Abstract
In the present study, we examined meal patterns during and after exposure to the visible burrow system (VBS), a rodent model of chronic social stress, to determine how the microstructure of food intake relates to the metabolic consequences of social subordination. Male Long-Evans rats were housed in mixed-sex VBS colonies (4 male, 2 female) for 2 wk, during which time a dominance hierarchy formed [1 dominant male (DOM) and 3 subordinate males (SUB)], and then male rats were individually housed for a 3-wk recovery period. Controls were individually housed with females during the 2-wk VBS period and had no changes in ingestive behavior compared with a habituation period. During the hierarchy-formation phase of VBS housing, DOM and SUB had a reduced meal frequency, whereas SUB also had a reduced meal size. However, during the hierarchy-maintenance phase of VBS housing, DOM meal patterns did not differ from controls, whereas SUB continued to display a reduced food intake via less frequent meals. During recovery, DOM had comparable meal patterns to controls, whereas SUB had an increased meal size. Hypothalamic neuropeptide Y (NPY) mRNA levels were not different between these groups during the experimental period. Together, the results suggest that exposure to chronic social stress alters ingestive behavior both acutely and in the long term, which may influence the metabolic changes that accompany bouts of stress and recovery; however, these differences in meal patterns do not appear to be mediated by hypothalamic NPY.
Keywords: adiposity, meal size, body composition, food intake
stress can have a major influence on food intake and body weight in humans and other animal species. As a generalization, humans subjected to daily psychological stressors such as school exams, public speaking, job stress, ego-threatening, and interpersonal situations tend to increase food intake and body weight (19, 40, 44), whereas stressors not experienced on an everyday basis, such as combat or traumatic grief, decrease food intake and body weight (25, 42, 43). Similarly, in animal models, metabolic changes are dependent on the type, severity, and duration of the stressor (53).
The visible burrow system (VBS) is a validated animal model of chronic social stress, inducing reproducible behavioral, endocrine, physiological, and neurochemical changes (1, 11, 15, 24, 38, 51). In a usual VBS experiment, laboratory rats are housed together in a mixed-sex colony for a period of 2 wk. Agonistic interactions occur among the males in the VBS and the males form a dominance hierarchy. Previous VBS studies determined that subordinate (SUB) males are severely stressed and develop profound weight loss, have reduced food intake, and lose adipose mass while in the VBS (11, 24, 38, 50, 51); however, when removed from the VBS and given the opportunity to recover, SUB are hyperphagic and have increased adiposity specifically in the visceral region (38, 52). These metabolic changes are not simply a consequence of weight cycling per se (i.e., food restriction/refeeding) (52), suggesting that some aspect of chronic stress contributes to the changes in body composition.
Alterations in the microstructure of ingestive behavior can have a major influence on physiology (39). For example, consuming many small meals throughout the day decreases body weight relative to consuming the same number of calories as a few large meals (21). Conversely, taking fewer, larger meals promotes the gain of fat mass and can increase plasma levels of triglycerides, lipids, and cholesterol independent of total caloric intake in both rats and humans, and weight gain from caloric overconsumption can be prevented by consuming smaller, more frequent meals in humans (14, 18, 20, 21, 35, 55). These studies indicate that meal number and size can affect metabolism, but whether exposure to social stress alters the microstructure of food intake is unclear.
Few studies have examined the effects of stress on meal patterns, and prior experiments using the VBS paradigm were not able to assess the eating patterns of individual animals. We have now circumvented this limitation by using microchips implanted in individual animals and, through the development of a software program, we can analyze individual meal patterns in single and group-housed animals.
The impact of stress on food intake along with the physiological changes in SUB animals after VBS-recovery cycles (38, 50–52) suggests that the pattern in which food is consumed may contribute to the metabolic consequences of VBS exposure. Accordingly, we tested the hypothesis that SUB adopt different feeding strategies during VBS and recovery, which are associated with the body weight and body composition changes observed in these animals. Furthermore, we examined the expression of the orexigenic peptide neuropeptide Y (NPY) in the hypothalamus to determine whether altered patterns of ingestion are associated with changes in brain neurochemistry. NPY plays a prominent role in stimulating food intake in times of negative energy balance (possibly by increasing meal size) and has a close relationship with the hypothalamic-pituitary-adrenal (HPA) axis (6, 13, 17, 27, 28) and, therefore, may be altered throughout VBS stress and recovery. Meal patterns, body weight, body composition, plasma corticosterone (CORT), and hypothalamic NPY expression were measured during 2 wk of VBS stress and the subsequent 3-wk recovery period.
MATERIALS AND METHODS
Animals.
Animals were maintained in a temperature- and humidity-controlled room on a 12:12-h light-dark cycle (lights off at 1800) and were housed and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (24a). All protocols, animal handling, and treatment were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Male and female Long-Evans rats (90-days old; Harlan; Indianapolis, IN) were individually housed in the animal facility for 3 wk prior to experimental testing. During this time, each animal was briefly anesthetized with isoflurane and implanted with a unique subcutaneous microchip just behind their ears using a hollow needle (Trovan, Electronic Identification Devices; Santa Barbara, CA), allowing for identification and monitoring of feeding behavior. All animals were then placed into individual DietMax cages (#45-DMCD2R; AccuScan Instruments, Columbus, OH) for 1 wk to habituate to consuming powdered chow. A subgroup (n = 12) was randomly selected and monitored with the DietMax-ID system (AccuScan Instruments; Columbus, OH) to determine baseline food intake and meal pattern behavior (habituation period).
VBS colonies were formed with 4 males and 2 females; males in each colony were weight-matched at the start of the experiment to within 25 g of one another. Control (CON) males were weight-matched to their assigned colony and individually housed in a DietMax cage with an adult female. Colonies were continuously housed together for 2 wk, and dominance was determined based on previously described methods (38, 51). Following 2 wk of VBS housing, a randomly selected subset of animals was individually housed in DietMax cages and recovered for up to 3 wk.
