Abstract
Nerve-released ACh is the main stimulus for contraction of urinary bladder smooth muscle (UBSM). Here, the mechanisms by which ACh contracts UBSM are explored by determining Ca2+ and electrical signals induced by nerve-released ACh. Photolysis of caged inositol 1,4,5-trisphosphate (IP3) evoked Ca2+ release from the sarcoplasmic reticulum. Electrical field stimulation (20 Hz) induced Ca2+ waves within the smooth muscle that were present only during stimulus application. Ca2+ waves were blocked by inhibition of muscarinic ACh receptors (mAChRs) with atropine and depletion of sarcoplasmic reticulum Ca2+ stores with cyclopiazonic acid (CPA), and therefore likely reflect activation of IP3 receptors (IP3Rs). Electrical field stimulation also increased excitability to induce action potentials (APs) that were accompanied by Ca2+ flashes, reflecting Ca2+ entry through voltage-dependent Ca2+ channels (VDCCs) during the action potential. The evoked Ca2+ flashes and APs occurred as a burst with a lag time of ∼1.5 s after onset of stimulation. They were not inhibited by blocking IP3-mediated Ca2+ waves, but by blockers of mAChRs (atropine) and VDCCs (diltiazem). Nerve-evoked contractions of UBSM strips were greatly reduced by blocking VDCCs, but not by preventing IP3-mediated Ca2+ signaling with cyclopiazonic acid or inhibition of PLC with U73122. These results indicate that ACh released from nerve varicosities induces IP3-mediated Ca2+ waves during stimulation; but contrary to expectations, these signals do not appear to participate in contraction. In addition, our data provide compelling evidence that UBSM contractions evoked by nerve-released ACh depend on increased excitability and the resultant Ca2+ entry through VDCCs during APs.
Keywords: muscarinic receptor, sarcoplasmic reticulum, IP3 receptor, voltage-dependent calcium channel
the urinary bladder serves two functions: storage and voiding of urine. Micturition (the elimination of urine) is triggered by the action of ATP and ACh, which are released from parasympathetic nerves, on purinergic receptors (P2X1Rs) and muscarinic ACh receptors (mAChRs), respectively (2, 20, 44). The relative contribution of each pathway to contraction depends on stimulation frequency and varies by species, but the muscarinic pathway is a major mediator of micturition in all species (2). In humans, mAChRs predominantly mediate micturition under normal conditions, but purinergic mechanisms become prominent under pathological conditions, such as overactive bladder (33, 38) or interstitial cystitis (34).
The prevailing view is that ACh contracts urinary bladder smooth muscle (UBSM) by inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release from the sarcoplasmic reticulum (SR) through activation of M3 mAChRs (3, 12). M3 mAChRs canonically couple to the G protein Gq, which activates PLC to produce IP3 (8). Stimulation of M3 mAChRs has been shown to activate IP3 production (32, 40) and to promote contraction (26, 28). IP3-mediated release of Ca2+ from the SR has been demonstrated by photolysis of caged IP3 in isolated colonic smooth muscle cells (29). However, a causal link between IP3-mediated Ca2+ release and nerve-evoked contraction under physiological conditions has not been established in UBSM.
On the other hand, there is evidence that an increase in excitability and consequent Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs) during action potentials (APs) are important for contraction. Creed et al. (5) have shown that ACh increases AP frequency in rabbit UBSM. In guinea pig, nerve-stimulation (with purinergic receptors disabled) causes a transient increase in AP frequency (14). In accord with these data, disabling VDCCs greatly reduces contractile force induced by muscarinic agonists (43, 47) or nerve stimulation (21). Hence, the mechanisms by which mAChRs increase intracellular Ca2+ to subsequently cause contraction are not fully resolved.
In the present study, we investigated the elementary signaling events evoked by nerve-released ACh and their contribution to contraction. Because bath application of muscarinic agonists lacks the spatial and temporal aspects of normal bladder physiology, we used electrical field stimulation (EFS) to evoke release of ACh from parasympathetic nerve fibers in UBSM strips and thereby physiological delivery of ACh to the smooth muscle cells. High-speed confocal Ca2+ imaging was used to investigate the Ca2+ events triggered by nerve-released ACh, and revealed two primary Ca2+ events: 1) Ca2+ waves, reflecting IP3R-mediated Ca2+ release from the SR, and 2) Ca2+ flashes, reflecting Ca2+ influx through VDCCs during APs. These two events can be distinguished by their temporal, spatial, and pharmacological properties. In addition, increases in UBSM excitability and AP frequency in response to nerve-released ACh were determined directly by measuring membrane potential (Vm). Contrary to prevailing assumptions, our results do not support an essential role for IP3-mediated Ca2+ signaling in nerve-evoked UBSM contractility. Instead, our results indicate that increased UBSM excitability, measured as Vm depolarization, APs, and Ca2+ flashes, plays a central role in mediating the contractions induced by nerve-released ACh.
MATERIALS AND METHODS
Solutions.
All experiments were performed in physiological saline solution (PSS in mM: 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 11 glucose, and 23 μM EDTA). HEPES buffered solution (HBS in mM: 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.3 with NaOH) was used for tissue dissection and loading of tissues with fluo-4 and caged IP3 [iso-Ins (1,4,5)P3-PM (iso-IP3)].
Animals and tissue.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Vermont. Male C57BL/6J mice (3 to 4 mo of age) were used for all experiments. Mice were euthanized by intraperitoneal injection of pentobarbital sodium followed by decapitation. The abdominal cavity was opened by a midline incision, and the urinary bladder was quickly removed and transferred to ice-cold HBS. The bladder was then cut open by a longitudinal incision, and residual urine was rinsed away with ice-cold HBS. Detrusor strips obtained from the area above the ureteric orifices and dissected free of mucosa were used in all experiments.
Spinning disc confocal microscopy.
