Abstract
Interest in the pathophysiological relevance of intramuscular triacylglycerol (IMTG) accumulation has grown from numerous studies reporting that abnormally high glycerolipid levels in tissues of obese and diabetic subjects correlate negatively with glucose tolerance. Here, we used a hindlimb perfusion model to examine the impact of obesity and elevated IMTG levels on contraction-induced changes in skeletal muscle fuel metabolism. Comprehensive lipid profiling was performed on gastrocnemius muscles harvested from lean and obese Zucker rats immediately and 25 min after 15 min of one-legged electrically stimulated contraction compared with the contralateral control (rested) limbs. Predictably, IMTG content was grossly elevated in control muscles from obese rats compared with their lean counterparts. In muscles of obese (but not lean) rats, contraction resulted in marked hydrolysis of IMTG, which was then restored to near resting levels during 25 min of recovery. Despite dramatic phenotypical differences in contraction-induced IMTG turnover, muscle levels of diacylglycerol (DAG) and long-chain acyl-CoAs (LCACoA) were surprisingly similar between groups. Tissue profiles of acylcarnitine metabolites suggested that the surfeit of IMTG in obese rats fueled higher rates of fat oxidation relative to the lean group. Muscles of the obese rats had reduced lactate levels immediately following contraction and higher glycogen resynthesis during recovery, consistent with a lipid-associated glucose-sparing effect. Together, these findings suggest that contraction-induced mobilization of local lipid reserves in obese muscles promotes β-oxidation, while discouraging glucose utilization. Further studies are necessary to determine whether persistent oxidation of IMTG-derived fatty acids contributes to systemic glucose intolerance in other physiological settings.
Keywords: skeletal muscle, contraction, exercise, intramuscular lipids, acylcarnitines, obesity
numerous studies have shown that elevated intramuscular triacylglycerol (IMTG) levels correlate negatively with glucose tolerance (11, 19). IMTG accumulation in obese human subjects is often accompanied by high levels of diacylglycerol (DAG), ceramides, and long-chain acyl CoA (LCACoA) (10, 16–18). A similar phenotype occurs in many rodent models of insulin resistance, including genetic obesity and diabetes, as well as diet-induced weight gain (8, 26, 31). Although the IMTGs themselves are widely viewed as benign molecules, they can give rise to the foregoing lipid intermediates, which are thought to directly antagonize insulin signal transduction (28). These findings have fueled intense investigation into the mechanisms controlling IMTG synthesis and turnover, as well as the connection between lipid imbalance and insulin resistance. Metabolic disturbances that contribute to intramuscular lipid accumulation include increased total body and perimuscular adiposity, oversupply of dietary fat, enhanced fatty acid transport, and reduced fatty acid oxidation (2, 15, 18).
Paradoxically, IMTG levels are also increased by exercise training, and IMTG content in elite endurance athletes is similar to or even exceeds that measured in diabetic subjects (34). Additionally, the highly insulin-sensitive type 1 (oxidative) muscle fibers amass more IMTG than type II (glycolytic) fibers (35). These findings indicate that expansion of the IMTG pool is not necessarily pathological; and, moreover, suggest that these local fuel reserves can provide an important source of oxidative substrate for aerobically active muscles. Fitting with this possibility, several studies have demonstrated that IMTG undergo lipolysis during exercise (20, 33, 35, 36) and that habitual training increases utilization of IMTG during moderate-intensity activities (33). Using methodologies, such as indirect calorimetry combined with stable isotope tracers, biochemical and/or histological evaluation of muscle biopsies, and magnetic resonance spectroscopy, previous studies have estimated that IMTG account for ∼40% of the long-chain fatty acids utilized during an acute bout of exercise (33, 35). Considering that muscle contraction can increase rates of fatty acid oxidation by as a much as 10-fold (33, 35), this locally available pool of lipid fuel could play a key role in sustaining prolonged activity. Thus, in aggregate, IMTG appears to confer an advantage to conditioned muscles by functioning in a manner analogous to muscle glycogen.
Whereas strong evidence suggests that intramuscular lipid reserves play a role in fueling physical activity in trained athletes (33), few studies have investigated exercise-induced IMTG metabolism in the context of obesity. Thus, the question of whether or not muscle contraction enhances mobilization and oxidation of IMTG-derived fatty acids in obese animals remains unclear. One study reported that an acute swimming exercise (240 min) failed to reduce IMTG levels in both lean and obese Zucker rats (4). Conversely, treadmill running lowered IMTG content in rats fed a high-fat diet, as well as those pretreated with the diabetogenic drug, streptozotocin (30). Human studies in endurance-trained athletes and in patients with type 2 diabetes showed that higher preexercise IMTG levels correlated with larger decrements after acute exercise (29, 35), suggesting that use of local lipids during exercise occurs regardless of fitness or training level. Whereas this earlier work focused mainly on IMTG per se, the present investigation sought to relate contraction-induced mobilization of IMTG in lean and obese Zucker rats to more global changes in the local metabolic milieu. To this end, we used an in situ hindlimb perfusion model, which permitted comparisons of changes in intramuscular lipids between an electronically stimulated, contracted limb and a contralateral control (rested) limb. Importantly, this model obviates obesity-related perturbations in circulating factors such as metabolic substrates, hormones, cytokines, and adipokines, all of which are known to influence fuel availability and selection. Phenotype-specific metabolic responses occurring immediately after contraction and after a 25-min recovery period were evaluated using conventional lipid chemistry and mass spectrometry-based metabolic profiling. We hypothesized that increased availability, mobilization, and utilization of IMTG-derived fatty acids in muscle of obese compared with lean rats would be accompanied by glucose sparing during and after contractile activity.
