Abstract
Synthetic glucocorticoids are commonly given to pregnant women when premature delivery threatens. Antenatal administration of clinically relevant doses of betamethasone to pregnant sheep causes sex-specific compromises of renal function and increases in blood pressure in adult offspring. However, it is unclear whether such effects are present in immature lambs. Therefore, the aims of the present study were to determine whether antenatal betamethasone at 80–81 days of gestation increases blood pressure and adversely impacts renal function in adolescent ewes and rams. Prenatal steroid exposure increased blood pressure significantly in the young male (84 ± 2 vs. 74 ± 3 mmHg) and female sheep (88 ± 5 vs. 79 ± 4), but it did not alter basal glomerular filtration rate, renal blood flow (RBF), or sodium excretion in either sex. However, antenatal betamethasone exposure blocked increases in RBF (P = 0.001), and enhanced excretion of an acute Na load (P < 0.05) in response to systemic infusions of angiotensin (ANG)-(1–7) at 10 pmol·kg−1·min−1 in males. In females, the natriuretic response to combined ANG-(1–7), and Na load was significantly altered by prenatal betamethasone exposure. These findings indicate that blood pressure is increased in immature animals in response to antenatal steroid exposure and that sex-specific effects on renal function also exist. These changes may reflect greater risk for further loss of renal function with age.
Keywords: prenatal steroid exposure, Na load, glomerular filtration rate, Na excretion, angiotensin-(1–7)
a significant development in perinatal care over the last 30 years has been the administration of antenatal steroid treatment to pregnant women at risk of premature delivery to facilitate fetal lung maturity (10, 34a). Although the benefits of such treatment are widely accepted, accumulating data from experimental animals and some clinical epidemiologic studies have led researchers to raise concerns about the possible untoward consequences of excess glucocorticoid exposure in the prenatal period (10, 34a).
We and others have demonstrated using rodent and sheep models that adult offspring from dams/ewes exposed to steroid during gestation have reductions in nephron number (36, 37, 58, 61), elevated blood pressure (13, 16, 20, 37, 47), and reductions in renal function (55). Interestingly, in some models of fetal programming, the reductions in renal function and/or hypertension in mature adults are less pronounced or even absent in younger animals (30, 42, 51). However, data from studies of human subjects suggest that antenatal steroid exposure increases blood pressure in adolescents (18). Therefore, a goal of the present studies was to determine whether administration of a clinically relevant dose of betamethasone to pregnant sheep at ∼0.6 gestation increases blood pressure in peripubertal offspring.
Data also indicate that components of the renal renin-angiotensin system (RAS) are altered by antenatal glucocorticoid exposure. For example, we have reported altered renocortical expression of angiotensin receptors with high angiotensin type 1 (AT1) and low angiotensin type 2 (AT2) receptors in nuclear and plasma membranes from proximal tubule cells obtained from offspring of prenatal steroid-treated ewes (23). Furthermore, we recently demonstrated a greater ratio of ACE/ACE2 activity induced by steroid exposure (48, 53), which would favor an increased ratio of renal ANG II to ANG-(1–7) (49). Such changes may explain the compromised Na excretion and ANG-(1–7)-induced natriuresis that we observed in adult sheep (55). Given that there could be fetal programming and age-related interactions affecting renal function (6, 11, 34), an additional goal of these studies was to determine whether antenatal betamethasone would alter Na excretion in adolescent animals. We studied both male and female lambs to see whether sex plays a role in young animals as sex-specific effects of fetal programming have been reported in adults (21, 22).
METHODS
Animals.
Mixed-breed, time-dated pregnant sheep obtained from local suppliers were maintained in open pasture with free access to food and water during pregnancy and lactation. The betamethasone dose given is analogous to that used in human pregnancy. Sheep were randomly assigned to two groups: one received two 0.17 mg/kg intramuscular injections of a 1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan, Schering, Kenilworth, NJ), while the other group received two vehicle injections, which contained 3.4 mg of monobasic sodium phosphate, 7.1 mg of dibasic sodium phosphate, 0.1 mg of sodium ethylenediaminetetraacetic acid, and 0.2 mg of benzalkonium chlorine per milliliter. Doses were given 24 h apart at days 80 and 81 of gestation (term is ∼145 days in our flock). Pregnancy was allowed to continue unimpeded and offspring were born naturally at term. Offspring were weaned at 3 mo of age and transferred to the laboratory at 6 mo of age for study. All procedures were approved by the Institutional Animal Care and Use Committee.
