Abstract
Dietary methionine restriction (MR) limits fat deposition and decreases plasma leptin, while increasing food consumption, total energy expenditure (EE), plasma adiponectin, and expression of uncoupling protein 1 (UCP1) in brown and white adipose tissue (BAT and WAT). β-adrenergic receptors (β-AR) serve as conduits for sympathetic input to adipose tissue, but their role in mediating the effects of MR on energy homeostasis is unclear. Energy intake, weight, and adiposity were modestly higher in β3-AR−/− mice on the Control diet compared with wild-type (WT) mice, but the hyperphagic response to the MR diet and the reduction in fat deposition did not differ between the genotypes. The absence of β3-ARs also did not diminish the ability of MR to increase total EE and plasma adiponectin or decrease leptin mRNA, but it did block the MR-dependent increase in UCP1 mRNA in BAT but not WAT. In a further study, propranolol was used to antagonize remaining β-adrenergic input (β1- and β2-ARs) in β3-AR−/− mice, and this treatment blocked >50% of the MR-induced increase in total EE and UCP1 induction in both BAT and WAT. We conclude that signaling through β-adrenergic receptors is a component of the mechanism used by dietary MR to increase EE, and that β1- and β2-ARs are able to substitute for β3-ARs in mediating the effect of dietary MR on EE. These findings are consistent with the involvement of both UCP1-dependent and -independent mechanisms in the physiological responses affecting energy balance that are produced by dietary MR.
Keywords: energy expenditure, metabolic efficiency, futile cycles, adipose tissue, dietary restriction, leptin, adiponectin, oxidative metabolism, uncoupling
dietary methionine restriction (MR) is a mimetic of chronic dietary restriction that produces a comparable increase in rodent longevity without restriction of food consumption. We report here that MR produces a rapid and persistent increase in total energy expenditure (EE) that limits fat deposition despite increasing weight-specific food consumption. In Fischer 344 (F334) rats studied 3, 9, and 20 mo after weaning onto control or MR diets, total EE was ∼1.5-fold higher in MR vs. Control rats at all ages, due primarily to higher O2 consumption during the fed state. The transition from fasted to fed state produced a twofold higher heat increment of feeding (HIF; 3.0°C vs. 1.5°C) in MR vs. Control rats, and an exaggerated increase in respiratory quotient (RQ) to 1.0, indicative of a complete shift to carbohydrate oxidation and increase in de novo lipogenesis. During the subsequent transition to fasting, the shift to fat oxidation was also more complete in MR (RQ: ∼0.75 vs. Control group: RQ ∼0.85). Imposition of MR in mature rats produced a similar enhancement of metabolic flexibility, but in this case, the increase in EE was matched to the increase in food consumption, such that rats were in energy balance and maintained both body weight and composition over the subsequent 6 mo. Similarly, imposition of dietary MR using high-fat diets with obesity-prone Osborne-Mendel rats prevented postweaning accretion of fat despite increasing weight-specific food consumption. Collectively, these findings demonstrate that the hyperphagic response to dietary MR is matched to a coordinated increase in uncoupled respiration, suggesting the engagement of a nutrient-sensing mechanism, which compensates for limited methionine availability through integrated effects on energy homeostasis.
Dietary MR increases food consumption while paradoxically limiting fat accretion (31, 33, 44). Pair-feeding studies comparing rats fed a methionine-replete control diet to the amount of MR diet consumed by the MR group establish that dietary MR decreases metabolic efficiency (31, 44). In our companion article (21a), we show that dietary MR increases total daily EE, core body temperature, uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT) and white adipose tissue (WAT), and increased plasma adiponectin, while decreasing leptin expression in both types of adipose tissue. These findings are consistent with the hypothesis that dietary MR increases sympathetic outflow to BAT and WAT, and increases adaptive thermogenesis, in part, through induction of UCP1 and adiponectin. To explore the significance of sympathetic stimulation of adipose tissue to the ability of dietary MR to increase EE and limit fat deposition, the present studies were undertaken with mice lacking the β3-adrenergic receptor (β3-AR). Although the β3-AR is considered the primary receptor conduit for chronic sympathetic nervous system (SNS) input into adipose tissue (8, 30), previous studies have shown that coexpressed β1- and β2-ARs can substitute, in part, for the β3-AR in mediating some effects of SNS input (2, 10, 39). Therefore, our goal was to examine whether the metabolic responses to dietary MR required signaling input through β-ARs, and if so, which components of the transcriptional response in BAT and WAT were linked to the physiological responses to the diet. Using β3-AR-null mice, pharmacological antagonists, and the tools of metabolic phenotyping, we find that the β3-AR is required for induction of UCP1 expression in BAT but not in WAT or to modify the endocrine function of WAT, increase EE, or limit fat deposition. However, we do find that a significant component of the effect of dietary MR on EE does require signaling input through the combined effects of β-AR subtypes expressed in adipose tissue.
MATERIALS AND METHODS
Animals and diets.
All experiments were reviewed and approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee on the basis of guidelines established by the National Research Council, the Animal Welfare Act, and the Public Health Service Policy on the humane care and use of laboratory animals. Breeding pairs of mice with targeted deletion of the β3-adrenergic receptor (β3-AR) were obtained from Dr. Brad Lowell (39) and used to establish a breeding colony within the Pennington Center Vivarium and produce the β3-AR−/− mice for the studies. The β3-AR−/− mice were originally produced on a FVB background, and wild-type FVB mice were obtained from Jackson Laboratories (Bar Harbor, ME) to produce age-matched control mice for our studies (WT). Two studies were conducted using 12-wk-old male mice of each genotype. At the initiation of each study, the mice were weighed, and body composition was determined by NMR using a Bruker Mouse Minispec (Bruker Optics, Billerica, MA). Before each use, the instrument was calibrated to an external standard, and body composition was determined according to manufacturer's instructions. Thereafter, mice in each experiment were randomly assigned to receive purified diets manufactured by Dyets (Bethlehem, PA) that contained 0.86% methionine (control diet) or 0.17% methionine (MR diet). The pelleted diets were provided ad libitum, and 48-h food consumption, body weight, and body composition were determined weekly for the duration of the 10- to 12-wk study. The mice were singly housed, and food consumption was measured at specific intervals in each experiment by weighing the food provided at the beginning of the feeding interval and weighing the unconsumed and wasted food 48 h later between 3 and 7 PM. The corncob bedding was sifted through wire mesh to retrieve and weigh any food pellets that were removed from the food dispenser but not consumed. There was no evidence that the food pellets were being shredded and not consumed by either group. The energy content of both control and MR diets was 15.96 kJ/g, with 18.9% of calories coming from fat (soybean oil), 64.9% from carbohydrate, and 14.8% from a custom mixture of l-amino acids as previously described (31, 33, 36). The amino acid content on a weight basis was 14.1%, as shown in Table 1 of our companion article (21a). Water was provided ad libitum to all treatment groups, and room temperature was maintained at 22–23°C, and lights were on 12 h/day from 7 AM to 7 PM.
