Abstract
To evaluate the contribution of neural pathways to the determination of the circadian oscillator phase in peripheral organs, we assessed lateralization of clock gene expression in Syrian hamsters induced to split rhythms of locomotor activity by exposure to constant light. We measured the ratio of haPer1, haPer2, and haBmal1 mRNA on the high vs. low (H/L) side at 3-h intervals prior to the predicted activity onset (pAO). We also calculated expression on the sides ipsilateral vs. contralateral (I/C) to the side of the suprachiasmatic nucleus (SCN) expressing higher haPer1. The extent of asymmetry in split hamsters varied between specific genes, phases, and organs. Although the magnitude of asymmetry in peripheral organs was never as great as that in the SCN, we observed significantly greater lateralization of clock gene expression in the adrenal medulla and cortex, lung, and skeletal muscle, but not in liver or kidney, of split hamsters than of unsplit controls. We observed fivefold lateralization of expression of the clock-controlled gene, albumin site D-element binding protein (Dbp), in skeletal muscle (H/L: 10.7 ± 3.7 at 3 h vs. 2.2 ± 0.3 at 0 h pAO; P = 0.03). Furthermore, tyrosine hydroxylase expression was asymmetrical in the adrenal medulla of split (H/L: 1.9 ± 0.5 at 0 h) vs. unsplit hamsters (1.2 ± 0.04; P < 0.05). Consistent with a model of neurally controlled gene expression, we found significant correlations between the phase angle between morning and evening components (ψme) and the level of asymmetry (H/L or I/C). Our results indicate that neural pathways contribute to, but cannot completely account for, SCN regulation of the phase of peripheral oscillators.
Keywords: clock genes, splitting, SCN, circadian, period, Bmal1, tyrosine hydroxylase, albumin site D-element binding protein
a central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus crowns the hierarchy of mammalian circadian oscillators. Not only does the SCN regulate endogenous oscillations of locomotor behavior, body temperature, and the secretion of many hormones, but it coordinates oscillations of gene expression in peripheral organs throughout the body. In both the SCN and the periphery of mice, rats, and hamsters, interlocked transcriptional-translational feedback loops involving a limited number of clock genes and their protein products control these oscillations (47, 49, 58, 61).
The pathways by which the SCN regulates the peripheral phase may include humoral, neural, and behavioral links. SCN projections to the paraventricular nucleus (PVN) may mediate regulation of pituitary-dependent endocrine organs by control of neurosecretory outputs, including corticotrophin-releasing hormone and thyrotropin-releasing hormone. The SCN may also regulate peripheral organs by way of hormonal signals that are relatively independent of the pituitary (10, 11). In addition, peripheral organs may receive a timing signal through their autonomic innervation. SCN efferents to the PVN likely regulate preautonomic neurons that control sympathetic preganglionic neurons of the intermediolateral column and the parasympathetic preganglionic motor neurons of the dorsal motor nucleus of the vagus (5, 9, 50). SCN projections to the dorsomedial hypothalamus, preoptic area, and brain stem may also engage neural and/or endocrine pathways that ultimately regulate the peripheral oscillators (1, 13, 53, 54). Circadian oscillations of clock gene expression in the periphery may also be regulated by food cues and temperature fluctuations that ultimately depend upon the SCN (7). For example, through its regulation of activity patterns, the SCN may determine the timing of ingestive behaviors and thus the pattern of food-associated cues that directly determine clock gene expression. Similarly, through regulation of thermogenic activity in skeletal muscle and/or vasodilation or vasoconstriction (42, 43), the SCN may generate temperature cues that determine, at least in part, the phase of clock gene expression in the periphery. Thus SCN-regulated autonomic pathways may influence the peripheral phase indirectly as well as directly.
To investigate the nature of SCN signals that regulate circadian oscillators in peripheral organs, we assessed clock gene expression in peripheral organs of Syrian hamsters induced to split rhythms of locomotor behavior by exposure to constant light (LL). In this condition, the locomotor activity pattern dissociates into two bouts per circadian cycle (41). This change in the locomotor output is paralleled by dissociation of clock gene expression of the two (left and right) nuclei comprising the SCN, such that they couple approximately in antiphase (14). The contralateral nuclei of the SCN simultaneously exhibit peak Per1 and Bmal1 expression, indicating that one side is in subjective day, while the other is in subjective night. The utility of this preparation in evaluating neural outputs of the pacemaker has been demonstrated by de la Iglesia et al. (15), who showed that efferent signals from the SCN that control GnRH cells that govern the luteinizing hormone surge are lateralized in split hamsters.
We sought to extend this type of analysis to assess the nature of SCN control of peripheral oscillations. We reasoned that to the extent that completely crossed or uncrossed neural pathways originating in the SCN determine the phase of clock gene expression in peripheral paired organs, expression of haPer1, haPer2, and haBmal1 should peak in antiphase on the left and right side of hamsters that split in LL. In light of evidence that expression of many functionally important genes in peripheral organs is regulated by circadian oscillators (2, 40, 45) we predicted that the phase of peak and nadir expression of physiologically relevant, clock-controlled genes in peripheral paired organs will also be lateralized if the SCN regulates these tissues by neural pathways. Alternatively, if SCN control of peripheral oscillators is predominantly humoral, or if it depends upon control of temperature, feeding, or behaviorally determined cues, the phase of clock gene expression in peripheral paired organs on the left and right sides should coincide in split hamsters.
METHODS
Animals.
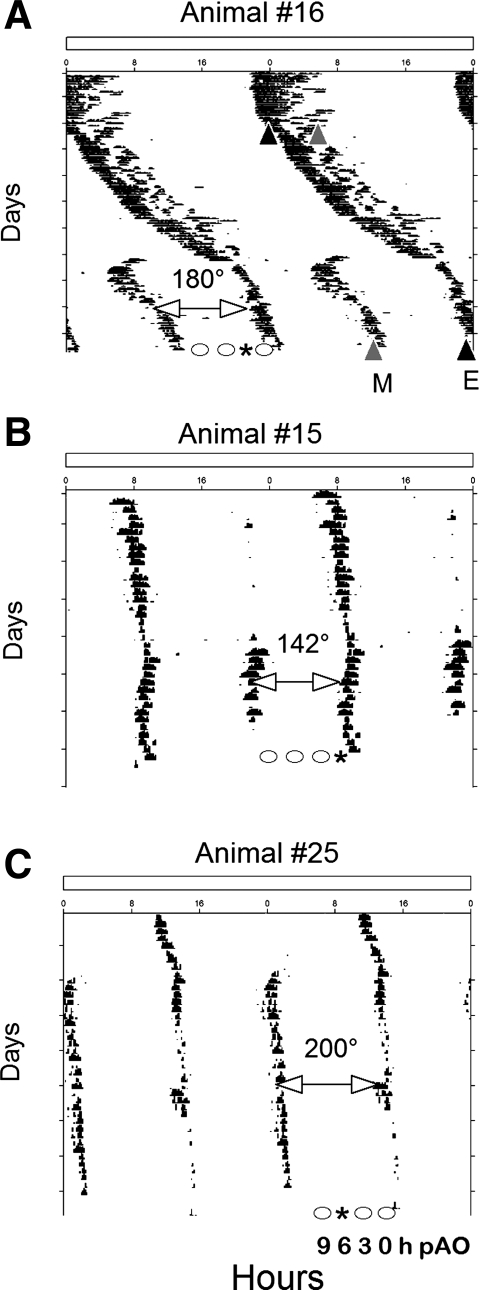
Adult male Syrian hamsters (Mesocricetus auratus, LVG strain) born and raised in a 14:10-h light-dark cycle were allowed ad libitum access to food and water throughout the experiment. All procedures were approved by the University of Massachusetts Institutional Animal Care and Use Committee. Syrian hamsters were transferred as young adults (∼10 wk of age) to a cage equipped with a running wheel (16.5 cm diameter) maintained in LL (white fluorescent light; ∼200 lux or ∼3 mmol·m2·s−1 at cage level). Wheel-running activity was monitored by computer (ClockLab Actimetrics Software). Actograms were reviewed, and a least-squares regression line was fit to the activity onsets. In addition, χ2 periodograms were analyzed for split locomotor patterns. Although hamsters were classified as split if two activity bouts with onsets ∼12 h apart were observed, some animals showed activity components that were separated by as few as 9 h (Fig. 1). A stable split was achieved ∼8 wk after transfer to LL (range of 4 to 13 wk). The limbs of activity were designated evening and morning based on the criteria of Pittendrigh and Daan (41).
