Abstract
Neutrophils are endowed with a highly active oxidative metabolism that is crucial for their antimicrobial functions but can produce oxidative conditions disruptive to the host. Opportunistic infections associated with HIV disease and ex-vivo studies of neutrophils from HIV patients suggest that neutrophil dysfunctions significantly contribute to HIV disease.
The calcium-binding proteins S100A8 and S100A9 are abundant cytosolic constituents of human neutrophils. Our previous work has shown that S100A8 and S100A9 inhibit neutrophil oxidative metabolism. In this study we tested the hypothesis that neutrophils from HIV infected subjects respond differently to S100A8 and S100A9 when compared to neutrophils isolated from control HIV naive subjects.
Neutrophils freshly isolated from whole blood were tested in a 96-well plate assay for their ability to oxidize the DCFH-DA probe. The neutrophils from HIV+ and HIV− subjects were stimulated with LPS and inhibited with recombinant S100A8 and S100A9. Our data indicate that when compared to neutrophils isolated from HIV− subjects, neutrophils from HIV+ subjects display an exaggerated response to LPS and a diminished response to S100A8 and S100A9 inhibition.
Our data support our hypothesis and signify that, in HIV disease, dysregulated neutrophil responses to endotoxins stimulation and S100A8/A9 inhibition may contribute to a higher risk for oxidative stress associated ailments. The mechanism for the observed differences in neutrophil response and their biological significance in the course of HIV disease should be addressed in further studies.
Keywords: neutrophil, hiv, oxidative burst, calprotectin
Introduction
Neutrophils are crucial cellular components of the innate immune system. They are the first cells recruited to sites of microbial challenges or injury. An essential function of neutrophils includes their ability to promptly generate and release copious amount of reactive oxygen species (ROS) in a process referred to as the oxidative burst. The production of ROS is critical to neutrophil anti-microbial activities. A deficiency in oxidative metabolism can result in immune impairment, as seen in chronic granulomatous disease (CGD) [1]. Conversely, an excess of ROS production can result in oxidative stress and tissue damage [2] [3], a process believed to contribute to the pathogenesis of several chronic viral infections inclusive of the human immunodeficiency virus (HIV) disease [4, 5].
People infected with HIV become progressively immunodeficient, a process that exposes infected individuals to an escalating risk of opportunistic infections. Even though, for the most part, HIV immunodeficiency is etiologically linked to CD4 lymphocytes, opportunistic infectious organisms observed during HIV disease (i.e. Pneumocystis carinii, Candida albicans or Mycobacterium avium) are suggestive of immunodeficiency in other immune cell type such as neutrophils [6, 7]. Ex-vivo, peripheral neutrophils isolated from HIV infected subjects display a dysfunctional phenotype of impaired chemotaxis and dysregulated production of ROS [8] [9]. ROS production in HIV+ individuals has been reported to be either exaggerated or reduced when compared to control HIV uninfected subjects. Dysregulated responses to stimulation and/or inhibition of neutrophil oxidative metabolism may therefore contribute to immune dysfunction and oxidative stress in HIV disease [10].
We recently demonstrated that S100A8 and S100A9, two immune-regulatory calcium binding proteins, exert an inhibitory effect on neutrophils oxidative metabolism in-vitro (Sroussi et al, Free radical Research; in-press). We have shown that the anti-oxidative effect exerted by S100A8 and S100A9 is mediated by adenosine metabolites, a finding in agreement with reports implicating adenosine in anti-oxidative and anti-inflammatory activities [11, 12]. The biological significance of the anti-oxidative effect exerted by S100A8 and S100A9 is supported by previous reports indicating that S100A9 may be involved in the down regulation of the oxidative metabolism of innate immunity cells in chronic granulomatous conditions [13] [14] [15].