Experiment 1.
Two groups of sixteen 12-wk-old WT and β3-AR−/− mice were randomly assigned to receive either the control or MR diet ad libitum for 10 wk. Food consumption and body composition were determined at weekly intervals, and body weight was measured twice a week. At the end of the 10-wk study, the mice were transferred to the Small Animal Phenotyping Core Facility at PBRC for measurement of voluntary activity and energy expenditure using a Comprehensive Laboratory Animal Monitoring System (CLAMS System, Columbus Instruments, Columbus, OH). The mice were acclimated in the metabolic chambers overnight prior to measurement of oxygen consumption (Vo2) and carbon dioxide production (Vco2) at 48-min intervals for 72 h. Physical activity was monitored, while the mice were in the chambers using an OPTO-M3 sensor system. At the end of the 4-day period, the mice were euthanized, and interscapular BAT was carefully dissected, and total RNA was isolated for measurement of UCP1 mRNA by quantitative RT-PCR or ribonuclease protection assay as before (42, 43). RQ was calculated as the ratio of Vco2 produced to Vo2 consumed, and EE was calculated as {Vo2 × [3.815 + (1.232 × RQ)] × 4.187} and expressed as kilojoules per kilogram fat-free mass (FFM) per hour (38).
Experiment 2.
Twelve-week-old β3-AR−/− mice were randomly assigned to receive either the control diet (n = 5), the control diet containing 500 μg/g dl-propranolol (n = 6), the MR diet (n = 5), or the MR diet containing 500 μg/g dl-propranolol (n = 6). At this concentration of propranolol in the diet, the dose averaged 40–50 μg dl-propranolol·day−1·g body wt−1 for mice on both diets, shown previously to block β1- and β2-ARs, but not the β3-AR (10). The respective diets for the four groups were provided ad libitum for 12 wk, with food consumption, body weight, and body composition determined at weekly intervals. Thereafter, voluntary activity and energy expenditure were measured as described in experiment 1, and BAT was harvested for measurement of UCP1 mRNA.
Methods of analysis.
Body weight and composition, food consumption, and UCP1 mRNA in experiment 1 were compared using a two-way ANOVA with genotype and diet as main effects. The genotype × diet interaction was tested using residual variance (animal within genotype × diet) as the error term, followed by post hoc testing for specific differences using the Bonferroni correction. The same variables were analyzed in experiment 2 using a two-way ANOVA, but here, dietary group and treatment were the main effects. The voluntary activity and calorimetric data (RQ and EE) in each phase of the light-dark cycle and spanning both phases of the diurnal cycle were tested for a diet × treatment interaction, and group means were compared as before using the Bonferroni correction. Protection against type I errors was set at 5% (α = 0.05).
RESULTS
Experiment 1.
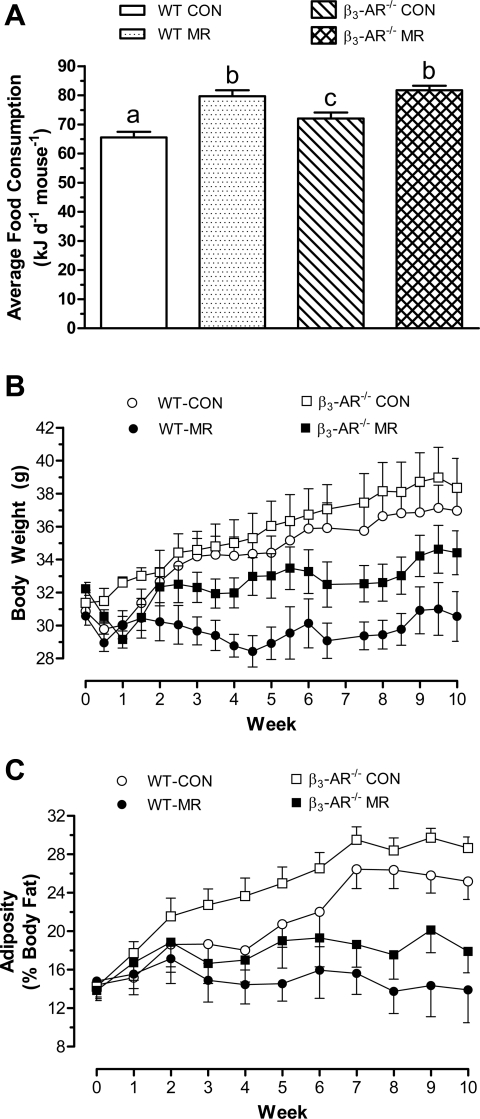
Our companion article (21a) shows that dietary MR produced coordinated changes in WAT endocrine function (i.e., decreased leptin and increased adiponectin), while increasing BAT UCP1 expression, core body temperature, food consumption, and total energy expenditure. Therefore, experiment 1 was undertaken to determine whether absence of the adipose tissue-specific β3-AR compromised the ability of dietary MR to affect the components of energy balance and modify the endocrine and metabolic function of WAT and BAT. Energy intake did not differ among the groups for the first 2–3 wk of the study, but thereafter, it was significantly higher in both MR groups compared with both groups on the control diet. Averaged over the entire study, energy intake in WT and β3-AR−/− mice on the MR diet was 21% and 25% higher than WT mice on the control diet (Fig. 1A). Energy intake in β3-AR−/− mice on the control diet was also significantly higher than WT mice on the control diet, making them intermediate between WT and β3-AR−/− mice on the MR diet and WT mice on the control diet (Fig. 1A). The initial body weights (BW) of WT and β3-AR−/− mice did not differ, and the BW of mice in both groups on the control diet increased in parallel over the 10-wk study (Fig. 1B). The β3-AR−/− mice appeared to be slightly larger at the end, but this difference was not significant (P > 0.05). Dietary MR resulted in a similar impairment of growth in both genotypes, with WT mice gaining essentially no weight and the 2 g increase in BW of β3-AR−/− mice during the last 2–3 wk, accounting for the group's entire increase in BW over the 10-wk study (Fig. 1B). Adiposity (fat mass expressed as a percentage of body weight) did not differ between WT and β3-AR−/− mice at the beginning of the study, but more than doubled over the 10-wk study in β3-AR−/− mice on the control diet (Fig. 1C). Fat accumulation in WT mice on the control diet occurred at a slower rate relative to β3-AR−/− mice during the first 4 wk of the study, but it increased substantially thereafter to finish the study with only slightly lower adiposity than the β3-AR−/− mice (Fig. 1C). Adiposity of WT mice on the MR diet at week 10 (13.9 ± 3.4%) was unchanged from their initial adiposity of 14.8 ± 2.0% (Fig. 1C), and although adiposity of β3-AR−/− mice on the MR diet appeared to be higher than WT mice for the last 5 wk of the study, the large standard errors attached to mean estimates precluded detection of this difference. Thus, the difference in adiposity between WT mice on the control and MR diets was comparable to the difference in adiposity between β3-AR−/− mice on the two diets (Fig. 1C). Together, these data show that despite fundamental differences between WT and β3-AR−/− mice in their size and nutrient partitioning during postweaning growth, the absence of the β3-AR did not compromise the ability of dietary MR to limit fat deposition or produce hyperphagia.
