Abstract
Cardiac contraction during systole is dependenton action potential-triggered Ca2+ release from the sarcoplasmic reticulum (SR) through ryanodine receptor (RyR) channels. SR Ca2+ release can also occur spontaneously during diastole, which causes a decrease in Ca2+ content within the SR and contributes to arrhythmogenesis. Here, we use measurements of cytosolic Ca2+ and intra-SR Ca2+ ([Ca2+]SR) to examine how RyR sensitization alters spontaneous SR Ca2+ release events in rabbit ventricular myocytes. RyR sensitization with caffeine (250 μM) increased the open probability of single RyR channels, increased the initial frequency and amplitude of local SR Ca2+ release events (Ca2+ sparks), and decreased the [Ca2+]SR level where Ca2+ sparks terminated. In intact myocytes, caffeine applied during rest after steady-state electrical stimulation increased the frequency of spontaneous Ca2+ waves and decreased the [Ca2+]SR level where waves terminated. These effects caused a marked loss of SR Ca2+ content. Therefore, increasing RyR activity has complex effects on cardiac function. Increased RyR activity during systole is beneficial as it increases SR Ca2+ release and contractile strength. However, increased RyR activity during diastole produces spontaneous, arrhythmogenic Ca2+ release events that lower SR Ca2+ content and subsequently decrease contractility.
Keywords: excitation-contraction coupling, Ca2+ spark, Ca2+ wave, ryanodine receptor, ventricular myocyte
Introduction
Cardiac excitation-contraction coupling (ECC) is triggered by the Ca2+-induced Ca2+ release (CICR) mechanism.1 During ECC, depolarization of the sarcolemma membrane opens L-type Ca2+ channels (LTCCs), and the subsequent Ca2+ influx activates sarcoplasmic reticulum (SR) Ca2+ release channels, ryanodine receptors (RyRs), and causes a massive release of Ca2+ into the cytosol. The global cytosolic Ca2+ transient that triggers contraction is composed of the temporal and spatial summation of thousands of individual CICR events that occur in a signaling microdomain called the dyadic cleft. In this region, the LTCCs in the t-tubule membrane (an invagination of the surface sarcolemma) come in close proximity to RyR clusters in the junctional SR, and form a specialized signaling complex termed the Ca2+ release unit (Fig. 1A). The concerted opening of a cluster of RyRs generates the fundamental CICR event of ECC, the Ca2+ “spark”,2 which is visualized using confocal fluorescence microscopy and high-affinity Ca2+ indicators (e.g., rhod-2) as a localized increase in cytosolic fluorescence (Fig. 1A and B upper). Alternatively, the Ca2+ released from the SR during a Ca2+ spark can be measured directly using low-affinity Ca2+ indicators (e.g., fluo-5N) entrapped within the SR; this reciprocal intra-SR Ca2+ depletion is termed a Ca2+ “blink”3,4 (Fig. 1A and B lower).
Figure 1.
The Ca2+ release unit of ventricular myocytes. (A) Illustration of a Ca2+ release unit highlighting the close proximity of L-type Ca2+ channels (LTCC) in the t-tubule membrane and RyR clusters in the junctional SR membrane. A spontaneous Ca2+ spark is observed using the high-affinity Ca2+ indicator rhod-2 as an increase in cytosolic fluorescence (red), or with the low-affinity Ca2+ indicator fluo-5N as an intra-SR Ca2+ depletion (Ca2+ blink, green). (B) 3-dimensional (time, width, amplitude) fluorescence profiles of a Ca2+ spark (red) and a Ca2+ blink (green). Profiles correspond to the average of 40 simultaneously recorded Ca2+ spark/Ca2+ blink pairs from permeabilized ventricular myocytes.