Surgery.
At 6 mo of age, vehicle and betamethasone-exposed sheep were brought into the air-conditioned, light-controlled laboratory, where temperature was maintained at 20 ± 2°C. Sheep were fed a standard commercial diet containing 0.75% NaCl and had ad libitum access to water. Prior to surgery, the animals were fasted for 24 h. General anesthesia was induced (isoflurane in oxygen), and polyvinyl catheters were inserted in the femoral arteries and veins and jugular vein. A Foley catheter was placed directly in the bladder via a suprapubic incision. The catheters were filled with 1,000 U/ml heparinized saline, closed with metal plugs, tunneled subcutaneously, exteriorized to the flank of the sheep and placed in a plastic ziplock bag. The Foley catheter allows continuous urine collection during the experiments without allowing volume accumulation in the bladder to promote spontaneous voiding.
Postoperative ampicillin (1 g) was administered for 3 days, and sheep were allowed to recover in large metal cages with free access to food and water for at least 5 days before experimentation.
Preparation of ANG-(1–7).
ANG-(1–7) was obtained from Bachem BioScience (King of Prussia, PA). The peptide was dissolved in distilled water and stored in 0.5 ml volumes (1 mg/ml) at −80°C until required. On the day of study, ANG-(1–7) stock was diluted in sterile isotonic saline to the required concentration.
Experimental protocol.
Sheep were housed in portable metabolic carts during the experiments and were studied on two separate days [day 1 control study, day 2 ANG-(1–7) study] with a 2- or 3-day washout period in between. All studies commenced between 0800 and 0900. During the first 2 h, urine was collected hourly. Intravenous infusions of inulin and p-aminohippuric acid (PAH) were then begun (details follow) and continued for 3 h. One hour into the inulin/PAH infusion, an infusion of hypertonic saline (0.0275 meq·kg−1·min−1) was started at a rate of 0.55 ml/min for 60 min. Following cessation of the saline infusion, the experiment continued for another hour. Blood and urine samples were carefully collected at intervals during the experiment for the measurement of inulin, PAH, and electrolytes. To ensure acquisition of fresh blood without saline dilution from the flush catheter, withdrawal of 4–5 ml of blood from the catheter preceded sample collection. After sample collection, this blood was returned to the animal. For the ANG-(1–7) study arm, the peptide was infused at the same time as PAH/inulin at a dose of 10 pmol·kg−1·min−1 for 2 h.
All blood and urine samples were centrifuged immediately after collection, and then they were aliquoted and stored at −80°C for later analysis. Urine volumes were noted.
Mean arterial blood pressure measurements.
Mean arterial pressure (MAP) was recorded through the femoral artery catheter between 0900 and 1500 using a Cobe transducer (World Precision Instruments, Sarasota, FL) connected to a DigiMed analyzer, which digitized the signal that was then recorded by computer using DMSI-400 software (DATAQ Instruments, Akron, OH). The equipment was balanced and calibrated every morning and afternoon.
Estimation of renal function indices.
Renal function was evaluated by assessing inulin and PAH steady-state clearance, as previously described (35, 46). Briefly, loading doses of 850 mg of inulin and 225 mg of PAH were given as bolus injections followed by constant intravenous infusions of 10 and 11 mg/min, respectively. Clearances of each were calculated by determining plasma concentrations of the indicators after the establishment of steady-state during the experiments. Under these conditions, the rate of renal elimination equals the rate of infusion when the plasma level reaches a steady-state (14, 29). The formula is expressed as C = (Ic × Iv)/S, where C is clearance, Ic is concentration of test substances in infusion fluid, Iv is velocity of infusion, and S is serum concentration. Plasma concentrations of inulin and PAH were measured using standard methods (14, 29). Clearances were calculated using 2–4 plasma samples taken 30 min apart. We assessed the accuracy of the steady state approach by comparing its results with those of the standard method (classic clearance method). These clearances and the classic clearances were similar during basal conditions and the Na load, e.g., clearances with PAH were 15.5 ± 1.0 vs. 14.5 ± 1.3 ml·min−1·kg−1 at baseline (P = 0.48, Student's t-test) and 16.5 ± 1.1 vs. 18.9 ± 1.4 ml·min−1·kg−1 (P = 0.14, Student's t-test) in Na load. The overall correlation in the results assessed by the two methods was significant (P < 0.0001, r = 0.7). Filtration fraction (FF) was calculated as glomerular filtration rate (GFR)/eRPF, where eRPF is estimated renal plasma flow. Renal vascular resistance (RVR) was estimated as [MAP × (1-Hct)]/eRPF, where Hct is hematocrit.
