard to extracellular glucose, the ANOVA indicated a significant effect of treatment [F (3, 211) = 7.27, P<0.001], time [F (7,211) = 22.14, P<0.001] and treatment × time interaction [F (21, 211) = 4.06, P<0.001]. However, there was no significant difference in the magnitude of the increase in extracellular glucose produced by MDMA in vehicle- or prazosin-treated animals (P=0.46). With regard to the effect of prazosin on MDMA-induced glycogenolysis, there was no difference in the magnitude of glycogen depletion produced by MDMA in vehicle and prazosin treated rats.
The effects of fluoxetine, propranolol and prazosin on MDMA-induced hyperthermia also were determined. Analysis of the data (2x2 ANOVA) indicated a significant effect of treatment [F(1, 48) = 109.47, P<0.001], pretreatment [F(3, 48), = 9.76, P<0.001] and a significant pretreatment × treatment interaction [F(3, 48) = 4.76, P=0.006]. Treatment with MDMA resulted in a significant increase in body temperature of approximately 1.6°C (Fig. 9). The MDMA-induced increase in body temperature was unaffected in rats treated with fluoxetine or propranolol. However, prazosin significantly (P<0.05) diminished the MDMA-induced hyperthermic response. There were no differences in the body temperatures of rats treated with vehicle, fluoxetine, propranolol or prazosin alone (Fig. 9).
Fig. 9. Effect of fluoxetine, propranolol or prazosin on the MDMA-induced increase in body temperature.
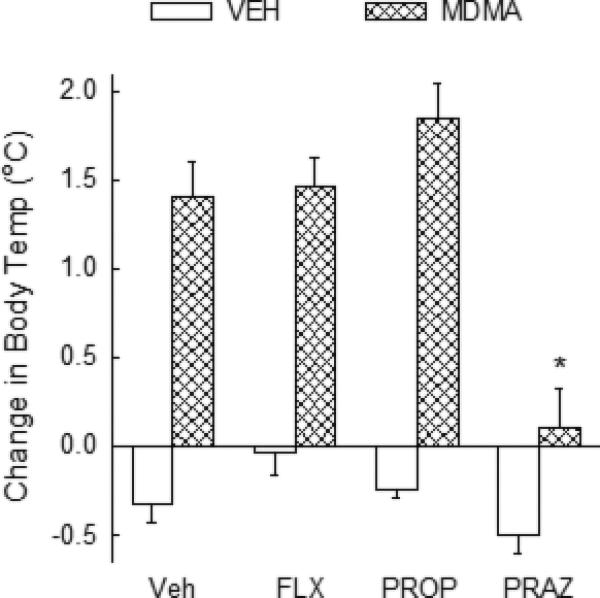
The effect of fluoxetine (FLX), propranalol (Prop), prazosin (Praz) or vehicle (Veh) on MDMA induced hyperthermia. Rats were treated with vehicle, propranolol (3 mg/kg, i.p.) or prazosin (1 mg/kg, i.p.) 30 min prior to treatment with MDMA (10 mg/kg, i.p.) at time 0. Fluoxetine (10 mg/kg) was injected i.p. 60 min prior to MDMA treatment. *P<0.05 compared to values for rats treated with Veh+MDMA. N=6-11 rats/group.
A summary of the interactions of fluoxetine, propranolol and prazosin with MDMA-induced increases in extracellular glucose, glycogenolysis and body temperature is presented in Table 1.
Table 1.