The intrinsic sensitivity of the RyR to cytosolic Ca2+ underlies the process of CICR and therefore cardiac contraction. However, recent evidence shows that channel gating is also regulated by the Ca2+ concentration within the lumen of the SR ([Ca2+]SR). In planar lipid bilayer experiments channel open probability is markedly enhanced by increasing Ca2+ on the luminal side of the channel,5 an effect that is removed following enzymatic digestion of the luminal side of the channel protein.6 In the cellular environment this property of the channel manifests as an increase in Ca2+ spark frequency and an increase in the number of Ca2+ release units that respond to an action potential as Ca2+ content within the SR rises (e.g., during adrenergic stimulation). However, elevated SR Ca2+ content also leads to spontaneous Ca2+ release events during diastole, including arrhythmogenic Ca2+ waves. Ca2+ waves occur when the Ca2+ released during a spontaneous Ca2+ spark diffuses to neighboring release junctions, triggers CICR, and forms a regenerative wave of CICR that travels from release junction to release junction.7 This form of diastolic Ca2+ release is highly arrhythmogenic as the Ca2+ released during the wave is extruded by the electrogenic Na+-Ca2+ exchanger (NCX), which depolarizes the sarcolemma and leads to spontaneous electrical activity such as delayed afterdepolarizations.8,9 Furthermore, diastolic Ca2+ release depresses cardiac contractility by impairing relaxation, as well as by decreasing SR Ca2+ content and Ca2+ release during subsequent systole.
In a recent study,10 we investigated how sensitization of RyR channels alters Ca2+ sparks and SR Ca2+ release in rabbit ventricular myocytes. Using a low dose of the RyR agonist caffeine (250 μM), we sensitized RyRs and monitored SR Ca2+ release using simultaneous measurements of cytosolic Ca2+ ([Ca2+]i) and [Ca2+]SR. These experiments showed that RyR sensitization alters both activation and termination of Ca2+ sparks, and both of these mechanisms contribute to an initial increase in global Ca2+ transient amplitude during ECC. However, during steady-state pacing, the initial increase in systolic SR Ca2+ release (positive inotropic effect) as well as the increased frequency and amplitude of diastolic Ca2+ sparks led to drastically reduced SR Ca2+ content and a subsequent decrease in systolic SR Ca2+ release (negative inotropic effect). In the follow-up study presented here, we tested whether RyR sensitization alters the properties of an additional form of diastolic SR Ca2+ release: spontaneous, arrhythmogenic Ca2+ waves. We find that RyR sensitization increases the occurrence of Ca2+ waves, lowers the [Ca2+]SR level where release terminates during a wave, and leads to a substantial loss of Ca2+ from the SR during diastole.
Results and Discussion
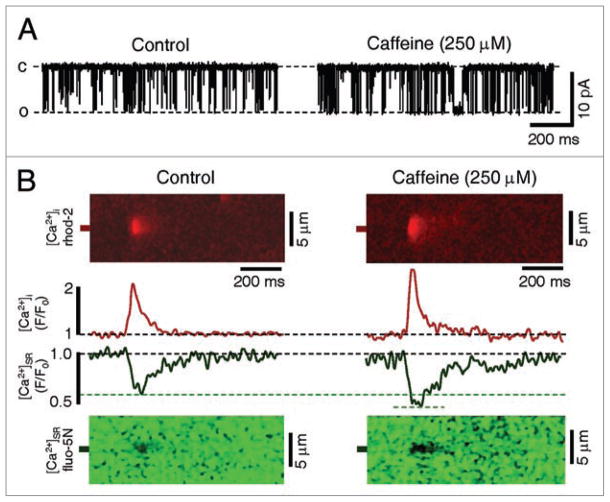
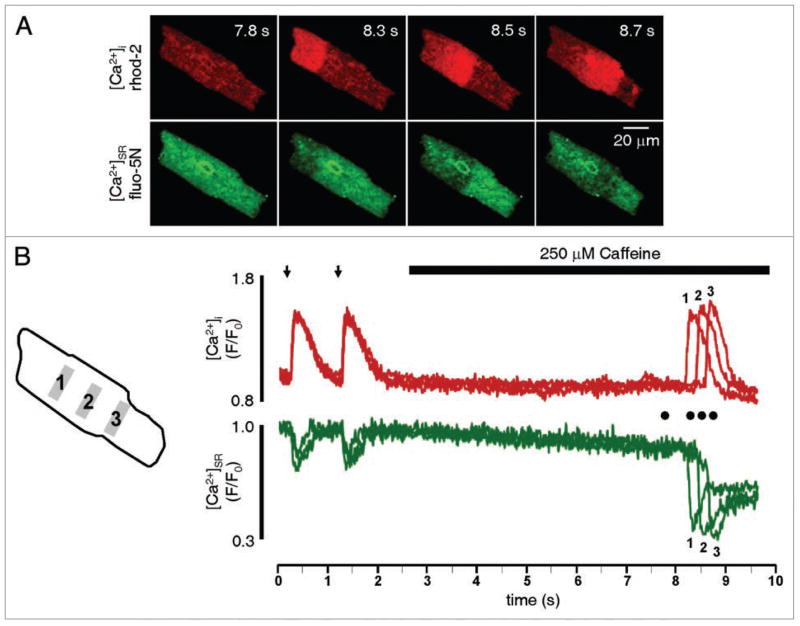