Fig. 1.
Postweaning energy intake (A), growth (B), and adiposity (C) of FVB wild-type (WT) and β3-adrenergic receptor (β3-AR)-null mice provided a control or methionine-restricted diet. Ten- to twelve-wk-old male FVB mice or FVB mice with targeted deletion of the β3-AR (27) were randomly assigned to groups that would receive a control diet containing 0.86% methionine (Control group) or a diet with methionine restriction (MR) to 0.17%. The diets were provided ad libitum, and food consumption, body weight, and body composition were measured in separate cohorts of 8 mice per genotype × diet combination. Energy intake (A), body weight (B), and adiposity (C) were measured at weekly intervals over the 10-wk duration of the study, as described in materials and methods. Response variables were analyzed by ANOVA. a,b,cMeans at each time point with letters that differ denote P < 0.05.
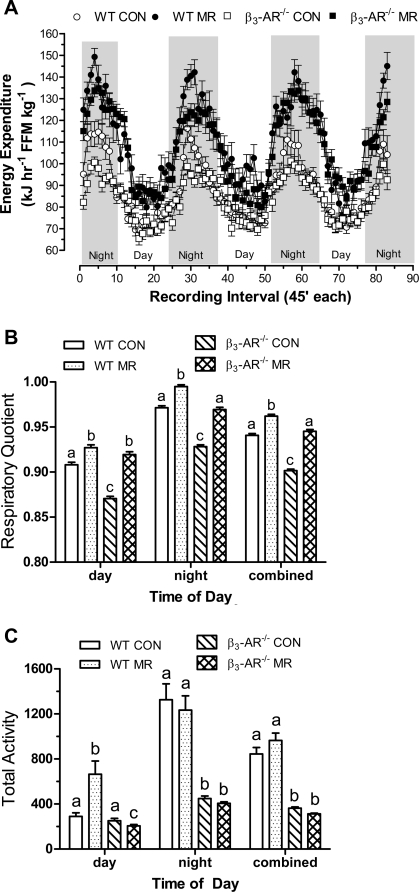
To test the role of the β3-AR in mediating the effects of MR on energy expenditure, indirect calorimetry was used to measure O2 consumption and CO2 production in WT and β3-AR−/− mice. Mean EE per unit of FFM changed during the diurnal cycle in a repeating pattern that was in phase with the light-dark cycle in all groups (Fig. 2A). EE was highest at night when mice were awake and eating, and lowest during the day when they normally sleep and consume small infrequent meals (Fig. 2A). In WT and β3-AR−/− mice on the control diet, mean EE was comparable during the day (WT: 75.0 ± 0.9 kJ·h−1·kg FFM−1; β3-AR−/−: 75.9 ± 0.7 kJ·h−1·kg FFM−1) but was significantly higher (P < 0.01) at night in WT (98.0 ± 1.6 kJ·h−1·kg FFM−1) compared with β3-AR−/− mice (90.7 ± 1.0 kJ·h−1·kg FFM−1). The difference represents a 31% increase in EE for WT mice during the day-to-night transition to the fed state compared with a 21% increase in EE for the β3-AR−/− mice (Fig. 2A). Mean EE during both day and night was significantly higher in WT and β3-AR−/− mice on the MR compared with the control diet (Fig. 2A), and dietary MR produced a comparable increase in EE in WT and β3-AR−/− mice during both day and night. In other words, the absence of the β3-AR did not compromise the ability of the MR diet to increase total daily EE in mice (Fig. 2A).
Fig. 2.
Energy expenditure (A), respiratory quotient (B), and total voluntary activity (C) in FVB wild-type (WT) and β3-AR-null mice provided a control or methionine-restricted diet for 10 wk. The mice were adapted to the calorimetry chambers for 24 h prior to measurement of O2 consumption, CO2 production, and voluntary activity for the following 4 days. Thereafter, blood and fat pads were harvested, and energy expenditure (EE) and respiratory quotient were calculated as described in materials and methods. Average EE was calculated for each animal in each group for the period when lights were on (7 AM–7 PM), when lights were off (7 PM–7 AM), and averaged over both periods to assess total daily EE. Total activity was averaged for each animal in each group for the period when lights were on or off and also averaged over both periods to assess total activity. For EE, RQ, and total activity, the day, night, and total averages for each response variable were compared by ANOVA. B and C: a,b,cMeans at each time of day with letters that differ denote P < 0.05.
The RQ provides a real-time index of fuel utilization during the metabolic cycle, and Fig. 2B shows that the measured changes in RQ over time were in phase with the diurnal fluctuation in EE among the groups. A ratio of CO2 produced to O2 consumed of 0.835 occurs when carbohydrate and fat utilization are equivalent (14, 26), with an increase or decrease in RQ indicative of a commensurate increase in utilization of carbohydrate or fat, respectively. Fig. 2B illustrates a fundamental difference between fuel selection in WT and β3-AR−/− mice in that β3-AR−/− mice use a higher proportion of fat during both day and night (Fig. 2B). Dietary MR increased carbohydrate use in both genotypes during both periods, but the overall increase in β3-AR−/− mice (4.4%) was twofold greater than in WT mice (2.1%, Fig. 2B). Despite this difference in response to MR, relative carbohydrate utilization in WT mice (RQ: 0.962 ± 0.002) was still significantly greater (P < 0.01) than β3-AR−/− mice (RQ: 0.945 ± 0.002) at night (Fig. 2B). Together, these data illustrate that the absence of the β3-AR did not compromise the ability of dietary MR to increase total daily EE in mice. However, they do indicate a key role for the β3-AR in overall fuel selection during the diurnal cycle that dietary MR appears to normalize during the day but not entirely at night.
Measures of voluntary activity were obtained to assess its potential contribution to differences in EE between genotypes and diets. During the day, total activity did not differ between WT and β3-AR−/− mice on the control diet (Fig. 2C), but the MR diet increased activity approximately twofold in WT mice, whereas in the β3-AR−/− mice, total activity was decreased by 13% (Fig. 2C). WT mice on the control diet increased their night-time voluntary activity by 4.6-fold, whereas the increase in β3-AR−/− mice was only 1.8-fold (Fig. 2C). The MR diet had no effect on night-time activity in either genotype (Fig. 2C), although the actual day-to-night fold increase in WT mice on the MR diet (1.9-fold) was less than WT mice on the control diet (4.6-fold). In β3-AR−/− mice, the day-to-night increase in activity was comparable between the control (1.6-fold) and MR diets (2.0-fold, Fig. 2C). When day and night activity was combined, the MR diet had no effect on voluntary activity in either genotype, but WT mice were 2.7-fold more active than β3-AR−/− mice (Fig. 2C).