Detrusor strips (∼1 × 4 mm) with only a few layers of muscle bundles were dissected from the serosal side of the bladder, pinned onto blocks made from Sylgard (Dow Corning, Midland, MI), and loaded with the fluorescent Ca2+ indicator fluo-4 AM (10 μM; Molecular Probes, Eugene, OR) for 60 to 90 min at 30°C in HBS. For experiments involving photolysis of caged IP3, the tissue was also loaded with 10 μM iso-IP3 (Axxora, San Diego, CA). Pluronic F-127 (0.04%; Invitrogen, Carlsbad, CA) was added to facilitate loading. After washing for at least 20 min in HBS, detrusor strips were placed in a tissue chamber, superfused with PSS (3 ml/min, 35–37°C, bubbled with 95% O2 and 5% CO2) containing 10 μM α,β-methylene ATP (α,β-meATP) to desensitize purinergic receptors, and allowed to equilibrate for 20 min. Fluo-4 was excited by a krypton-argon laser at 488 nm, and emission above 495 nm was recorded with a charge-coupled device camera (model XR Mega10; Stanford Photonics, Palo Alto, CA) coupled to an upright microscope (model E600FN; Nikon, Japan) equipped with a QCL 100 scanhead (Solamere, Salt Lake City, UT) and a ×60 water-immersion objective (numerical aperature 1.0; Nikon). Images of 512 × 512 pixels (119 × 119 μm) were acquired at a rate of 15 or 60 images/s using InVivo software (Media Cybernetics, Bethesda, MD). Ca2+ events were evoked by EFS or photolysis of iso-IP3. EFS (0.2-ms pulse duration, 20 Hz, single train of 1-s duration) was generated by an S-44 stimulator (Grass Technologies, West Warwick, RI) and delivered to the tissue via two platinum wires parallel to the detrusor strip. A UV flash (∼1-ms duration) from a xenon arc lamp-based flash unit (Rapp OptoElectronics, Hamburg, Germany) coupled to a custom-made condenser (Till Photonics, Gräfelfing, Germany) was used for photolysis of iso-IP3 throughout the entire field of view. Concentrated stock solutions of atropine, diltiazem, and cyclopiazonic acid (CPA), used as indicted in the text, were added to the superfusing PSS. The number and lag time of EFS-induced Ca2+ events, as well as the maximum increase in fractional fluorescence intensity (ΔF/F0) of events caused by photolysis of iso-IP3 were analyzed with custom-written software (SparkAn; Dr. Adrian Bonev, Nelson Laboratory, University of Vermont, Burlington, VT). An average fluorescence value obtained from 10 images immediately prior to stimulation was used as F0. Strips that did not respond to EFS after application of CPA or diltiazem and those in which excessive tissue movement prevented accurate quantification of Ca2+ events were excluded from the analysis.
Intracellular recording.
Small strips (∼1 × 2 mm) containing a single layer of smooth muscle bundles were carefully dissected from the serosal side of the bladder and pinned onto the bottom of a recording chamber. The tissue was permanently superfused with PSS (3 ml/min, 35–37°C, bubbled with 95% O2-5% CO2) containing 10 μM α,β-meATP. After equilibrating for ∼30 min, individual smooth muscle cells were impaled with KCl (0.5 M)-filled sharp microelectrodes having a resistance of ∼225 MΩ. APs were evoked by EFS (0.2-ms pulse duration, 20 Hz, 1-s train duration), and the tissue was stimulated twice before (control) and 15 min after application of drugs. Atropine, CPA, and diltiazem were added as concentrated stock solutions to the superfusing PSS.
Data were acquired at a rate of 2 or 20 kHz, and the signal was amplified with an Axoclamp 2A amplifier (MDS Analytical Technologies, Sunnyvale, CA). pClamp software (MDS Analytical Technologies) was used to record the data and analyze the time from the onset of EFS to each individual AP as well as the number of APs in a burst following EFS. To determine Vm changes in response to EFS, Vm during Ca2+ wave activity (average of four time points between 250 and 450 ms after onset of EFS) or during Ca2+ flash activity (average of four time points between 2 and 2.5 s after onset of EFS) was compared with Vm before stimulation.
Myography.
After removal of the bladder base and mucosa, the remaining tissue was cut into eight strips (∼1.5 × 6 mm) oriented from the dome to the base of the bladder. A loop of silk suture (5-0) was attached to either end of the strip, and the tissue was transferred to the organ bath of a myograph system (Med Associates, Georgia, VT) filled with PSS (37°C, bubbled with 95% O2-5% CO2). One end of the strip was attached to a force transducer, and contractile force was recorded using MED Myograph software (Med Associates, St. Albans, VT). Initially, a tension of 10 mN was applied, and the tissue was allowed to equilibrate for 1 h. During equilibration, PSS was changed every 20 min; after equilibration, α,β-meATP (10 μM) was added to the bath to desensitize purinergic receptors. After a 20-min incubation period, contractions were evoked by EFS generated by a PHM-152V stimulator and delivered to the tissue via platinum electrodes parallel to either side of the detrusor strip. Frequency response curves were obtained by applying trains of pulses (0.2-ms pulse duration, 20 V, 2-s train duration) every 3 min with increasing frequency (0.5, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, 50, and 75 Hz). Atropine, diltiazem, CPA, and U73122 were added directly to the bath as concentrated stock solutions 5 min after the first frequency response curves and a second frequency response curves was obtained after incubating for 20 min. Force amplitude, force integral, and half-amplitude duration, as well as the lag time of peak force of EFS-evoked contractions were analyzed using MyoViewer (Med Associates) and MiniAnalysis (Synaptosoft, Fort Lee, NJ) software. Force integral was measured as area under the curve from the onset of contraction to 95% relaxation.
Chemicals and reagents.
Fluo-4 and Pluronic F-127 were purchased from Molecular Probes (Eugene, OR), iso-IP3 from Axxora (San Diego, CA), and U73122 and CPA from Calbiochem (San Diego, CA). All other chemicals and reagents were obtained from Sigma (St. Louis, MO). Stock solutions of CPA, fluo-4, and iso-IP3 were prepared in DMSO; α,β-meATP and diltiazem were prepared in water. U73122 was dissolved in chloroform, aliquoted, desiccated under argon, and reconstituted in DMSO prior to each experiment.
Analysis and statistics.
Prism software (GraphPad Software, La Jolla, CA) was used for statistical tests and preparation of graphs. Data are expressed as means ± SE unless otherwise noted. Student's t-test and two-way repeated-measures ANOVA followed by Bonferroni post tests were used for comparison of two or more groups, as appropriate, and P < 0.05 was considered statistically significant. Unless stated otherwise, the number of experiments (n) refers to the number of tissue strips.
RESULTS
Photolysis of caged IP3 releases Ca2+ from SR Ca2+ stores, but does not induce Ca2+ flashes.
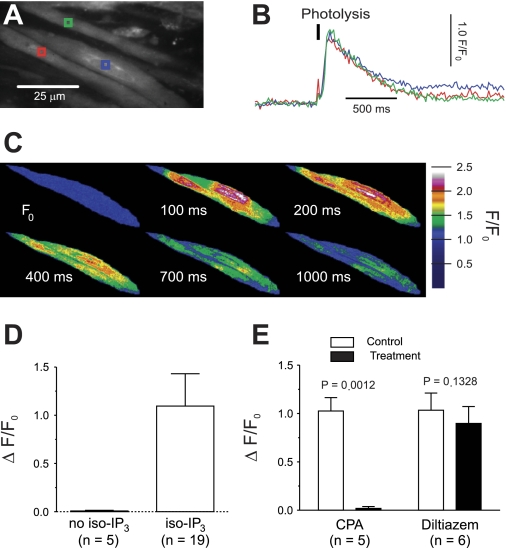
Stimulation of M3 mAChRs activates PLC to produce IP3 (23, 32, 40), and IP3, released by photolysis of caged IP3, acts on IP3Rs to release Ca2+ from the SR in isolated colonic smooth muscle cells (29). Using a similar approach to study IP3-mediated Ca2+ release in intact strips of UBSM, we found that photolysis of iso-IP3 by a short pulse of UV light (∼1-ms duration) significantly increases intracellular Ca2+ (ΔF/F0: 1.10 ± 0.34, mean ± SD; Fig. 1, A–D). Exposure to UV did not elicit any measurable response in tissue strips not loaded with iso-IP3 (ΔF/F0: 0.01 ± 0.01, mean ± SD; Fig. 1D), indicating that the increase in Ca2+ was due to photolysis of iso-IP3. The response to IP3 photolysis after a resting period of 15 min was not different from the initial response (P = 0.4170; paired t-test, n = 8). To deplete the SR of Ca2+, UBSM strips were incubated with CPA (10 μM), a specific inhibitor of sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA), for 15 min. CPA abolished the increase in intracellular Ca2+ following photolysis of iso-IP3 (ΔF/F0: 1.03 ± 0.14 in control vs. 0.02 ± 0.02 with CPA; Fig. 1E). These data indicate that IP3 releases Ca2+ from the SR of smooth muscle cells in UBSM strips and confirm that CPA effectively prevents IP3-induced SR Ca2+ release.