In this investigation we sensitized the cardiac RyR using 250 μM caffeine, and examined spontaneous Ca2+ release using measurements of [Ca2+]i and [Ca2+]SR. As shown in Figure 2A, application of 250 μM caffeine to a single cardiac RyR channel incorporated into a planar lipid bilayer increased the open probability of the channel without altering single channel conductance. This concentration of caffeine increased the open probability of RyR channels (127% increase from 0.026 ± 0.009 to 0.059 ± 0.013, p < 0.05, n = 5) by reducing the mean closed time (49% decrease from 128.7 ± 25.3 ms to 65.6 ± 46.4 ms, p < 0.05, n = 5) without altering the mean open time (3.6 ± 1.1 ms to 3.9 ± 1.5 ms). In the cellular environment, where RyRs function cooperatively as release clusters, this increase in channel sensitivity in the presence of 250 μM caffeine is observed as an initial increase in Ca2+ spark frequency10–12 and amplitude (Fig. 2B, red traces), and a decrease in the [Ca2+]SR level where Ca2+ sparks terminate (Fig. 2B and green traces). We next examined the effects of RyR sensitization on spontaneous Ca2+ waves in intact rabbit ventricular myocytes. As shown in the sequence of two-dimensional confocal images in Figure 3A, a Ca2+ wave is observed as a propagating increase in [Ca2+]i (red images) or as the reciprocal propagating [Ca2+]SR depletion (green images). Figure 3B presents the [Ca2+]i and [Ca2+]SR fluorescence profiles from 3 regions of the cell shown in Figure 3A (each separated by 20 μm) during electrical stimulation (1 Hz) as well as during rest after stimulation. Following cessation of electrical stimulation the cell was exposed to 250 μm caffeine and a spontaneous Ca2+ wave was observed (Fig. 3A). The fluorescence profiles from different areas of the cell superimpose during electrical stimulation, due to the synchronous nature of ECC in ventricular myocytes. As expected, this effect is evident in both the [Ca2+]i and [Ca2+]SR fluorescence profiles. In contrast, during the spontaneous Ca2+ wave the fluorescence profiles from the different regions no longer superimpose, and are temporally dissociated as the wave propagates through successive regions of the cell. This example also illustrates the marked loss of Ca2+ from the SR following a Ca2+ wave, which can be seen by comparing the initial [Ca2+]SR prior to the Ca2+ wave with the [Ca2+]SR remaining after the wave (Fig. 3B). Under control conditions, Ca2+ waves were not typically observed after rest from steady state pacing (2 waves in 24 cells). However, in the presence of 250 μM caffeine Ca2+ waves were more frequent (16 waves in 24 cells), which caused a substantial decrease in total SR Ca2+ content after 10 s of rest from pacing (48 ± 9% decrease in [Ca2+]SR in the presence of 250 μM caffeine versus 15 ± 4% under control conditions, n = 5, p < 0.05). Similar to the effect of increased Ca2+ spark frequency in the presence of caffeine, the increased propensity for spontaneous Ca2+ waves reflects RyRs which are sensitized to release activation.13 To examine if release termination is also altered during spontaneous Ca2+ waves, we examined the minimum [Ca2+]SR level observed during Ca2+ waves. As rabbit ventricular myocytes did not typically exhibit Ca2+ waves under control conditions, we added 25 nM of the β-adrenoreceptor agonist isoproterenol to increase activity of the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase, elevate SR Ca2+ content, and increase the probability of spontaneous Ca2+ waves. In an individual cell in the presence of 25 nM isoproterenol, the [Ca2+]SR level where Ca2+ waves terminated was variable, independent of initial SR Ca2+ content (data not shown). This is in contrast to spontaneous Ca2+ sparks and Ca2+ release during ECC, where Ca2+ release terminates at a set [Ca2+]SR irrespective of initial [Ca2+]SR, 4,10 and suggests that Ca2+ wave termination is a more complex phenomenon. As shown in the line-scan image and [Ca2+]SR profile of a spontaneous Ca2+ wave in Figure 4A, the Ca2+ wave terminated with a substantial amount of Ca2+ remaining in the SR. Similar results were reported in atrial myocytes,14 where 45% of the original [Ca2+]SR remained in the SR immediately following a Ca2+ wave. This observation contrasts with experimental evidence in permeabilized rabbit ventricular myocytes15 which showed nearly complete depletion of [Ca2+]SR during spontaneous Ca2+ waves. However, our results are from intact cells, while the investigation of MacQuaide et al.15 used permeabilized conditions with elevated cytosolic Ca2+ (600 nM), which may promote RyR opening and more substantially deplete [Ca2+]SR. 16 Furthermore, similar to our previous results on Ca2+ sparks and Ca2+ transients, RyR sensitization with 250 μM caffeine decreased the [Ca2+]SR where release terminated during a Ca2+ wave (Fig. 4B and C). This is an important observation, because decreasing the [Ca2+]SR level of release termination during a Ca2+ wave will increase cytosolic Ca2+ wave amplitude, increase Ca2+ extrusion via NCX, and exacerbate loss of Ca2+ from the SR. Furthermore, as the NCX is an electrogenic transporter (3 Na+ for 1 Ca2+), increased Ca2+ extrusion via NCX will depolarize the sarcolemmal membrane and increase the propensity for spontaneous electrical activity.
Figure 2.
RyR sensitization increases single channel activity and Ca2+ spark amplitude, and decreases the [Ca2+]SR termination level of Ca2+ sparks. (A) Representative traces of a single RyR channel incorporated into a planar lipid bilayer under control conditions (left) and in the presence of 250 μM caffeine (right). C represents the closed state and O represents the open state of the channel. (B) Representative confocal line scan images of a cytosolic Ca2+ spark (red image) and its corresponding intra-SR Ca2+ blink (green image) recorded in a permeabilized ventricular myocyte in the absence (left) and presence (right) of 250 μM caffeine. Presented between images are averaged (n = 10) fluorescence (F/F0) profiles of Ca2+ sparks (red) and Ca2+ blinks (green) in the presence and absence of 250 μM caffeine.
Figure 3.
Simultaneous cytosolic and intra-SR Ca2+ measurements during spontaneous Ca2+ waves. (A) 2-Dimensional images of [Ca2+]i (rhod-2, upper, red) and [Ca2+]SR (fluo-5N, lower, green) at rest (t = 7.8 s) and during propagation of a spontaneous Ca2+ wave in an intact ventricular myocytes in the presence of 250 μM caffeine (shown at 8.3, 8.5 and 8.7 s). (B) Cytosolic (red traces) and intra-SR (green traces) fluorescence profiles of 2 Ca2+ transients during ECC (arrows denote electrical stimulation), followed by rest from pacing. During rest, 250 μM caffeine was applied to sensitize RyRs, and a spontaneous Ca2+ wave propagated through the cell (this wave is shown in (A)). Profiles in (B) correspond to fluorescence from 5 μm-wide regions of interest (gray boxes 1, 2, 3 in illustration to left of profiles). Each region of interest was separated by 20 μm. Circles in (B) represent time where images in (A) were acquired.
Figure 4.