Elevated serum levels of S100A8, S100A9 and their hetero-complex calprotectin have been reported in association with HIV infection [16, 17] especially during disease exacerbation. Increased cerebrospinal fluid (CSF) levels of calprotectin have been shown to be associated with opportunistic central nervous system (CNS) infections [18]. To date, whether increased S100A8/A9 levels in association with inflammation or infection, contribute to enhanced immune response as suggested by some [19, 20] or instead, represent a native anti-inflammatory process as suggested by others [21, 22] [23, 24] [25] remain to be fully elucidated. Gene deletion studies of S100A8 and S100A9 have unfortunately not helped resolved this apparent discrepancy as the S100A8 knock-out mice are not viable [26] and mice in which S100A9 gene was deleted present only a mild degree of immune dysfunctions [27, 28]. In humans, a familial syndrome of exceptionally elevated plasma calprotectin levels has been described [29, 30]. This syndrome includes recurrent opportunistic infections supporting the hypothesis that S100A8 and S100A9 may act to depress immune system rather than activate it.
In this work, we tested the hypothesis that neutrophils isolated from asymptomatic HIV infected subjects (HIV+) respond differently to S100A8 and S100A9 when compared to neutrophils isolated from control HIV negative subjects (HIV−). We investigated the ability of S100A8 and S100A9 to inhibit the spontaneous and lipopolysaccharides (LPS) induced stimulation of neutrophil in standard 96-well plate assays with dichlorofluorescin-diacetate (DCFH-DA) as a probe for oxidation. The data indicated that neutrophils from asymptomatic HIV+ subjects are more responsive to LPS stimulation but less responsive to S100A8/A9 inhibition of their oxidative metabolism when compared to neutrophils from HIV− subjects. Our data supports our hypothesis and implicate a dysregulated response to S100A8/A9 as a potential source for immune dysfunction and oxidative stress in HIV disease.
Methods
Subjects
This study was approved by the Institutional Review Board at the University of Illinois at Chicago (UIC). 17 HIV+ and 10 HIV− subjects were recruited from the outpatient dental clinical at the College of Dentistry at UIC. After written consent, approximately 10 cc of venous blood was collected by venipuncture from each subject in heparin containing Vacutainer (Becton Dickinson, Franklin Lakes, NJ). Blood was immediately subjected to the process of granulocytes isolation.
Isolation of peripheral neutrophils
Granulocytes were isolated using 1119/1077 Histopaque gradients (Sigma-Aldrich, St. Louis, Mo) according to the manufacturer’s instructions. Cell viability and identity was confirmed by tryptan blue staining. Live cells and neutrophils represented at least 95% of isolated leukocytes.
Expression and purification of recombinant S100 proteins
Recombinant S100A8 and S100A9 protein were produced and purified based on standard methods as previously described [23, 24]. Briefly, both proteins were cloned in a pGEX-2T GST vector (Amersham, Piscataway, NJ). The proteins were expressed in Top-10 F’ E-coli as GST fusion proteins. The GST tag was cleaved during the purification process. Protein concentration was assessed through a Bradford protein assay (Pierce, Rockford, IL).
Reagents
Dichlorofluorescin diacetate (DCFH-DA) was purchased from EMD Calbiochem (San Diego, California). Lipopolysaccharides (LPS) from Escherichia coli 055:B5 was purchased from Sigma-Aldrich (St. Louis, Mo). Dubelco’s Phosphate Buffered Saline (DPBS) was purchased from Biowhittaker (Walkersville, MD).
Assay for oxidative activation of neutrophils
The method for the measurement of oxidative activation of neutrophils was based on the ROS-dependent oxidation of DCFH-DA to DCF and was adapted from Ciapetti et al [31]. DCFH-DA crosses the cell membrane and is hydrolyzed by nonspecific esterases to nonfluorescent DCFH. Its oxidation by ROS results in the generation of highly fluorescent DCF [32]. The assays were run in clear bottom black 96-well plate. “Edge effects” (a higher fluorescence in edge wells) were avoided by using only center wells. Briefly, 50 µl of DPBS containing DCFH-DA was added to each well with the final concentration of 10µg/ml. LPS and or S100 proteins were added at various concentrations. 50,000 to 100,000 neutrophils in 50 µl PBS were placed in each well. 96-well plates were incubated at 37°C and 5% CO2 and were read at baseline (immediately after cell addition to the plates) and at specified time points in a Spectra Max Gemini XS fluorescent plate reader. The excitation wavelength was 485 and the reading was done at 530 nm. Wells with no DCFH-DA were used to measure background fluorescence which was subtracted from each reading. Controls with no cells were also analyzed and display minimal or no increased fluorescence over time. All assays were conducted in triplicate wells.