Twelve food intake monitoring tunnels (see description below) and 8 modified VBS set-ups were used to monitor food intake and meal patterns of individual animals during group housing. VBS colonies were run in cohorts, and each cohort consisted of at least four colonies with corresponding CONs. All cohorts were subjected to the same protocol and maintained on powdered chow with the same tunnel-scale setup for the duration of the experiment; therefore, there were no differences among cohorts.
Body weight and body composition.
Body weight was recorded every other day for the duration of the experiment. During VBS housing, males were removed, weighed under red light illumination, and immediately returned to the same VBS chamber. Whole-body composition was measured using the EchoMRI whole body composition analyzer system (Echo Medical Systems, Houston, TX), which provides individual estimates of fat mass, lean mass, and water content. Male rats were placed into a plastic restraint tube, inserted into the EchoMRI, and scanned for ∼50 s. Time in the restraint tube was minimized to reduce stress. Body composition was evaluated during the habituation period (at which time all animals had comparable levels of fat and lean mass), at the end of the 2-wk VBS exposure and following 1- and 3-wk recovery. Values are expressed as change from the previous housing transition (e.g., VBS-habituation, 1 wk-VBS, and 3 wk-VBS).
Food intake monitoring system and modified VBS.
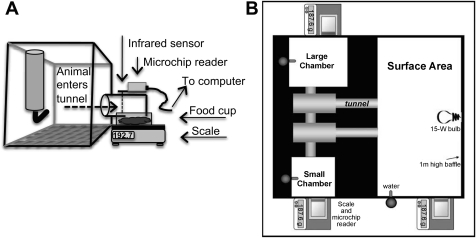
Each DietMax-ID cage was equipped to monitor individual animal's food intake using a microchip-scale system. A food cup containing powdered chow was placed upon a scale outside of the cage. The tunnel (10 cm long, 5.5 cm wide), attached to the cage (6.5 cm above flooring), was positioned above the food cup and scale, allowing the animal's head to enter the tunnel (via a 4-cm-wide opening) and to reach and consume the food. Each tunnel contained a scanner that was activated when the animal's head entered and broke an infrared beam triggering the microchip reader to identify the individual animal. Microchip readers and scales were connected to a central analyzer, which recorded time and duration of entry and changes in food cup weight. (Fig. 1A).
Fig. 1.
Schematics of experimental set up. A: microchip-scale system (L × W × H: 32 cm × 22 cm × 21 cm). B: visible burrow system (VBS) (L × W: 1.0 m2).
Each colony was continuously housed for 14 days in a modified VBS (Fig. 1B); the original model has been previously described in detail (38, 51). Each modified VBS was equipped with the same microchip-scale system described above and shown in Fig. 1A. Food tunnels were connected to the outer side of the VBS at three different locations, where 3-inch holes had been created to allow the animals to insert their heads (and break the infrared beam to activate the microchip reader) and reach the food cups on the scale. All tunnels and scales are connected to a central analyzer as described above.
Standard powdered laboratory chow was provided ad libitum (5% fat, 19% protein, 5% fiber; 3.46 kcal/g Teklad Lab Animal Diets #7012), and food intake was monitored throughout habituation, VBS housing, and recovery. Feeding data were examined in two phases of VBS housing: hierarchy-formation and hierarchy-maintenance. It is well established that a dominance hierarchy forms within the first few days of VBS housing and remains stable throughout the 2-wk period [from behavioral analysis, data not shown (10, 11, 51)]. Furthermore, body weight and feeding behavior stabilize within status groups on a day-to-day basis once the hierarchy is established, and DOM neuroendocrine measures (CORT and testosterone) are similar to CON by day 7 (24). In the current study, it was determined that the differences between days within status groups were lost following day 6 of VBS housing; therefore, data for feeding behavior were analyzed as overall averages for the hierarchy formation phase (days 1–6) and hierarchy-maintenance phase (days 7–14), as well as on a day-to-day basis.
Meal patterns.
Meal patterns were determined using data obtained from the DietMax-ID system for 22 h each day (1 PM–11 AM; 2 h allowed for daily husbandry). Further data processing details have been previously described (35).
Meals were defined as ingestion bouts having a consumption rate (grams consumed per minute) of less than 0.50 g/min, as this is the maximum rate at which an adult rat is able to consume powdered chow (35). Ingestive events that did not reach this criterion were not analyzed. Ingestive bouts were combined into meals if the interbout interval was 5 min or less. Food intake was calculated by summing the sizes of all meals for each subject per day. Intrameal interval (Intra-MI) is the time during the meal when the animal was not engaged in ingestive behavior. Meal duration is the time of the entire meal event (time eating + Intra-MIs in that meal). Intermeal interval (Inter-MI) is the time between successive meals. These criteria accounted for greater than 95% of the daily food intake.
Meal pattern characteristics were measured for 7 days during habituation. Data were calculated for each animal for each day and then averaged together to provide an overall habituation measure as a baseline for all of the conditions. Meal patterns were determined in VBS- and CON-housed animals on each of the 14 days of the VBS period, as well as for each day of the recovery period. Meal pattern characteristics were calculated for each animal on a daily basis and then averaged together with those animals of the same status group. Further analyses included separation into light-dark segments and an overall measure of the hierarchy-formation, hierarchy-maintenance, and recovery phases.
Plasma CORT analysis.
A tail-nick blood sample was obtained to assess basal CORT on day 13 of VBS housing and following 1 and 3 wk of recovery. At 1000, when CORT secretion is normally at its diurnal nadir, animals were removed from their cage (CON) or VBS (SUB and DOM), a blood sample (∼50 μl) was immediately taken, and the animal was returned to its assigned housing. Samples were kept on ice until cold centrifugation, and plasma was stored at −20°C until analyzed. The basal sample for VBS-housed animals was collected under red light, whereas the basal samples of CON and all samples during recovery were taken in normal light conditions. Total CORT was assessed by radioimmunoassay using a commercially available kit (CORT DA; MP Biomedicals, Solon, OH).
In situ hybridization.