Summary of interactions of fluoxetine, propanolol and prazosin with MDMA-induced increases in extracellular glucose, glycogenolysis and hyperthermia.
| MDMA-Induced Elevation of Glucose | MDMA-Induced Glycogenolysis | MDMA-Induced Hyperthermia | |
|---|---|---|---|
| Fluoxetine | Suppressed | No change | No change |
| Propranolol | Suppressed | Suppressed | No change |
| Prazosin | No change | No change | Suppressed |
4. Discussion
The purpose of the present study was to assess the role of serotonergic and noradrenergic mechanisms in the MDMA-induced increase in extracellular glucose and reduction in brain glycogen. It was of further interest to delineate the relationship between MDMA-induced hyperthermia and the effects of MDMA on glucose and glycogen. The key findings of the present study include: 1) MDMA increases the extracellular concentration of glucose in multiple brain regions, 2) fluoxetine prevents the MDMA-induced increase in brain glucose but not glycogenolysis or hyperthermia, 3) propranolol antagonizes both the MDMA-induced increase in brain glucose and glycogenolysis, but not hyperthermia and 4) prazosin does not alter MDMA-induced increases in brain glucose or glycogenolysis but does suppress the hyperthermic response.
In the present study, the systemic administration of MDMA increased extracellular concentrations of glucose in the striatum, hippocampus and prefrontal cortex by 65-100%. These results are consistent with previous reports that extracellular concentrations of glucose in the striatum are increased following MDMA (Darvesh et al., 2002; Gramsbergen and Cumming, 2007).
The administration of MDMA also resulted in a modest increase in peripheral blood glucose. Previous studies on the effect of MDMA on peripheral glucose have provided inconsistent results. Soto-Montenegro et al (2007) demonstrated that MDMA produces a 35% reduction in blood glucose, whereas Gramsbergen and Cumming (2007) and Banks et al (2009) have reported that relatively large doses of MDMA administered i.v. result in a transient increase in blood glucose. MDMA appears to increase extracellular concentrations of glucose in the brain at doses that do not increase peripheral glucose (Gramsbergen and Cumming, 2007). In addition, the duration of elevation of glucose in brain, e.g., greater than 5 hrs (Darvesh et al., 2002), greatly exceeds the duration of the increase in peripheral blood, e.g., 60-120 min (Gramsbergen and Cumming, 2007). Thus, it is unclear to what extent the MDMA-induced increase in extracellular glucose in the brain is a reflection of an effect of MDMA to increase circulating blood glucose in the periphery.
Alternatively, Gramsbergen and Cumming (2007) have suggested that increased glucose uptake by the brain may account for the effect of MDMA on extracellular glucose in the brain. Serotonin enhances the uptake of glucose into several peripheral tissues (Moore et al., 2005; Fisher et al., 1995; Hajduch et al., 1999), and this has been attributed to an increased translocation of the glucose transporter. It is conceivable that MDMA produces an up-regulation of glucose transport across the blood-brain barrier. Effects of MDMA to increase peripheral blood glucose and increase glucose transport across the blood-brain barrier may account for the increase in extracellular glucose in the brain following treatment with MDMA.
The MDMA-induced increase in extracellular glucose in the brain may be indicative of complex changes in local cerebral blood flow and cerebral glucose utilization. Results from studies on the effects of MDMA on cerebral glucose metabolism have not been consistent. Although Quate et al (2004) have reported that MDMA acutely increases local cerebral glucose utilization in many brain regions, the finding has not been replicated by others (Wilkerson and London, 1989; Soto-Montenegro et al., 2007). Thus, it is unclear whether the MDMA-induced increase in extracellular glucose is reflective of an increase in neuronal energy demand. Moreover, since MDMA has been reported to substantially decrease local cerebral blood flow (Quate et al., 2004), it seems unlikely that increased brain glucose following MDMA is the result of an increased delivery of glucose to the brain through increased blood flow.
Although it has been speculated previously that increases in extracellular glucose in the brain may result from increased glycogenolysis (Darvesh et al., 2002), Dringen et al (1993) have concluded that the breakdown of brain glycogen, which is predominately stored in astrocytes, results in the release of lactate rather than glucose. In the present study, fluoxetine suppressed the MDMA-induced increase in brain glucose without altering MDMA-induced glycogenolysis. This further suggests that the increased breakdown of glycogen and increased extracellular glucose following MDMA are not causally related.