Data analysis
Comparisons between the HIV+ and HIV− groups for the S100A8, S100A9 proteins and LPS dose effect were calculated with two-way ANOVA using SPSS (SPSS Inc., Chicago, IL) with alpha set to ≤ 0.05 to determine statistical relevance. Comparisons between the HIV+ and HIV− groups for the S100A8 and S100A9 proteins were calculated with two sample t-tests and Bonferroni corrections using SPSS (SPSS Inc., Chicago, IL) with alpha set to ≤ 0.025 to determine statistical relevance.
Results
Differential response to LPS by neutrophils isolated from HIV positive and negative subjects
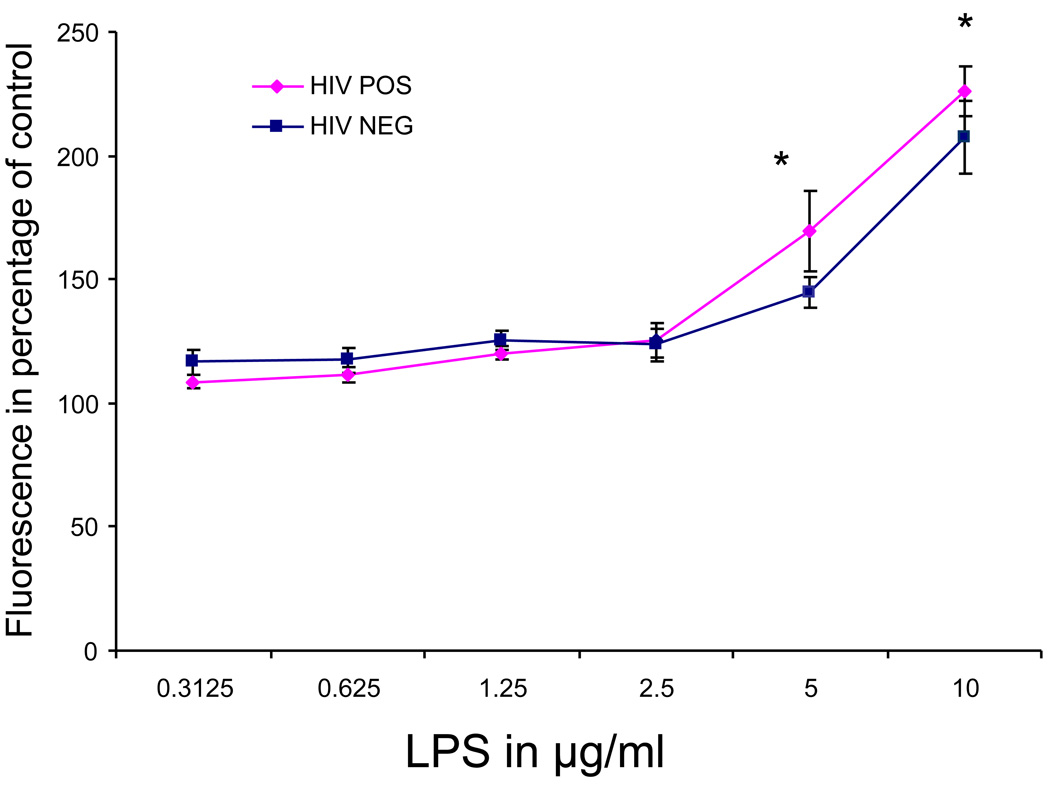
Incubation of peripheral neutrophils with the DCFH-DA probe resulted in rising fluorescence readings indicative of a constitutive production of ROS by neutrophils independently of any controlled activation. This constitutive oxidative metabolism was observed in HIV positive and negative subjects at similar rates (data not shown). We tested the ability of LPS at a concentration ranging from 0.3125 µg/ml to 10 µg/ml to accelerate the production of ROS by peripheral neutrophils. The data indicated that both HIV+ and HIV− subjects responded by an accelerated oxidative metabolism when compared to untreated control. When compared to the response in HIV− subjects, the magnitude of the response to LPS was higher in HIV+ subjects at concentrations of 5 and 10 µg/ml (P<0.025) (Fig. 1). Although a significant main effect was not found between the HIV+ and HIV− groups overall, a significant main effect was found for dose (F = 34.195, p = .001) and a significant interaction between the HIV status and dose was found (F = 3.321, p = .006).