Fig. 1.
A–C: representative double-plotted running records of hamsters whose locomotor activity split in exposure to constant light (LL). E (black triangle in A) and M (gray triangle in A) are evening and morning components, respectively. The phase angle between morning and evening components (ψme) of 180 degrees (A), 142 degrees (B), and 200 degrees (C) are illustrated. Animals were killed at 9, 6, 3, or 0 h before the predicted activity onset (pAO; phases indicated as circles in each actogram). *Time of sampling of these individuals at 3-h (A), 0-h (B) and 6-h (C) pAO.
In our experiment 1, four hamsters were rapidly decapitated between 4 and 9 wk after their locomotor activity patterns had split in LL. These animals were killed at 3 h prior to the expected onset of one of the predicted activity bouts (pAO). In experiment 2, five hamsters were killed 2–4 wk after their activity had split, at 3–4 h pAO. Four other hamsters that did not split their activity in LL were killed at the same time for use as controls. In experiment 3, 26 animals were killed 7 wk after a stable split was established. Groups of hamsters were rapidly decapitated at 0-h (n = 5), 3-h (n = 5), 6-h (n = 5), or 9-h (n = 6) pAO. Another five hamsters that displayed a single activity bout per 24 h (nonsplit controls) were killed at 3 h pAO. Observations from the first two experiments led to the development of the third experiment to examine more systematically the variation of peripheral asymmetry with phase. Only the data from the experiment 3 was used for statistical analysis.
Brains, kidneys, and adrenal glands were rapidly dissected. Samples (∼500 mg) of the extreme left and right lobes of the liver were collected and placed in separate tubes. In experiment 3, lungs were also collected. Skeletal muscle samples were obtained from the vastus group of the hind leg. Organs were frozen on dry ice or in methyl butane immediately upon collection and stored at −80°C until time of analysis. Brains were notched on the animal's left side and sectioned on a cryostat (−20°C) at a thickness of 20 μ in series of four, cold-mounted on slides (Superfrost-Plus), and kept at −80°C until in situ hybridization for haPer1. Adrenal glands were sectioned on a cryostat (−20°C) at 20 μ in series of four, warm-mounted on slides, and kept at −80°C until in situ hybridization.
Quantification of mRNA.
In situ hybridization histochemistry was carried out using procedures described previously (21, 49). Slides were warmed briefly to room temperature, fixed in 4% paraformaldehyde for 15 min, deaminated in acetic anhydride/TEA, and then dehydrated in an alcohol series, delipidated in chloroform, partially rehydrated, and air dried. Following prehybridization, slides were sealed and stored at −20°C until the hybridization step.
cRNA probes were generated with [35S]UTP-labeled antisense and sense probes transcribed in vitro from appropriate templates. For haPer1, AF249882 nt 215–1336, homologous to AF02292 nt 337–1120, ∼730 bp in pBluescript II vector, was cut with HindIII or SmaI (57). The haBmal1 template (AF070917 ∼1881 bp, nt 1–1881) cloned in pcDNA3 vector, was cut with HindIII or Xba1 (18). The template for haPer2 [homologous to AF035830 nt 841–1620, 780 bp], in pGEM-T Easy vector, was cut with SpeI or ApaI (22). Probe for tyrosine hydroxylase (rTH; NM_012740; nt 14–1165) was transcribed from template cloned in pBS(−) vector and cut with Pvu1 or HindIII for antisense or sense, respectively.
Sections were hybridized with antisense or sense probe (1 × 106 cpm probe/25 μl of hybridization buffer) overnight at 57°C. Slides were rinsed in 1× SSC followed by 2× SSC/50% Formamide at 52°C, and incubated with RNAse A (50 μg/μl; USB, Cleveland, OH) at 37°C. Slides were rinsed in 2× SSC, dehydrated, and air-dried. Slides were then apposed to Kodak Biomax MR film for appropriate durations.
Following film development, brain sections were stained with toluidine blue and adrenals with hematoxylin and eosin, and coverslipped with Permount. Clock gene expression in the rostral, middle, and caudal SCN and in the adrenal medulla and cortex was quantified on film autoradiograms by using a DAGE charge-coupled device camera and National Institutes of Health Image software. Adrenal cortex layers were determined by characterizing the shape and clustering of cells of the zona glomerulosa, zona fasiculata, and zona reticularis (17).
The expression of haPer1, haPer2 and haBmal1 was analyzed in the lungs, skeletal muscle, liver, and kidneys of animals from the third experiment only, by quantitative real-time PCR (qRTPCR). Gapdh was similarly quantified for purposes of normalization. We also quantified expression in the clock-controlled gene, Dbp, in organs in which clock gene expression showed significant asymmetry. Approximately 200 mg of each sampled organ was homogenized, and RNA was extracted with Ultraspec II RNA reagent (Friendswood, TX), following the manufacturer's instructions, and treated with DNase 1 (0.2 U/ml; USB). RNA was quantified spectrophotometrically and checked for integrity by gel electrophoresis. A working stock of RNA was stored at −80°C until analyzed.
Standard curves were generated from a 10× dilution series using plasmids containing the respective target sequences (haPer1, haPer2, haBmal1, haDbp, and haGapdh). Clock gene and clock-controlled gene expression was normalized to Gapdh levels in the same tissue extract. Reactions were carried out on 96-well plates in a thermal cycler (model Mx3000p; Stratagene). Primer specificity was confirmed by dissociation (melting) curve analysis of the products.
Each unknown sample was normalized to a plate control. qRTPCR reactions were carried out by using 100 ng of total RNA (Quantitect qRT-PCR kit; Qiagen). Samples were incubated in a 10-μl final volume of 2× SYBR-green master mix containing 1.3 μl (0.5 ng) each of forward and reverse primer. The haPer1 forward and reverse primers (5′-AGCCATGCTGCCTACTCATTG-3′ and 5′-TCTTGTCAGGAGGGATGCG-3′, respectively) yield a 72-bp product. The haBmal1 forward (ACCAACATGCAATGCG-3′) and reverse (5′-TCAGTTCGTCATCGGAG-3′) primers generate a 119-bp product. Gapdh forward (5′-TGCACCACCAACTGCTTAG-3′) and reverse (5′-GTGGATGCAGGGATGATGTTC-3′) primers yield a 143-bp product. haPer2 forward (5′-GAGAACGAGATTCGCTACCA-3′) and reverse (5′-GGAATCCTAGGGGCTTCATA-3′) primers generate a 140-bp product. Dbp forward (5′-AAGGCAAGGAAAGTCCAGGT-3′) and reverse (5′-GAAGGCAGCCCT-CACAGATA-3′) primers generate a 141-bp product.