Plasma and urine electrolytes measurements.
Plasma and urinary concentrations of electrolytes were determined using a Medical Easy Lyte instrument (Bedford, MA). Electrolyte excretion was calculated by multiplying the urine volume by the urine electrolyte concentration. The percentage of the Na load excreted was calculated by dividing the quantity of Na excreted from the start of the hypertonic saline infusion until the end of the experiment by the total amount of Na given in the infusion and expressing that quotient as a percentage. The filtered sodium load (FLNa) was calculated as GFR × plasma sodium concentration and fractional reabsorption of sodium (FRNa) as (FLNa − UNaV)/ FLNa, where UNaV is urinary sodium excretion.
Statistical analysis.
Data are presented as means ± SE. Because there were three time points in the control and ANG-(1–7) infusion experiments, for statistical analyses, we used two-way ANOVA to examine the effect of betamethasone compared with vehicle group and the effect of ANG-(1–7) compared with control study. Comparisons of the three time points with each group (vehicle or betamethasone) were made by one-way ANOVA. The t-test was used to assess the difference in the means of two time points within one group. The level of significance was set at a probability of ≤0.05 for all tests.
RESULTS
Animal characteristics.
Body weights at birth and at the time of the experiments (6 mo of age) were not different in the vehicle and steroid groups (Table 1). Singletons comprised 22% of the vehicle-treated groups and 50% of the betamethasone-treated groups.
Table 1.
Animal characteristics
| Body Weight, kg |
|||
|---|---|---|---|
| S, T | Birth | Six-Month | |
| Males | |||
| Vehicle | 1, 4 | 4.0 ± 0.4 | 34.3 ± 3.7 |
| Beta | 3, 3 | 3.8 ± 0.3 | 37.8 ± 3.1 |
| Females | |||
| Vehicle | 1, 5 | 4.4 ± 0.4 | 46.6 ± 3.8 |
| Beta | 3, 3 | 4.0 ± 0.4 | 44.1 ± 1.6 |
Values are expressed as means ± SE. Beta, prenatal betamethasone exposed; S, T, singeleton, twins.
Hemodynamics.
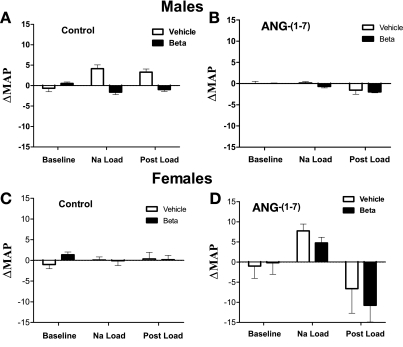
Basal MAP was significantly elevated in both female and male lambs exposed antenatally to betamethasone (males: 83.9 ± 1.6 vs. 73.7 ± 2.9 mmHg; females: 87.9 ± 4.8 vs. 78.6 ± 4.5 mmHg, P < 0.05). Figure 1 shows the changes in MAP (ΔMAP) during the experiments. In the males, there was a group (betamethasone, Beta) effect (F = 27.2, P < 0.0001), and a group × time interaction (F = 14.1, P < 0.0001) in the control experiments, while in the ANG-(1–7) studies only a time effect was noted (F = 7.2, P = 0.003). In females, there was no significant change in MAP during the control study in either group; however, there was an effect of time in the ANG-(1–7) infusion experiments (F = 7.1, P = 0.005).
Fig. 1.
Change in mean arterial pressure (ΔMAP) representing individual MAP changes over the baseline during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in male and female sheep prenatally exposed to vehicle (open bars, n = 5 or 6) or betamethasone (Beta; solid bars, n = 6). In males, there were effects of Beta during the control study (A), (F = 27.2, P < 0.0001) and a group × time interaction (F = 14.1, P < 0.0001). In the ANG-(1–7) study (B), there was an effect of time in both groups (F = 7.2, P = 0.003); In females, there was no change in MAP in the control study in either group (C). In the ANG-(1–7) study, there was an effect of time in both groups (D) (F = 7.1, P = 0.005).