Figure 1. Oxidative metabolism of neutrophils stimulated with different concentrations of LPS.
The data is expressed as fluorescence emission of the DCFH-DA probe in percentage of unstimulated controls. A significant interaction between the HIV status and dose was found (F = 3.321, p = .006). *=P<0.025
Inhibition of the constitutive oxidative metabolism of neutrophils by S100A8 and S100A9 in HIV positive and negative subjects
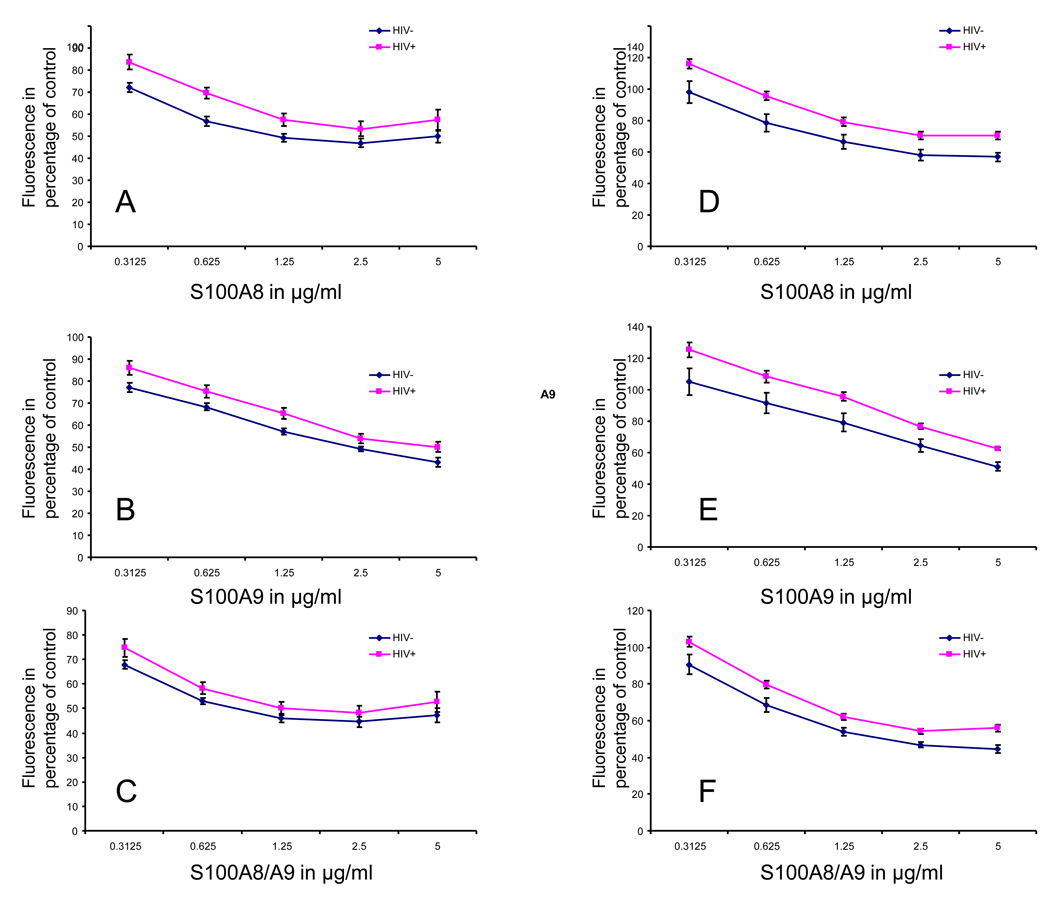
We next tested the ability of S100A8 and S100A9 to inhibit the constitutive and LPS stimulated oxidative metabolism of neutrophils. Following one hour of incubation, S100A8, S100A9 or both proteins at a concentration ranging from 0.3125 to 5 µg/ml were tested for their ability to inhibit the rate of DCFH-DA oxidation by neutrophils. The data indicated that both the constitutive and the LPS stimulated oxidative metabolism of neutrophils from HIV+ and HIV− subjects were inhibited by S100A8 and S100A9 in a dose-dependent manner. The anti-oxidative effect of S100A8, S100A9 and S100A8/A9 were significantly weaker on neutrophils isolated from HIV+ subjects when compared to HIV− subjects. Statistical analysis revealed a significant main effect of HIV status on S100A8, S100A9 and S100A8/A9 anti-oxidative effect on neutrophil constitutive oxidative metabolism with a respective F=34.381, 88.223 and 30.858, p=0.000 (Fig 2 panel A–C) and on LPS stimulated oxidative metabolism with a respective F=30.051, 33.595 and 71.181, p=0.000 (Fig 2 panel D–F).
Figure 2. Inhibition of neutrophil oxidative metabolism by different concentrations of S100A8, S100A9 and both proteins together (S100A8/A9).
The inhibition of constitutive (panel A–C) and LPS (5µg/ml) stimulated neutrophil oxidative metabolism (Panel D–F) is presented in percentage of controls with no S100 proteins. A significant main effect of HIV status on S100A8, S100A9 and S100A8/A9 anti-oxidative effect on the constitutive oxidative metabolism (F=34.381, 88.223 and 30.858, p=0.000) and on the LPS stimulated metabolism (F=30.051, 33.595 and 71.181, p=0.000) was found.
Discussion