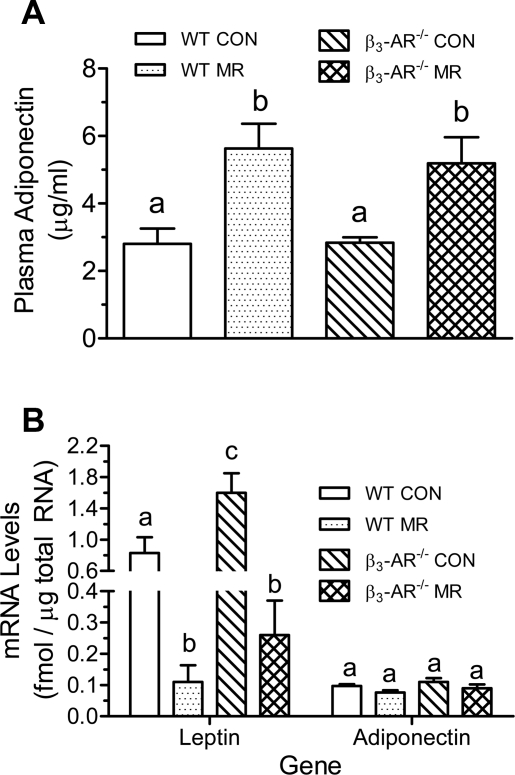
Our companion study (21a) and previous studies (31) have shown that in rats, dietary MR alters endocrine function of WAT by reciprocally regulating adiponectin and leptin. We measured plasma adiponectin to test for comparable changes in mouse WAT and whether the β3-AR is required to affect these changes. After 10 wk, plasma adiponectin did not differ between WT and β3-AR−/− mice on the control diet but was increased twofold by dietary MR in both genotypes (Fig. 3A). The change in plasma adiponectin occurred without a change in transcription of the gene, as adiponectin mRNA in inguinal WAT did not differ between WT and β3-AR−/− mice on either the control or MR diets (Fig. 3B). In contast, leptin mRNA was increased in WAT from β3-AR−/− mice on the control diet, but the absence of the β3-AR did not compromise the ability of MR to reduce leptin expression in WAT, as MR produced a comparable 6- to 8-fold reduction in leptin mRNA in both WT and β3-AR−/− mice (Fig. 3B).
Fig. 3.
Alteration of white adipose tissue endocrine function in FVB WT and β3-AR null mice provided a control or methionine-restricted diet for 10 wk. Plasma adiponectin (A) and mRNA levels for adiponertin and leptin in inguinal white adipose tissue (B) were measured in tissues harvested after 10 wk on the diets, as described in materials and methods. Response variables were analyzed by ANOVA. a,b,cMeans with letters that differ denote P < 0.05.
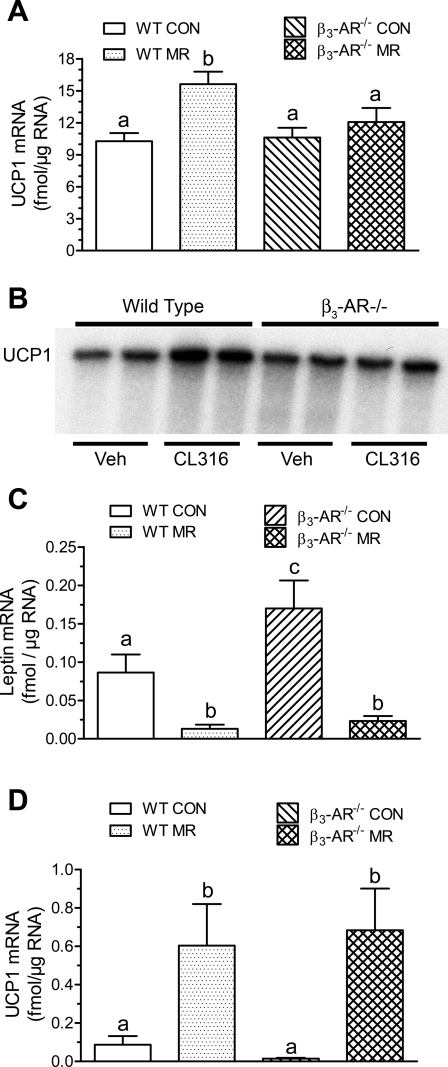
We also examined UCP1 expression in BAT and WAT, as the β3-AR is known to link SNS stimulation of BAT with transcriptional activation of the UCP1 gene under conditions of chronic sympathetic input (30). Given the increase in UCP1 expression produced by dietary MR in both tissues from rats in our companion study (21a), the objective of the present study was to assess the involvement of β3-AR signaling in the response in mouse adipose tissue. Fig. 4A shows that UCP1 mRNA did not differ in BAT from WT or β3-AR−/− mice on the control diet, but it was significantly increased in BAT from WT mice on the MR diet. In contrast, dietary MR failed to increase UCP1 mRNA in BAT of β3-AR−/− mice. To further assess the contribution of β3-AR signaling to UCP1 induction in BAT, the response of WT and β3-AR−/− mice to a selective agonist of the β3-AR (CL-316,243) was examined. In BAT from each genotype treated for 24 h with vehicle or CL-316,243, UCP1 mRNA expression was comparable in WT and β3-AR−/− mice treated with vehicle (Fig. 4B). However, the β3-AR agonist produced a twofold induction of UCP1 mRNA in WT mice but had no effect in β3-AR−/− mice (Fig. 4B). This agrees with findings published in the original description of the β3-AR−/− mice (39), which also showed that short-term cold exposure induced UCP1 in BAT via β1-AR/β2-ARs. To test the possibility that chronic consumption of the MR diet led to desensitization of β1-AR/β2-ARs in β3-AR−/− mice, we examined leptin mRNA expression in BAT, which is negatively regulated by SNS input in both BAT and WAT. Consistent with Fig. 3B and as previously reported for WAT (10), leptin mRNA was significantly increased in BAT from β3-AR−/− on the control diet (Fig. 4C). This increase illustrates the important role of signaling through the β3-AR in regulating leptin expression in both BAT and WAT. However, as also shown in Fig. 4C, dietary MR produced a significant decrease in leptin mRNA in BAT of WT mice that was comparable to that seen in WAT (Fig. 3B). More importantly, the absence of the β3-AR did not compromise this response to the diet in BAT (Fig. 4C), supporting the view that signaling through β1-AR/β2-ARs was uncompromised by 10 wk of dietary MR. Lastly, to test whether the failure of dietary MR to induce UCP1 mRNA in BAT was gene specific rather than tissue specific, we examined the effect of dietary MR on UCP1 mRNA in WAT. Fig. 4D shows that dietary MR produced a 6- to 10-fold induction of UCP1 mRNA in inguinal WAT of both WT and β3-AR−/− mice. This finding is consistent with our interpretation of Figs. 3B and 4C, namely that signaling through β1-AR/β2-ARs remained intact in both BAT and WAT and was unlikely to be responsible for the failure of dietary MR to induce UCP1 mRNA in BAT from β3-AR−/− mice.
Fig. 4.