Normalized quantities of mRNA in left and right kidney and lung, in skeletal muscle obtained from the left and right hind legs, and in the left and right lobes of the liver were calculated (MxPro Mx3000P version 4.0; Stratagene). The average interplate coefficient of variation for a pooled mRNA sample was 2.5%. The average coefficient of variation within plates was 1.4%.
Statistical analysis.
Asymmetry was assessed as a ratio of clock gene or TH expression between the high and low sides. For peripheral organs, the I/C ratio relative to the SCN expressing higher haPer1 was also calculated. Values from split hamsters were evaluated across groups by using the Kruskal-Wallis test and, where significance was observed, the Mann-Whitney U-test. The control group was compared with split groups by using Mann-Whitney U-test. Significance was accepted at P ≤ 0.05.
Model parameters.
To predict effects of ψme on symmetry of clock gene expression in peripheral organs, we generated a model in which standard sine wave equations were used. The model parameters incorporated empirical data to set clock gene expression phase relative to activity onset. For this model we designated the activity onset to be driven by one of the nuclei, one being the morning and the other, the evening oscillator. Houben et al. (24) observed that the decline in SCN firing rate to approximately the half-maximum value of electrical activity, occurs at activity onset. Yamamoto et al. (57) reported Per1 expression in the hamster SCN falls from a maximum at CT4 to a minimum at CT16 with an approximate half-maximal value at CT12. Thus, we set activity onset at the half-maximum value of declining Per1 expression within the nucleus driving the evening bout. Guo et al. (21) observed a maximum of Per1 expression in the adrenal medulla of Syrian hamsters between CT9 and CT15, with a phase lag of ∼6 h to the SCN maximum discussed above. For this reason, we imposed a 6-h lag between the SCN and theoretical peripheral organ in our model. Based on empirical observation of Per1 expression within the SCN, the Per1 expression rhythm was modeled to have an amplitude of 150 units with a nadir value of 2.6 units. A standard sine wave equation, sine{2*π*[(time/24)+1]*(150/2)}+2.6, was used to generate our model of the SCN. On empirical grounds, we estimate the amplitude of the oscillation in the periphery to be half that observed in the SCN and modeled Per1 expression within the adrenal medulla with a peak of 75 units and a nadir value at 25 units. A 6-h lag was imposed on the peripheral oscillators, so that the equation used was sine{2*π*[(time−6)/24]+1}*(75/2)+25. The predictions are sensitive to parameter settings of the phase difference between peripheral organs and SCN, the maximum and minimum values of the peripheral organ's oscillation, and the waveform itself.
RESULTS
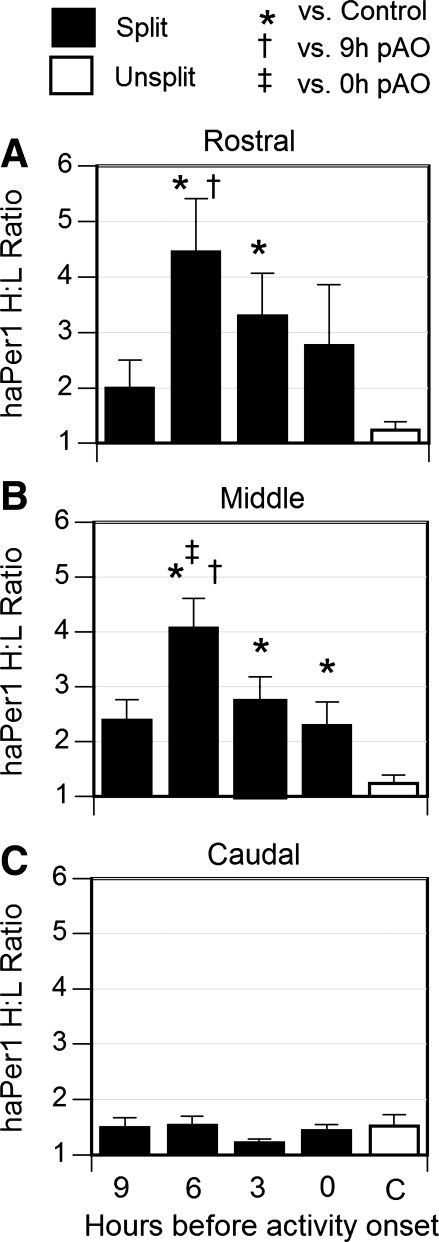
Expression of haPer1 at 3-h pAO did not differ between the left and right SCN of control hamsters that failed to split after 10–15 wk of exposure to LL. In contrast, haPer1 expression was asymmetrical in the SCN of hamsters that split in LL, and the distribution of mRNA was anatomically and temporally restricted (Fig. 2). Although SCN Per1 expression differed at least twofold in the rostral and middle SCN at all times sampled, the asymmetry reached statistical significance only at 6- and 3-h pAO in these two planes. The middle plane was also asymmetric at 0-h pAO (Fig. 2). In three hamsters (1 from 9-h and 2 from 0-h pAO), the side expressing higher haPer1 differed between the rostral and the middle plane.
Fig. 2.
High-to-low (H/L) ratio (mean ± SE) of haPer1 expression in rostral (A), middle (B), and caudal (C) coronal planes of the suprachiasmatic nucleus (SCN) was determined by measurement of optical density of autoradiograms generated by in situ hybridization. Filled bars, ratios calculated in hamsters that split in LL killed at the indicated times before pAO. Open bar, unsplit controls killed 3-h pAO. *P < 0.05 vs. unsplit control, †P < 0.05 vs. 9-h pAO, ‡P < 0.05 vs. 0-h pAO.
In addition to inducing asymmetry, protracted exposure to LL may alter absolute levels and rhythmicity of clock gene expression in the periphery. To evaluate this, clock gene mRNA values in peripheral organs were averaged between the left and right side. Levels of expression of at least one clock gene differed significantly between phases in lung, liver, and kidney (see Supplemental Fig. 1, A, C, and D; Supplemental data for this article are available online at the American Journal of Physiology–Regulatory, Integrative and Comparative Physiology website.). haPer1 expression tended to rise at the time of activity onset in skeletal muscle of split hamsters, but this trend did not reach statistical significance (P < 0.07; Supplemental Fig. 1B). At 3-h pAO, split animals had higher haBmal1 levels in each of these organs than did unsplit controls (Supplemental Fig. 2). LL may thus alter the absolute levels, the amplitude of rhythmicity, and the phase angle of peripheral clock gene expression relative to values in constant darkness (DD).
Asymmetry of peripheral gene expression in split hamsters was evaluated in two different ways. First, the H/L ratio of mRNA levels was compared across phases and with that of unsplit controls. Second, we sought to determine whether any asymmetry was systematically related to the lateralization of clock gene expression in the SCN. Thus we determined for each hamster whether the left or right SCN had higher haPer1 expression. We then calculated the ratio of clock gene expression in the peripheral organs on the side ipsilateral relative to the side contralateral to this side of the SCN.