In this study, we hypothesized that neutrophils isolated from asymptomatic HIV infected subjects respond differently to S100A8 and S100A9 when compared to neutrophils isolated from control HIV negative subjects. Our data support our hypothesis and indicate that neutrophils from HIV+ subjects have an exaggerated response to LPS and a diminished inhibitory response to S100A8/A9 proteins.
Exaggerated neutrophil responses to stimulation by endotoxins and dimished inhibitory responses to S100A8 and S100A9 may combine to result overall in disproportionately high neutrophil oxidative metabolism. Those dysregulated responses would contribute to the previously reported oxidative stress in HIV disease [10, 33]. Dysregulated oxidative metabolism of neutrophils in HIV disease does not only contribute to the onset of bacterial or fungal infections but also to increased transactivation of HIV [34] and potentially to a higher risk for cardiovascular disease [35].
Consequently, repeated microbial challenges and/or inflammatory conditions in the course of HIV disease is likely to result in disproportionate neutrophil oxidative activity which in turn would aggravate oxidative stress resulting in infections, increased HIV viremia and cardiovascular disease. For that reason, a strong argument can be made that avoiding infection and inflammation is a crucial aspect in the management of HIV disease.
The HIV+ subjects enrolled in this study were asymptomatic patients with controlled viremia (<50 copies/ml) and stable or rising CD4 counts. The relevancy of their dysregulated neutrophil oxidative metabolism to their long-term prognosis remains unclear and requires further investigations. The overall effect of S100A8 and S100A9 on neutrophil functions in the course of HIV infections is also unclear as it is conceivable that a diminished dose-response to the two proteins could be compensated by increased S100 protein levels in the circulation [16, 17]. Finally, more should be done to investigate at the cellular and molecular level the causes for the differences in neutrophil responses observed in HIV disease. Identifying those causes and normalizing neutrophil responses may improve the overall immune status and prognosis of HIV infected individuals.