Subsets of males were euthanized on the morning of day 14 of VBS housing, and on the morning of days 7 and 21 of recovery. Brains were immediately removed, flash frozen, and stored at −20°C. Brains were coronally sectioned at 14 μm on a Leica 3050 cryostat, mounted on Fisherbrand Superfrost-Plus-charged glass slides (Hampton, NH), and stored at −20°C until further analysis. Brain sections were fixed in 4% paraformaldehyde solution, rinsed in 5 mM potassium PBS, acetylated in 0.25% acetic anhydride, delipidated in chloroform, and dehydrated through an ethanol rinse series. Antisense rat NPY riboprobes were generated by in vitro transcription using 35S-labeled UTP. Riboprobe 35S percent incorporation was determined with TCA precipitation.
Slides were hybridized with the NPY riboprobe (1.0 × 106 cpm/50 μl buffer), combined with hybridization buffer [50% dextran sulfate, 5× hybridization stock, formamide, fish sperm (ssDNA), tRNA, and dithiothreitol (DTT)] and covered with glass coverslips. Slides were then placed into hybridization chambers, which were moistened with 50% formamide and incubated overnight at 55°C. The following morning slides were posttreated following the removal of the coverslips beginning with a wash in 2× SSC. Next, slides were incubated in RNase A (from bovine pancreas; Fisher Scientific, Pittsburgh, PA) for 30 min at 37°C, washed three times in 0.2× SSC, placed in 65°C 0.2× SSC for 1 h, dehydrated through an ethanol series and air-dried.
Image analysis.
Hybridized slides were exposed to Kodak BioMax MR film for 4–6 days and subsequently developed. Film images of brain sections were captured by digital camera. Semiquantitative microdensitometery analysis for autoradiograph images was performed using Scion Image (Alpha 4.0.3.2; Scion, Frederick, MD) software.
Hypothalamic brain regions were identified using the Paxinos and Watson rat brain atlas (41). Each identified region of interest was analyzed by subtracting the nonhybridized tissue (background) from the hybridized signal within the same brain section, and data were expressed as corrected gray level (CGL). Twenty-four brain sections were analyzed per region per animal. Average CGL values were calculated in series for the arcuate nucleus (Arc) and dorsomedial nucleus (DMH) of the hypothalamus, and the highest average value was used for that individual animal. 14C standards were developed with each film and analyzed for CGL to confirm that all measured gray levels were within the linear range of the film.
Statistics.
Repeated-measures ANOVA, 1-way ANOVA, 2-way ANOVA, and paired t-tests were used where appropriate (SigmaStat v.3.1). Holm-Sidak post hoc analysis was used when differences reached significance (P < 0.05). Data more than three standard deviations from the mean were discarded (less than 2% of the possible values).
RESULTS
Body weight and composition.
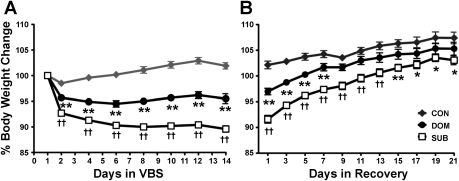
All colonies formed dominance hierarchies as documented by behavioral video analysis, and individual rats displayed the typical body weight changes associated with the VBS model (11, 51), including a significant loss of weight in the SUB population compared with CON and DOM (P < 0.001) and an intermediate weight loss by DOM animals compared with CON (P < 0.001) (Fig. 2A). Weights of DOM and SUB reached a reduced plateau by VBS day 6. DOM recovered their body weight to CON levels after 1 wk of recovery. Although SUB regained weight, they did not reach CON levels during the 3-wk recovery period (P < 0.001) (Fig. 2B).
Fig. 2.
Percent body weight change during VBS and recovery housing. A: dominant males (DOM) and subordinate males (SUB) lose weight during VBS housing; however, SUB lose significantly more than DOM. Following day 6, body weight did not significantly change within each group [n = 28 (CON), 26 (DOM), 76 (SUB)]. B: both DOM and SUB regained lost body weight; however, SUB did not reach CON levels. [week 1; n = 17 (CON), 19 (DOM), 40 (SUB); weeks 2 and 3; n = 14 (CON), n = 11 (DOM), n = 25 (SUB)]. Data are expressed as means ± SE. *P < 0.01 vs. CON; **P < 0.001 vs. CON; ††P < 0.001 vs. CON and DOM.
Both DOM and SUB lost significant adipose mass during VBS housing compared with CON (P < 0.001). SUB also lost significant lean mass compared with DOM and CON (P < 0.001), whereas DOM and CON maintained or gained lean mass (Table 1). During recovery, both DOM and SUB regained both lean and adipose mass; however, adipose gain was more pronounced in SUB and was significant after week 1 compared with CON (P < 0.05) and after week 3 compared with CON and DOM (P < 0.001) (Table 1).
Table 1.
Body composition and basal corticosterone levels
| Recovery |
|||
|---|---|---|---|
| VBS | Week 1 | Week 3 | |
| Change in Adipose Mass, g | |||
| CON | −4.4 ± 0.97 | 6.3 ± 1.21 | 5.7 ± 1.74 |
| DOM | −26.7 ± 1.56** | 7.7 ± 2.33 | 13.4 ± 1.08**‡ |
| SUB | −34.5 ± 1.40†† | 12.4 ± 1.52* | 26.1 ± 1.54††‡ |
| Change in Lean Mass, g | |||
| CON | 8.7 ± 1.76 | −10.4 ± 2.62 | 3.2 ± 3.57‡ |
| DOM | 2.4 ± 3.65 | 14.7 ± 4.70** | 27.2 ± 2.42**‡ |
| SUB | −20.4 ± 2.54†† | 25.3 ± 2.77** | 34.1 ± 3.37** |
| Basal Plasma Corticosterone, ng/ml | |||
| CON | 31.8 ± 4.80 | 29.5 ± 7.83 | 32.4 ± 15.4 |
| DOM | 26.4 ± 8.51 | 14.6 ± 2.06 | 26.3 ± 13.8 |
| SUB | 80.6 ± 13.9† | 14.0 ± 0.80 | 15.4 ± 1.79 |
Data are expressed as means ± SE. Adipose and lean mass values are expressed as a change from the previous housing transition (e.g., visible burrow system (VBS)—habituation; 1 Week—VBS; 3 Weeks—VBS).