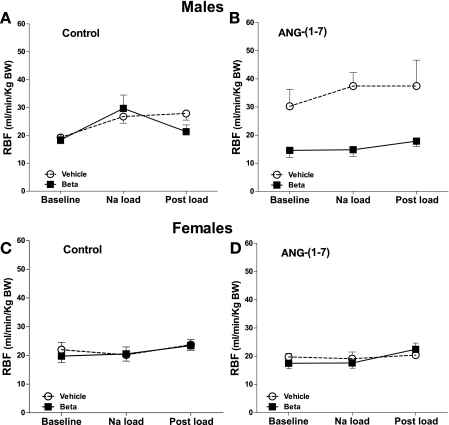
There were no significant differences in effective renal blood flow (RBF) adjusted by body weight between vehicle and betamethasone-treated males in basal conditions. An acute sodium load in the control study increased RBF in both vehicle and betamethasone-treated males (F = 5.2, P = 0.01) (Fig. 2A). There was a significant increase in RBF during the ANG-(1–7) infusion study in the vehicle-treated group compared with the control study (F = 6.1, P = 0.02) (Fig. 2B), but the Na load did not cause an additional increase The betamethasone-treated males showed no RBF responses to ANG-(1–7) or the Na load during the peptide infusion; thus, betamethasone exposure blocked the response to ANG-(1–7) (F = 24.6, P < 0.0001). Renal blood flow in the females was not different between the groups and did not change significantly with time (Fig. 2, C and D).
Fig. 2.
Estimated renal blood flow (eRBF) normalized for body weight during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in male (A, B) and female (C, D) sheep prenatally exposed to vehicle (○, n = 5 or 6) or Beta (■, n = 6). In males, there was an effect of time (Na load) on RBF in the control study (F = 5.2, P = 0.01). In ANG-(1–7) study, RBF increased in the vehicle-treated animals (F = 6.1, P = 0.02), and there was an effect of Beta (F = 24.6, P < 0.0001). No effects of Beta were noted in the females.
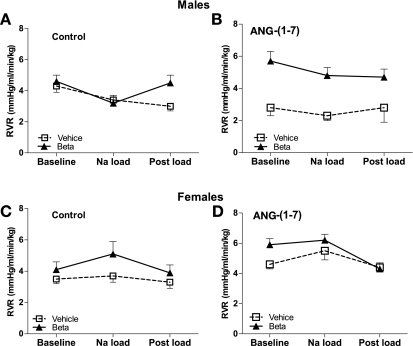
Figure 3 depicts RVR during control and ANG-(1–7) infusion studies. In the males, RVR decreased with the Na load in the control experiments (F = 3.8, P = 0.04). RVR was lower in the vehicle-treated animals receiving ANG-(1–7) compared with the betamethasone-exposed animals in the ANG-(1–7) experiments (F = 26.5, P < 0.0001) or the vehicle-treated animals (F = 5.0, P = 0.03) in the control experiments (Fig. 3, A and B). In females, there were minimal effects of Beta, but there was an effect of Na load (F = 6.7, P = 0.004) in the ANG-(1–7) experiments (Fig. 3, C and D).
Fig. 3.
Renal vascular resistance (RVR) during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in male (A, B) and female (C, D) sheep prenatally exposed to vehicle (□, n = 5 or 6) or Beta (▴, n = 6). In males, RVR decreased with the Na load in the control study (F = 3.8, P = 0.04). Compared with the control study, ANG-(1–7) decreased RVR in vehicle-treated animals (F = 5.0, P = 0.03). RVR was lower in vehicle than in Beta animals in the ANG-(1–7) studies (F = 26.5, P < 0.0001); In females, there was an effect of Na load in both groups in ANG-(1–7) studies (F = 6.7, P = 0.004).
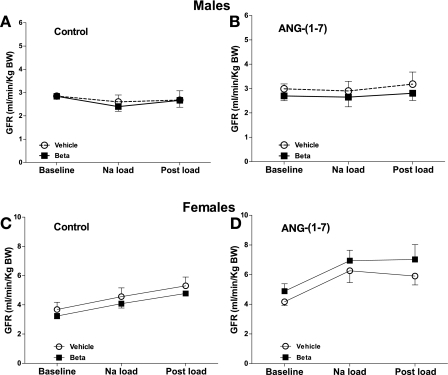
GFR.