Acknowledgments
This work was funded by NIDCR/NIH grant number K22 DE017161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckert JW, Abramson SL, Starke J, Brandt ML. The surgical implications of chronic granulomatous disease. Am J Surg. 1995 Mar;169(3):320–323. doi: 10.1016/S0002-9610(99)80167-6. [DOI] [PubMed] [Google Scholar]
- 2.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186(3):259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 3.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8(5–6):691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 4.Hayani KC, Verral SC, Pitrak DL. Impaired phagocyte oxidative capacity in human immunodeficiency virus-infected children. J Infect Dis. 1999 Mar;179(3):584–589. doi: 10.1086/314622. [DOI] [PubMed] [Google Scholar]
- 5.Stehbens WE. Oxidative stress in viral hepatitis and AIDS. Exp Mol Pathol. 2004 Oct;77(2):121–132. doi: 10.1016/j.yexmp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Pitrak DL. Neutrophil deficiency and dysfunction in HIV-infected patients. Am J Health Syst Pharm. 1999 Dec 15;56 Suppl 5:S9–S16. doi: 10.1093/ajhp/56.suppl_5.S9. [DOI] [PubMed] [Google Scholar]
- 7.Pitrak DL, Mullane KM, Bilek ML, Stevens P, Allen RC. Impaired phagocyte oxidative capacity in patients with human immunodeficiency virus infection. J Lab Clin Med. 1998 Oct;132(4):284–293. doi: 10.1016/s0022-2143(98)90041-5. [DOI] [PubMed] [Google Scholar]
- 8.Valone FH, Payan DG, Abrams DI, Goetzl EJ. Defective polymorphonuclear leukocyte chemotaxis in homosexual men with persistent lymph node syndrome. J Infect Dis. 1984 Aug;150(2):267–271. doi: 10.1093/infdis/150.2.267. [DOI] [PubMed] [Google Scholar]
- 9.Chen TP, Roberts RL, Wu KG, Ank BJ, Stiehm ER. Decreased superoxide anion and hydrogen peroxide production by neutrophils and monocytes in human immunodeficiency virus-infected children and adults. Pediatr Res. 1993 Oct;34(4):544–550. doi: 10.1203/00006450-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Elbim C, Pillet S, Prevost MH, Preira A, Girard PM, Rogine N, et al. The role of phagocytes in HIV-related oxidative stress. J Clin Virol. 2001 Feb;20(3):99–109. doi: 10.1016/s1386-6532(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 11.Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Inhibition of fMLP-triggered respiratory burst of human monocytes by adenosine: involvement of A3 adenosine receptor. J Leukoc Biol. 1999 Sep;66(3):495–501. [PubMed] [Google Scholar]
- 12.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008 Mar;83(3):447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delabie J, de Wolf-Peeters C, van den Oord JJ, Desmet VJ. Differential expression of the calcium-binding proteins MRP8 and MRP14 in granulomatous conditions: an immunohistochemical study. Clin Exp Immunol. 1990;81(1):123–126. doi: 10.1111/j.1365-2249.1990.tb05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariano M. The experimental granuloma. A hypothesis to explain the persistence of the lesion. Rev Inst Med Trop Sao Paulo. 1995 Mar–Apr;37(2):161–176. doi: 10.1590/s0036-46651995000200012. [DOI] [PubMed] [Google Scholar]
- 15.Aguiar-Passeti T, Postol E, Sorg C, Mariano M. Epithelioid cells from foreign-body granuloma selectively express the calcium-binding protein MRP-14, a novel down-regulatory molecule of macrophage activation. J Leukoc Biol. 1997;62(6):852–858. doi: 10.1002/jlb.62.6.852. [DOI] [PubMed] [Google Scholar]
- 16.Muller F, Froland SS, Aukrust P, Fagerhol MK. Elevated serum calprotectin levels in HIV-infected patients: the calprotectin response during ZDV treatment is associated with clinical events. J Acquir Immune Defic Syndr. 1994 Sep;7(9):931–939. [PubMed] [Google Scholar]