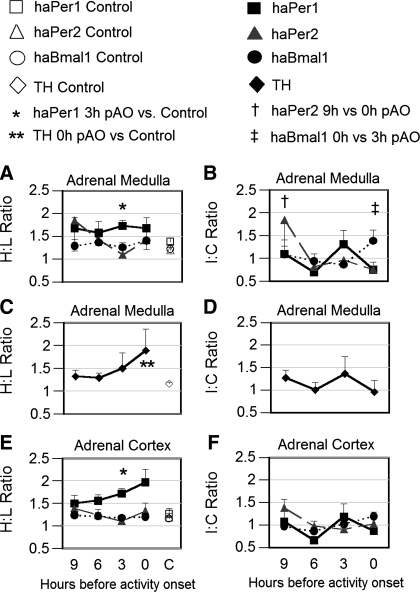
In the adrenal glands of unsplit control hamsters, clock gene expression was relatively symmetrical (H/L ratios of 1.2 to 1.5). Asymmetry of haPer1 expression in the adrenal gland was significantly greater in split hamsters than in controls, although the extent of the difference between the left and right side differed with phase. When examined at 3-h pAO in experiment 1, H/L ratios of haPer1 expression as high as 4 were found in the adrenal medulla (Fig. 3), although ratios between 1.5 and 2 were more typical. When asymmetry of haPer1 expression was examined at a variety of time points (experiment 3) we found a significantly greater H/L ratio in split hamsters than in unsplit controls at 3-h pAO (Fig. 4A). haPer2 and haBmal1 expression were consistently symmetric when assessed as H/L ratio in the adrenal medulla and did not differ from unsplit controls at any phase (Fig. 4A). At activity onset, the H/L ratio of TH expression in the adrenal medulla was significantly higher in split hamsters than in unsplit controls (P < 0.05; Fig. 4C). A different pattern emerged when asymmetry was considered as I/C ratios, i.e., relative to the haPer1 expression in the SCN. No significant influence of phase on an I/C ratio was detected for haPer1 or TH. Thus asymmetry of haPer1 expression in the adrenal medulla was not consistently related to the side of the SCN showing higher haPer1 expression at the time of death. However, haPer2 and haBmal1 expression was greater in the ipsilateral than the contralateral adrenal medulla at 9-h pAO and 0-h pAO, respectively (P < 0.05; Fig. 4B).
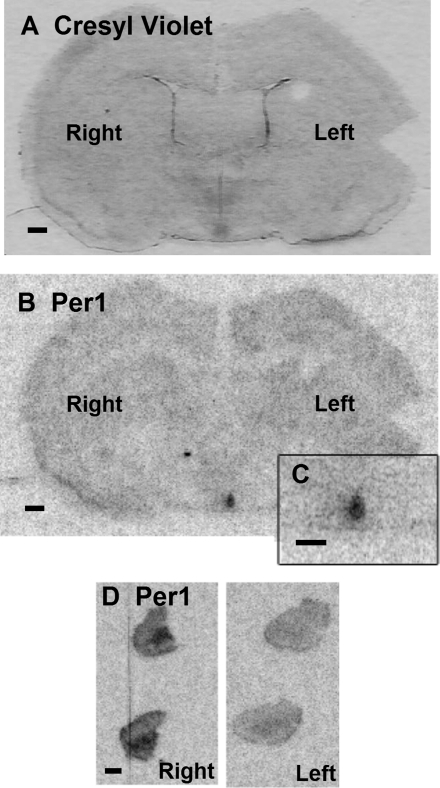
Fig. 3.
haPer1 expression in brain and adrenal gland of split hamsters. A: coronal brain section stained with cresyl violet demonstrating the left and right nuclei of the SCN of an animal killed 3-h pAO in experiment 1. B: corresponding autoradiogram of the brain section in A showing haPer1 expression assessed by in situ hybridization (scale bar = 0.05 cm). Inset, C: haPer1 mRNA in SCN at higher magnification (scale bar = 0.05 cm). Striking lateralization of haPer1 expression was evident in both SCN (B and C) and adrenal gland (D). Two serial sections of whole adrenal from each side are shown (scale bar = 0.05 cm).
Fig. 4.
Levels of gene expression in adrenal medulla (A–D) and adrenal cortex (E and F) of hamsters exposed to LL in experiment 3. Filled symbols represent data from split hamsters killed at 9-h (n = 6), 6-h (n = 5), 3-h (n = 5), and 0-h (n = 5) pAO. Open symbols represent data of unsplit controls (n = 5) killed at 3-h pAO. Left: asymmetry assessed as H/L ratio (A, C, and E); Right: asymmetry expressed as ratio of mRNA on sides ipsilateral vs. contralateral to the side of the SCN showing higher haPer1 expression in the same hamster (I/C; B, D, and F). Serial sections of the adrenals from each animal were processed by in situ hybridization for haPer1 (squares), haPer2 (triangles), haBmal1 (circles; A, B, E, and F), and for tyrosine hydroxylase (diamonds; C and D). Statistical significance: *P < 0.05 vs. unsplit control for haPer1 in adrenal medulla and cortex; tyrosine hydroxylase expression **P < 0.05 vs. unsplit control, †P < 0.05 vs. 0-h pAO for haPer2 only, and ‡P < 0.05 vs. 3-h pAO for haBmal1 only in adrenal medulla.
In the adrenal cortex, haPer1 H/L asymmetry was significantly greater at 3-h pAO in split hamsters than in unsplit controls (Fig. 4E). Upon quantifying clock gene expression by cell layer, we found that haPer1 I/C differed at 9-h vs. 6-h pAO in the zona glomerulosa (P < 0.05; data not shown). In the zona fasciculata, haPer1expression did not differ between the sides ipsilateral vs. contralateral to the peak SCN. However, the I/C ratio of haPer2 expression was greater at 9-h than at 3-h pAO (P < 0.05). Asymmetry of I/C haPer2 expression was significantly higher in the zona reticularis at 9-h pAO than at all other phases measured (P < 0.05). The haBmal1 I/C ratio was significantly greater at 0-h pAO than at 6-h and 3-h pAO (P < 0.05) in the zona fasciculata.
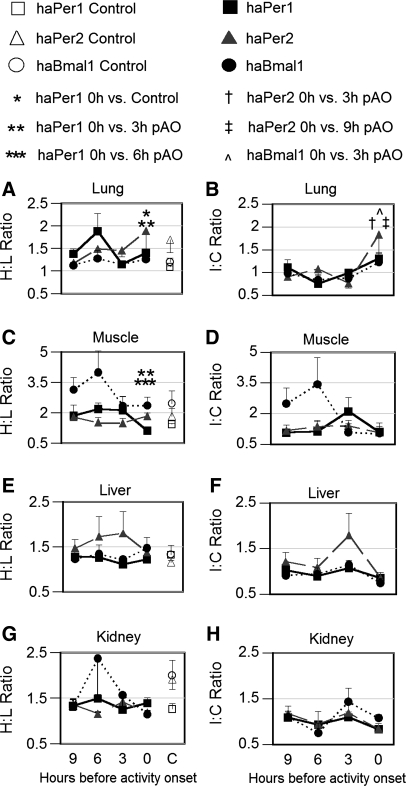
In the lung of unsplit controls, H/L ratios of clock gene expression ranged from 1.0 to 1.7. In split hamsters, the H/L ratio of haPer1 expression significantly exceeded this baseline at activity onset (P < 0.05; Fig. 5A). No significant asymmetry of haPer2 or haBmal1 expression was evident from H/L ratios at any phase in this tissue. As in the adrenal, a different pattern emerged when clock gene expression was evaluated on the sides ipsilateral vs. contralateral to the peak SCN in split animals. Although no significant I/C asymmetry was observed in haPer1 mRNA (Fig. 5B), I/C asymmetry of haPer2 expression in the lung was significantly greater at the time of running onset than at 3-h or 9-h pAO (P < 0.05). Asymmetry of haBmal1 expression in the lung, assessed as I/C ratio, was significantly greater (P < 0.05) at 0-h than at 3-h pAO. No significant asymmetry of Dbp expression was observed in lung (Fig. 6, A and B).