MATERIALS AND METHODS
Animals
All experiments were approved by the Animal Care and Use Committee at East Carolina University. Male obese Zucker rats (fa/fa) (12 wk of age) and lean Zucker littermates were obtained from Harlan (Indianapolis, IN). Animals were maintained on a 12:12-h light-dark cycle, provided with ad libitum standard chow (Purina rodent chow) and water until the time of death. Animals were fasted the night before the experimental procedure (∼10 h). For all experiments, n = 6–8 animals were examined. The obese Zucker rats were significantly heavier than their lean littermates (479 ± 11 vs. 332 ± 7 g; P < 0.001). Skeletal muscle (total gastrocnemius) was taken at baseline, immediately after muscle contraction, and 25 min after muscle contraction to determine changes in the endogenous lipid (IMTG, DAG, and LCACoA) and glycogen stores of both lean and obese rats (Fig. 1). To control for the effects of perfusion, the same measures were made in skeletal muscle taken from the nonexercising leg (no treatment) of the 25-min postcontraction perfusion prep (Fig. 1). Changes in the glucose concentration of the perfusion media during and following muscle contraction were also measured to aid in determining differences in substrate utilization between phenotypes.
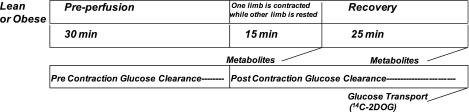
Fig. 1.
Both lean and obese Zucker rats underwent the following procedures. Preperfusion occurred for a period of 30 min followed by the contraction of one limb for 15 min via electrical stimulation. Metabolites were measured in muscle (gastrocnemius) removed from rats either immediately postcontraction or 25 min of recovery from contraction. To control for the effects of perfusion, the same measures were made in muscle taken from the nonexercising limb. Glucose clearance from the circulating perfusion media was also assessed separately during precontraction and postcontraction, and glucose transport was determined in muscle taken after 25 min of recovery using 14C 2-deoxyglucose (14C 2DOG).
Hindlimb Perfusions
Hindlimb perfusions were performed as described previously (5, 7, 31). In all perfusions, one limb was electrically stimulated to contract, and the other limb served as a noncontracted control as previously detailed (31). Following an overnight fast, rats were anesthetized intraperitoneally with ketamine/xylazine (10 mg/100 g body wt). The aorta and vena cave were ligated, and a hemicorpus preparation was created. The aorta and vena cava of the hemicorpus were quickly catheterized in succession and a prepared media [4% BSA, 100 mg/dl glucose, 33% washed bovine red blood cells and Krebs-Henseleit buffer (KHB)] was perfused at a rate of 18 ml/min, gassed with O2/CO2 (95:5) to maintain a Po2 of >90% saturation, and maintained at a chamber temperature of 37°C. Although the perfusion medium was not supplemented with fatty acids, we have found that a 4% solution of fraction IV BSA yields a fatty acid concentration of 100–150 μM in the medium. The first 50 ml of fluid collected from the perfusion prep was discarded, while the remaining 100 ml was recirculated. After a 30-min preperfusion period, one limb was stimulated to contract for a total of 15 min. The entire gastrocnemius skeletal muscle was excised immediately postcontraction or 25 min after contraction and snap frozen for the later analysis of intramuscular metabolites, including lipid content, acylcarnitine-profiling, pyruvate, lactate, and glycogen content (Timeline: Fig. 1). Gastrocnemius muscle was also taken for measurements at the same timepoints from the contralateral rested limb. After being snap frozen, the entire gastrocnemius was powdered with a pestle with liquid nitrogen, then mixed, and stored at −80°C, as performed previously (31). Media were sampled and measured for glucose concentration every 5 min throughout the perfusion. Small doses of 50% liquid dextrose was added when needed to maintain a media glucose concentration of ∼100 mg/dl (±5 mg/dl) throughout the experiment perfusion. Both the media glucose concentrations and the amount of 50% liquid dextrose added to the media were recorded prior to and after contraction and used to estimate the rate of glucose clearance from the circulating media.
Electrical Stimulation
During surgery, the sciatic nerve of one limb was isolated and connected to an electrode, and electrical stimulation was delivered by a Grass SD9 stimulator (West Warwick, RI), as done previously (31) The sciatic nerve was not severed for the procedure, rather the electrode was clamped onto the nerve. For the contraction treatment, skeletal muscle was stimulated for three 5-min periods separated by 1-min rest periods. Stimulation consisted of a double pulse per second frequency (duration of each pulse was 1 mS with a delay of 1 mS) delivered at 4 V. The double pulse is similar to the often-used train stimulation. Previous preliminary studies performed by our group have shown that the stimulation protocol had similar effects on muscle fatigue in lean and obese animals, as judged by tension curves (6).
Glucose Transport
Glucose transport was measured in gastrocnemius from the contracted limb and the contralateral control limb 25 min following the contraction. Radioactive label was added to the media at a final concentration of 20 mM sorbitol containing 0.1 μCi of [U-14C] sorbitol and 0.2 μCi of 2-deoxy-[3H]glucose. Tissue was removed immediately after perfusion and freeze clamped in liquid nitrogen. Powdered tissue (50–100 mg) was added to 0.4 ml of distilled water and then dispersed via heat (40°) and sonication, followed by the addition of 5 ml of liquid scintillation fluid, as performed previously (31). 3H/14C radioactivity (dpm) and glucose concentrations were analyzed at 5-min intervals and used to determine specific radioactivity. [14C] sorbitol was utilized to evaluate the amount of glucose in the interstitial space.