Serotonergic mechanisms appear to mediate, at least in part, the MDMA-induced increase in extracellular glucose. This contention is based on the findings that fluoxetine (present study) and the 5-HT2 antagonist LY 53857 (Darvesh et al., 2002) suppress the stimulatory effect of MDMA on brain glucose. The present finding also is in accord with the report of Gramsbergen and Cumming (2007) in which the stimulatory effect of MDMA on brain glucose was markedly suppressed in rats treated with the 5-HT synthesis inhibitor PCPA. In the present study, fluoxetine alone produced a modest increase in extracellular glucose. This effect of fluoxetine may be the result of an elevation of extracellular 5-HT and the subsequent activation of 5-HT2 receptors. This supposition is based on the finding that DOI, a 5-HT2 agonist, has been shown to increase extracellular glucose in the brain (Darvesh and Gudelsky, 2003).
Noradrenergic mechanisms, in particular β-adrenergic receptors, also appear to contribute to the MDMA-induced increase in extracellular glucose, since this response was greatly diminished in rats treated with propranolol. Fray et al (1996) also have reported that noradrenergic mechanisms contribute to the regulation of extracellular glucose based on the finding that local perfusion with isoproterenol increased extracellular glucose in the brain.
Consistent with previous reports (Darvesh et al., 2002; Darvesh and Gudelsky, 2004), MDMA reduced brain concentrations of glycogen by approximately 35%. This finding also is in accord with the reports that amphetamine and parachloroamphetamine also reduce brain glycogen (Heuther et al., 1997; Nahorski and Rogers, 1975). In the present study, MDMA-induced glycogenolysis was not altered by fluoxetine treatment, suggestive of the lack of involvement of serotonergic mechanisms in this response. However, it was previously reported that 5-HT2 receptor blockade suppressed MDMA-induced glycogenolysis (Darvesh et al., 2002). One explanation for these seemingly discrepant findings is that MDMA may act directly as a 5-HT2 agonist (Nash et al., 1994) to promote glycogenolysis within astrocytes (Poblete and Azmitia, 1995). Such an action of MDMA would not be antagonized by fluoxetine.
The finding that propranolol attenuated MDMA-induced glycogenolysis is supportive of the view β-adrenergic receptors also contribute to this effect of MDMA. The present finding is consistent with earlier reports that 1) cerebral glycogenolysis is increased by isoproterenol (Nahorski et al., 1975) and 2) amphetamine-induced glycogenolysis is suppressd by propranolol (Nahorski and Rogers, 1975). Thus, it appears that MDMA promotes glycogenolysis in the rat brain through the activation of both 5-HT2 and β-adrenergic receptors.
It has been suggested that the MDMA-induced hyperthermia contributes to increased glycogenolysis, and possibly, a subsequent increase in extracellular glucose (Huether et al., 1999; Darvesh et al., 2002). However, results from the present study illustrate dissociation between MDMA-induced hyperthermia and its effects on glycogen and glucose. For example, the α1-antagonist prazosin attenuated MDMA-induced hyperthermia, in accord with previous reports (Sprague et al., 2003), but did not alter the stimulatory effect of MDMA on extracellular glucose or on glycogenolysis. Conversely, propranolol suppressed both the increased extracellular glucose and glycogenolysis elicited by MDMA without altering MDMA-induced hyperthermia. Similarly, fluoxetine attenuated the MDMA-induced increase in extracellular glucose without altering the drug induced hyperthermia. These data further illustrate the dissociation between the effects of MDMA on cellular energetics and body temperature. However, it is conceivable that fluoxetine and/or propranolol intercede “down-stream” from MDMA-induced hyperthermia to suppress the stimulatory effect of MDMA on brain glucose and/or glycogen. Nevertheless, MDMA-induced hyperthermia appears to be neither necessary nor sufficient for the effects of this drug on extracellular glucose or glycogen.