P < 0.05 vs. CON;
P < 0.001 vs. CON;
P < 0.05 vs. CON and DOM;
P < 0.001 vs. CON and DOM;
P < 0.05 vs. week 1 of same status. CON, control; DOM, dominant; SUB, subordinate. VBS: CON, n = 14 or 15; DOM, n = 10–14, SUB, n = 30–40; Week 1: CON, n = 4–6, DOM, n = 3–4, SUB, n = 9–12; Week 3: CON, n = 6–10, DOM, n = 3–7, SUB, n = 9–13.
Plasma CORT.
Following 2 wk of VBS housing, SUB had increased basal CORT levels compared with CON and DOM (P < 0.05), and all groups had similar basal CORT levels during recovery (Table 1).
Food intake.
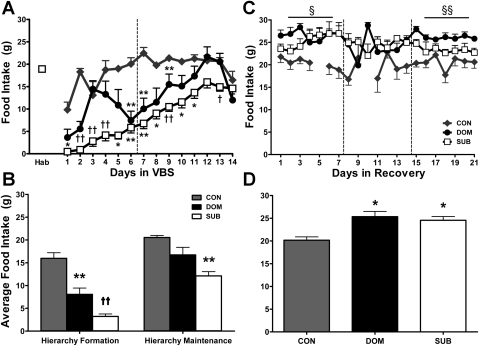
When first exposed to VBS housing, DOM and SUB immediately reduced their food intake compared with intake during the habituation period (P < 0.001). DOM recovered their food intake to CON and habituation levels once the hierarchy was stable (hierarchy-maintenance phase). After their initial drop in food intake, SUB caloric consumption steadily increased throughout the duration of the VBS housing period; however, it remained significantly reduced compared with CON (P < 0.001) (Fig. 3, A and B).
Fig. 3.
Food intake. Hab indicates the average consumption during the 7-day habituation period prior to VBS exposure. The vertical line separates the hierarchy-formation and hierarchy-maintenance phase of VBS housing and the 3 wk of the recovery period (see materials and methods). The horizontal lines above the weeks of recovery indicate a significant difference was found across that week, and the symbol indicates which groups were different and is defined in each figure legend. These time points and line indicators will be represented on each of the following meal pattern graphs [Hab: n = 12; VBS: n = 6 (CON), n = 7 (DOM), n = 21 (SUB); Recovery: n = 6 (CON), n = 6 (DOM), n = 18 (SUB)] A and B: CON food consumption is comparable to Hab. DOM and SUB are hypophagic during the initial VBS housing period; however, SUB food intake remains suppressed throughout the hierarchy-maintenance phase, where DOM express CON levels of energy consumption. C and D: CON consumed values comparable to habituation and hierarchy-maintenance values throughout recovery. DOM and SUB were hyperphagic during recovery compared with CON and to both the habituation and hierarchy-maintenance phase. Data are expressed as means ± SE. *P < 0.01 vs. CON; **P < 0.001 vs. CON; †P < 0.01 vs. CON and DOM; ††P < 0.001 vs. CON and DOM; §P < 0.05 CON vs. SUB and DOM; §§P < 0.001 CON vs. SUB and DOM.
Compared to CON, DOM and SUB were hyperphagic during recovery (P < 0.01) (Fig. 3D). CON food intake was similar to that during habituation, whereas DOM and SUB consumed more food during recovery than during habituation or hierarchy-maintenance (DOM: P < 0.01, P < 0.01; SUB: P < 0.05, P < 0.001, respectively) (Fig. 3C).
Meal pattern analysis.
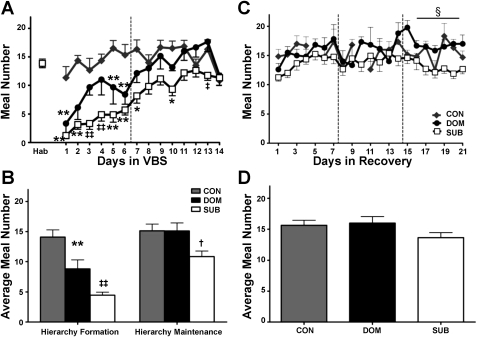
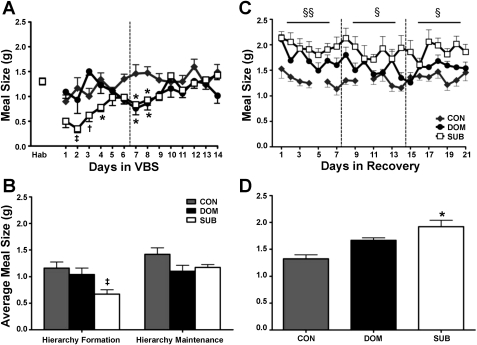
CON had comparable meal frequency, meal size, and meal duration values as during the habituation phase throughout the experiment. Within the VBS, during the hierarchy-formation phase, the rats that ultimately became DOM had a decreased meal number compared with CON (P < 0.001), but other meal characteristics were similar to CON. During hierarchy-formation, the rats that became SUB took fewer (P < 0.001) and smaller (P < 0.01) meals compared with CON and DOM (Figs. 4, A and B, 5, A and B). Additionally, SUB meal duration was decreased (P < 0.01) and Inter-MI increased (P < 0.01) compared with CON (Table 2).
Fig. 4.
Meal frequency. A: DOM and SUB have a reduced meal frequency upon VBS exposure, and meal number remained suppressed in SUB. B: Overall, meal frequency was reduced in DOM and SUB during the hierarchy-formation phase, but only remained decreased in SUB through the duration of VBS housing. C: meal frequency was similar among groups during the recovery period except during the last week when SUB consumed fewer meals than DOM. D: there were no overall significant differences in meal number during recovery. Data are expressed as mean ± SE. *P < 0.01 vs. CON; **P < 0.001 vs. CON; †P < 0.01 vs. DOM; ‡P < 0.01 vs. CON and DOM; ‡‡P < 0.001 vs. CON and DOM; §P < 0.01 SUB vs. DOM.
Fig. 5.