RyR sensitization decreases the minimum [Ca2+]SR level during a Ca2+ wave. Example line-scan images and fluorescence profiles of 2 Ca2+ transients during ECC (arrows denote electrical stimulation) followed by an intra-SR Ca2+ depletion wave in an intact ventricular myocyte in the absence (A) and presence (B) of 250 μM caffeine. Profiles represent spatially averaged fluorescence over the width (y-axis) of the line-scan images. For analysis of wave kinetics and wave nadir, fluorescence was averaged along a single line positioned parallel to the wavefront, as indicated by break marks. (C) Summary data showing [Ca2+]SR prior to waves and the minimum [Ca2+]SR level (wave nadir) during waves in the absence and presence of 250 μM caffeine. All experiments shown were performed in the presence of 25 nM isoproterenol to increase [Ca2+]SR and induce Ca2+ waves. F/F0 of 1 corresponds to diastolic [Ca2+]SR during electrical stimulation. *p < 0.05 versus control, n = 6.
In summary, our previous study10 and this work show that RyR sensitization has effects on both release activation and release termination. During systole in response to the action potential, RyR sensitization increases release junction recruitment and makes SR Ca2+ release highly synchronized. In addition, at each activated release junction during ECC, the amount of Ca2+ release increases due to a decrease in the [Ca2+]SR level where release terminates. The effects of RyR sensitization during systole are therefore beneficial, and cause an increase in SR Ca2+ release and contractility. On the contrary, the effects of RyR sensitization during diastole have detrimental effects on ECC and cardiac function. Increasing sensitivity to release activation causes an increase in the frequency of diastolic Ca2+ release events such as spontaneous Ca2+ sparks and waves. The increased frequency of diastolic release events, combined with the increased amount of Ca2+ released during each release event (via the altered release termination level), causes substantial loss of [Ca2+]SR during diastole. Therefore, when [Ca2+]SR reaches a new steady-state following RyR sensitization, Ca2+ release is depressed even though RyRs are sensitized.10 Furthermore, the increased frequency and amplitude of diastolic release events increases electrogenic Ca2+ extrusion via NCX and increases the likelihood of arrhythmogenic delayed afterdepolarizations. As increasing evidence points to “sensitized” RyRs following activation of neurohormonal signaling pathways and during the progression of disease,17–21 these results have important implications for the altered cellular Ca2+ homeostasis associated with these conditions.
Methods
Myocyte isolation
Rabbit ventricular myocytes were isolated from male New Zealand White Rabbits (2.5 kg) using retrograde Langendorff perfusion with Liberase Blendzyme 4 solution as previously described.10 All protocols were approved by the Institutional Animal Care and Use Committee.
Confocal microscopy
Ventricular myocytes were incubated with 5 μM fluo-5N/AM (Molecular Probes/Invitrogen, Carlsbad, CA) with 0.25% Pluronic F-127 for 2.5 hours at 37°C to load the SR with fluo+5N. For Ca2+ spark and blink measurements, myocytes were permeabilized with 0.005% saponin for 30 s, followed by application of the internal solution containing rhod-2.4 The internal cell solution contained (in mM): 100 K+ aspartate, 15 KCl, 5 KH2PO4, 5 MgATP, 0.35 EGTA, 0.12 CaCl2, 0.75 MgCl2, 10 phosphocreatine, 5 U/ml creatine phosphokinase, 8% dextran (Mr: 40,000), 10 HEPES, 0.04 rhod-2 tripotassium salt (Molecular Probes/Invitrogen); pH 7.2 with KOH. Free [Ca2+] and [Mg2+] of this solution were 150 nM and 1 mM, respectively.
Intact (fluo-5N loaded) myocytes were incubated with 5 μM rhod-2/AM (Molecular Probes/Invitrogen) for 10 minutes, followed by a 10 minute wash. The external solution contained (in mM): 135 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 D-Glucose,10 HEPES; pH 7.4 with NaOH. For Ca2+ wave measurements, intact myocytes were electrically stimulated at 1 Hz for 20 s, followed by 10 s of rest. During the rest period, cytosolic and/or intra-SR Ca2+ dynamics during spontaneous Ca2+ waves were measured in the presence or absence of 250 μM caffeine. In some experiments, 25 nM isoproterenol was added to increase [Ca2+]SR and induce waves. Fluo-5N signals were corrected for the Ca2+-insensitive component of the fluorescence (FMin) obtained after complete SR Ca2+ depletion with 10 mM caffeine, and are presented as F/F0 (F − FMin/F0 − FMin), where F0 is resting or diastolic (1 Hz) fluorescence.