Fig. 1.
Depletion of sarcoplasmic reticulum (SR) Ca2+ stores abolishes inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release. A: fluo-4-loaded urinary bladder smooth muscle (UBSM) strip with regions of interest (ROIs, colored boxes). B: increase in Ca2+ recorded from the ROIs shown in A following photolysis of iso-Ins (1,4,5)P3-PM (iso-IP3). C: color-coded F/F0 recordings showing the increase in cytosolic Ca2+ after photolysis of iso-IP3. Warmer color temperatures indicate higher concentration of Ca2+. D: F/F0 increased by 1.10 ± 0.34 following photolysis of iso-IP3. In tissue not loaded with iso-IP3, exposure to UV flash did not trigger any Ca2+ release. Data are means ± SD. E: depletion of SR Ca2+ stores by cyclopiazonic acid (CPA) prevented IP3-induced Ca2+ release. Inhibition of voltage-dependent Ca2+ channels (VDCCs) by diltiazem for 15 min had no effect. Significance by paired t-test; data are means ± SE.
IP3-mediated release of Ca2+ is dependent on the state of SR Ca2+ stores, which in turn depends on refilling processes, including those involving Ca2+ influx through VDCCs (46). To determine whether IP3-induced Ca2+ signals depend on VDCC activity in the context of our experimental setting, the Ca2+ signaling response to photolysis of iso-IP3 was measured after treatment with diltiazem (50 μM) for 15 min. Diltiazem did not affect IP3-mediated Ca2+ release (ΔF/F0: 1.04 ± 0.18 in control vs. 0.90 ± 0.17 after diltiazem; Fig. 1E), indicating that SR Ca2+ stores can be maintained for the duration of the experiment independently of functional VDCCs. Conversely, opening of VDCCs was not induced by IP3-mediated Ca2+ release, as evidenced by the fact that photolysis of iso-IP3 did not elicit Ca2+ flashes (15 strips), which represent Ca2+ influx through VDCCs during APs (19). Hence, photolysis of iso-IP3 does not significantly increase excitability.
Nerve-released ACh acts through mAChRs to induce Ca2+ waves followed by Ca2+ flashes.
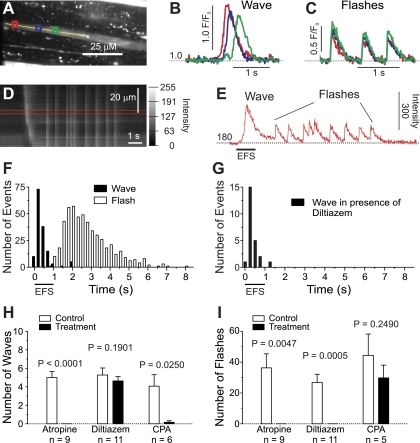
We have previously shown that, under conditions in which purinergic receptors are disabled (i.e., with only muscarinic signaling intact), EFS induces two distinct nerve-evoked Ca2+ events: Ca2+ waves and Ca2+ flashes (20). Thus, nerve-released ACh, which acts on Gq-coupled M3 mAChRs to promote contraction of UBSM (26), is responsible for inducing both types of Ca2+ events. Ca2+ waves precede Ca2+ flashes (Fig. 2, E and F), and can be distinguished from Ca2+ flashes by their local initiation, progression through the cell as a spreading wave front (Fig. 2, B–D), and slower rise in Ca2+ concentration (20). Here, we further characterized the relationship between nerve-released ACh and evoked Ca2+ signals in UBSM, focusing on the temporal properties and potential interdependence of these signals, as well as Ca2+ sources and release mechanisms.
Fig. 2.
Stimulation of muscarinic receptors causes Ca2+ waves followed by Ca2+ flashes. A: fluo-4 loaded UBSM strip with ROIs (colored boxes) in which F/F0 (B and C) was measured. The line scan (D) was performed offline along the yellow line. B: representative recording of F/F0 during a Ca2+ wave. Note the sequential rise in F/F0 in the 3 ROIs indicating propagation. C: representative recording of F/F0 during Ca2+ flashes. Note the simultaneous rise in F/F0 in all three ROIs. D: line scan showing the different Ca2+ events evoked by nerve-released ACh. Ca2+ waves were characterized by propagation (sloped line), whereas Ca2+ flashes did not show any propagation (vertical lines). E: average intensity measured between the red lines in D; a Ca2+ wave is followed by 9 Ca2+ flashes. F: histogram showing the lag time of Ca2+ events. Ca2+ waves occurred during stimulation, whereas Ca2+ flashes followed in a burst ∼1–6 s after the onset of stimulation (n = 145 waves and 521 flashes from 21 strips). G: blocking Ca2+ flashes with diltiazem (50 μM) revealed that Ca2+ wave activity stops with cessation of stimulation (n = 24 waves from 7 strips). H: Ca2+ waves were sensitive to atropine (10 μM) and CPA (10 μM), but not to diltiazem (50 μM). I: Ca2+ flashes were blocked by atropine and diltiazem, but not by CPA. Significance by paired t-test; data are means ± SE.
With purinergic receptors inhibited by α,β-meATP, EFS (20 Hz, 1-s train duration) very rapidly induced Ca2+ waves (lag time after onset of stimulation: 428 ± 376 ms, mean ± SD; 25th percentile: 222 ms, 75th percentile: 498 ms; n = 145 Ca2+ waves from 21 strips); these Ca2+ waves were followed by a burst of Ca2+ flashes that began after a lag of 1.6 ± 0.5 s (mean ± SD, n = 20) and lasted for ∼5 s (Fig. 2, E and F). Fifty percent of observed Ca2+ flashes occurred between 1.9 s and 3.4 s after the onset of stimulation (25th and 75th percentile, respectively; n = 521 Ca2+ flashes from 21 strips). Time controls, after an interval of 15 min without stimulation, showed no difference in the number of EFS-evoked Ca2+ events (Ca2+ waves: 5.3 ± 0.5 initially vs. 5.5 ± 0.8 after 15 min, P = 0.7412; Ca2+ flashes: 16.3 ± 4.9 initially vs. 24.8 ± 6.4 after 15 min, P = 0.1231; paired t-test, n = 6). Ca2+ waves and Ca2+ flashes were completely abolished by treatment with the muscarinic antagonist atropine (10 μM; Ca2+ waves: 5.0 ± 0.7 in control vs. 0 ± 0 after atropine; Ca2+ flashes: 38.4 ± 9.9 initially vs. 0 ± 0 after atropine; Fig. 2, H and I), indicating that ACh-activated mAChRs were responsible for initiating these events.