Meal size. A: SUB have a decreased meal size upon VBS housing. B: overall, average meal size was reduced in SUB only during the hierarchy-formation phase. C: SUB animals consumed larger meals during every week of the recovery period compared with CON. D: meal size was increased in SUB throughout the recovery period. Data are expressed as means ± SE. *P < 0.01 vs. CON; †P < 0.01 vs. DOM; ‡P < 0.01 vs. CON and DOM; §P < 0.05 CON vs. SUB; §§P < 0.01 CON vs. SUB.
Table 2.
Average meal pattern characteristics during VBS and recovery
| VBS |
|||
|---|---|---|---|
| HF | HM | Recovery | |
| Duration, min | |||
| CON | 5.14 ± 0.30 | 5.55 ± 0.49 | 5.87 ± 0.35 |
| DOM | 3.67 ± 0.46 | 4.06 ± 0.40 | 5.90 ± 0.37 |
| SUB | 3.77 ± 0.47* | 5.86 ± 0.38† | 8.48 ± 0.60‡ |
| Inter-MI, min | |||
| CON | 68.2 ± 3.99 | 66.3 ± 3.21 | 65.4 ± 4.95 |
| DOM | 114 ± 18.4 | 65.6 ± 4.08 | 51.7 ± 2.64 |
| SUB | 184 ± 19.4* | 120 ± 8.55‡‡ | 74.1 ± 4.34† |
| Intra-MI, min | |||
| CON | 1.16 ± 0.23 | 0.75 ± 0.12 | 1.15 ± 0.24 |
| DOM | 0.66 ± 0.21 | 0.70 ± 0.13 | 0.69 ± 0.08 |
| SUB | 1.34 ± 0.17 | 1.54 ± 0.13‡‡ | 1.23 ± 0.13 |
Data are expressed mean ± SE.
P < 0.01 vs. CON;
P < 0.05 vs. DOM;
P < 0.01 vs. CON and DOM;
P < 0.001 vs. CON and DOM. CON, control; DOM, dominant; SUB, subordinate; VBS, visible burrow system; HF, hierarchy-formation; HM, hierarchy-maintenance; Inter-MI, intermeal interval; Intra-MI, intrameal interval.
During hierarchy-maintenance, DOM had meal characteristics similar to those of CON. In contrast, SUB continued to have disrupted feeding, with a reduced meal number compared with DOM (P < 0.01), but normal meal size (Fig. 4, A and B, 5, A and B). SUB also had increased meal duration compared with DOM (P < 0.05), Intra-MI compared with CON and DOM (P < 0.001) and Inter-MI compared with CON and DOM (P < 0.001) (Table 2).
During recovery, SUB displayed a larger meal size compared with CON (P < 0.01) and longer meal duration compared with CON and DOM (P < 0.01) and Inter-MI compared with DOM (P < 0.05) (Figs. 4 and 5, Table 2).
Meal patterns analysis in the light and dark cycle.
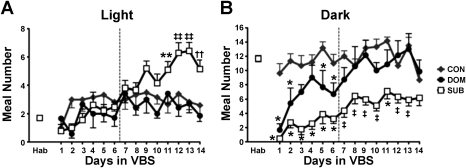
Compared with the habituation period, DOM and SUB ate fewer meals in both the dark and the light during hierarchy-formation (P < 0.01). Once the colony was stable, DOM were similar to CON, whereas SUB continued to eat fewer meals in the dark (P < 0.001) and also ate more meals during the light (P < 0.01) compared with CON and DOM (Fig. 6, Table 4). SUB took smaller meals compared with CON and DOM in the dark phase of the hierarchy-formation (P < 0.01) and compared with CON in the dark phase of hierarchy-maintenance (P < 0.05). Detailed light-dark microstructural data are presented in Table 4.
Fig. 6.
Light vs. dark analysis: meal frequency. A: SUB consumed significantly more meals during the light phase once the VBS hierarchy was stable. B: during VBS housing DOM initially took fewer meals during the dark but recovered to CON levels. SUB had a decreased meal frequency in the dark throughout VBS housing. Data are expressed as means ± SE. *P < 0.01 vs. CON; **P < 0.001 vs. CON; ††P < 0.001 vs. DOM; ‡P < 0.01 vs. CON and DOM; ‡‡P < 0.001 vs. CON and DOM.
Table 4.
Meal pattern characteristics during the light and dark phase
| VBS |
||||||
|---|---|---|---|---|---|---|
| HF |
HM |
Recovery |
||||
| Light | Dark | Light | Dark | Light | Dark | |
| Average Meal Number | ||||||
| CON | 2.90 ± 0.39 | 11.3 ± 0.68 | 3.06 ± 0.44 | 12.1 ± 0.68 | 2.81 ± 0.23 | 12.8 ± 0.74 |
| DOM | 1.45 ± 0.37* | 6.40 ± 1.55** | 2.66 ± 0.43 | 10.9 ± 1.69 | 1.92 ± 0.12 | 14.0 ± 1.14 |
| SUB | 1.73 ± 0.17* | 2.90 ± 0.44‡‡ | 4.97 ± 0.39‡ | 5.91 ± 0.77‡‡ | 3.14 ± 0.37 | 10.2 ± 0.71† |
| Average Meal Size, g | ||||||
| CON | 1.00 ± 0.11 | 1.20 ± 0.14 | 1.11 ± 0.07 | 1.48 ± 0.14 | 1.12 ± 0.04 | 1.35 ± 0.07 |
| DOM | 1.58 ± 0.23* | 1.11 ± 0.06 | 1.19 ± 0.11 | 1.15 ± 0.13 | 1.40 ± 0.06 | 1.65 ± 0.07 |
| SUB | 0.99 ± 0.12 | 0.61 ± 0.09‡ | 1.39 ± 0.07 | 0.96 ± 0.08* | 1.51 ± 0.13* | 1.79 ± 0.15* |
Data are expressed as means ± SE.
P < 0.05 vs. CON;
P < 0.001 vs. CON;
P < 0.05 vs. DOM;
P < 0.01vs. CON and DOM;
P < 0.001 vs. CON and DOM.
During the initial recovery period SUB continued to have increased meal frequency during the light period (P < 0.02) and reduced meal frequency during the dark period (P < 0.01) compared with CON and DOM (data not shown); however, throughout recovery SUB took larger meals compared with CON in both the light (P < 0.05) and dark (P < 0.05) and ate less frequently during the dark compared with DOM (P < 0.05) (Table 4).