Western Blot Analysis
Serine67 phosphorylation of acetyl CoA carboxylase (ACC) and total ACC was assessed at rest and immediately following muscle contraction as reported previously (31). Powdered muscle was homogenized in buffer [50 mM HEPES, 50 mM Na pyrophosphate, 100 mM Na fluoride, 10 mM EDTA, 10 mM Na orthovanadate, 1% Triton X-100, and protease and phosphatase (1 and 2) inhibitor cocktails (Sigma, St. Louis, MO)] on ice. After centrifugation for 25 min at 15,000 g, supernatants were extracted and protein content was detected using a BCA protein assay (Pierce, Rockford, IL), separated by SDS-PAGE and then transferred to PVDF membranes for probing with antibodies. ACC-phosphoserine67, and ACC were purchased from Cell Signaling (Beverly, MA).
Malonyl CoA
Malonyl CoA levels in gastrocnemius skeletal muscle were measured as performed previously (1), as modified by procedures developed by McGarry et al. (24).
Intramuscular Lipids
Triacylglycerol and diacylglycerol.
Triacylglycerol and diacylglycerol were measured as reported previously (31). Briefly, powdered muscle (∼25 mg) was added to 3 ml of lipid extraction solution [(1:2) (vol/vol) methanol:chloroform containing 0.05 mg/ml BHT] in addition to IMTG and DAG internal standards [(IMTG: 1 mg Tri-C17:0/ml chloroform); DAG (0.1 mg of 1,30 dipentadecanoyl-glycerol/ml chloroform)]. The samples were vortexed and stored for 24 h at 4°C for extraction of lipids. The samples were then centrifuged at 3,500 rpm for 30 min at 4°C. The supernatant was extracted and dried under a flow of N2. IMTG and DAG were separated by TLC, the bands were scraped into individual tubes, and 50 μl hexane and 1 ml 14% BF3-CH3OH were added. Samples were then vortexed and heated at 100°C for 4 min followed by cooling on ice. One milliliter of double-distilled H2O and 2 ml hexane were added, and the solution was shaken for 15 min, then centrifuged for 15 min at 2,000 rpm. The upper phase containing the hexane and sample was extracted and dried, then brought up in 100 μl hexane for DAG and 200 μl for IMTG in preparation for analysis by GC. Both total IMTG and DAG were calculated as the addition of the following species: 14:0, 16:0, 16:1, 18:0, 18:1, 18:2, and 18:3.
Long-chain fatty acyl CoA.
Muscle LCACoA content was analyzed using HPLC/MS as referenced previously (31). Briefly, HPLC/MS analysis was performed with an Agilent 1100 series liquid chromatograph-1956B SL single-quadropole mass spectrometer (Agilent Technologies, Palo Alto, CA) with a binary gradient pump, heated column compartment, and electrospray ionization source with LC/MSD Chem-Station software Rev. A.10.02 (Agilent) controlling the system. The analytical column was composed of a reversed-phase Zorbax Extend-C18 2.1′ 150 mm, 5 mm with guard cartridge. All standard and tissue samples were analyzed in duplicate by LC/MS, and the average values were used for the calculations. Total LCACoA were calculated as the addition of the following species: 14:0, 16:0, 16:1, 18:0, 18:1, 18:2, and 18:3.
Fatty acylcarnitines.
Acylcarnitine esters were measured as previously described (21, 31). Briefly, 10 mg of powdered muscle were added to a 1 ml Potter-Elvejhem homogenizer containing 0.3 ml dd-H2O. After 20 strokes, samples were transferred to 1.5-ml tubes, sonicated at 2.5 W for 5 s, and derivatized. Acylcarnitines were measured by direct-injection electrospray tandem mass spectrometry, using a Micromass Quattro Micro system equipped with a model 2777 autosampler, a model 2777 autosampler, a model 1525 HPLC solvent delivery stem, and 4.0 MassLynx software (Waters, Milford, MA).
Intramuscular Pyruvate and Lactate Content
Lactate and pyruvate were quantified from cleared tissue extracts using 0.2 mM of 2H3-lactate (C/D/N Isotopes, Pointe-Claire, Quebec) and 13C3-pyruvate (Sigma Aldrich, St. Louis, MO) as internal standards. Trimethylsilyl derivatives of these analytes were separated on a Thermo Finnigan Trace GC Ultra and quantified by a Thermo Finnigan Trace DSQ MS operating under Excalibur 1.4 (Thermo Fisher Scientific, Austin, TX) employing stable isotope dilution as previously described (23).
Glycogen Content
Glycogen content was assessed in the muscle samples using a glucose reagent kit (Thermo Electron, Louisville, CO), as performed previously (31).
Statistical Analysis
Statistical changes were determined with a two-way type × treatment ANOVA. Follow-up statistical measures included an independent t-test for type comparisons and a Tukey post hoc test for type or treatment conditions, where appropriate. Univariate analyses were performed with the Statistical Package for the Social Sciences (v. 11.0, SPSS, Chicago, IL). Multivariate pairwise correlations and unsupervised cluster analysis were performed using JMP version 8.0 (SAS Institute, Cary, NC). Data are presented as means ± SE, and P values of <0.05 were considered significant.
RESULTS
Changes in Circulating Glucose Clearance, Glucose Transport Rates, and Muscle Glycogen
The rate of glucose clearance from the perfusion medium was greater in the lean than obese rats, both prior to (P = 0.001) and after the initiation of muscle contraction (P = 0.002) (Fig. 2), suggesting that skeletal muscle glucose utilization is diminished in the obese state. Because all perfusions were performed with one rested leg and a contralateral contracted limb, we could not determine the rate of glucose clearance across the entire hindlimb preparation in the absence of muscle contraction (Fig. 1). Interestingly, a negative glucose clearance rate was measured in some of the obese hindlimb preparations prior to muscle contraction. This likely occurred because the obese rats were hyperglycemic (glucose >200–300 mg/dl) before being perfused with a media with a glucose concentration of 100 mg/dl. Therefore, it is likely that elevated glucose levels in the interstitial space of the obese Zucker rats was released into the perfusion media, causing an increase in media glucose concentrations, which resulted in negative glucose clearance values. This only occurred in a few of the obese Zucker rats prior to muscle contraction, but importantly, it shows that perfusion media concentrations were different than what would be circulating in normal in vivo conditions of the obese Zucker rat.