Our results indicate that nerve-released ACh induces Ca2+ waves during stimulation that are supplanted by Ca2+ flashes after stimulation ceases. The inability to detect Ca2+ waves after Ca2+ flash activity begins could mean that Ca2+ waves actually cease after the stimulation period has ended; alternatively, Ca2+ waves may continue to occur but are obscured by Ca2+ flashes. To distinguish between these two possibilities, we selectively eliminated Ca2+ flashes by inhibiting VDCCs with diltiazem. In the presence of diltiazem, EFS-induced Ca2+ waves abruptly ceased with cessation of stimulation (Fig. 2G), indicating that Ca2+ waves persist only as long as EFS is applied.
The rapid induction of atropine-sensitive Ca2+ waves following the onset of EFS suggests that IP3 levels rise rapidly to stimulate IP3R-mediated release of SR Ca2+. In support of this, prevention of SR Ca2+ uptake with the SERCA inhibitor CPA (10 μM) eliminated Ca2+ waves (4.2 ± 1.3 in control vs. 0.2 ± 0.2 after CPA; Fig. 2H). In contrast, inhibition of VDCCs with diltiazem (50 μM) did not prevent Ca2+ waves (5.3 ± 0.8 in control vs. 4.6 ± 0.5 after diltiazem; Fig. 2H), consistent with the interpretation that Ca2+ waves reflect IP3-mediated Ca2+ release from the SR. On the other hand, Ca2+ flashes were completely prevented by application of diltiazem (26.8 ± 5.3 in control vs. 0 ± 0 after diltiazem; Fig. 2I), as we have shown previously (19), but depletion of SR Ca2+ with CPA did not reduce the number of Ca2+ flashes (44.4 ± 13.9 in control vs. 29.8 ± 8.3 after CPA; Fig. 2I), even in the absence of purinergic receptor inhibitors (P = 0.3850; paired t-test, n = 5). Combined application of diltiazem and CPA abolished all Ca2+ events (P = 0.0130 for Ca2+ waves, P = 0.0243 for Ca2+ flashes; paired t-test, n = 6).
Collectively, these data show that, with purinergic receptors desensitized, nerve stimulation by EFS evokes rapid Ca2+ release from SR Ca2+ stores, which manifests as Ca2+ waves, and Ca2+ influx through VDCCs in the form of Ca2+ flashes. These two events occur independently of each other since CPA did not abolish Ca2+ flashes and diltiazem did not affect the number of Ca2+ waves. Accordingly, CPA can be used to disable SR Ca2+ release without inhibiting Ca2+ entry, and diltiazem to selectively block Ca2+ influx.
Activation of mAChR by nerve-released ACh elicits a burst of APs.
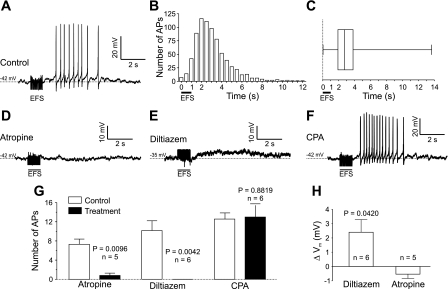
To explore the involvement of SR Ca2+ release in neurally mediated increases in excitability, intracellular recording techniques were used to determine the electrical response of UBSM strips to EFS. The Vm recorded in individual smooth muscle cells in UBSM strips was −43 ± 5 mV (mean ± SD, n = 26), a value similar to that previously reported for mouse UBSM (20, 30), and few cells exhibited spontaneous APs. In the presence of α,β-meATP (10 μM) to block purinergic signaling, EFS (20 Hz, 1-s train duration) caused a burst of APs (Fig. 3A), with the first AP occurring 1.2 ± 0.5 s after the beginning of EFS (mean ± SD, n = 36). Each burst consisted of 10.3 ± 6.0 APs and had a duration of 3.4 ± 2.0 s (mean ± SD, n = 36). The number of APs evoked by EFS did not change significantly over time (11.4 ± 3.0 vs. 14.8 ± 5.3 initially and after a resting period of 15 min, respectively; P = 0.2434; paired t-test, n = 4). Atropine greatly reduced the number of APs triggered by EFS (7.3 ± 1.1 in control vs. 0.8 ± 0.5 after atropine; Fig. 3, D and G), confirming that the burst of APs was due to activation of mAChRs. The upstroke of APs in UBSM is caused by Ca2+ influx through VDCCs (14, 15, 18, 19); thus, as expected, application of 50 μM diltiazem completely abolished APs (10.2 ± 2.0 APs in control vs. 0 ± 0 after diltiazem; Fig. 3, E and G).
Fig. 3.
Nerve-released ACh increases urinary bladder smooth muscle excitability to evoke action potentials independently of SR Ca2+. A: representative recording of membrane potential in mouse UBSM. Electrical field stimulation (EFS) in the presence of α,β-methylene ATP (α,β-meATP; 10 μM) triggered a burst of APs. B: histogram showing the distribution of nerve-induced APs over time. Most APs occurred between 1 and 6 s after the onset of EFS (n = 751 APs from 72 strips). C: summary data showing the lag time of APs after onset of EFS (n = 751 APs from 72 strips). D: inhibition of muscarinic ACh receptors (mAChRs) with atropine (10 μM) abolished nerve-induced APs. E: blockade of VDCCs with diltiazem (50 μM) prevented nerve-induced APs. F: depletion of SR Ca2+ stores by CPA (10 μM) did not inhibit nerve-induced APs. G: summary data of the effects of atropine, diltiazem, and CPA on the number of evoked action potentials. Significance by paired t-test; data are means ± SE. H: in strips treated with diltiazem, EFS caused a membrane depolarization (measured 2 s after onset of EFS and compared with hypothetical mean 0; 1-sample t-test) that was absent in strips treated with atropine.
Because IP3-mediated Ca2+ waves precede Ca2+ flashes/APs (Fig. 2, E and F), it is conceivable that SR Ca2+ release could modulate excitability and affect nerve-evoked APs. However, blocking Ca2+ waves with CPA did not affect the induction of Ca2+ flashes (Fig. 2I), suggesting that Ca2+ waves neither suppress APs during stimulation nor trigger them following stimulation. Similarly, depletion of SR Ca2+ stores with the SERCA blocker CPA (10 μM) did not inhibit APs (12.0 ± 1.4 APs in control vs. 11.7 ± 2.4 after CPA; Fig. 3, F and G), indicating that release of Ca2+ from the SR is not involved in temporal aspects of increased excitability. Consistent with this, Ca2+ waves appearing during the 1-s stimulation interval did not cause membrane depolarization, but instead were associated with a small, but significant, hyperpolarization (ΔVm: −1.8 ± 0.7 mV, P = 0.0325 compared with the hypothetical mean 0, n = 15) that was absent after depletion of SR Ca2+ stores with CPA (ΔVm: 0.2 ± 0.3 mV, P = 0.5707 compared with the hypothetical mean 0, n = 6). This lends further support to the hypothesis that IP3-mediated Ca2+ release does not increase excitability.