Hypothalamic NPY mRNA expression.
Although there were differences in food intake and meal patterns between DOM and SUB during VBS housing and recovery, this was not reflected by changes in hypothalamic NPY expression. At the termination of VBS housing, both DOM and SUB had increased NPY mRNA expression in the Arc relative to CON (P < 0.001), whereas there were no differences among groups in NPY mRNA expression in the DMH (Table 5). After 1 and 3 wk of recovery, NPY mRNA was similar among groups in the Arc and DMH (Table 5).
Table 5.
Hypothalamic NPY mRNA expression
| Recovery |
|||
|---|---|---|---|
| VBS | Week 1 | Week 3 | |
| Arcuate Nucleus (average CGL) | |||
| CON | 63.4 ± 3.9 | 52.2 ± 5.2 | 44.2 ± 5.4 |
| DOM | 94.9 ± 4.3** | 50.6 ± 11.2 | 61.0 ± 9.1 |
| SUB | 91.1 ± 3.1** | 55.2 ± 5.7 | 56.5 ± 4.6 |
| Dorsomedial Nucleus (average CGL) | |||
| CON | 24.8 ± 1.8 | 9.90 ± 2.7 | 16.6 ± 2.4 |
| DOM | 27.7 ± 2.4 | 20.3 ± 2.4 | 22.3 ± 5.0 |
| SUB | 25.2 ± 1.8 | 12.7 ± 2.0 | 20.7 ± 2.8†† |
Data are expressed as means ± SE.
P < 0.001 vs. CON;
P < 0.001 vs. week 1 of same status. CGL, corrected gray level. CON: n = 6–13, DOM: n = 6–10, SUB: n = 14–25.
DISCUSSION
This report presents the first thorough examination of the microstructure and food intake patterns of animals during a period of social stress and recovery, and provides insight as to how these behavioral changes may predispose animals, especially the more stressed subordinate animals, to develop deleterious metabolic consequences. We also provide information on changes in hypothalamic regulatory systems that influence feeding and metabolism. Consistent with previous studies (51), both DOM and SUB lost body weight and adipose mass while in the VBS. After an initial short period of status determination, DOM had essentially normal feeding patterns, whereas SUB exhibited hypophagia, decreased meal frequency, increased meal duration, and Inter-MI and an altered circadian pattern of feeding. Upon removal of the social stress situation, both DOM and SUB regained body weight lost during VBS housing but differentially altered their body composition (i.e., the SUB became significantly fatter). These results confirm previous reports (38, 52) and further suggest that some of the effects of chronic social stress on feeding patterns are long lasting.
During hierarchy-formation, the rats that ultimately proved to be DOM ate fewer meals than CON. Once the social hierarchy was established and stable, the feeding patterns of DOM were similar in all respects to those of CON. CON feeding patterns were similar throughout the entire experiment, including when they were housed with a female, suggesting that social housing itself does not necessarily alter meal patterns when food is freely available. However, in a social situation, such as the VBS, which more closely resembles a rat's natural environment, many changes in feeding behavior become apparent. These are relatively short lived in DOM but persist in SUB and may contribute to their long-term metabolic health.
SUB ate less food than CON and had altered meal patterns throughout the period of VBS housing, and 3 wk of recovery was not sufficient to normalize meal patterns to those exhibited prior to VBS housing. During hierarchy-formation, SUB consumed fewer meals that were smaller and of shorter duration than normal. During hierarchy-maintenance, when DOM had resumed normal feeding patterns, SUB continued to take fewer meals of longer duration and had elevated Intra-MI and Inter-MI. Thus, DOM and SUB utilize different feeding strategies that are presumably a consequence of differential social status and stress levels.
DOM and SUB were both in negative energy balance throughout the period of VBS housing, and both lost significant adipose mass, consistent with previous studies (36, 51). DOM animals were likely maintaining a lower body weight due to increased activity in the VBS, and, therefore, may have had increased energy expenditure, although direct measures need to be performed to confirm these predictions. Other studies of social hierarchies indicate that dominant and subordinate animals have different activity levels and that dominant rats may maintain their rank at higher energy costs (4, 37). DOM typically maintain or increase their lean mass, supporting this theory.
During recovery, both DOM and SUB were hyperphagic. This result was somewhat unexpected, as previous reports indicated that only recovering SUB are hyperphagic (52). Nonetheless, the hyperphagic behavior manifested differentially in SUB and DOM, confirming the importance of examining meal patterns. DOM were comparable to CON in both meal number and meal size, and their increased overall food intake resulted from taking slightly more frequent meals. Although this increase was statistically significant only during the final week of recovery, other studies in humans have found that a small alteration such as one additional meal a day can significantly impact body weight and composition (14). Additionally, following the first week of recovery DOM body weight was similar to CON despite remaining hyperphagic, consistent with findings that body weight in humans can be maintained on a hypercaloric diet if meals are taken more frequently (20, 21). Together, these data suggest that despite being hyperphagic throughout the recovery period, the pattern of food consumption in DOM likely contributes to the maintenance of normal body weight and recovery of lean mass.
The hyperphagia of SUB during recovery resulted from an increase in meal size and a slight decrease in meal frequency, specifically during the dark cycle when they consumed the majority of their calories. This combination has been reported to predispose both humans and animals to weight gain and fat accumulation (14, 20, 21, 55). Although SUB recovered some body weight during recovery, their body weight never reached that of CON despite being hyperphagic. Other studies have reported similar findings following stress (5, 16), implying that food intake alone does not govern body weight (16). Furthermore, the weight gained by SUB during recovery was predominantly as adipose mass, and we have previously observed that this occurs mainly in the visceral adipose depot (52). Chronic stress in humans and Syrian hamsters also leads to the accumulation of fat in the abdominal region (12, 22, 49), and one contributing factor is likely the pattern of food consumption. As discussed above, SUB consumed fewer but larger meals during recovery. In humans, reducing meal frequency by one meal per day led to increased fat accumulation, and this occurred even under hypocaloric conditions (14). (See Table 3 for meal pattern summary.)