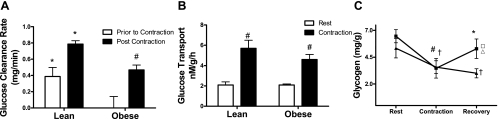
Fig. 2.
Glucose clearance rates (A) from the circulating media were measured in the period prior to and after muscle contraction. *Lean vs. obese; #contraction-induced change. B: glucose transport rates measured by 14C 2DOG uptake. #Contraction-induced change. C: changes in muscle glycogen were measured in whole gastrocnemius muscle of (▴) lean and (■) obese Zucker rats in resting conditions, immediately after contraction and after 25 min of recovery. †,#Contraction-induced change in both groups. *Lean vs. obese. Metabolites measured in the rested (no contraction) contralateral limbs at the 25-min recovery time point are displayed to show the effects of perfusion on both (▵) lean and (□) obese groups.
Assessment of radiolabeled 2-deoxyglycose uptake into each limb (Fig. 2B) revealed higher rates of glucose transport in contracted muscles but no difference between the lean and obese groups. This finding suggests that the low glucose clearance rates in the obese group were secondary to differences in glucose metabolism rather than deficits in glucose transport and/or hexokinase activity. Contraction-induced lowering of muscle glycogen was similar between groups; 33% and 46% in the gastrocnemius muscle of both lean and obese groups, respectively (Fig. 2C). However, during the 25-min recovery period glycogen resynthesis occurred only in the obese group (Fig. 3A); whereas conversely, muscle glycogen content in the lean group continued to decline. Glycogen content was unchanged in the perfusion/rested control limbs of both groups.
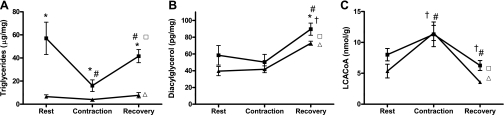
Fig. 3.
Changes in IMTG, DAG, LCACoA, and glycogen were measured in whole gastrocnemius muscle of (▴) lean and (■) obese Zucker rats in resting conditions, immediately after contraction, and after 25 min of recovery. A: *Obese vs. lean; #contraction-induced change. B: †,#Elevated after 25 min of recovery. C: †Contraction-induced change. Metabolites measured in the rested (no contraction) contralateral limbs at the 25-min recovery time point are displayed to show the effects of perfusion on both lean (Δ) and obese (□) groups. Significance was set at P < 0.05; values are expressed as means ± SE.
Intramuscular Lipids
Intramuscular triacylglycerol content was higher in the obese than the lean group at all time points. Contraction caused a robust 68% (P = 0.013) reduction in IMTG in the obese rats (Fig. 3A). A more modest, nonsignificant reduction (−39%, P = 0.28) was observed in the lean group. During the 25-min recovery period, IMTG content in the obese muscles increased 156%, nearly returning to baseline levels (Fig. 3A). A similar recovery to baseline occurred in the lean group. Thus, contraction-induced lipolysis was apparently followed by rapid reesterification of the hydrolyzed fatty acids. IMTG content in the rested control limbs was similar between the baseline and 25-min recovery time points.
In both the lean and obese groups, total intramuscular DAG content was unchanged immediately after contraction. By contrast, DAG levels increased 72–78% during the recovery period compared with the baseline condition, regardless of group (Fig. 3B), suggesting that this metabolite was affected by the perfusion conditions. Because this specific lipid molecule is derived only from intracellular sources, we speculate that the perfusion conditions activated adipose triglyceride lipase or perhaps a phospholipase, without eliciting a parallel activation of hormone-sensitive lipase, thereby resulting in the generation and accumulation of DAG.
Measures of LCACoAs provided further evidence of IMTG lipolysis and reesterification. Immediately after contraction total intramuscular LCACoAs were increased in both groups (Fig. 3C). Surprisingly, the relative change was actually more robust in the lean (115%; P = 0.003) than the obese muscles (41%; P = 0.146), and peak LCACoAs were similar between groups despite the marked hydrolysis of IMTG in the obese rats. During the recovery period LCACoA content was restored to baseline levels in both groups, indicating these lipid molecules were quickly utilized for mitochondrial metabolism and/or reesterification. LCACoA levels measured in the resting, contralateral legs of both lean and obese rats were similar to the baseline measures.
Acetyl CoA Carboxylase and Malonyl CoA
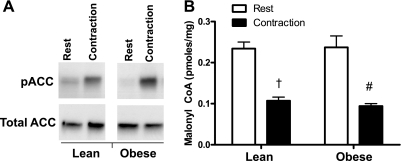
Exercise is known to promote fat oxidation by inactivating (phosphorylating) acetyl-CoA carboxylase (ACC) and lowering its product, malonyl-CoA, which functions as a negative regulator of carnitine palmitoyltransferase I (CPT-1). Phosphorylation of ACC on serine67 was dramatically increased immediately following muscle contraction in both groups (Fig. 4A). The resulting inhibition of ACC activity was evidenced by a greater than 50% reduction in muscle malonyl-CoA content, which likewise occurred in both groups (Fig. 4B). Thus, contraction-induced changes in phosphorylated ACC and malonyl-CoA content favored increased CPT-1 activity and LCACoA entry into the mitochondria, regardless of obesity. Malonyl CoA content was not measured in muscles after 25 min of recovery.
Fig. 4.
Acetyl, CoA carboxylase (ACC) phosphorylation and malonyl CoA content was measured in whole gastrocnemius muscle in a rested limb and in the contracted limb immediately after contraction. A: ACC phosphorylation67 was increased significantly by contraction in both groups. †,#Contraction-induced changes.