In summary, the present results suggest that the MDMA-induced increase in extracellular glucose and glycogenolysis appear to be dependent on serotonergic and/or noradrenergic mechanisms, and that MDMA-induced glycogenolysis does not appear to contribute to the increase in extracellular glucose. Finally, the effects of MDMA on extracellular glucose and glycogen are independent of its hyperthermic effect.
Fig. 7. Effect of prazosin on the MDMA-induced increase in extracellular glucose in the striatum.
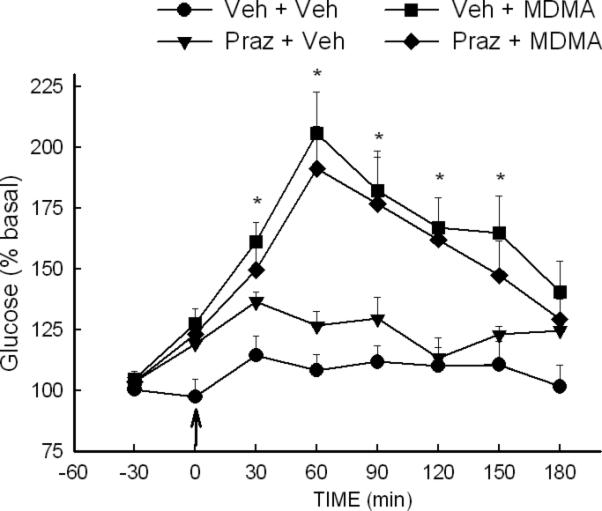
Rats were treated with vehicle (veh) or prazosin (Praz) (1 mg/kg, i.p.) 30 min prior to treatment with MDMA (10 mg/kg, i.p.) at time 0. *P<0.05 compared to values at corresponding time for Veh+Veh rats. N=8-10 rats/group.
Fig. 8. Effect of prazosin on MDMA-induced glycogenolysis.
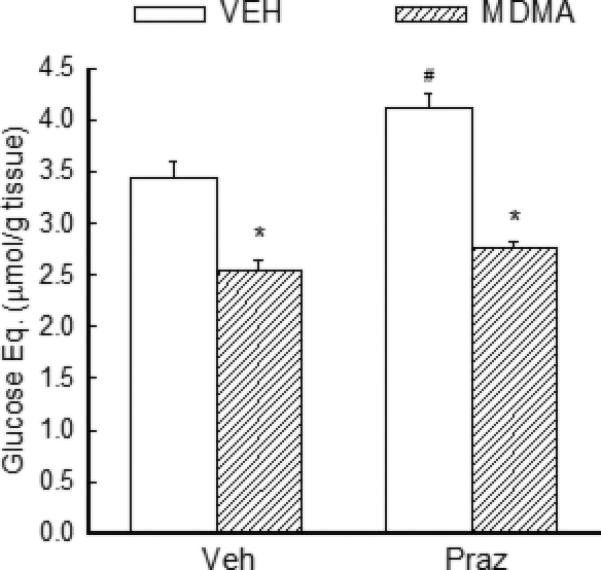
Rats received vehicle (veh) or prazosin (Praz) (1 mg/kg, i.p.) 30 min prior to treatment with MDMA. Rats were sacrificed 1 h after treatment with MDMA (10mg/kg, i.p.). *P<0.05 compared to the values for the appropriate vehicle treated control animals. #P<0.05 compared to the values for Veh+Veh rats. N=5-6 rats/group.