GFR adjusted by body weight in male and female sheep is shown in Fig. 4. There was no significant effect of Beta on the adjusted GFR in the males or females. There was no response to sodium load and ANG-(1–7) infusion in males. In females, Na load significantly increased GFR in the control (F = 6.6, P = 0.004) and ANG-(1–7) infusion studies (F = 5.8, P = 0.01).
Fig. 4.
Glomerular filtration rate (GFR) normalized for body weight during saline (control) (A) or ANG-(1–7) (B) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in male (A, B) and female (C, D) sheep prenatally exposed to vehicle (○, n = 5 or 6) or Beta (■, n = 6). In females, there was an effect of Na load on GFR in both the control (F = 6.6, P = 0.004) and ANG-(1–7) infusion studies (F = 5.8, P = 0.01).
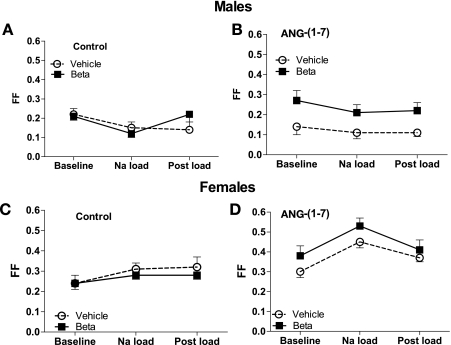
Filtration fraction.
Figure 5 shows filtration fraction (FF), during control and ANG-(1–7) infusion studies. Filtration fraction was higher in the Beta males than in the vehicle-treated males in the ANG-(1–7) experiments (F = 13.9, P = 0.001). Na load did not affect FF significantly in the males, although there was a tendency for it to decline in the control experiments (F = 3.3, P = 0.053). In the females, there was no effect of Beta in the control or ANG-(1–7) studies. In the ANG-(1–7) experiments, Na load elevated FF in both female groups (F = 7.1, P = 0.003), presumably reflecting increases in GFR.
Fig. 5.
Filtration fraction (FF) during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in female sheep prenatally exposed to vehicle (○, n = 5 or 6) or Beta (■, n = 6). In males, there was a Beta effect during ANG-(1–7) study (F = 13.9, P = 0.001); In females, Na load increased FF in both groups during ANG-(1–7) study (F = 7.1, P = 0.003).
Plasma and urinary electrolytes.
Tables 2 and 3 illustrate plasma and urinary sodium and potassium levels in males and females during the experiments. Overall, in males and females, plasma electrolyte levels were similar; no sex, group, time, or ANG-(1–7) effects were noted. ANG-(1–7) reduced urinary potassium levels in the vehicle (F = 11.8, P = 0.002) and Beta groups (F = 27.1, P < 0.0001). The [Na]/[K]urine ratio significantly increased after Na load in vehicle and Beta animals in the control study (male: F = 6.1, P = 0.007; female: F = 11.9, P = 0.002); ANG-(1–7) infusion increased the ratio only in females (vehicle: F = 8.4, P = 0.008; Beta: F = 8.5, P = 0.007). In males, vehicle but not Beta animals increased their [Na]/[K] ratio (F = 6.1, P = 0.007) following Na load during ANG-(1–7) infusion. In contrast, no Beta effect was seen in female animals.
Table 2.