Fig. 5.
Left: H/L ratio (mean ± SE) of haPer1 (squares), haPer2 (triangles) and haBmal1 (circles) expression in lung (A), skeletal muscle (C), liver (E), and kidney (G) of split animals (filled symbols) killed at 9-h (n = 6), 6-h (n = 5), 3-h (n = 5), and 0-h (n = 5) pAO and in unsplit controls (n = 5; open symbols) in experiment 3. Gene expression was assessed by quantitative real-time PCR (qrtPCR) and normalized to GAPDH (see methods). Statistical significance: *P < 0.05 vs. unsplit control and **P < 0.05 vs. 3-h pAO for haPer1 only in lung; ***P < 0.05 vs. 6-h pAO and **P < 0.05 vs. 3-h pAO for haPer1 only in muscle. Right: ratio of clock gene expression (I/C; mean ± SE) between sides of lung (B), skeletal muscle (D), liver (F), and kidney (H) ipsilateral vs. contralateral to side of SCN in which haPer1 expression was higher. Statistical significance: †P < 0.05 haPer2 vs. 3-h pAO and ‡P < 0.05 haPer2 vs. 9-h pAO; P < 0.05 haBma1 vs. 3-h pAO.
Fig. 6.
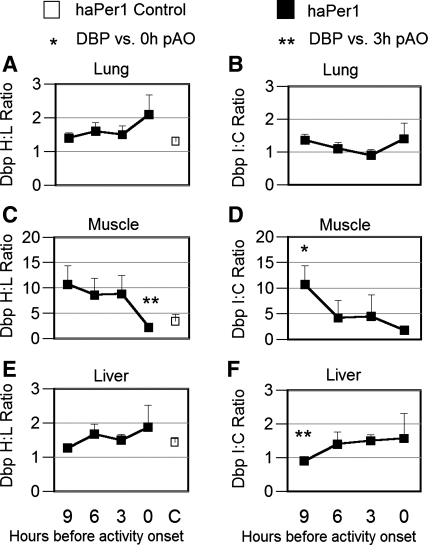
Left: H/L ratio (mean ± SE) of D-element binding protein (Dbp) expression in lung (A), skeletal muscle (C), and liver (E) of split animals (filled symbols) killed at 9-h (n = 6), 6-h (n = 5), 3-h (n = 5), and 0-h (n = 5) pAO and in unsplit controls (n = 5; open symbols) in experiment 3. Right: I/C ratio of the clock-controlled gene, Dbp, between sides of lung (B), skeletal muscle (D), and liver (F) ipsilateral vs. contralateral to the side of the SCN in which haPer1 expression was higher. Gene expression was assessed by qrtPCR and normalized to GAPDH (see methods). Statistical significance: *P < 0.05 vs. 0-h pAO and **P < 0.05 vs. 3-h pAO.
In skeletal muscle, the H/L ratio of unsplit controls ranged from 1.4 to 2.4. Among split experimental hamsters, lateralization of H/L haPer1 expression in this tissue was significantly greater at 6-h and 3-h pAO than at the time of activity onset (P < 0.05; Fig. 5C). Nevertheless, no significant I/C asymmetry was observed at any phase. No statistically significant asymmetry of either haPer2 or haBmal1 expression was observed in either the H/L or I/C ratios in skeletal muscle. A fivefold H/L asymmetry in expression of Dbp occurred in skeletal muscle at 3 h, and this differed significantly from the ratio at 0-h pAO (P = 0.03; Fig. 6C). Consistent with this finding, a similar peak of I/C asymmetry in Dbp was observed (9-h vs. 0-h pAO P = 0.04; Fig. 6D).
In liver and kidney of unsplit controls, the H/L ratio of clock gene expression ranged between 1.1 and 2.0. No statistically significant asymmetry of expression of haPer1, haPer2, or haBmal1 was observed in either the liver or the kidney of split hamsters at any phase relative to running onset (Fig. 5, E and G). The I/C ratio of clock gene expression in the liver and kidney did not differ between phases. Nevertheless, the I/C ratio of Dbp expression was more asymmetric at 3-h pAO than at 9-h pAO in the liver (P = 0.03; Fig. 6F).
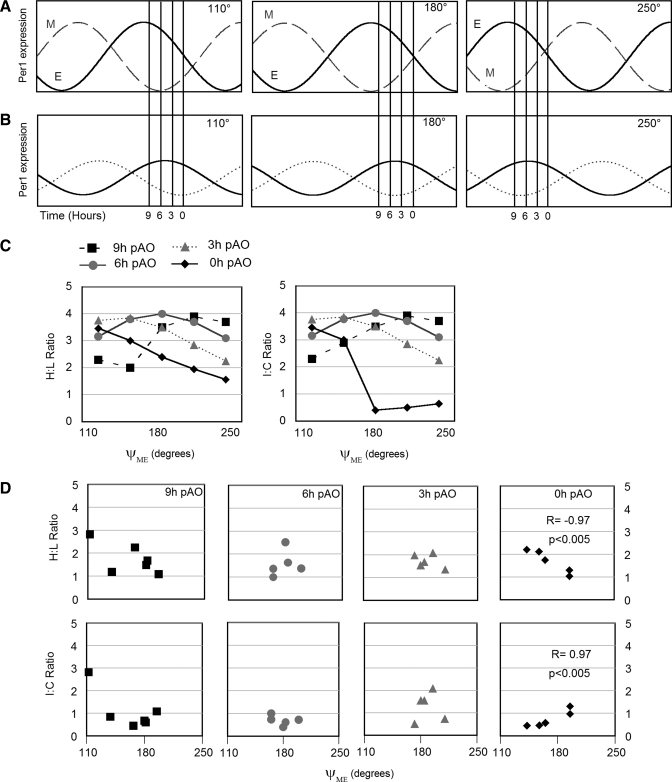
In many animals the split bouts of activity did not couple exactly in antiphase (Fig. 1, A–C and 7A). We hypothesized that the phase angle difference of activity bouts is systematically correlated with patterns of clock gene expression in peripheral organs (Fig. 7B). To evaluate this, we examined the H/L and I/C ratios of haPer1, haPer2, haBmal1 and Dbp in peripheral organs of hamsters showing different intervals between evening and morning running bouts (Fig. 1A). The phase angle was calculated as the interval between the onset of the morning bout and the onset of the evening bout (ψme). The mean ψme in the 21 split hamsters was 175 degrees and ranged between 112 and 210 degrees. In the adrenal medulla (Fig. 7D), the kidney, and the lung (Supplemental Fig. 3) we found a statistically significant correlation between ψme and the I/C ratio of haPer expression. Such correlations were restricted to particular phases and expression of particular genes. In the adrenal medulla, haPer1 I/C asymmetry was positively correlated, but the H/L ratio was negatively correlated, with ψme at the time of activity onset (P < 0.01; Fig. 7D). This reflects the fact that the high side tended to be contralateral to the high side of the split SCN. A negative correlation between ψme and haPer1 H/L asymmetry was observed at 9-h pAO (P < 0.05) in the lung. haPer2 I/C asymmetry was negatively correlated with ψme at 6-h pAO in the kidney (P < 0.01). In the lung, Dbp H/L asymmetry was positively correlated with ψme at activity onset (P < 0.05).