Acknowledgements
This work was supported by grant DA07427.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Marrocu P, Pisanu A, Cadoni C, Zernig G, Saria A, DiChiara G. Intravenous administration of ecstasy (3, 4-methylenedioxymethamphetamine) enhances cortical and striatal acetylcholine release in vivo. Eur. J. Pharmacol. 2001;418:207–2011. doi: 10.1016/s0014-2999(01)00937-2. [DOI] [PubMed] [Google Scholar]
- Banks ML, Buzare SK, Gehret CM, Monroy AN, Kenaston MA, Mills EM, Sprague JE. Pharmacodynamic characterization of insulin on MDMA-induced thermogenesis. Eur. J. Pharmacol. 2009;615:257–261. doi: 10.1016/j.ejphar.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, Murray TK, Green AR. 5-HT lossin rat brain following 3,4-methylenedioxymethamphetamine (MDMA), p-chloroamphetamine and fenfluramine administration and effects of chlormethiazole and dizolcilpine. Br J Pharmacol. 1993;108:583–589. doi: 10.1111/j.1476-5381.1993.tb12846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvesh AS, Shankaran M, Gudelsky GA. 3, 4-Methylenedioxymethamphetamine produces glycogenolysis and increases the extracellular concentration of glucose in the rat brain. J. Pharmacol. Exp. Ther. 2002;300:138–144. doi: 10.1124/jpet.301.1.138. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Gudelsky GA. Activation of 5-HT2 receptors induces glycogenolysis in the rat brain. Eur. J. Pharmacol. 2003;464:135–140. doi: 10.1016/s0014-2999(03)01432-8. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Gudelsky GA. The relationship between hyperthermia and glycogenolysis in 3, 4-methylenedioxymethamphetamine-induced serotonin depletion in rats. Neurotoxicology and Tetralogy. 2004;26:571–577. doi: 10.1016/j.ntt.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Fischer HS, Zernig G, Schatz DS, Humpel C, Saria A. MDMA (ecstasy) enhances basal acetylcholine release in brain slices of the rat striatum. Eur. J Neurosci. 2000;12:1385–1390. doi: 10.1046/j.1460-9568.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Thomas J, Kamp J, Jungling E, Rose H, Carpene, Kammermeier H. 5-Hydroxytryptamine stimulates glucose transport in cardiomyocytes via a monoamine oxidase-dependent reaction. Biochem. J. 1995;311(Pt 2):575–583. doi: 10.1042/bj3110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray AE, Forsyth RJ, Boutelle MG, Fillenz M. The mechanism controlling physiologically stimulated changes in rat brain glucose and lactate: a micodialysis study. J. Physiol. 1996;496:49–57. doi: 10.1113/jphysiol.1996.sp021664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen JB, Cumming P. Serotonin mediates rapid changes of striatal glucose and lactate metabolism after systemic 3, 4-methylenedioxymethamphetamine (MDMA. “Ecstasy”) administration in awake rats. Neurochemistry International. 2007;51:8–15. doi: 10.1016/j.neuint.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol. Biochem. Behavior. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Rencurel F, Balendran A, Batty IH, Downes CP, Hundal HS. Serotonin (5-hydroxytryptamine), an novel regulator of glucose transport in rat skeletal muscle. J. Biol. Chem. 1999;274:13563–13568. doi: 10.1074/jbc.274.19.13563. [DOI] [PubMed] [Google Scholar]
- Herrin DV, Liu S, Ullrich T, Rice KC, Cunningham KA. Role of the serotonin 5-HT2A receptor in the hyperlocomotive and hyperthermic effects of 3,4-methylenedioxymethamphetamine. Psychopharmacology. 2005;178:505–513. doi: 10.1007/s00213-004-2030-4. [DOI] [PubMed] [Google Scholar]