Table 3.
Summary of meal pattern changes
| VBS |
|||
|---|---|---|---|
| HF | HM | Recovery | |
| Food Intake | |||
| DOM | ↓** | ↑* | |
| SUB | ↓†† | ↓** | ↑* |
| Meal Number | |||
| DOM | ↓** | ||
| SUB | ↓†† | ↓‡ | |
| Meal Size | |||
| DOM | |||
| SUB | ↓† | ↑* | |
P < 0.01 vs. CON;
P < 0.001 vs. CON;
P < 0.01 vs. DOM;
P < 0.01 vs. CON and DOM;
P < 0.001 vs. CON and DOM.
The altered meal patterns observed while animals were in the VBS suggests that signals normally controlling ingestive behavior become impaired or overridden during social stress. Direct (e.g., stimulation of preabsorptive receptors by ingesta) and indirect factors (e.g., insulin, environment) act within (or modulate) the central nervous system, particularly within the hypothalamus, to initiate and terminate meals (34, 48, 57). NPY is a well-known peptide, particularly within the hypothalamus, that stimulates food intake and also has an intricate relationship with the stress axis. Glucocorticoids stimulate food intake and increase the expression of Arc NPY, which, in turn, can activate the HPA axis stimulating further CORT release (26, 27, 34, 46, 56). Activation of the HPA axis relies on negative feedback from CORT to suppress the expression of corticotropin-releasing hormone (CRH), thus ending the stress response. However, under conditions of chronic stress, CRH can be up-regulated, escaping the negative feedback of glucocorticoids (33). DOM had increased Arc NPY mRNA directly following VBS housing. This result was unexpected as DOM had similar food intake as CON, although the elevated expression may be secondary to the increased activity that occurs in the VBS itself. Wheel running has been reported to have a comparable effect on Arc NPY mRNA (8). NPY expression in the DMH was not different among groups, consistent with prior studies where Arc NPY was increased following stress and DMH NPY was unchanged (30, 32). It has been suggested that the role of NPY in the DMH is to maintain energy homeostasis by specifically increasing meal size following long-term alterations in energy balance (7). We, therefore, expected elevated DMH NPY expression in SUB; however, the unchanged expression of NPY in the DMH following VBS housing was not surprising, as there were no differences in meal size among groups at that time.
Although increased NPY was not associated with increased food intake in SUB during VBS housing, SUB immediately became hyperphagic upon removal from the VBS, and continued to overeat despite a rapid return of hypothalamic NPY mRNA levels to normal during recovery. Collectively, these data suggest that in our model NPY does not directly mediate the hyperphagia of DOM or SUB, nor the increased meal size of SUB, despite the close relationship of NPY and stress circuitry in the hypothalamus.
Meal pattern alterations during VBS housing likely represent behavioral and neuroethological adaptations to the VBS environment. For example, SUB took longer meals and had a longer Intra-MI. The latter may be a result of pausing many times during a meal to gauge the risk of continuing to eat; i.e., if the DOM is near and threatening to interact. SUB also had an altered circadian pattern of ingestive behavior. Once the social hierarchy was established, SUB consumed more meals during the light phase and fewer during the dark. While CON and DOM ate the majority of their daily calories during the dark, SUB consumed most food during the light phase, and this pattern continued into the initial recovery phase. Rats are nocturnal and therefore take the majority of their meals during the dark (3, 47). When food-deprived, rats voluntarily recover their food intake in the dark (31). However, stress can alter the predominantly nocturnal food consumption and increase intake during the light (45, 54). Therefore, SUB may have a shifted sleep-wake cycle, interrupted sleep sessions resulting from social stress, and/or a potential lack of light exposure while in the VBS. The latter possibility is due to the fact that SUB spend the majority of their time in the smaller VBS chambers, which are kept in constant darkness. This pattern presumably develops since the DOM rat is less likely to stray from the open surface chamber into the inner chambers. It is unknown if sleep cycles are disrupted in SUB during the VBS period or in the recovery period; however, the present data suggest that at least some behavioral alterations are sustained during the initial stages of recovery.
This is this first study of its kind to examine meal patterns in real-time during exposure to chronic social stress and during a subsequent recovery period, as well as to begin to evaluate the neuroendocrine and neurochemical underpinnings of the altered ingestive patterns observed. VBS stress and recovery induce changes in body weight and composition, and the alterations in meal patterns reported here may contribute to these physiological changes. Current mechanisms behind the altered meal pattern behavior are unknown; however, increased basal levels of CORT and disrupted or overridden effects of NPY may be involved during the VBS housing period, and other, as yet unknown, factors may drive increased meal size during the recovery period, ultimately resulting in the altered meal patterns and body composition during the VBS stress recovery paradigm.
Perspectives and Significance
Stress is experienced on a daily basis, particularly psychological (e.g., social stress) stress, suggesting that many individuals experience cycles of stress and recovery throughout the day, or on a weekly basis. If, following stress, we consume larger and less frequent meals, and have greater than normal levels of glucocorticoids circulating in our blood, a situation would be created that promotes weight gain and adiposity, particularly abdominal adiposity. Furthermore, abdominal adiposity, as well as stress, can contribute to the development of cardiovascular disease, immune dysfunction, as well as other metabolic disorders (9, 23, 29). As a result, understanding the relationship between stress and obesity is essential to potentially treat and prevent the further development of the associated comorbidities, and the VBS model allows for such studies.
GRANTS
These studies were supported by the National Institute of Diabetes, Digestive and Kidney Diseases Grant RO1 DK066596–06 (to R. R. Sakai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Clinton T. Elfers, Amanda Jones, and Annette DeKloet for their excellent technical assistance.