Regulation of uncoupling protein (UCP1) and leptin mRNA in brown adipose tissue (BAT; A, C) and white adipose tissue (WAT; D) by dietary MR or selective agonist of β3-AR (B) in WT and β3-AR-null mice. UCP1 and leptin mRNA levels were measured by real-time PCR (A, C, D) or ribonuclease protection assay (B), as described in materials and methods in BAT or inguinal WAT harvested at the end of experiment 1. The increase in UCP1 mRNA in response to CL316,243 was quantitated by densitometry and increased 2.1 ± 0.2-fold in WT mice and 1.1 ± 0.3-fold in β3-AR null mice (B). Means of UCP1 and leptin mRNA measured by real-time PCR were compared by ANOVA. a,b,cMeans within each experiment with letters that differ denote P < 0.05.
Experiment 2.
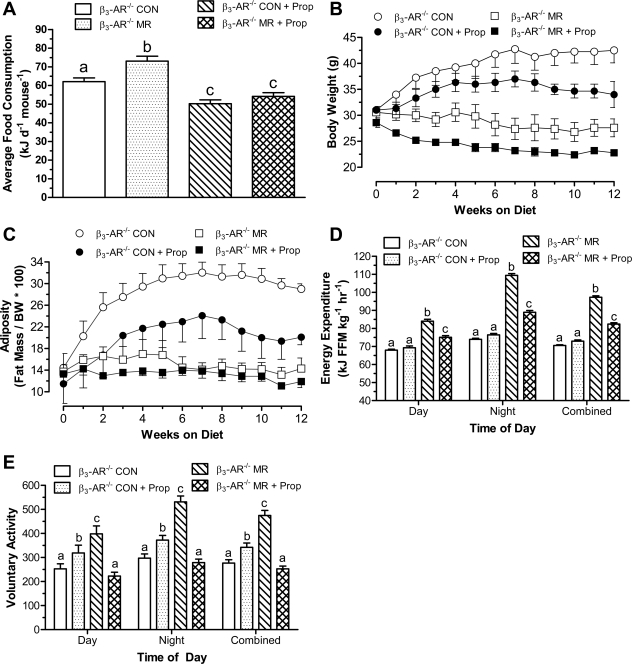
An additional experiment was conducted with β3-AR−/− mice treated with the β-AR antagonist propranolol to test whether blocking all β-adrenergic input to adipose tissue would alter the ability of dietary MR to affect energy homeostasis. Propranolol decreased consumption of the control diet by 21% over the 12-wk study in β3-AR−/− mice, while total energy intake increased 18% in vehicle-treated mice on the MR diet (Fig. 5A). Propranolol completely blocked the hyperphagic response to MR, such that energy intake did not differ between mice on the control and MR diets with propranolol (Fig. 5A). Mice consuming the control diet with vehicle increased BW from 31.0 ± 1.9 g to 42.5 ± 2.4 g over the course of the 12-wk study (Fig. 5B). The 21% reduction in consumption of the control diet containing propranolol translated into a 25% reduction in BW accumulation, with mice in this group ending the study at 34.0 ± 2.5 g (Fig. 5B). In contrast, the 18% higher energy intake of mice consuming the MR diet without propranolol resulted in no increase in BW over the same period (Fig. 5B). Stated another way, the 18% higher intake of the MR diet translated into ∼15 g less BW gain (Fig. 5B). Dietary MR also decreased metabolic efficiency in the presence of propranolol. This effect is clearly seen in the comparison between mice on control and MR diets with propranolol, where energy intake did not differ (Fig. 5A), but mice on the MR diet ended the study weighing 7 g less than mice on the control diet (Fig. 5B). Notwithstanding the effect of propranolol to reduce overall consumption of both diets, these findings show that propranolol diminished the efficacy of dietary MR to limit BW accumulation of β3-AR−/− mice.
Fig. 5.
Post-weaning energy intake (A), growth (B), adiposity (C), energy expenditure (D), and voluntary activity (E) of β3-AR-null mice provided a control or methionine-restricted diet with or without propranolol. Twenty-four 10- to 12-wk-old male FVB mice with targeted deletion of the β3-AR (27) were randomly assigned to four groups. Two of the groups received either a control diet containing 0.86% methionine (Control group) or the control diet formulated to contain 500 μg propranolol per gram of diet (Control + propranolol). The remaining two groups received either a diet with methionine restricted to 0.17% (MR) or the MR diet formulated to contain 500 μg propranolol per gram of diet (MR + propranolol). The diets were provided ad libitum for 12 wk. Energy intake (A), body weight (B), body composition (C) were measured at weekly intervals for 12 wk, as described in materials and methods. Thereafter, mice were adapted to calorimetry chambers for 24 h prior to measurement of O2 consumption, CO2 production, and voluntary activity for the following 4 days. Thereafter, fat pads were harvested. Average EE was calculated for each animal in each group for the period when lights were on (7 AM–7 PM), when lights were off (7 PM–7 AM), and averaged over both periods to assess total daily EE. Total activity was averaged for each animal in each group for the period when lights were on or off and also averaged over both periods to assess total activity. Response variables were analyzed by ANOVA. a,b,cMeans at each time point with letters that differ denote P < 0.05.
Weekly measurements of body composition showed that dietary MR completely abrogated the fat accretion observed in β3-AR−/− mice on the control diet (Fig. 5C), with adiposity that was essentially unchanged over the course of the 12-wk study. The reduction in consumption (21%) of the control diet by propranolol translated into a 17% reduction of fat accumulation over the course of the study (Fig. 5C). The proportionality of the decrease in energy intake, BW, and fat accumulation in this group suggests that the antagonist had little or no effect on energy balance beyond its effect to reduce energy intake. In contrast, adiposity was essentially unchanged in mice consuming the MR diet without propranolol, despite their 18% higher rate of energy intake (Fig. 5, A and C). As noted above for BW, the contrast between mice consuming the control and MR diets with propranolol is particularly instructive because it shows that at equivalent energy intakes, adiposity of mice on the MR diet was 5.2% less (Fig. 5C). The difference in adiposity between mice consuming the control and MR diets without propranolol was 14.7% (Fig. 5C), but the 18% higher energy intake of the MR diet in the absence of propranolol must be considered when assessing the full impact of the diet on energy balance (Fig. 5A). Overall, after accounting for these differences in energy intake, the findings provide strong support for the conclusion that propranolol blocked at least half of the effect of dietary MR to limit BW and fat accumulation.
In the absence of propranolol, dietary MR increased total daily EE by an average of 36%, and the addition of propranolol to the MR diet blocked greater than 50% of this increase during both day and night (Fig. 5D). In contrast, propranolol had no effect on EE during either the day or night in mice consuming the control diet (Fig. 5D). This finding is consistent with our earlier suggestion that propranolol had no metabolic effect in β3-AR−/− mice independent of its effect on energy intake. Collectively, these findings suggest that the combined signaling through β-ARs is an important component of the mechanism used by dietary MR to increase EE and limit fat accretion.