Acylcarnitines
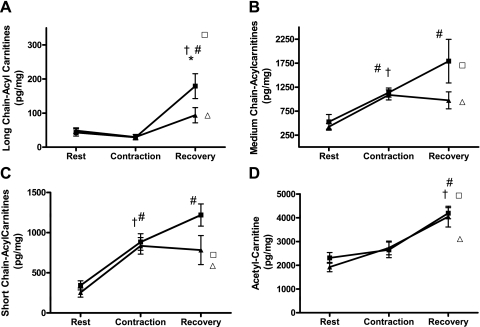
We surmised that some of the LCACoAs generated during lipolysis of IMTG were used as substrates for mitochondrial β-oxidation. To gain insight into changes in mitochondrial energy metabolism, we used mass spectrometry to profile acylcarnitine intermediates of various chain lengths. These metabolites are byproducts of fuel degradation that reflect shifts in substrate availability and/or flux limitations at specific catabolic enzymes. LC acylcarnitines accumulate when their production by mitochondrial CPT-1 exceeds flux through β-oxidation enzymes, such as long-chain acyl-CoA dehydrogenase and/or β-OH-acyl-CoA dehydrogenase. Despite the robust increase in LCACoA immediately after contraction, which was accompanied by diminished malonyl-CoA levels and a presumptive increase in CPT-1 activity, LC acylcarnitines trended downward; suggesting that metabolic flux through the early steps of β-oxidation was uninhibited at this time point (Fig. 5A). By contrast, LC acylcarnitines increased dramatically during the recovery period. In the lean group, LC acylcarnitine content at 25 min after recovery was comparable to that in the rested/contralateral control muscles. In the obese group, the rise in LC acylcarnitines during the recovery period was more robust than that observed in their lean counterparts (522% in obese vs. 217% in lean); however, contraction lowered these metabolites compared with the rested controls. Taken together with the results shown in Fig. 3, these findings imply that the high lipid content of obese muscles fueled an increase in substrate delivery to CPT-1. It is yet unclear whether accumulation of LC acylcarnitines in the rested muscles reflected local glycerolipid hydrolysis that occurred in response to the hindlimb perfusion; or alternatively, acylcarnitine efflux from the contracted muscles, as previous studies have shown plasma acylcarnitines to increase during high-intensity exercise (12). In support of the latter possibility, the six-fold increase in LC acylcarnitines in the rested muscle of the obese rats paralleled the dramatic contraction-induced drop in IMTG content in the contralateral limb. However, we were unable to confirm the presumed increase in acylcarnitine efflux because measurements of these metabolites in the perfusion medium were not obtained.
Fig. 5.
Acyl-carnitine metabolites were measured in whole gastrocnemius muscle of lean (▴) and obese (■) Zucker rats in resting conditions, immediately after contraction, and after 25 min of recovery. A: †,#Increased after recovery. *Obese vs. lean. B: †,#Contraction-induced change increased (#) during recovery. C: †,#Contraction-induced change continued to increase (#) during recovery period. D: †,#Increased during the 25-min recovery period. Metabolites measured in the rested (no contraction) contralateral limbs at the 25-min recovery time point are displayed to show the effects of perfusion on both lean (▵) and obese (□) groups. Both long- and medium-chain acylcarnitines increased in the rested limb of the obese but not the lean group. Significance was set at P < 0.05; Values are expressed as means ± SE. Metabolites measured in the rested (no contraction) contralateral limbs at the 25-min recovery time point are displayed to show the effects of perfusion on both lean (▵) and obese (□) groups. Significance was set at P < 0.05; values are expressed as means ± SE.
In both the lean and obese groups, total short- and medium-chain acylcarnitines (SC and MC acylcarnitines) were increased two- to threefold immediately after contraction (Fig. 5, B and C). During the 25-min postcontraction recovery period concentrations of short- and medium-chain acylcarnitines remained constant in the lean muscles, but continued to increase in the obese group. At the 25-min time point, MC acylcarnitines were similar between the contracted and rested muscles, whereas contraction increased SC acylcarnitines, but only in the obese group. Muscle concentrations of acetylcarnitine (C2), derived from mitochondrial acetyl-CoA, were initially similar between the lean and obese muscles and remained unchanged immediately after contraction, suggesting that production of acetyl-CoA did not exceed flux into the TCA cycle (Fig. 5D). By contrast, C2 levels rose ∼60% in both groups during the recovery period. A similar increase was observed in the rested control muscles, such that C2 levels at the 25-min time point were increased by 48% and 58% in the lean and obese groups, respectively, compared with the baseline measures. Here, again, this metabolite might have been produced locally and/or could have originated in the contracted limb.
Contraction increased the ratio of SC relative to LC acylcarnitines (SC/LC) in both groups (seven-fold in lean and three-fold in obese) (Table 1). This outcome, which was most pronounced immediately after contraction, suggests a shift in flux limitation from the early to the latter steps of β-oxidation. In addition, the ratio of acetyl carnitine to SC acylcarnitines (C2/SC) (Table 1) decreased immediately following contraction, suggesting that increased mitochondrial substrate catabolism was accompanied by a high rate of acetyl CoA entry into the TCA cycle.
Table 1.