Similar to Ca2+ flashes, 50% of observed APs occurred between 1.9 and 3.8 s after onset of EFS (25th and 75th percentile, respectively; Fig. 3, B and C). Interestingly, during this interval (2 to 2.5 s after onset of EFS), we observed a small, but statistically significant depolarization in the presence of diltiazem (ΔVm: 2.4 ± 0.9 mV, P = 0.0420 compared with the hypothetical mean 0, n = 6; Fig. 3H), but not after pretreatment with the muscarinic antagonist atropine (ΔVm: −0.5 ± 0.3 mV, P = 0.1531 compared with the hypothetical mean 0, n = 5; Fig. 3H). These data suggest that nerve-released ACh induces a delayed depolarization, which could contribute to the increase in action potential frequency.
Ca2+ influx, but not IP3-mediated Ca2+ release, is essential for contraction.
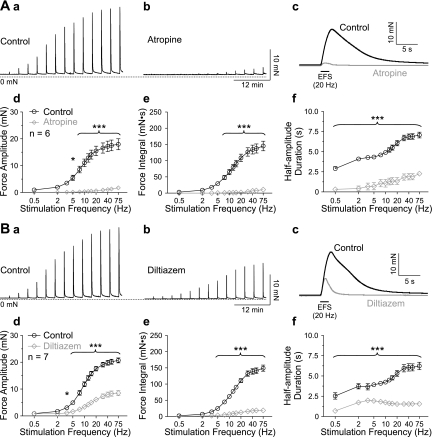
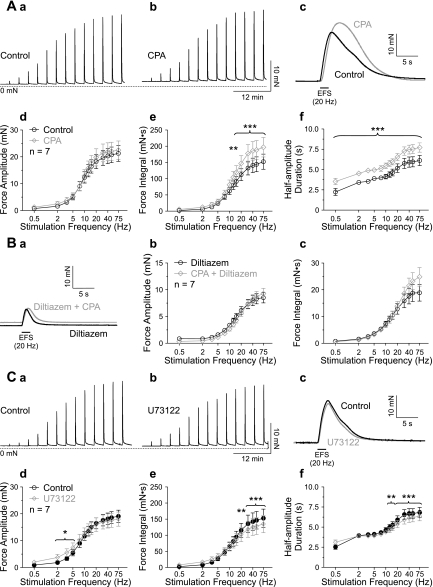
Previously, we have shown that the combined block of muscarinic and purinergic receptors completely abrogates nerve-evoked contractions in mouse UBSM, and inhibition of mAChRs alone is sufficient to eliminate nerve-evoked contractions in the absence of P2X1Rs (20), demonstrating that mouse urinary bladder contraction is mediated exclusively by purinergic and muscarinic receptors. Here, we found that, after inhibition of purinergic receptors with α,β-meATP (10 μM), EFS caused frequency-dependent contractions ranging from 2.57 ± 1.46 mN·s at a stimulation frequency of 0.5 Hz to 151.51 ± 47.12 mN·s at 75 Hz (force integral, mean ± SD, n = 41). Contractions evoked by 20 Hz stimulation reached peak force 2.75 ± 0.32 s after initiating EFS (mean ± SD, n = 56), and half-amplitude duration was 5.7 ± 1.1 s (mean ± SD, n = 41). Pretreatment with the specific muscarinic antagonist atropine (10 μM) almost completely abolished the contractile response (Fig. 4A); it significantly reduced force amplitude at stimulation frequencies ≥ 5 Hz (Fig. 4, A, d), force integral ≥ 7.5 Hz (Fig. 4, A, e), and half-amplitude duration at all stimulation frequencies (Fig. 4, A, f).
Fig. 4.
Ca2+ influx through VDCC is crucial for contraction. A: representative recordings of contractions evoked by EFS with increasing frequency in the presence of α,β-meATP (10 μM) before (A, a) and after (A, b) application of atropine (10 μM). Contractions in response to 20 Hz stimulation are shown on a faster time scale (A, c). Atropine significantly reduced force amplitude (A, d), force integral (A, e), and half-amplitude duration (A, f). B: representative recordings of nerve-evoked contractions in the presence of α,β-meATP (10 μM) before (B, a) and after (B, b) application of diltiazem (50 μM). Contractions in response to 20 Hz stimulation are shown on a faster time scale (B, c). Diltiazem significantly reduced force amplitude (B, d), force integral (B, e), and half-amplitude duration (B, f). *P < 0.05 and ***P < 0.001 by two-way repeated-measures ANOVA; data are means ± SE.
To investigate the roles of Ca2+ waves and Ca2+ flashes in nerve-evoked contractions of UBSM, we used CPA (10 μM) and diltiazem (50 μM), which can be used to selectively inhibit Ca2+ waves and Ca2+ flashes/APs, respectively, as shown by imaging and electrical data (Figs. 1–3). Blocking VDCCs significantly inhibited contractions evoked by nerve-released ACh (Fig. 4B), reducing force amplitude at stimulation frequencies ≥ 3.5 Hz (Fig. 4, B, d), force integral ≥ 5 Hz (Fig. 4, B, e), and half-amplitude duration at all stimulation frequencies (Fig. 4, B, f). In contrast, inhibition of SR Ca2+ release by blocking SERCA with CPA did not reduce contractile force (Fig. 5A); instead, it increased force integral at stimulation frequencies ≥ 12.5 Hz (Fig. 5, A, e) due to an increase in half-amplitude duration (Fig. 5, A, f) without any change in force amplitude (Fig. 5, A, d). Combined application of CPA and diltiazem reduced force amplitude significantly at stimulation frequencies ≥ 3.5 Hz, force integral ≥ 5 Hz, and half-amplitude duration at all stimulation frequencies (P < 0.05, two-way repeated-measures ANOVA; n = 7), but the effects on force amplitude and integral were not significantly different from that obtained with diltiazem alone (Fig. 5B). These data indicate that SR Ca2+ release is not required for the elevation of force evoked by nerve-released ACh, but do not strictly preclude a functional role for IP3. To further examine this possibility, contractile responses were measured in the presence of the PLC inhibitor U73122 (Fig. 5C), which has been shown to prevent mAChR-induced increases in IP3 (37). U73122 (10 μM) augmented force amplitude slightly at stimulation frequencies of 2, 3.5, and 5 Hz (Fig. 5, C, d). At stimulation frequencies ≥ 20 Hz, U73122 reduced force integral (Fig. 5, C, e) and decreased half-amplitude duration at stimulation frequencies ≥ 12.5 Hz (Fig. 5, C, f). Although statistically significant, the maximum reduction of the force integral in the presence of U73122 was only 14.6 ± 4.8% (at 30 Hz stimulation frequency, n = 7). The minimal effects of the PLC inhibitor U73122 also support the concept that IP3-mediated Ca2+ release does not have a major role in neurally evoked contractions of UBSM.