Fig. 7.
Model of the dependence of neurally driven rhythms of clock gene expression on the phase relationship between evening (E) and morning (M) oscillators. Hypothetical model of asymmetrical CG expression in SCN (A) and peripheral organs (B). A: Per1 mRNA abundance in the SCN of split animals is represented as a sinusoid, with the nuclei driving the E and M activity bouts shown separately (solid and dashed lines, respectively). B: Per1 oscillations in peripheral organs ipsilateral (solid line) and contralateral (dotted) to the E nucleus of the SCN. A lag of 6 h between pacemaker and slave is depicted. For both A and B, phase relationships are illustrated for ψme of 110° (left), 180° (middle), and 250° (right; see Fig. 1 for actual data). Vertical lines at 9-h, 6-h, 3-h, and 0-h pAO before the half-maximum of Per1 within the E nucleus of the SCN represent phases of organ collection. Amplitude of oscillation in peripheral organs is depicted as half that of SCN due to nonneural contribution and/or partial crossing of pathways descending from SCN. C: relationship between the H/L ratio (left) and I/C ratio (right) as a function of ψme at 9-h (squares), 6-h (circles), 3-h (triangles), and 0-h (diamonds) pAO predicted from the model. Note that the effect of ψme on H/L or I/C ratio can be diametrically opposite when sampling is done at different phases. D: empirically measured asymmetry of Per1 expression in adrenal medulla of split hamsters is plotted as a function of phase relationship between the E and M components. The dependence of H/L and I/C ratios on ψme at 0-h pAO was statistically significant in the adrenal medulla (see Supplemental Fig. 3). In groups showing a full range of ψme at particular pAO, the empirical data correspond to the model.
The characteristics of the activity bout may reflect the strength of the timing signal from the SCN regulating motor activity. The morning and evening activity bouts were similar in duration, volume (revolutions per bout) and intensity (average revolutions per minute). The evening activity bout used to project activity onset of each split group (pAO) was similar in duration, volume, and intensity across groups. In the kidney, evening bout intensity was negatively correlated to the H/L ratio of TH expression in adrenal medulla at 3-h pAO (r = −1.00; P < 0.05) and negatively correlated with haPer1 expression at 3-h pAO (r = −0.9; P < 0.05).
DISCUSSION
The asymmetry of clock gene expression in peripheral organs of behaviorally split hamsters indicates that neural inputs contribute to the regulation of circadian phase. The extent of this influence, however, differs between these organs. Previous work indicates that the degree and type of neural regulation of local clocks by the SCN may differ by organ (4, 48, 52). In some cases, sympathetic input may predominate (48), while in others the parasympathetic input may have the more important role (4). Furthermore, light-stimulated autonomic regulation of clock gene expression differs by phase and organ (12, 25). Differences between organs in the phase of peak clock gene expression have been reported in mice (6, 34, 39, 42) and hamsters (21). The present experiments show that the phase of peak lateralization also differs between organs in hamsters maintained in LL. In combination with tract tracing data (8, 51), these findings suggest the existence of multisynaptic pathways by which the SCN may regulate peripheral organs. Differences in the number of synapses, the signal transduction pathways, or other factors may account for variation between organs in the lag time between peak clock gene expression in the pacemaker and the periphery. This likely contributes to our finding of different patterns of lateralization of haPer1, haPer2, and haBmal1 mRNAs in peripheral organs.
The symmetry we observed in clock gene expression in the liver of hamsters held in constant light is most consistent with the hypothesis that nonneural signal(s) are more important in the setting phase in this organ. Guo et al. (20) reported that rhythms of mPer1, mPer2, and mBmal1 expression were restored in the liver and kidney but not in the heart, muscle, and spleen, of SCN lesioned mice parabiosed to SCN intact partners. Together these findings suggest a greater degree of nonneural control of phase in the liver than in other organs, but do not establish whether hormonal cues or other influences contribute. Our data are equivocal regarding the role of neural signals regulating the phase of clock gene in the kidney. Endocrine signals clearly regulate peripheral clocks: the glucocorticoid agonist dexamethasone sets the circadian phase of clock gene expression in liver (3) and lung (19). Physiologically relevant temperature changes are also sufficient to maintain and synchronize oscillations of peripheral tissues (7) and the SCN (23). In the present study, such an influence of body temperature may have reduced asymmetry of clock gene expression in peripheral organs.
In the lung, skeletal muscle, and adrenal medulla and cortex of split hamsters, H/L ratios support the contribution of neural control of haPer1 expression but do not indicate consistent asymmetry in the expression of haPer2 or haBmal1. In contrast, the I/C ratio more consistently supports neural control of expression of haPer2 and haBmal1 than of haPer1 in the same organs. In many instances the asymmetry, as indicated by the H/L ratio, did not consistently correspond to higher clock gene expression on the side ipsilateral or contralateral to the SCN side on which haPer1 expression was greater at the time of death. Several considerations are relevant to this apparent inconsistency. The lag imposed by the number of synaptic or other links between the pacemaker and periphery complicate our ability to relate asymmetries in the SCN to those in organs of the same individual. In addition, if tissue is collected at a time other than the maximum of the peak or nadir difference, the ability to relate the asymmetry to that of the pacemaker is compromised (Fig. 7). At the time of death, the asymmetry of either the SCN or the periphery may be increasing or decreasing, and at different phases marked as pAO groups, these relationships may vary. A high and low side can be assigned, but if the amplitude of the oscillation or the difference in clock gene expression between the left and right sides is small, there is uncertainty in this designation and a less consistent relationship to the SCN may result. In addition, different clock genes showed different patterns of asymmetry. Phase relations between clock genes may differ between the SCN and peripheral organs and between different peripheral organs. A nonparametric model of entrainment suggests oscillator components change phase as slaves advance or delay to match the period of the master pacemaker (41). The degree to which transcription is controlled by signals originating outside each organ may vary, and this may also contribute to differences between genes and between organs in the extent of lateralization of per1, per2, and bmal1. This idea is supported by the observation that interruption of tissue-specific clock function may compromise rhythmicity of expression of some genes but not others (27). Lilley (33) reported hamsters split in LL have a bimodal pattern of circulating corticosterone levels. Whether two daily pulses of corticosterone may attenuate Per1 expression rhythms or uncouple clock gene expression is yet to be tested. The loss of antiphasic relationship of Per1/2 and Bmal1 in the kidney (Supplemental Fig. 1) may be a result of such an altered in vivo environment.
Many issues arise when split animals are used to address neural control of peripheral clock gene expression. First, split hamsters varied in the phase angle between the evening and morning activity bouts (Fig. 1). While the basis of this variation is not understood, it may contribute to differences between animals in lateralization of clock gene expression among animals killed at the same phase relative to activity onset. For example, we noted that in the adrenal medulla of split animals sampled at the time of activity onset, those with larger ψme showed greater lateralization. The range of ψme between animals may reflect the phase angle of gene expression in the SCN, and this may also be maintained in peripheral paired organs (Fig. 7). This range may have increased the variance between pAO groups and made it more difficult to detect lateralization of gene expression within peripheral organs. Variation in ψme may also complicate efforts to designate either side as ipsilateral or contralateral to the high haPer1 expressing SCN. Nevertheless, our finding that the I/C ratio correlates with the ψme supports a neural contribution to circadian phase in adrenal medulla, kidney, and lung.