REFERENCES
- 1.Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci 17: 4895–4903, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bare JK, Cicala G. Deprivation and time of testing as determinants of food intake. J Comp Physiol Psychol 53: 151–154, 1960 [DOI] [PubMed] [Google Scholar]
- 4.Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell'Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One 4: e4331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 18: 13–24, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides 28: 352–356, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol 285: R1030–R1036, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev 2: 73–86, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res 58: 113–121, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20: 117–134, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Branth S, Ronquist G, Stridsberg M, Hambraeus L, Kindgren E, Olsson R, Carlander D, Arnetz B. Development of abdominal fat and incipient metabolic syndrome in young healthy men exposed to long-term stress. Nutr Metab Cardiovasc Dis 17: 427–435, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Campbell RE, ffrench-Mullen JM, Cowley MA, Smith MS, Grove KL. Hypothalamic circuitry of neuropeptide Y regulation of neuroendocrine function and food intake via the Y5 receptor subtype. Neuroendocrinology 74: 106–119, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Chapelot D, Marmonier C, Aubert R, Allegre C, Gausseres N, Fantino M, Louis-Sylvestre J. Consequence of omitting or adding a meal in man on body composition, food intake, and metabolism. Obesity (Silver Spring) 14: 215–227, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav 89: 301–310, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Coccurello R, D'Amato FR, Moles A. Chronic social stress, hedonism and vulnerability to obesity: Lessons from rodents. Neurosci Biobehav Rev 33: 537–550, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology 148: 3666–3673, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Drewnowski A, Cohen AE, Faust IM, Grinker JA. Meal-taking behavior is related to predisposition to dietary obesity in the rat. Physiol Behav 32: 61–67, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26: 37–49, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Fabry P, Hejda S, Cerny K, Osancova K, Pechar J. Effect of meal frequency in schoolchildren. Changes in weight-height proportion and skinfold thickness. Am J Clin Nutr 18: 358–361, 1966 [DOI] [PubMed] [Google Scholar]
- 21.Fabry P, Tepperman J. Meal frequency—a possible factor in human pathology. Am J Clin Nutr 23: 1059–1068, 1970 [DOI] [PubMed] [Google Scholar]
- 22.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 290: R1284–R1293, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12: 1–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod 67: 1750–1755, 2002 [DOI] [PubMed] [Google Scholar]
- 24a.Institute of Laboratory Animal Resources Guide for the Care, and Use of Laboratory Animals, edited by Institute of Laboratory Animal Resources Commission on Life Science Washington DC: National Academy Press, 1996 [Google Scholar]
- 25.Jacobson IG, Smith TC, Smith B, Keel PK, Amoroso PJ, Wells TS, Bathalon GP, Boyko EJ, Ryan MA. Disordered eating and weight changes after deployment: longitudinal assessment of a large U.S. military cohort. Am J Epidemiol 169: 415–427, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Jeanrenaud B, Rohner-Jeanrenaud F. CNS-periphery relationships and body weight homeostasis: influence of the glucocorticoid status. Int J Obes Relat Metab Disord 24Suppl 2: S74–S76, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kakui N, Kitamura K. Direct evidence that stimulation of neuropeptide Y Y5 receptor activates hypothalamo-pituitary-adrenal axis in conscious rats via both corticotropin-releasing factor- and arginine vasopressin-dependent pathway. Endocrinology 148: 2854–2862, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kalra SP, Kalra PS. NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides 38: 201–211, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kyrou I, Tsigos C. Stress mechanisms and metabolic complications. Horm Metab Res 39: 430–438, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Liang S, Byers DM, Irwin LN. Chronic mild stressors and diet affect gene expression differently in male and female rats. J Mol Neurosci 33: 189–200, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Lima FB, Hell NS, Timo-Iaria C. Carbohydrate metabolism and food intake in food-restricted rats Relationship between the metabolic events during the meal and the degree of food intake. Physiol Behav 35: 695–700, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Makino S, Asaba K, Nishiyama M, Hashimoto K. Decreased type 2 corticotropin-releasing hormone receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology 70: 160–167, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 73: 147–158, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci 7: 271–280, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol Behav 99: 33–39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel C, Duclos M, Cabanac M, Richard D. Chronic stress reduces body fat content in both obesity-prone and obesity-resistant strains of mice. Horm Behav 48: 172–179, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D'Amato FR. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology 31: 623–633, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Nguyen MM, Tamashiro KL, Melhorn SJ, Ma LY, Gardner SR, Sakai RR. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology 148: 6145–6156, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Nicklas TA, Baranowski T, Cullen KW, Berenson G. Eating patterns, dietary quality and obesity. J Am Coll Nutr 20: 599–608, 2001 [DOI] [PubMed] [Google Scholar]
- 40.O'Connor DB, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychol 27: S20–S31, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- 42.Popper R, Smits G, Meiselman HL, Hirsch E. Eating in combat: a survey of U.S. Marines. Mil Med 154: 619–623, 1989 [PubMed] [Google Scholar]
- 43.Prigerson HG, Bierhals AJ, Kasl SV, Reynolds CF, 3rd, Shear MK, Day N, Beery LC, Newsom JT, Jacobs S. Traumatic grief as a risk factor for mental and physical morbidity. Am J Psychiatry 154: 616–623, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Roberts C, Troop N, Connan F, Treasure J, Campbell IC. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity (Silver Spring) 15: 3045–3055, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol Regul Integr Comp Physiol 273: R1612–R1622, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology 149: 4544–4553, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Siegel PS. Food intake in the rat in relation to the dark-light cycle. J Comp Physiol Psychol 54: 294–301, 1961 [Google Scholar]
- 48.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev 20: 41–46, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 292: R283–R290, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav 91: 440–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav 80: 683–693, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol 293: R1864–R1874, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition 23: 887–894, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Varma M, Chai JK, Meguid MM, Gleason JR, Yang ZJ. Effect of operative stress on food intake and feeding pattern in female rats. Nutrition 15: 365–372, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Wheeler J, Martin R, Lin D, Yakubu F, Hill JO. Weight cycling in female rats subjected to varying meal patterns. Am J Physiol Regul Integr Comp Physiol 258: R124–R129, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Wilding JP, Gilbey SG, Lambert PD, Ghatei MA, Bloom SR. Increases in neuropeptide Y content and gene expression in the hypothalamus of rats treated with dexamethasone are prevented by insulin. Neuroendocrinology 57: 581–587, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition 16: 894–902, 2000 [DOI] [PubMed] [Google Scholar]