Changes in the endogenous lipid and glycogen stores of both lean and obese rats
| Lean |
Obese |
|||||
|---|---|---|---|---|---|---|
| Basal | Contraction | 25 min Postcontraction | Basal | Contraction | 25 min Postcontraction | |
| β-oxidative flux | ||||||
| Short chain/long chain | 5.0 ± 0.4 | 38.1 ± 9.4 | 16.4 ± 0.8 | 9.3 ± 2.5 | 27.1 ± 1.1 | 7.4 ± 1.0 |
| Entry into TCA | ||||||
| Acetyl (C2)/Short chain | 9.4 ± 1.9 | 3.4 ± 0.3 | 3.2 ± 0.6 | 11.09 ± 3.3 | 3.1 ± 0.5 | 3.6 ± 0.5 |
Pyruvate and Lactate
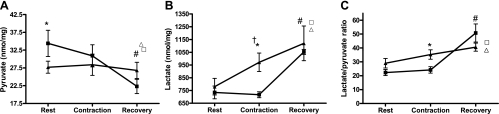
We next measured intramuscular pyruvate and lactate levels as markers of glycolytic metabolism. At baseline, pyruvate levels were greater in the obese than the lean state, whereas lactate content was similar between groups (Fig. 6B). Immediately after contraction, lactate levels increased 24% in the lean muscles but were unchanged in the obese state, implying that the latter group was less dependent on glycolytic ATP production (Fig. 6A). However, after 25 min of recovery, lactate levels rose to similarly high levels in all groups, including the rested control muscles, suggesting that the perfusion conditions promoted glycolytic metabolism and/or that lactate released by the contracted muscles was delivered to the contralateral muscle. The latter possibility was supported by the finding that lactate levels gradually increased in perfusate throughout the duration of the experiment (data not shown). In the lean muscles, pyruvate concentrations remained stable throughout the experiment, whereas in the obese group, pyruvate dropped dramatically during the recovery period. Likewise, the intramuscular lactate-pyruvate ratio was lower in the obese group immediately after contraction, but rose markedly during the recovery period.
Fig. 6.
Changes in pyruvate and lactate were measured in whole gastrocnemius muscle of lean (▴) and obese (■) Zucker rats in resting conditions, immediately after contraction, and after 25 min of recovery. A: *Obese vs. lean difference; #contraction-induced change. B: †Contraction-induced change increased (#) during recovery; *Lean vs. obese difference. C: *Lean vs. obese difference. Metabolites measured in the rested (no contraction) contralateral limbs at the 25-min recovery time point are displayed to show the effects of perfusion on both lean (▵) and obese (□) groups. Metabolites measured in the rested (no contraction) contralateral limbs at the 25-min recovery time point are displayed to show the effects of perfusion on both lean (▵) and obese (□) groups. Significance was set at P < 0.05; values are expressed as means ± SE.
Relationship Between IMTG and Muscle Glycogen
Lastly, we questioned why postcontraction muscle glycogen resynthesis occurred in the obese but not lean muscles (Fig. 2C), despite lower rates of glucose clearance (Fig. 2A). To evaluate interactions between glycogen and other metabolites, we generated a multivariate pairwise correlation matrix of the 25-min recovery time point, followed by an unsupervised cluster analysis. Interestingly, we identified a large cluster of lipid metabolites that correlated positively with muscle glycogen content, including total IMTG (R = 0.84, P < 0.002) and oleolycarnitine (C18:1) (R = 0.80, P < 0.006) (Table 2).
Table 2.
Multivariate cluster analysis of lipid metabolites that correlated positively with muscle glycogen levels 25 min postrecovery
| Variable | R | Lower 95% CI | Upper 95% CI | P |
|---|---|---|---|---|
| Total TAG | 0.838 | 0.441 | 0.961 | 0.002 |
| TAG C16:1 | 0.834 | 0.430 | 0.960 | 0.003 |
| TAG C18:1 | 0.832 | 0.425 | 0.959 | 0.003 |
| TAG C16:0 | 0.813 | 0.376 | 0.954 | 0.004 |
| DAG C16:1 | 0.811 | 0.318 | 0.959 | 0.008 |
| LCAC C18:1 | 0.796 | 0.333 | 0.950 | 0.006 |
| DAG C18:1 | 0.784 | 0.249 | 0.952 | 0.012 |
| LCAC C18:2 | 0.784 | 0.304 | 0.946 | 0.007 |
| LC-CoA 14 | 0.765 | 0.260 | 0.941 | 0.010 |
| Total DAG | 0.762 | 0.198 | 0.947 | 0.017 |
| LC-CoA C16:1 | 0.758 | 0.245 | 0.939 | 0.011 |
| LCAC C14:2 | 0.757 | 0.243 | 0.939 | 0.011 |
| LC-CoA C18:1 | 0.708 | 0.142 | 0.925 | 0.022 |
| Total LC-CoA | 0.708 | 0.141 | 0.925 | 0.022 |
| TAG C18:0 | 0.703 | 0.132 | 0.924 | 0.023 |
| LCAC C14:1 | 0.697 | 0.119 | 0.922 | 0.025 |
| Total LCAC | 0.654 | 0.042 | 0.909 | 0.040 |
| MCAC C10:1 | 0.650 | 0.034 | 0.908 | 0.042 |
| LC-CoA C16 | 0.642 | 0.021 | 0.906 | 0.045 |
DAG; diacylglycerol; TAG, triacylglycerol; LCAC, long-chain acylcarnitine; LC-CoA, long-chain acyl-CoA; CI, confidence interval. C(n) refers to the species-specific subset of each lipid class comprising the indicated chain length and degree of saturation.