Second, evaluation of clock function in LL must take into account the degree of asymmetry (actual or due to assay error) in unsplit animals. We observed up to a twofold asymmetry ratio in some organs in the control group (Fig. 5, C and G). These low-amplitude left-right differences exceed assay variation and thus reflect authentic lateralization of clock gene expression. Although the unsplit control animals were housed in LL for a similar length of time as those animals that had split, they may not have been a completely homogeneous group. De la Iglesia et al. (15) reported one case in which a hamster housed in LL exhibiting a single activity bout had a dissociated SCN, suggesting the SCN falls out of phase prior to behavioral splitting. We do not know whether splitting of locomotor patterns precedes, coincides with, or follows dissociation of peripheral rhythms. Although the phase of peak SCN lateralization was at 6-h pAO in split hamsters, the control animals in our study were killed at 3-h pAO. Thus we may have overestimated the level of lateralization in the peripheral organs of unsplit hamsters.
Uncertainties concerning anatomical relationships contribute a third consideration about the split model. We found some cases in which the asymmetry of haPer1 expression was reversed between the rostral and middle planes of the SCN. Yan et al. (59) reported that Per1, c-Fos and p-erk in the core and contralateral shell of each side of the SCN of split hamsters may be expressed in antiphase. While we were unable to find evidence of such regional differences in our film autoradiograms, heterogeneity of cell groups within the nucleus complicate attempts to determine whether peripheral asymmetry of gene expression is ipsilateral or contralateral to that in the SCN. Furthermore, descending projections that regulate peripheral clock gene expression may be partly crossed. Between 72 and 96 h after injecting pseudorabies virus into the left adrenal cortex, bilateral labeling is found in neurons of the intermediolateral column (9). Vrang et al. (51) observed significant contralateral cholera toxin-ir at the level of the paraventricular nucleus following unilateral SCN injection, although the largest population of labeled cells was ipsilateral. Thus, crossing of neural projections at any of a number of points between the pacemaker and the periphery may contribute to the reduced level of asymmetry below the level of the SCN. This may explain, in part, our observation that in the adrenal gland, skeletal muscle, and lung, mean asymmetries of clock gene expression levels were no greater than twofold. This is only about half of the amplitude of asymmetry in the SCN of hamsters that split in LL. In considering the amplitude of asymmetries in the periphery, it is important to keep in mind that our experiments assessed only total tissue mRNA content of peripheral organs. It is possible that rhythmicity of clock gene expression (and its asymmetry) varies between cell types within an organ (19). For example, we observed differences in the patterns of clock gene expression between the zones of the adrenal cortex (see results). In many peripheral organs, a striking asymmetry in one cell type may be difficult to detect if obscured by symmetrical gene expression in other types of cells in the same organ.
A fourth concern in the use of the split model is the action of constant light to suppress behavioral rhythms and oscillations of peptide expression in SCN (26). LL can compromise rhythmicity of clock gene expression in the SCN (37), and LL used in the present study may have had an influence on peripheral organs through such pathways. We were thus concerned that hamsters exposed to LL might experience a suppression of rhythms of clock gene expression in the periphery, which would make it difficult to assess neuronal control in split animals. It is difficult to compare fluctuations of clock gene expression sampled over a 12-h interval, in hamsters that split in LL, with those fluctuations in hamsters that free run in DD. Rhythmicity in LL hamsters was most easily assessed in liver, in which clock gene expression tended to be bilaterally symmetrical in split animals. Although significant differences in clock gene expression persisted over time, they generally appeared smaller than those we have observed in hamsters maintained in constant darkness (21, 49). Furthermore, the inverse relationship between haPer1/2 and haBmal1 expression characteristic of both entrained animals and those studied during DD free runs was weak or absent in hamsters that split in LL (Supplemental Fig. 1). Nevertheless, expression of one or more of the clock genes differed with phase in kidney, liver, and lung of the split animals. Comparisons between the experimental and control animals sampled at the same phase (3 h pAO) allow some assessment of effects of splitting as opposed to LL on peripheral gene expression: haPer1 mRNA was reduced, and haBmal1 mRNA elevated, in the kidney and lung of split animals compared with unsplit hamsters. For organs in which lateralization of clock gene expression was observed, the persistence of rhythmicity is difficult to assess in the absence of longitudinal measures. The absence of change with phase in clock gene expression levels in skeletal muscle, taken as the mean of the left and right sides, may result from the significant lateralization observed in H/L ratio of haPer1.
Finally, pathways for pacemaker control of behavior and peripheral rhythms likely diverge. Rhythmicity of locomotor activity is controlled in part by diffusible factors released from the SCN (44), while physiological rhythms require axonal projections (15, 35). A minimum number of SCN cells of particular phenotype must be intact to regulate activity bout characteristics (28). Variability in the duration and intensity of the evening vs. the morning bouts, although slight, may reflect an imbalance in the neurally driven signal. This imbalance of the lateralized temporal signal may be reflected in the level of asymmetry observed in the peripheral organs. Conversely, a more intense activity bout may provide a stronger systemic signal (e.g., higher levels of circulating hormones or greater increase in core body temperature) which results in a global synchronizing cue that lowers the level of laterality in gene expression of peripheral paired organs. This speculation is supported by the negative correlations between the H/L ratios of TH and haPer1 expression in the kidney at 3-h pAO with α intensity. The dissociated behavioral bouts of split hamsters are quite stable (Fig. 1) but it is difficult to assess whether lateralized SCN control over left or right peripheral organs remains consistent or how closely gene expression in the periphery reflects the phase and intensity of bouts of running activity.
It remains to be determined whether the twofold level of lateralization of clock gene expression we observed in some peripheral tissues is physiologically important. Clock-controlled gene expression in the retina (46), lung (19), liver, and pancreas (29, 36) constitutes an important level of physiological regulation. Disruption of normal phase relationships between the pacemaker and peripheral organs is believed to underlie decrements in physiological function during jet lag (58). Zone-specific lateralization (see results) indicates differential neural regulation of clock gene phase between layers of the adrenal cortex. Mineralocorticoid synthesis in the zona glomerulosa is disrupted in the absence of a functional molecular clock, resulting in excessive electrolyte and water retention (16). The role of neural input in control of the clock gene phase for coordination of the synthesis of mineralocorticoids, glucocorticoids, and/or androgens within the adrenal cortex warrants further investigation. Our finding of significant asymmetry in TH and Dbp expression in split hamsters is also provocative in this regard. Some clock-controlled genes appear to be directly regulated by the core circadian loop, as indicated by the presence of E-box motifs in their promoters (32). A majority of clock-controlled genes, however, are regulated only indirectly by protein products of the core clock genes. Instead, other transcription factors (including DBP and other members of the proline and acidic amino acid-rich family of basic leucine zipper transcription factors), may influence the degree of asymmetry of physiological functions in split animals. As an important relay between the core circadian clock and many physiologically significant proteins, DBP regulates circadian rhythms of expression of a variety of genes critical to hepatic (30, 55) and renal (62) function. Dbp seems a particularly sensitive indicator of neural drive, in that H/L and I/C ratios of up to fivefold were evident even in organs that showed only low degrees of asymmetry of clock gene expression. Lateralization of tyrosine hydroxylase expression in the adrenal medulla may also have important physiological consequences. Indeed, the diurnal pattern of tyrosine hydroxylase expression within chromaffin cells is likely due to an SCN signal rather than a local molecular clock (31). Medullary secretion of epinephrine into the vasculature would be expected to eliminate lateralization at the level of target tissues. Lateralized neural drive of other sympathetic ganglia, however, is likely to induce asymmetry of physiological functions.