Laser scanning confocal microscopy was used to monitor changes in [Ca2+]i (rhod-2) and [Ca2+]SR (fluo-5N) in either frame-scan mode (60 fps at 512 × 128, A1R, Nikon, Japan) or line-scan mode (3 ms/line, Radiance 2000/MP, Biorad, UK). Fluo-5N was excited at 488 nm with emission collected at 515 ± 15 nm, while rhod-2 was excited at 543 nm with emission collected at >600 nm. All experiments were performed at room temperature (20–23°C).
Ryanodine receptor single channel recordings
Single RyR channel activity was measured using SR vesicles isolated from ventricular tissue, which were incorporated into planar lipid bilayers.22 Cs+ was used as the charge carrier for recordings, with the cis (cytosolic) and trans (luminal) chambers containing (in mM): 400 CsCH3SO3, 0.1 CaCl2, 20 HEPES; pH 7.3. Free [Ca2+] in the cis-chamber was adjusted to 3 μM with EGTA. Single channel currents were sampled at 5 kHz using an Axopatch 200B amplifier (Axon Instruments/Molecular Devices Corporation) at a holding potential of −20 mV, and filtered at 1 kHz.
Statistics
Data are presented as individual observations or as mean ± SEM of n measurements. Statistical comparisons between groups were performed with the Student’s t test. Differences were considered statistically significant at p < 0.05.
Acknowledgments
Supported by National Institutes of Health Grants P01HL80101 and R01HL62231 (to Lothar A. Blatter) and F32HL090211 (to Timothy L. Domeier). The authors would like to thank Dr. Elisa Bovo for assistance with myocyte isolation.
Abbreviations
- [Ca2+]i
cytosolic calcium concentration
- [Ca2+]SR
intra-sarcoplasmic reticulum free calcium concentration
- CICR
calcium-induced calcium release
- ECC
excitation-contraction coupling
- LTCC
L-type Ca2+ channel
- NCX
Na+/Ca2+ exchanger
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
References
- 1.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 2.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–4. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 3.Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci USA. 2005;102:3099–104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux and intra-SR Ca2+ diffusion. Circ Res. 2008;103:105–15. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–10. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–6. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:148–59. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 8.Fedida D, Noble D, Rankin AC, Spindler AJ. The arrhythmogenic transient inward current iTI and related contraction in isolated guinea-pig ventricular myocytes. J Physiol. 1987;392:523–42. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass RS, Lederer WJ, Tsien RW, Weingart R. Role of calcium ions in transient inward currents and after-contractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol. 2009;587:5197–209. doi: 10.1113/jphysiol.2009.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–98. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh H, Katoh H, Velez P, Fill M, Bers DM. Bay K 8644 increases resting Ca2+ spark frequency in ferret ventricular myocytes independent of Ca2+ influx: contrast with caffeine and ryanodine effects. Circ Res. 1998;83:1192–204. doi: 10.1161/01.res.83.12.1192. [DOI] [PubMed] [Google Scholar]
- 13.Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol. 1999;518:173–86. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domeier TL, Blatter LA. Intra-SR Ca2+ measurements in rabbit cardiomyocytes during Ca2+ transients and waves. Biophys J. 2008;94:500. [Google Scholar]
- 15.MacQuaide N, Dempster J, Smith GL. Assessment of sarcoplasmic reticulum Ca2+ depletion during spontaneous Ca2+ waves in isolated permeabilized rabbit ventricular cardiomyocytes. Biophys J. 2009;96:2744–54. doi: 10.1016/j.bpj.2008.12.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens SC, Terentyev D, Kalyanasundaram A, Periasamy M, Gyorke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol. 2009;587:4863–72. doi: 10.1113/jphysiol.2009.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–8. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 18.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 19.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–4. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 20.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 21.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–72. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zima AV, Kockskamper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003;550:765–83. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]