Fig. 5.
SR Ca2+ release does not promote contractility. A: representative recordings of nerve-evoked contractions in the presence of α,β-meATP (10 μM) before (A, a) and after (A, b) application of CPA (10 μM). Contractions in response to 20 Hz stimulation are shown on a faster time scale (A, c). CPA had no effect on force amplitude (A, d), but significantly increased force integral (A, e) and half-amplitude duration (A, f). B: contractions evoked by 20 Hz stimulation in the presence of diltiazem alone and diltiazem with CPA (B, a). Combined inhibition of SR Ca2+ release and Ca2+ influx was not different from blocking only Ca2+ influx. Note that the graphs for force amplitude (B, b) and force integral (B, c) coincide (P > 0.05 by two-way ANOVA). C: representative recordings of nerve-evoked contractions in the presence of α,β-meATP (10 μM) before (C, a) and after (C, b) application of U73122 (10 μM). Contractions in response to 20 Hz stimulation are shown on a faster time scale (C, c). U73122 slightly increased force amplitude at 2, 3.5, and 5 Hz stimulation frequency (C, d), but reduced force integral by maximally ∼15% (C, e) and half-amplitude duration (C, f) at stimulation frequencies > 20 Hz and 12.5 Hz, respectively. *P < 0.05, **P < 0.01 and *** P < 0.001 by two-way repeated-measures ANOVA; data are means ± SE.
DISCUSSION
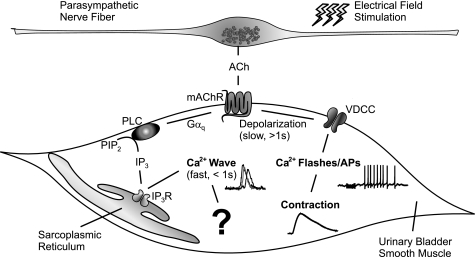
Micturition depends on a forceful and coordinated contraction of UBSM that is brought about by activation of muscarinic and purinergic receptors in response to nerve-released ACh and ATP, respectively (2). Here, we determined the UBSM Ca2+ signals induced by neurally mediated release of ACh, showing that two major Ca2+ signals are evoked by nerve-mediated muscarinic signaling: IP3-mediated Ca2+ waves during stimulation, and Ca2+ flashes, which reflect Ca2+ influx through VDCCs during APs, after stimulation. In addition, we investigated the contribution of these events to contractions evoked by nerve-released ACh. Inhibition of Ca2+ influx through VDCCs by diltiazem abolished Ca2+ flashes and APs and greatly reduced contractile force. Depletion of SR Ca2+ stores by CPA abolished SR Ca2+ release, but did not inhibit nerve-evoked Ca2+ flashes, APs, or contractions. Furthermore, preventing the production of IP3 by inhibiting PLC with U73122 had little effect on nerve-evoked contractions. Interestingly, EFS induced a small, but significant ACh-dependent Vm depolarization (2.4 mV), which may be sufficient to trigger APs (18). Taken together, our data indicate that nerve-released ACh promotes UBSM contraction by increasing excitability and inducing APs independently of IP3-mediated SR Ca2+ release (Fig. 6).
Fig. 6.
Illustration of proposed muscarinic signaling. Nerve-released ACh activates mAChRs to 1) rapidly activate PLC to produce IP3 and release Ca2+ from SR via IP3 receptors (IP3Rs) (Ca2+ wave), and 2) with a slower onset, increase excitability to promote opening of VDCCs (Ca2+ flash/AP). Both events occur independently of each other. Ca2+ waves do not contribute to excitability and contractility; their function remains unknown. Ca2+ flashes/APs, on the other hand, are crucial for contraction. PIP2, phosphatidylinositol-4,5-bisphosphate; Gαq, a G protein.
Muscarinic paradigm.
UBSM expresses two types of mAChRs: M2 and M3 (17). Although the M2 subtype is more abundant, M3 mAChRs mediate ACh-dependent contraction, as evidenced by studies employing selective inhibitors of mAChR subtypes (9, 16, 25, 26) and mAChR knockout mice (28, 39). Canonically, M3 mAChRs signal through the G protein Gαq, which activates PLC-mediated IP3 production and thereby induces IP3R-mediated release of Ca2+ from the SR (8). Because contraction is directly dependent on cytosolic Ca2+ (6, 27), this cascade has traditionally been presumed to constitute a major signaling pathway to cause contraction (3, 12). It has been shown that stimulation of UBSM M3 mAChRs by muscarinic agonists increases IP3 production (40) and causes contraction (28, 39), and Ji et al. (24) reported EFS-evoked atropine-sensitive Ca2+ waves in intact UBSM strips. Here, we show that photolysis of iso-IP3 increases intracellular Ca2+ in intact UBSM strips. This response was prevented by depletion of SR Ca2+ stores by CPA. In addition, we show that stimulation of mAChRs by nerve-released ACh induces CPA-sensitive Ca2+ waves. Although it is well established that mAChRs evoke IP3R-mediated Ca2+ waves, a causal relationship between this event and contraction has not been established.
PLC, IP3, and IP3-mediated Ca2+ release.
IP3R-mediated Ca2+ wave activity, induced by stimulation of mAChRs by nerve-mediated release of ACh, was only present during EFS and stopped with cessation of stimulus application. This suggests a rapid termination of ACh-dependent signaling through the Gαq/PLC/IP3 pathway, possibly reflecting rapid degradation of IP3. In contrast, contractions evoked by nerve-released ACh reached peak force after cessation of EFS, and their half-amplitude duration was approximately three times longer than the stimulus duration. This suggests that IP3-mediated Ca2+ signals are too short-lived to sustain contractions after cessation of stimulus application.
Although contraction is initiated by cytosolic Ca2+, it depends on phosphorylation of the regulatory myosin light chain (RLC), which is determined by the balance of myosin light chain kinase (MLCK) and phosphatase (MLCP) activities. MLCP activity can be dynamically regulated by the Rho kinase and PKC pathways, and as such can lead to maintained force with decaying Ca2+ (Ca2+ sensitization). Recently, Ding et al. (6) provided evidence that contraction of mouse UBSM strips to EFS (50 Hz, 3-s train duration) is strictly dependent on Ca2+, MLCK activation, and RLC phosphorylation without any apparent involvement of the Rho kinase or PKC pathway. Therefore, IP3-mediated Ca2+ release does not appear capable of initiating and sustaining contractions evoked by nerve-released ACh.
Depleting the SR of Ca2+ by blocking SERCA abolished Ca2+ waves, but did not decrease contractile force. On the contrary, it increased contractile force, possibly by slowing the rate of Ca2+ removal from the cytosol or by preventing Ca2+ sparks and thereby reducing large-conductance Ca2+-activated K+ channel activity (22). Thus, our data do not support an essential role for SR Ca2+ stores in nerve-evoked contraction in UBSM. This conclusion is in accord with several studies that did not find an inhibitory effect of CPA on contractions in urinary bladder (31, 35, 50). The failure of CPA to inhibit contractile force excludes a significant role for SR in providing Ca2+ for contraction.