In the future, use of reporter constructs (such as Per2::luc knockins) for in vitro studies may prove useful in assessing neural control of clock gene expression in peripheral organs collected from split animals (38). However, changing of the medium can reinstigate a damped rhythm in vitro (58). Furthermore, organs differ in their response to death and culture preparation (60), making it difficult to assess the phase relationship peripheral organs had established with the SCN prior to ex vivo preparation. Thus setting up the culture of paired organs from a split animal may influence the relative phase of the left and right side, complicating our ability to infer in vivo asymmetry from organ culture approaches. Although attempts to characterize clock gene expression within living animals in real time has met with limited success (25, 56), such an in vivo approach has not yet become available for studies of asymmetry in split animals.
Perspectives and Significance
The organization of the circadian system is revealed by its tendency to split in constant light, and this preparation is a tool to reveal neural, endocrine, and other pathways for hierarchical control of peripheral oscillators by the central pacemaker. The extent to which peripheral oscillators rely on neural pathways to receive timing information from the SCN differs between organs. The degree of neural influence on expression levels of specific components of the molecular clock (Per1 vs. Per2 vs. Bmal1) and on clock-controlled genes, such as Dbp may also differ and warrants further investigation. Coordination of physiological events is accomplished by temporal signals of the SCN that are conveyed to periphery by autonomic nervous, neuroendocrine, and behavioral effectors. Further work is needed to determine the extent of integration by peripheral tissues of humoral and neural signals that determine the phase of local molecular clocks.
GRANTS
This study was supported by National Institute of Mental Health Grant RO1-MH-070019.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the University of Massachusetts animal care personnel for their dedication to the animals used in this study, William J. Schwartz for his initial contributions to the development of these experiments and his critical comments regarding the data and manuscript, and Tanya Leise for discussion of the model depicted in Fig. 7.
REFERENCES
- 1.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916: 172–191, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540–550, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci 27: 4359–4365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartness TJ, Song CJ, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms 16: 196–204, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am J Physiol Regul Integr Comp Physiol 285: R561–R569, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 12: 1574–1583, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11: 1535–1544, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Buijs RM, La Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 464: 36–48, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pévet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci 22: 2531–2540, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Cailotto C, van Heijningen C, van der Vliet J, van der Plasse G, Habold C, Kalsbeek A, Pevet P, Buijs R. Daily rhythms in metabolic liver enzymes and plasma glucose require a balance in the autonomic output to the liver. Endocrinology 149: 1914–1925, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pévet P, Buijs RM. Effects of nocturnal light on (Clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS ONE 4: e5650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci 23: 10691–10702, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Iglesia HO, Meyer J, Carpino A, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290: 799–801, 2000 [DOI] [PubMed] [Google Scholar]
- 15.de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 23: 7412–7414, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanernatsu A, Ogawa O, Todo T, Tsutsui K, van der Horst G, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16: 67–74, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Fawcett DW. A Textbook of Histology (12th ed.). New York: Chapman and Hall, 1994 [Google Scholar]
- 18.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahshi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon ASI. Circadian timing in the lung: a specific role for bronchiolar epithelial cells. Endocrinology 150: 268–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA 102: 3111–3116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci 26: 6406–6412, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada T, LeSauter J, Venuti JM, Silver R. Expression of period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci 21: 7742–7750, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog ED, Huckfeldt RM. Circadian entrainment to temperature, but not light, in isolated suprachiasmatic nucleus. J Neurophysiol 90: 763–770, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Houben T, Deboer T, van Oosterhout F, Meijer JH. Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. J Biol Rhythms 24: 477–487, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2: 297–307, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Isobe Y, Nishino H. AVP rhythm in the suprachiasmatic nucleus in relation to locomotor activity under constant light. Peptides 19: 827–832, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol 5: e34, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegsfeld LJ, LeSauter J, Silver R. Targeted microlesions reveal novel organization of the hamster suprechiasmatic nucleus. J Neurosci 24: 2449–2457, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavery DJ, Schibler U. Circadian transcription of the cholesterol 7-α hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev 7: 1871–1884, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Lemos DR, Goodspeed L, Tonelli L, Antoch MP, Ojeda SR, Urbanski HF. Evidence for circadian regulation of activating transcription factor 5 but not tyrosine hydroxylase by the chromaffin cell clock. Endocrinology 148: 5811–5821, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Li R, Yue J, Zhang Y, Zhou L, Hao W, Yuan J, Qiang B, Ding JM, Peng X, Cao JM. CLOCK/BMAL1 regulates human nocturnin transcription through binding to the E-box of nocturnin promoter. Mol Cell Biochem 317: 169–177, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Lilley T, Wotus C, de la Iglesia HO. Circadian regulation of the cortisol release in behaviorally split golden hamsters (Abstract). Society for Research on Biological Rhythms: 12th Biennial Meeting, Destin, FL, May 2010, p. 139 [Google Scholar]
- 34.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140: 207–218, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Muhlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol 606: 61–71, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci 8: 267–269, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Ohta H, Mitchell AC, McMahon DG. Constant light disrupts the developing mouse biological clock. Pediatr Res 60: 304–308, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms 21: 350–361, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: V. Pacemaker structure: a clock for all seasons. J Comp Physiol 106: 333–355, 1976 [Google Scholar]
- 42.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol 27: 1694–1705, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Satinoff E, Kent S, Li H, Megirian D, Tomkowiak JM. Circadian rhythms of body temperature and drinking and responses to thermal challenge in rats after PCPA. Pharmacol Biochem Behav 38: 253–257, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382: 810–813, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell 130: 730–741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms 21: 494–506, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA 100: 6795–6800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong Y, Guo H, Brewer JM, Lee H, Lehman MN, Bittman EL. Expression of haPer1 and haBmal1 in Syrian hamsters: heterogeneity of transcripts and oscillations in the periphery. J Biol Rhythms 19: 113–125, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Ueyama T, Krout KE, Van Nguyen X, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 2: 1051–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Vrang N, Mikkelsen JD, Larsen PJ. Direct link from the suprachiasmatic nucleus to hypothalamic neurons projecting to the spinal cord: a combined tracing study using cholera toxin subunit B and Phaseolus vulgaris-leucoagglutinin. Brain Res Bull 44: 671–680, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Vujovic N, Davidson AJ, Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol 295: R355–R360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warren WS, Champney TH, Cassone VM. The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol Behav 55: 1091–1099, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology 145: 529–540, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Wuarin J, Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell 63: 1257–1266, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi S, Kobayashi M, Mitsui S, Ishida Y, van der Horst GT, Suzuki M, Shibata S, Okamura H. View of a mouse clock gene ticking (Abstract). Nature 409: 684, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol 430: 518–532, 2001 [PubMed] [Google Scholar]
- 58.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci 25: 9017–9026, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshikawa T, Yamazaki S, Menaker M. Effects of perparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms 20: 500–512, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.