DISCUSSION
Whereas IMTG accumulation is well recognized as a key feature of obesity, the impact of this specific lipid reservoir on skeletal muscle substrate selection is incompletely understood. The present study combined an isolated hindlimb perfusion preparation with comprehensive metabolic profiling to examine exercise-induced hydrolysis, catabolism, and recycling of intramuscular lipids in lean and obese rats. Although this approach does not provide definitive measures of metabolic flux, it does yield a comprehensive snapshot of intermediary metabolism that can be used to infer changes in mitochondrial selection and degradation of specific carbon substrates. Importantly, the longitudinal aspect of the study design allowed us to track metabolic events occurring immediately and 25 min after contractile activity. Electrically stimulated contraction caused profound depletion of IMTG in the obese rats, which corresponded with an increase LCACoAs. Surprisingly, however, LCACoAs, DAG, and total acylcarnitines measured immediately after contraction were similar between the lean and obese muscles. In both groups, short- and medium-chain acyl-carnitines were increased at the postcontraction time point, whereas LC acylcarnitines and acetyl-carnitine remained low. This profile is in accord with the idea that flux limitations during contraction occur at the medium- and short-chain acyl-CoA dehydrogenases. These findings also raise intriguing questions about the immediate fate of the fatty acids released from the large IMTG pool in skeletal muscles of obese rats. Perhaps many of the extra LCACoA molecules generated during contraction were used as mitochondrial substrates, but without causing increased accumulation of CoA and carnitine intermediates. This interpretation fits with the glucose-sparing effect implied by the low glucose clearance and enhanced glycogen resynthesis evident in obese compared with lean muscles. Obesity-related alterations in substrate selection were also implicit in the measurement of skeletal muscle pyruvate and lactate levels. For example, baseline pyruvate levels were higher in obese compared with the lean muscles, consistent with reduced activity of pyruvate dehydrogenase. Additionally, muscle lactate levels measured immediately after contraction were 21% lower in obese compared with lean rats, adding further evidence that the obese muscles were less dependent on glycolytic metabolism.
In contrast to the metabolic profile observed immediately after contraction, the recovery period was characterized by robust resynthesis of IMTG, marked increases in LC acylcarnitines and modest elevations in most short and medium carnitine esters. Thus, as ATP demand declined, LCACoAs were rapidly repackaged back into lipid droplets. It also appeared that substrate supply to CPT1 exceeded flux through the distal arms of the β-oxidation pathway, suggested by the robust rise in LC acylcarnitines. Although observed in both groups, accumulation of acylcarnitines was more pronounced with obesity, particularly in the rested limbs, which reflected a condition of high lipid availability but low ATP turnover. Also noteworthy is that glycogen resynthesis during recovery occurred only in the obese state, which correlated positively with intramuscular levels of several lipid metabolites, including IMTG and LC acylcarnitines. The dramatic rise in IMTG and LC acylcarnitine levels during the recovery phase in the obese group indicates that long-chain acyl-CoAs were available to the glycerolipid biosynthetic enzymes, as well as CPT1 . Thus, presuming that IMTG content at this time point reflects fatty acid availability during recovery, these findings support the notion that high intramuscular lipid levels promote continued β-oxidation after exercise, which might, in turn, permit glucose sparing and more rapid glycogen resynthesis. Interestingly, in the obese group, the recovery period also encompassed a dramatic upward shift in the lactate/pyruvate ratio, due both to an increase in lactate and a fall in pyruvate. Although the biochemical underpinnings of this shift remain unclear, the reciprocal regulation of these two metabolites implies a corresponding change in redox state that favors net forward flux through lactate dehydrogenase.
The leptin receptor defect in the obese Zucker rats renders these animals hyperphagic with extreme hyperlipidemia, which not only increases systemic fat supply but also enhances muscle capacity for fatty acid transport (22, 32). As a result, skeletal muscles from obese Zucker rats have massively elevated IMTG levels that are accompanied by increased activity of the mitochondrial enzymes and enhanced capacity for fat oxidation (13, 32). In the present study, glucose clearance rates from the medium were lower in the obese compared with lean hindlimb preparations, both before and after the initiation of muscle contraction. This was observed in the absence of insulin administration, implicating factors other than insulin resistance. Likewise, the finding that glucose clearance but not 2-deoxyglucose uptake was diminished by obesity implies metabolic regulation at a step downstream of hexokinase. Taken together with the metabolite profiles, these results strongly imply that obesity lowers contraction-induced glucose utilization while favoring oxidation of IMTG-derived fatty acids.
In the context of this discussion, it is necessary to consider some important caveats of the hindlimb perfusion system. First, the fiber recruitment pattern and contraction force elicited by electrical stimulation are distinct from that which occurs during moderate-intensity exercise. Thus, 15 min of electrically stimulated contraction does not mimic 15 min of exercise, but it might resemble more prolonged sessions of intermittent, high-intensity exercise. Herein, contraction caused robust depletion of IMTG and glycogen. The ATP-producing potential of the fatty acids and glucose generated from these fuel reserves appears to have exceeded the estimated costs of the contractile activity, suggesting that IMTG and glycogen breakdown during exercise is not tightly coupled to energy demands. This observation, which is consistent with another study showing that intramuscular accumulation of acetyl-CoA and LCACoA occurred in excess of energy requirements during exercise (36) leads us to question the fate of the excess fuel. In the obese group, a substantial portion of the hydrolyzed triacylglycerol and glycogen molecules appear to be resynthesized within 25 min of recovery. Additionally, we reason that some glucose- and fatty acid-derived carbons that were not used to support muscle contraction might have been processed to, and/or exported as, metabolic intermediates such as pyruvate, alanine, lactate, and acylcarnitines. Indeed, previous work has shown that acylcarnitines are readily exported from skeletal muscle during conditions of increased fuel supply (25). These intermediates, along with lactate and alanine, provide a mechanism whereby excess carbons derived from glycogen and lipid breakdown are rerouted to the liver and/or other organs. Accordingly, it seems feasible that some of the substrate coming from degradation of intracellular fuel reserves is exported from the muscle rather than being converted into ATP locally.