Total voluntary activity was increased 23% by propranolol in β3-AR−/− mice on the control diet, and the increase was similar during both day and night (Fig. 5E). Relative to mice on the control diet, Fig. 5E shows that voluntary activity was also higher in the MR group during both day (64%) and night (79%). However, propranolol completely blocked the effect of MR to increase voluntary activity, and activity levels in this group did not differ from mice on the control diet without propranolol. In the presence of propranolol, activity of mice on the control diet was modestly higher than the MR group (Fig. 5E).
Lastly, the ability of dietary MR to induce UCP1 mRNA expression in both BAT and WAT was examined in β3-AR−/− mice after blocking signaling through β1-AR/β2-ARs with propranolol. As noted in experiment 1, UCP1 mRNA was not induced in BAT of β3-AR−/− mice by dietary MR (6.5 ± 0.6 fmol/μg RNA) relative to the control diet (6.1 ± 0.8 fmol/μg RNA), and the addition of propranolol to the control and MR diets reduced UCP1 mRNA to 5.4 ± 0.8 fmol/μg and 5.4 ± 0.6 fmol/μg RNA, respectively. In contrast to BAT, dietary MR produced a 10-fold induction of UCP1 mRNA in inguinal WAT of β3-AR−/− mice in the absence of propranolol (control: 0.076 ± 0.049 fmol/μg RNA; MR: 0.86 fmol/μg RNA). The addition of propranolol to the control and MR diets reduced UCP1 mRNA in inguinal WAT from the two groups of mice to 0.051 ± 0.021 and 0.089 ± 0.022 fmol/μg RNA, respectively. These data illustrate that after pharmacologic inhibition of β1-AR/β2-ARs in β3-AR−/− mice, induction of UCP1 mRNA in response to dietary MR does not occur in either BAT or WAT.
DISCUSSION
The findings from this and our companion article (21a) show that dietary MR produced a coordinated set of changes in WAT (i.e., decreased leptin and increased UCP1) and BAT (increased UCP1) that are indicative of a diet-induced increase in SNS stimulation of adipose tissue (9, 10). Moreover, dietary MR produced a coordinated set of physiological changes (increased EE and core body temperature) that effectively limited fat accretion despite also increasing energy intake. The sensing, signaling, and effector systems that coordinate this integrated set of responses are poorly defined and are the subject of the present work. The complementary findings from our rat and mouse studies make a compelling case that dietary MR limits fat deposition by uncoupling respiration and decreasing metabolic efficiency. An important unresolved question is whether the transcriptional responses in BAT and WAT are linked to and necessary for the physiological effects of the diet on energy balance. To explore this hypothesis, we examined the ability of dietary MR to recapitulate each element of the overall response in mice lacking the β3-AR, in conjunction with experiments involving pharmacological blockade of the remaining β1-AR and β2-AR subtypes. The original description of the β3-AR−/− mouse line used here showed that the β3-AR and β1-AR/β2-ARs were interchangeable with respect to mediating acute effects of SNS input in adipose tissue (39), whereas deletion of all three β-AR subtypes produced mice that were cold-sensitive, refractive to sympathomimetics, and obesity-prone (2). A second component of our rationale is that β3-ARs lack the phosphorylation site in the third intracellular loop of β1-AR/β2-ARs that render them sensitive to desensitization by β-AR kinases and PKA (5, 40). Third, our companion article (21a) provides evidence that dietary MR produces a chronic long-term increase in SNS input to adipose tissue, suggesting a physiological environment in which signaling through the β3-AR could become essential if desensitization of the β1-AR/β2-ARs occurred. Therefore, examining the responses of β3-AR−/− and WT mice to dietary MR after 3 mo tested whether the absence of the β3-AR under these conditions compromised any of the physiological or molecular components of the response. Given the metabolic phenotype of β-AR-less mice (2), extending our design to remove signaling through the remaining β1-AR/β2-AR subtypes with propranolol was intended to test the overall significance of SNS input and β-AR signaling in mediating the responses produced by dietary MR.
The most significant findings from the present study are that the absence of the β3-AR did not compromise the ability of dietary MR to increase energy intake, increase EE, or limit fat deposition. The absence of the β3-AR did block the induction of UCP1 mRNA in BAT but not WAT, whereas inhibition of signaling through the remaining β1- and β2-AR subtypes with propranolol blocked over 50% of the effect of dietary MR on EE and fat accumulation, and blocked the induction of UCP1 in WAT. Interpreted alongside results presented in our companion article (21a), the findings support the view that 1) dietary MR produced a chronic increase in SNS outflow to adipose tissue, 2) a significant component of the mechanism engaged by dietary MR to increase EE involves signaling through β-ARs, 3) transcriptional activation of the UCP1 gene by dietary MR may contribute to but is not essential to the ability of the diet to increase EE and limit fat deposition, 4) the absence of the β3-AR does not compromise the effect of dietary MR on EE or most of the transcriptional effects in WAT or BAT, induction of UCP1 mRNA in BAT being the exception, and 5) the increase in EE produced by dietary MR does not require an increase in energy intake.
Rats, mice, golden hamsters, and hibernating animals are among a subgroup of species that express the β3-AR in BAT and WAT at high levels relative to the β1-AR and β2-AR (8, 18, 30), whereas other species (humans, monkeys, guinea pigs, dogs) are partially or minimally responsive to selective agonists of the β3-AR (25, 30, 40). In the subgroup that expresses all three β-AR subtypes, the combination of unequal expression and their differing affinities for endogenous ligands makes it difficult to assess the relative contribution of each subtype to signal translation, but the higher affinity of β1-AR and β2-AR subtypes for endogenous catecholamines results in their selective recruitment at low norepinephrine concentrations (7, 30). The higher synaptic concentrations of norepinephrine produced by intense or chronic SNS activation recruits the β3-AR to the response (6, 7, 30) and can also lead to desensitization of β1-AR and β2-AR subtypes (3, 15, 17). The response of adipocytes to catecholamines is also influenced by signaling input from α-adrenergic receptor subtypes, where activation of α2-ARs by norepinephrine inhibits adenylcyclase (29). In primates and various other species, increased expression of α2-ARs relative to β-ARs can transform SNS input from a primarily lipolytic into an antilipolytic signal (29, 30). Although antagonistic input from α-ARs in adipocyte signaling appears minimal in rodents (7, 16), it follows that translation of SNS input and therefore the response to dietary MR could differ in species that express a significant complement of α2-ARs in adipose tissue. To our knowledge, species heterogeneity in responsiveness to dietary MR has not been evaluated, although a preclinical trial of dietary MR in adult humans was reported to produce a sustained decrease in body mass index over the 17-wk course of the study (13). Its limitations notwithstanding, this study suggests that the efficacy of dietary MR to reduce fat accretion may not be limited to rodents.