The products of PLC-mediated hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2), IP3, and diacylglycerol (DAG), could cause contraction directly by increasing UBSM excitability. For example, IP3 has been shown to activate canonical transient receptor potential (TRP)3 channels independently of SR Ca2+ release in cerebral arteries (48), and DAG could increase excitability through PKC-dependent inhibition of potassium channels (e.g., ATP-sensitive potassium channels) (4) or activation of TRP channels (41). In addition, PIP2 has been shown to inhibit TRP melastatin channels (49) and to activate large-conductance Ca2+-activated K+ channels (42). Accordingly, depletion of PIP2 could increase excitability. The common element in these mechanisms involving IP3, DAG, and depletion of PIP2 is PLC-mediated hydrolysis of PIP2. However, the PLC inhibitor U73122 had little effect on nerve-evoked contractions. This observation is in agreement with the findings of Schneider et al., (37) who showed that U73122 completely blocks carbachol-induced accumulation of inositol phosphates, but does not inhibit carbachol-induced contractions. Our data and those of others (11) do not support a major role of PLC in muscarinic-induced increases in excitability or contractility to nerve stimulation or bath-applied agonists. The role of PKC in muscarinic-induced contractions also appears to be minimal (11). Taken together, these data provide solid evidence that IP3-mediated Ca2+ release does not directly contribute to contractions evoked by nerve-released ACh.
Although muscarinic receptor activation stimulates PLC and increases IP3-mediated Ca2+ waves, their functional role in UBSM remains unclear. Ca2+ waves do not appear to trigger APs and Ca2+ flashes, because photolysis of caged IP3 did not induce flashes, and blocking waves with CPA did not affect nerve-evoked AP or Ca2+ flashes. It is, however, possible that IP3-mediated Ca2+ waves are responsible for the small CPA-sensitive membrane hyperpolarization that we observed. We have recently reported that purinergic signaling during stimulation suppresses the subsequent, cholinergic-driven Ca2+ flashes (20). It is possible that during stimulation, the IP3-mediated Ca2+ waves, in conjunction with purinergic-induced increases in excitability, contribute to suppression of cholinergic-mediated delayed Ca2+ flashes. The PLC pathway could also affect excitability under conditions when other target ion channels are active, e.g., the KATP channel. Longer-term processes, such as activation of Ca2+-dependent transcription factors, could also be a target for IP3-mediated Ca2+ release. For example, nuclear factor of activated T-cells activation as well as proliferation of smooth muscle have been shown to be dependent on IP3-mediated Ca2+ release (13, 45). This area warrants further investigation.
Excitability and Ca2+ influx through VDCCs.
The main trigger for contraction is intracellular Ca2+ (2, 6, 27). Here, we provide direct evidence that stimulation of mAChRs increases cytosolic Ca2+ by two different mechanisms: 1) IP3-mediated Ca2+ release from the SR, manifested as Ca2+ waves, and 2) increased excitability and associated opening of VDCCs, which gives rise to Ca2+ flashes and APs. IP3-mediated Ca2+ release was not crucial for contraction; instead, we found that inhibition of VDCCs by diltiazem, which abolished Ca2+ flashes and APs, greatly reduced contractile force mediated by nerve-released ACh. In rabbit UBSM, ACh has been shown to increase AP frequency (5), and EFS (with P2X receptors blocked) causes an increase in AP frequency in guinea pig UBSM (14). In addition, we have shown previously that diltiazem inhibits nerve-evoked contractions when both purinergic and muscarinic receptors are available (21). The residual increase in force observed in diltiazem and CPA may reflect a contraction in response to a small, and therefore undetected, Ca2+ signal [e.g., PKC-dependent opening of TRP channels (7)] or Ca2+-independent phosphorylation of MLC by Rho kinase, integrin-linked kinase, or zipper-interacting protein kinase (36). Our results, taken together with these previous observations, suggest that mAChRs increase excitability to trigger APs and that Ca2+ influx during the AP provides Ca2+ for contraction.
Wu et al. (46) have proposed that Ca2+ influx through VDCCs contributes to filling of SR Ca2+ stores. It has also been suggested that stimulation of mAChRs leads to Ca2+ influx through VDCCs, followed by uptake of Ca2+ into the SR and subsequent IP3-mediated release from the SR to cause contraction (35). However, our data do not support this model. First, in the present study, inhibition of VDCCs reduced neither SR Ca2+ release in response to photolysis of caged IP3 nor the frequency of Ca2+ waves evoked by EFS. Secondly, CPA would be expected to have an effect on contraction similar to that of diltiazem, because both inhibitors should interfere with refilling of SR Ca2+ stores. Finally, mAChR-mediated Ca2+ waves always precede Ca2+ flashes/APs. Therefore, an increase in excitability, which occurs independently of IP3-mediated Ca2+ signaling and results in Ca2+ influx during APs, mediates contraction induced by nerve-released ACh.
Neurally released ACh could increase excitability through Vm depolarization. Indeed, we observed an atropine-sensitive depolarization of ∼2.4 mV following EFS in the presence of diltiazem that may be sufficient to trigger APs (18). The delayed onset of this depolarization seems independent of previous changes of membrane excitability, because, as we have shown previously (20), the muscarinic response has a slow onset in the presence as well as in the absence of P2X1R-induced changes in excitability. Although several studies have shown that activation of mAChRs causes an increase in excitability (5, 14), the mechanisms by which this occurs remain incompletely defined. One mechanism that has received considerable attention is the RhoA/Rho kinase pathway. The small GTPase RhoA can be activated by Gq-coupled receptors, such as M3 mAChRs (1), and studies have shown that the Rho kinase inhibitor Y27632 reduces carbachol-induced contractions in rat UBSM (22% reduction with 3 μM Y27632) (10). Similar inhibitory effects of Y27632 have been observed in mouse UBSM (43). However, Ding et al. (6) did not detect phosphorylation of the Rho kinase target (myosin phosphatase targeting subunit 1) in response to nerve stimulation of UBSM strips. Therefore, the mechanism by which ACh increases UBSM excitability remains elusive, including the identity of the ion channels that are the presumed targets of this pathway. In addition, other pathways, such as phospholipase A2 and phospholipase D (37), may also contribute.
Conclusions.
In the present study, we present strong evidence that stimulation of mAChRs by nerve-released ACh causes rapid generation of IP3R-dependent Ca2+ waves during stimulation, and a profound increase in VDCC-dependent Ca2+ flashes/APs that have a much slower onset. Ca2+ waves cease after the stimulus ends, possibly reflecting rapid breakdown of IP3 and ACh or activation of intracellular mechanisms that terminate G protein signaling (e.g., RGS proteins). We also show that nerve-released ACh increases UBSM excitability that does not depend on IP3-mediated Ca2+ release, and may be caused by membrane depolarization. Although the mechanisms linking mAChR activation with increased excitability in UBSM are not known, our data suggest that IP3, DAG, and depletion of PIP2 are not involved. In addition, and contrary to conventional wisdom, our data do not support a role for IP3-mediated Ca2+ release in promoting contractility. Instead, our data strongly support increased excitability and subsequent APs in response to mAChR activation as the primary mechanism underlying contraction induced by stimulation of parasympathetic nerves.