- Huether G, Zhou D, Ruther E. Causes and consequences of the loss of serotonerhgic presynapses elicited by the consumption of 3, 4-methylendioxymethamphetamine (MDMA, “ecstasy”) and its congeners. J. Neural. Transm. 1997;104:771–794. doi: 10.1007/BF01285547. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000;22:513–522. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Moore MC, DiCostnazo CA, Dardevet D, Lautz M, Farmer B, Cherrington AD. Interaction of a selective serotonin reuptake inhibitor with insulin in the control of hepatic glucose uptake in conscious dogs. Am. J. Physiol. Endocrinol. Metab. 2005;288:E556–E663. doi: 10.1152/ajpendo.00405.2004. [DOI] [PubMed] [Google Scholar]
- Nahorski SR, Rogers KJ. An enzymatic fluorimetric micro method for determination of glycogen. Anal. Biochem. 1972;49:492–497. doi: 10.1016/0003-2697(72)90453-8. [DOI] [PubMed] [Google Scholar]
- Nahorski SR, Rogers K,J, Edwards C. Cerebral glycogenolysis and stimulation of beta-adrenoreceptors and histamine H2 receptors. Brain Research, 1975. 1975;92:529–533. doi: 10.1016/0006-8993(75)90342-x. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gudelsky GA. 3, 4-Methylenedioxymethamphetamine enhances the release of acetylcholine in the prefrontal cortex and dorsal hippocampus of the rat. Psychopharmacology. 2006;184:182–189. doi: 10.1007/s00213-005-0271-5. [DOI] [PubMed] [Google Scholar]
- Nash JF, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after administration of 3, 4-methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA. Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci. Lett. 1994;177:111–115. doi: 10.1016/0304-3940(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Poblete JC, Azmitia EC. Activation of glycogen phosphorylase by serotonin and 3, 4-methylenedioxymethamphetamine in astroglial-rich primary cultures: involvement of 5-HT2A receptor. Brain Res. 1995;680:9–15. doi: 10.1016/0006-8993(95)00201-z. [DOI] [PubMed] [Google Scholar]
- Quate L, McBean DE, Ritchie IM, Olverman HJ, Kelly PAT. Acute methylenedioxymethamphetamine administration: effects on local cerebral blood flow and glucose utilization in the dark agouti rat. Psychopharmacology. 2004;173:287–295. doi: 10.1007/s00213-004-1784-z. [DOI] [PubMed] [Google Scholar]
- Schimdt CJ, Black CK, Abbate GM, Taylor VL. Methylenedioxymethamphetamine- induced hyperthermia and neurotoxicity are independently mediated by 5HT2 receptors. Brain Res. 1990;529:85–90. doi: 10.1016/0006-8993(90)90813-q. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Involvement of the serotonin transporter in the formation of hydroxyl radicals induced by 3,4-methylenedioxymethamphetamine. Eur. J. Pharmacol. 1999;385:103–110. doi: 10.1016/s0014-2999(99)00728-1. [DOI] [PubMed] [Google Scholar]
- Soto-Montenegro ML, Vaquero JJ, Arango C, Ricaurte G, Garcia-Bareno P, Desco M. Effects of MDMA on blood glucose levels and brain glucose metabolism. Eur. J. Nucl. Mol. Imaging. 2007;34:916–925. doi: 10.1007/s00259-006-0262-8. [DOI] [PubMed] [Google Scholar]
- Spanos LJ, Yamamoto BK. Acute and subchronic effects of methylenedioxymethamphetamine (MDMA) on locomotion and serotonin syndrome behavior in the rat. Pharmacol. Biochem. Behavior. 1989;32:835–840. doi: 10.1016/0091-3057(89)90044-0. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) J. Pharmacol. Exp. Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Sprauge JE, Moze P, Caden D, Rusyniak DE, Holmes C, Goldstein DS, Mills EM. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) in an animal model. Crit. Care Med. 2005;33:1311–1316. doi: 10.1097/01.ccm.0000165969.29002.70. [DOI] [PubMed] [Google Scholar]
- Wilkerson G, London ED. Effects of methylenedioxymethamphetamine on local cerebral glucose utilization in the rat. Neuropharmacology. 1989;28:1129–1138. doi: 10.1016/0028-3908(89)90128-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto B, Pehek E. A neurochemical heterogeneity of the rat striatum as measured by in vivo electrochemistry and microdialysis. Brain Res. 1990;506:236–242. doi: 10.1016/0006-8993(90)91256-g. [DOI] [PubMed] [Google Scholar]