On its surface, the failure of dietary MR to increase UCP1 mRNA in BAT of β3-AR−/− mice suggests that UCP1 is not involved in the diet-induced decrease in metabolic efficiency. However, two related observations merit consideration. First, our finding that dietary MR decreased leptin mRNA in BAT of β3-AR−/− mice indicates that signaling through β1-AR/β2-ARs is uncompromised by the diet in these mice. The data further suggest that the difference in BAT of β3-AR−/− mice, which prevented induction of UCP1 mRNA, was downstream of MR-induced β-AR and PKA activation, and it would not prevent the same diet-induced activation of β1-AR/β2-ARs in BAT from increasing uncoupled oxidation of lipid by posttranslational activation of endogenous UCP1 in that tissue. In contrast, dietary MR increased UCP1 mRNA by 5- to 10-fold in WAT of β3-AR−/− mice, as it did in all four dissectable WAT depots in rats. This response is also seen in rats and mice after cold exposure or treatment with β3-AR agonists, and the translated protein can be detected in islands of multilocular adipocytes within WAT depots (19, 20, 22–23). The physiological significance of UCP1 induction in WAT is difficult to assess directly, but a substantial literature supports the conclusion that increases in ectopic UCP1 expression in WAT have a significant effect on energy balance (4, 11, 27–28, 37). The common finding in these studies was that the observed increase in cAMP signaling or β-AR sensitivity, which induced UCP1 expression in WAT produced a similar lean, obesity-resistant phenotype. The metabolic phenotype in gain of function models expressing UCP1 from the aP2 promoter in WAT was also lean and obesity resistant (27, 28). However, our findings also suggest that dietary MR works through UCP1-independent mechanisms, and the metabolic phenotype of UCP1-null mice clearly illustrate that alternative, energy-wasting thermogenic mechanisms exist and can be readily engaged (12, 41). Thus, the overall role of UCP1 in regulating body composition and defending body weight remains an open question (12, 24), as does the extent to which its induction and/or activation plays a role in the effects of dietary MR on EE. Our findings that propranolol blocked over 50% of the MR-induced increase in EE while also blocking the induction, and presumably the activation of UCP1 in β3-AR−/− mice is consistent with the conclusion that UCP1 mediates part of the increase in EE produced by MR. However, this conjecture is based on correlation of the two events and the known effects of SNS input in adipose tissue. The best test of this hypothesis will require evaluating the ability of dietary MR to increase EE and limit fat deposition in the absence of UCP1. This approach will provide a direct assessment of the relative contributions of UCP1-dependent and independent mechanisms, the latter inferred from the retention of part of the diet-induced increase in EE in mice receiving propranolol. Overall, the data are uniformly consistent with the view that dietary MR engages multiple mechanisms to decrease the efficiency of fuel oxidation and increase total EE.
A potential caveat in our studies with propranolol was that it decreased energy intake in β3-AR−/− mice on both control and MR diets. The antagonist prevented the hyperphagic response to dietary MR in β3-AR−/− mice, but it also reduced consumption of the control diet in β3-AR−/− mice, such that energy intake in these two groups did not differ. The associated decrease in BW and fat accumulation in the Control group was proportional to the decrease in EE, suggesting no metabolic effect of propranolol independent of its anorexigenic effect. In contrast, at the same level of intake, β3-AR−/− mice on the MR diet with propranolol accumulated 13 g less fat and 11 g less BW. Although the mechanism of this component of the diet's effect on energy balance is unclear, future experiments with UCP1-null mice will provide a less confounded background for identification of the affected systems.
A key goal of our studies was to test whether dietary MR produced changes in voluntary activity that could contribute to the increase in total EE produced by the diet. In each experiment, activity was continuously measured over a 3- to 4-day period, while mice were in the metabolic chambers. Given that mice typically sleep during the day and feed at night, we expected a day-to-night increase in ambulatory activity. In experiment 1, voluntary activity of WT mice on the control diet increased four-fold between day and night, whereas night-time activity in β3-AR−/− mice on the control diet increased less than twofold. Averaged over day and night, total activity was three-fold higher in WT than β3-AR−/− mice, and the MR diet had no effect on total activity of either genotype. In experiement 2, day, night, and total voluntary activity were increased by ∼70% by dietary MR in β3-AR−/− mice without propranolol, whereas with the addition of propranolol, dietary MR produced a modest reduction in activity (Fig. 5D). This differs from experiment 1, where there was no effect of dietary MR on activity in β3-AR−/− mice. It is difficult to assess the contribution of differences in voluntary activity to differences in EE, particularly at night when the diurnal increase in EE is confounded by the heat increment of feeding and the effect of dietary MR on metabolic efficiency. Measurements of day-time EE are less confounded by the heat increment of feeding, so we initially compared the relationship between diet-induced effects on EE and diet-induced effects on activity in experiments 1 and 2. MR increased day-time EE by 23% in WT mice in experiment 1, and by 22% and 24% in β3-AR−/− mice in experiments 1 and 2, respectively. MR increased day-time activity by twofold in WT mice, had no effect in β3-AR−/− mice in experiment 1, and increased activity by 64% in experiment 2. The comparisons reveal no relationship between EE and activity among the groups, which would suggest that dietary MR is affecting EE by affecting activity. A similar analysis of night-time activity and EE among the groups supports the same conclusion, namely that dietary MR increases EE through a mechanism that does not involve changes in activity. These data and data from our companion article (21a) in rats provide no evidence that an increase in voluntary activity is the basis for the increase in EE by MR.
Two responses to dietary MR common to both rats and mice are increased plasma adiponectin and decreased leptin. Both responses are uncompromised in β3-AR−/− mice but appear to be mediated through separate mechanisms. The increase in total plasma adiponectin produced by dietary MR in rats and mice does not appear to involve a change in transcription of the gene. In both species and in all WAT depots surveyed to date, the consistent increase in circulating adiponectin has never been associated with any increase in adiponectin mRNA. Collectively, our findings are most consistent with a posttranscriptional effect of dietary MR that produces a rapid and persistent increase in circulating adiponectin without any change in adiponectin mRNA. It is also possible that the MR diet increases plasma adiponectin through effects on clearance, although recent work suggests this as an unlikely mechanism (21). In addition, we do not know whether the increase in total adiponectin measured by our assay represents an increase in the biologically active, high molecular form (1). However, the improved insulin sensitivity and metabolic flexibility observed in our earlier studies with dietary MR (31) are consistent with an increase in the biologically active form of the protein.
The biological responses to dietary MR are also consistent in many ways with the specific responses of peripheral tissues to adiponectin (32, 35) and include enhanced insulin sensitivity, reduced circulating triglycerides, and enhanced AMPK activation in WAT (31, 34). The present work builds upon these findings by showing that the enhanced oxidation of metabolic fuels in both fed and fasted states is uncoupled to a degree that restricts deposition of fat despite a significant increase in energy intake. Previous work has shown that intracerebroventricular or intravenous injection of adiponectin increased oxygen consumption and fat oxidation and reduced body weight in mice without affecting food consumption (35). Thus, it is attractive to propose that some of the metabolic effects of dietary MR are mediated by increasing adiponectin release from adipose tissue. The effects of dietary MR on leptin, adiponectin, and UCP1 suggest roles of these adipocyte genes as partial mediators of the physiological responses to dietary MR. It will be important in future studies to evaluate the role of each using loss-of-function approaches with the diet.