- 17.Strasser F, Gowland PL, Ruef C. Elevated serum macrophage inhibitory factorrelated protein (MRP) 8/14 levels in advanced HIV infection and during disease exacerbation. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(4):230–238. doi: 10.1097/00042560-199712010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Dunlop O, Bruun JN, Myrvang B, Fagerhol MK. Calprotectin in cerebrospinal fluid of the HIV infected: a diagnostic marker of opportunistic central nervous system infection? Scand J Infect Dis. 1991;23(6):687–689. doi: 10.3109/00365549109024294. [DOI] [PubMed] [Google Scholar]
- 19.Ryckman C, McColl SR, Vandal K, de Medicis R, Lussier A, Poubelle PE, et al. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003 Aug;48(8):2310–2320. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- 20.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638. Epub 2007 Sep 2. [DOI] [PubMed] [Google Scholar]
- 21.Hsu K, Passey RJ, Endoh Y, Rahimi F, Youssef P, Yen T, et al. Regulation of S100A8 by Glucocorticoids. J Immunol. 2005 February 15;174(4):2318–2326. doi: 10.4049/jimmunol.174.4.2318. 2005. [DOI] [PubMed] [Google Scholar]
- 22.Ikemoto M, Murayama H, Itoh H, Totani M, Fujita M. Intrinsic function of S100A8/A9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clin Chim Acta. 2007;376(1–2):197–204. doi: 10.1016/j.cca.2006.08.018. Epub 2006 Aug 24. [DOI] [PubMed] [Google Scholar]
- 23.Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 Triggers Oxidation-sensitive Repulsion of Neutrophils. J Dent Res. 2006;85(9):829–833. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81(3):818–824. doi: 10.1189/jlb.0706433. Epub 2006 Nov 30. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka K, Terasaki F, Ikemoto M, Fujita S, Tsukada B, Katashima T, et al. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail. 2009 Mar;11(3):229–237. doi: 10.1093/eurjhf/hfn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, et al. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999 Aug 15;163(4):2209–2216. [PubMed] [Google Scholar]
- 27.Hobbs JA, May R, Tanousis K, McNeill E, Mathies M, Gebhardt C, et al. Myeloid cell function in MRP-14 (S100A9) null mice. Mol Cell Biol. 2003 Apr;23(7):2564–2576. doi: 10.1128/MCB.23.7.2564-2576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeill E, Conway SJ, Roderick HL, Bootman MD, Hogg N. Defective chemoattractant-induced calcium signalling in S100A9 null neutrophils. Cell Calcium. 2006;28:28. doi: 10.1016/j.ceca.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, et al. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet. 2002 Nov 30;360(9347):1742–1745. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- 30.Saito Y, Saito K, Hirano Y, Ikeya K, Suzuki H, Shishikura K, et al. Hyperzincemia with systemic inflammation: a heritable disorder of calprotectin metabolism with rheumatic manifestations? J Pediatr. 2002 Feb;140(2):267–269. doi: 10.1067/mpd.2002.121699. [DOI] [PubMed] [Google Scholar]
- 31.Ciapetti G, Granchi D, Verri E, Savarino L, Cenni E, Savioli F, et al. Fluorescent microplate assay for respiratory burst of PMNs challenged in vitro with orthopedic metals. J Biomed Mater Res. 1998;41(3):455–460. doi: 10.1002/(sici)1097-4636(19980905)41:3<455::aid-jbm15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2',7'- dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5(2):227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 33.Israel N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci. 1997 Dec;53(11–12):864–870. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legrand-Poels S, Vaira D, Pincemail J, van de Vorst A, Piette J. Activation of human immunodeficiency virus type 1 by oxidative stress. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1389–1397. doi: 10.1089/aid.1990.6.1389. [DOI] [PubMed] [Google Scholar]
- 35.Parra S, Coll B, Aragones G, Marsillach J, Beltran R, Rull A, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2009 Oct 21; doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]