Additionally, in the present study, the perfusate supplied only a low level of circulating fatty acids, thereby necessitating increased use of glucose, glycogen, or other endogenously available fuels. Thus, the dramatic hydrolysis of IMTG observed in the perfusion model might not apply to a bona fide exercise challenge in which adipocyte lipolysis and plasma free fatty acids are elevated. Nonetheless, our findings are consistent with previous studies in humans. For example, increased reliance on IMTG has been observed in exercising obese compared with nonobese subjects (9). In this report, which combined indirect calorimetry with stable isotope tracer methods, estimated IMTG use during exercise was 50% higher in obese men compared with age- and fitness-matched nonobese control subjects (9). A similar but more striking outcome was observed in obese women, who used three-fold more muscle IMTG during exercise than the nonobese controls (14). Although elevated IMTG use by obese subjects might simply reflect a mass action effect, results obtained by Braun et al. (3) implied otherwise, as insulin-resistant women used less carbohydrate as a fuel source during low-intensity exercise than insulin-sensitive women matched for body mass index and body composition. Taken together, these investigations support the notion that the relative contribution of blood glucose and muscle glycogen to the overall energy cost of exercise is lower in obese and/or insulin-resistant subjects compared with nonobese healthy controls (3, 9, 14).
Perspectives and Significance
To our knowledge, this study represents the first comprehensive analysis of contraction-induced changes in skeletal muscle lipid metabolites comparing lean and obese conditions. Our findings show that obesity is associated with enhanced IMTG turnover and diminished use of circulating glucose and muscle glycogen during and after muscle contraction. Thus, the rich local lipid supply in the obese state appears to discourage glucose utilization by providing an alternative oxidative substrate. These results extend the concepts originally proposed by Randlev et al. (27) and suggest that large reservoirs of IMTG impact fuel selection during and immediately after exercise. This study also underscores the importance of accounting for IMTG-derived fatty acids when estimating skeletal muscle lipid oxidation using radiolabeled or stable isotope tracers. Further work is needed to translate these findings to a more physiological model of exercise and to better understand whether or not persistent oxidation of IMTG-derived fatty acids contributes to glucose intolerance at the whole body level.
GRANTS
This work was funded by National Institutes of Health (NIH) DK 46121 (to G. L. Dohm), NIH DK56112 and the American Diabetes Association (to D. M. Muoio) and a VA CD Award (to J. P. Thyfault). Part of this work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1.Bell JA, Volpi E, Fujita S, Cadenas JG, Rasmussen BB. Dysregulation of muscle fatty acid metabolism in type 2 diabetes is independent of malonyl-CoA. Diabetologia 49: 2144–2152, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Braun B, Sharoff C, Chipkin SR, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J Appl Physiol 97: 991–997, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bruce CR, Lee JS, Hawley JA. Postexercise muscle glycogen resynthesis in obese insulin-resistant Zucker rats. J Appl Physiol 91: 1512–1519, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Dohm GL, Kasperek GJ, Tapscott EB, Beecher GR. Effect of exercise on synthesis and degradation of muscle protein. Biochem J 188: 255–262, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolan PL, Boyd SG, Dohm GL. Differential effect of maturation on insulin- vs. contraction-stimulated glucose transport in Zucker rats. Am J Physiol Endocrinol Metab 268: E1154–E1160, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Dolan PL, Tapscott EB, Dorton PJ, Dohm GL. Contractile activity restores insulin responsiveness in skeletal muscle of obese Zucker rats. Biochem J 289: 423–426, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franch J, Knudsen J, Ellis BA, Pedersen PK, Cooney GJ, Jensen J. Acyl-CoA binding protein expression is fiber type- specific and elevated in muscles from the obese insulin-resistant Zucker rat. Diabetes 51: 449–454, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res 10: 575–584, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 51: 144–151, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 178: 373–383, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hiatt WR, Regensteiner JG, Wolfel EE, Ruff L, Brass EP. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. J Clin Invest 84: 1167–1173, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway GP, Benton CR, Mullen KL, Yoshida Y, Snook LA, Han XX, Glatz JF, Luiken JJ, Lally J, Dyck DJ, Bonen A. In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. Am J Physiol Endocrinol Metab 296: E738–E747, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz JF, Klein S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. J Appl Physiol 89: 2276–2282, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol 294: R1111–R1116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houmard JA, Tanner CJ, Yu C, Cunningham PG, Pories WJ, MacDonald KG, Shulman GI. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes 51: 2959–2963, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2: 251–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284: E741–E747, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hulver MW, Lynis Dohm G. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc 63: 375–380, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86: 205–243, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Luiken JJ, Arumugam Y, Dyck DJ, Bell RC, Pelsers MM, Turcotte LP, Tandon NN, Glatz JF, Bonen A. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem 276: 40567–40573, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Makowski L, Noland RC, Koves TR, Xing W, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Muoio DM. Metabolic profiling of PPARα−/− mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. FASEB J 23: 586–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarry JD, Stark MJ, Foster DW. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem 253: 8291–8293, 1978 [PubMed] [Google Scholar]
- 25.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284: 22840–22852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakes ND, Bell KS, Furler SM, Camilleri S, Saha AK, Ruderman NB, Chisholm DJ, Kraegen EW. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes 46: 2022–2028, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963 [DOI] [PubMed] [Google Scholar]
- 28.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Standl E, Lotz N, Dexel T, Janka HU, Kolb HJ. Muscle triglycerides in diabetic subjects. Effect of insulin deficiency and exercise. Diabetologia 18: 463–469, 1980 [DOI] [PubMed] [Google Scholar]
- 30.Straczkowski M, Kowalska I, Gorski J, Kinalska I. The effect of a single bout of exhaustive exercise on muscle carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus. Acta Diabetol 37: 47–53, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol 292: C729–C739, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Turcotte LP, Swenberger JR, Zavitz Tucker M, Yee AJ. Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats. Diabetes 50: 1389–1396, 2001 [DOI] [PubMed] [Google Scholar]
- 33.van Loon LJ. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol 97: 1170–1187, 2004 [DOI] [PubMed] [Google Scholar]
- 34.van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflügers Arch 451: 606–616, 2006 [DOI] [PubMed] [Google Scholar]
- 35.van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553: 611–625, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol 541: 969–978, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.