Perspective and Significance
A fundamental function of the urinary bladder is to void urine. This occurs through activation of parasympathetic nerves to release ACh (and ATP) to contract UBSM. It has been accepted that nerve-released ACh acts on M3 mAChRs to cause contraction through IP3-mediated SR Ca2+ release. However, the linkage between this Ca2+ signal and UBSM contraction has been through association. Here, we challenge this dogma by examining the effects of neurally released ACh on Ca2+ signals and excitability and contractility of UBSM. Our data exclude a significant role for IP3-mediated Ca2+ signals in nerve-evoked contraction, and support the concept that ACh contracts UBSM through an increase in excitability (AP), which depends on VDCCs. Therefore, our data have important implications for urinary bladder function, and indicate new avenues of research to understand UBSM excitation-contraction coupling in health and disease.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-053832, DK-065947, National Heart, Lung, and Blood Institute Grants HL-44455, HL-098243, and HL-077378, and the Totman Trust for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. David Hill-Eubanks, Gary Mawe, George Wellman, and Lydia Nausch for comments on the manuscript.
REFERENCES
- 1.Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol Pharmacol 77: 111–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonev AD, Nelson MT. Muscarinic inhibition of ATP-sensitive K+ channels by protein kinase C in urinary bladder smooth muscle. Am J Physiol Cell Physiol 265: C1723–C1728, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338: 149–164, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding HL, Ryder JW, Stull JT, Kamm KE. Signaling processes for initiating smooth muscle contraction upon neural stimulation. J Biol Chem 284: 15541–15548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J 9: 619–625, 1995 [PubMed] [Google Scholar]
- 9.Fetscher C, Fleichman M, Schmidt M, Krege S, Michel MC. M(3) muscarinic receptors mediate contraction of human urinary bladder. Br J Pharmacol 136: 641–643, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleichman M, Schneider T, Fetscher C, Michel MC. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. II. Protein kinases. J Pharmacol Exp Ther 308: 54–58, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Frazier EP, Peters SL, Braverman AS, Ruggieri MR, Sr, Michel MC. Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and β-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 377: 449–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154: 3–13, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem 277: 37756–37764, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524: 565–579, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashitani H, Suzuki H. Electrical and mechanical responses produced by nerve stimulation in detrusor smooth muscle of the guinea-pig. Eur J Pharmacol 284: 177–183, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol 120: 1409–1418, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci 64: 419–428, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564: 201–212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol 587: 5275–5288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Iacovou JW, Hill SJ, Birmingham AT. Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J Urol 144: 775–779, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Ji G, Feldman ME, Deng KY, Greene KS, Wilson J, Lee JC, Johnston RC, Rishniw M, Tallini Y, Zhang J, Wier WG, Blaustein MP, Xin HB, Nakai J, Kotlikoff MI. Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle. J Biol Chem 279: 21461–21468, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kories C, Czyborra C, Fetscher C, Schneider T, Krege S, Michel MC. Gender comparison of muscarinic receptor expression and function in rat and human urinary bladder: differential regulation of M2 and M3 receptors? Naunyn Schmiedebergs Arch Pharmacol 367: 524–531, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Longhurst PA, Leggett RE, Briscoe JA. Characterization of the functional muscarinic receptors in the rat urinary bladder. Br J Pharmacol 116: 2279–2285, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malmqvist U, Arner A, Uvelius B. Mechanics and Ca2+-sensitivity of human detrusor muscle bundles studied in vitro. Acta Physiol Scand 143: 373–380, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA 97: 9579–9584, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarron JG, MacMillan D, Bradley KN, Chalmers S, Muir TC. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J Biol Chem 279: 8417–8427, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Meng E, Young JS, Brading AF. Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn 27: 79–87, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Munro DD, Wendt IR. Effects of cyclopiazonic acid on [Ca2+]i and contraction in rat urinary bladder smooth muscle. Cell Calcium 15: 369–380, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Nelson CP, Gupta P, Napier CM, Nahorski SR, Challiss RA. Functional selectivity of muscarinic receptor antagonists for inhibition of M3-mediated phosphoinositide responses in guinea pig urinary bladder and submandibular salivary gland. J Pharmacol Exp Ther 310: 1255–1265, 2004 [DOI] [PubMed] [Google Scholar]
- 33.O'Reilly BA, Kosaka AH, Knight GF, Chang TK, Ford AP, Rymer JM, Popert R, Burnstock G, McMahon SB. P2X receptors and their role in female idiopathic detrusor instability. J Urol 167: 157–164, 2002 [PubMed] [Google Scholar]
- 34.Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol 150: 2007–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Rivera L, Brading AF. The role of Ca2+ influx and intracellular Ca2+ release in the muscarinic-mediated contraction of mammalian urinary bladder smooth muscle. BJU Int 98: 868–875, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil 20 Suppl 1: 39–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider T, Hein P, Michel MC. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J Pharmacol Exp Ther 308: 47–53, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Sjogren C, Andersson KE, Husted S, Mattiasson A, Moller-Madsen B. Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol 128: 1368–1371, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Stengel PW, Yamada M, Wess J, Cohen ML. M(3)-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol 282: R1443–R1449, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Tran JA, Matsui M, Ehlert FJ. Differential coupling of muscarinic M1, M2, and M3 receptors to phosphoinositide hydrolysis in urinary bladder and longitudinal muscle of the ileum of the mouse. J Pharmacol Exp Ther 318: 649–656, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Trebak M, St J Bird G, McKay RR, Birnbaumer L, Putney JW., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem 278: 16244–16252, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol 132: 13–28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegener JW, Schulla V, Lee TS, Koller A, Feil S, Feil R, Kleppisch T, Klugbauer N, Moosmang S, Welling A, Hofmann F. An essential role of Cav1.2 l-type calcium channel for urinary bladder function. FASEB J 18: 1159–1161, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Wilkerson MK, Heppner TJ, Bonev AD, Nelson MT. Inositol trisphosphate receptor calcium release is required for cerebral artery smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol 290: H240–H247, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Sui G, Fry CH. The role of the l-type Ca2+ channel in refilling functional intracellular Ca2+ stores in guinea-pig detrusor smooth muscle. J Physiol 538: 357–369, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wuest M, Hiller N, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Contribution of Ca2+ influx to carbachol-induced detrusor contraction is different in human urinary bladder compared to pig and mouse. Eur J Pharmacol 565: 180–189, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res 102: 1118–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing J, Strange K. Phosphatidylinositol 4,5-bisphosphate and loss of PLCγ activity inhibit TRPM channels required for oscillatory Ca2+ signaling. Am J Physiol Cell Physiol 298: C274–C282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziganshin AU, Hoyle CH, Ziganshina LE, Burnstock G. Effects of cyclopiazonic acid on contractility and ecto-ATPase activity in guinea-pig urinary bladder and vas deferens. Br J Pharmacol 113: 669–674, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]