Perspectives and Significance
The cloning of the ob gene in 1994 identified leptin as a key component of the complex network of sensing, signaling, and effector systems that function in an integrated manner to regulate energy balance in higher animals. The findings presented herein show that dietary MR produces an integrated series of molecular, cellular, and physiological responses that have a profound impact on both the energy intake and expenditure components of the energy balance equation. The net effect of these responses is a decrease in metabolic efficiency and a corresponding decrease in fat accretion in adipose tissue. The studies reported here describe our initial efforts to identify the signaling systems that link the cellular and molecular responses to dietary MR to the physiological responses to the diet. Important future goals are to unravel the mechanisms engaged by dietary MR that simultaneously enhance fuel oxidation and uncouple respiration, understand where and how the reduction in dietary methionine content is initially sensed, and identify the communication networks that affect and coordinate the integrated set of physiological responses to the diet.
GRANTS
This work was supported by the Orentreich Family Foundation, and, in part, by the National Center for Complementary and Alternative Medicine (P50-AT 002776-01) and by National Institutes of Health (NIH) grant P20-RR 021945 (T. W. Gettys) from the National Center for Research Resources, NIH Nutrition Obesity Research Center Grant 1P30 DK 072476, and NIH RO1 074772 (T. W. Gettys).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Aaron Adamson, Amanda Laque, Jeri Gomez, Meghan Vial, Daniel Cason, and Dean Listi (Pennington Biomedical Research Center) for excellent technical contributions, Dr. Michael Pellizzon for advice in formulating the diets (Research Diets), and Anne Gooch and Meghan Greeley for outstanding administrative support.
REFERENCES
- 1.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, Inukai T, Nakano Y. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes 55: 1954–1960, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. Beta-AR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Carpéné C, Galitzky J, Collon P, Esclapez F, Dauzats M, Lafontan M. Desensitization of β-1 and β-2, but not β-3, adrenoceptor-mediated lipolytic responses of adipocytes after long-term norepinephrine infusion. J Pharmacol Exp Ther 265: 237–247, 1993 [PubMed] [Google Scholar]
- 4.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106: 563–573, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhry A, Granneman JG. Influence of cell type upon the desensitization of the beta 3- adrenergic receptor. J Pharmacol Exp Ther 271: 1253–1258, 1994 [PubMed] [Google Scholar]
- 6.Chaudhry A, Granneman JG. Differential regulation of functional responses by β-adrenergic receptor subtypes in brown adipocytes. Am J Physiol Regul Integr Comp Physiol 277: R147–R153, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the β-3 and β-1 adrenergic receptor in adipose tissue of congenitally obese (C57BLJ6-ob/ob) mice. Mol Endocrinol 8: 518–527, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Collins S, Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res 56: 309–328, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter RD, Gettys TW. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology 140: 4772–4776, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different β-adrenoceptor subtypes. J Biol Chem 275: 33059–33067, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda R, McKnight GS. Genetically lean mice result from targeted disruption of the RIIβ subunit of protein kinase A. Nature 382: 622–626, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Epner DE, Morrow S, Wilcox M, Houghton JL. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr Cancer 42: 158–166, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Flatt JP. Dietary fat, carbohydrate balance, and weight maintenance. Ann NY Acad Sci 683: 122–140, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. Phosphorylation and desensitization of the human beta 1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J Biol Chem 270: 17953–17961, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Gettys TW, Rohlfs EM, Prpic V, Daniel KW, Taylor IL, Collins S. Age-dependent changes in β-adrenergic receptor subtypes and adenylyl cyclase activation in adipocytes from Fischer 344 rats. Endocrinology 136: 2022–2032, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Granneman JG. Effects of agonist exposure on the coupling of beta1 and beta3 adrenergic receptors to adenylyl cyclase in isolated adipocytes. J Pharmacol Exp Ther 261: 638–642, 1992 [PubMed] [Google Scholar]
- 18.Granneman JG, Lahners KN. Differential adrenergic regulation of β-1 and β-3 adrenoceptor messenger ribonucleic acids in adipose tissues. Endocrinology 130: 109–114, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor aActivation. Am J Physiol Endocrinol Metab 289: E608–E616, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control: Effects on body weight and adiposity. J Clin Invest 102: 412–420, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 58: 1961–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, Shin J, Huypens P, Malloy VL, Plaisance EP, Krajcik RA, Orentreich N, Gettys TW. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol (June10, 2010). doi:10.1152/ajpregu.00837.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himms-Hagen J, Cui J, Danforth E, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic β3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol 266: R1371–R1382, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem 276: 12460–12465, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Ito M, Grujic D, Abel ED, Vidal-Puig A, Susulic VS, Lawitts J, Harper ME, Himms-Hagen J, Strosberg AD, Lowell BB. Mice expressing human but not murine β-adrenergic receptors under the control of human gene regulatory elements. Diabetes 47: 1464–1471, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Jequier E, Felber JP. Indirect calorimetry. Baillieres Clin Endocrinol Metab 1: 911–935, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 96: 2914–2923, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopecky J, Hodny Z, Rossmeisl M, Syrovy I, Kozak LP. Reduction of dietary obesity in aP2-UCP transgenic mice: physiology and adipose tissue distribution. Am J Physiol Endocrinol Metab 270: E768–E775, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lafontan M. Inhibition of epinephrine-induced lipolysis in isolated white adipocytes of aging rabbits by increased alpha-adrenergic responsiveness. J Lipid Res 20: 208–216, 1979 [PubMed] [Google Scholar]
- 30.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res 34: 1057–1091, 1993 [PubMed] [Google Scholar]
- 31.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5: 305–314, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 281: 2654–2660, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 123: 269–274, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Perrone CE, Mattocks DA, Hristopoulos G, Plummer JD, Krajcik RA, Orentreich N. Methionine restriction effects on 11-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J Lipid Res 49: 12–23, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med 10: 524–529, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8: 1302–1307, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Soloveva V, Graves RA, Rasnick MM, Spiegelman BM, Ross SR. Transgenic mice overexpressing the β1-adrenergic receptor in adipose tissue are resistant to obesity. Mol Endocrinol 11: 27–38, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A, Majewski M, Cefalu WT, Gettys TW. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metab Clin Exp 57: S39–S46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 270: 29483–29492, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Thomas RF, Liggett SB. Lack of beta 3-adrenergic receptor mRNA expression in adipose and other metabolic tissues in the adult human. Mol Pharmacol 43: 343–348, 1993 [PubMed] [Google Scholar]
- 41.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem 281: 31894–31908, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Watson PM, Commins SP, Beiler RJ, Hatcher HC, Gettys TW. Differential regulation of leptin release and function in A/J versus C57BL/6J mice during diet-induced obesity. Am J Physiol Endocrinol Metab 279: E356–E365, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, Mynatt RL, Gettys TW. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J 19: 1482–1491, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol 38: 47–52, 2003 [DOI] [PubMed] [Google Scholar]