Urinary and plasma Na and K concentrations in male sheep before and after Na load and Na load with ANG-(1–7) infusion
| Baseline |
Na load |
Post Na load |
||||
|---|---|---|---|---|---|---|
| Vehicle | Beta | Vehicle | Beta | Vehicle | Beta | |
| [Na]Urine, mmol/l | ||||||
| Control | 125.3 ± 42.0 | 110.8 ± 26.0 | 127.8 ± 28.0 | 95.1 ± 16.6 | 142.7 ± 20.0 | 150.2 ± 15.4 |
| ANG-(1–7) | 89.9 ± 15.7 | 155.2 ± 20.8 | 121.3 ± 15.6 | 119.8 ± 18.1 | 153.8 ± 49.0 | 144.0 ± 27.7 |
| [K]Urine, mmol/l | ||||||
| Control | 141.8 ± 60.0 | 157.9 ± 41.0 | 144.2 ± 54.0 | 156.0 ± 32.0 | 136.0 ± 52.0 | 152.2 ± 21.9 |
| ANG-(1–7) | 188.1 ± 36.0 | 173.3 ± 18.9 | 182.9 ± 33.0 | 174.2 ± 29.9 | 118.1 ± 18.0 | 189.2 ± 38.0 |
| [Na]Plasma, mmol/l | ||||||
| Control | 145.0 ± 0.9 | 140.0 ± 3.7 | 144.7 ± 1.1 | 145.5 ± 0.8 | 144.5 ± 0.7 | 142.2 ± 1.9 |
| ANG-(1–7) | 144.5 ± 0.9 | 142.9 ± 0.5 | 145.3 ± 1.5 | 144.6 ± 0.5 | 144.4 ± 1.1 | 144.8 ± 0.8 |
| [K]Plasma, mmol/l | ||||||
| Control | 4.37 ± 0.1 | 4.03 ± 0.2 | 3.99 ± 0.17 | 3.85 ± 0.1 | 4.04 ± 0.2 | 3.91 ± 0.1 |
| ANG-(1–7) | 4.14 ± 0.1 | 4.17 ± 0.1 | 4.05 ± 0.2 | 3.89 ± 0.2 | 4.18 ± 0.3 | 4.07 ± 0.2 |
| [Na]/[K]Urine ratio | ||||||
| Control | 0.79 ± 0.13 | 0.60 ± 0.10 | 0.80 ± 0.14 | 0.58 ± 0.09 | 1.05 ± 0.13 | 0.94 ± 0.10 |
| ANG-(1–7) | 0.49 ± 0.10 | 0.92 ± 0.19 | 0.68 ± 0.11 | 0.60 ± 0.12 | 1.22 ± 0.26 | 0.79 ± 0.16 |
Values are expressed as means ± SE for n =5 or 6 sheep. Group effect in [Na]/[K]urine ratio: Na load effect in control study in both groups (F = 6.1, P = 0.007) and in ANG-(1–7) study only in vehicles (F = 7.2, P = 0.009).
Table 3.
Urinary and plasma Na and K concentrations in female sheep before and after Na load and Na load with ANG-(1–7) infusion
| Baseline |
Na load |
Post Na load |
||||
|---|---|---|---|---|---|---|
| Vehicle | Beta | Vehicle | Beta | Vehicle | Beta | |
| [Na]Urine, mmol/l | ||||||
| Control | 145.2 ± 30.0 | 190.6 ± 33.2 | 185.9 ± 28.1 | 196.6 ± 29.1 | 238.2 ± 33.0 | 243.8 ± 25.3 |
| ANG-(1–7) | 188.3 ± 15.9 | 184.0 ± 12.5 | 177.5 ± 19.7 | 156.1 ± 31.1 | 169.2 ± 20.8 | 169.9 ± 33.9 |
| [K]Urine, mmol/l | ||||||
| Control | 187.8 ± 10.2 | 198.9 ± 16.0 | 145.5 ± 19.2 | 203.9 ± 15.4 | 135.4 ± 20.1 | 161.4 ± 13.0 |
| ANG-(1–7) | 128.4 ± 15.8 | 143.9 ± 21.5 | 75.1 ± 11.3 | 88.4 ± 19.0 | 115.9 ± 25.2 | 97.8 ± 23.1 |
| [Na]Plasma, mmol/l | ||||||
| Control | 155.8 ± 2.5 | 152.5 ± 3.1 | 156.0 ± 2.9 | 155.0 ± 2.7 | 154.3 ± 2.9 | 153.1 ± 2.2 |
| ANG-(1–7) | 155.2 ± 3.6 | 149.4 ± 0.4 | 153.6 ± 3.1 | 150.9 ± 0.6 | 155.0 ± 3.3 | 151.8 ± 1.5 |
| [K]Plasma, mmol/l | ||||||
| Control | 4.31 ± 0.1 | 4.09 ± 0.1 | 4.23 ± 0.1 | 3.94 ± 0.1 | 4.28 ± 0.2 | 4.06 ± 0.1 |
| ANG-(1–7) | 4.31 ± 0.1 | 3.95 ± 0.1 | 4.08 ± 0.2 | 3.87 ± 0.1 | 4.02 ± 0.3 | 3.67 ± 0.2 |
| [Na]/[K]Urine Ratio | ||||||
| Control | 0.75 ± 0.15 | 0.93 ± 0.13 | 1.23 ± 0.17 | 0.98 ± 0.14 | 1.81 ± 0.30 | 1.51 ± 0.15 |
| ANG-(1–7) | 1.69 ± 0.21 | 1.78 ± 0.56 | 2.12 ± 0.43 | 1.74 ± 0.26 | 1.74 ± 0.24 | 1.72 ± 0.26 |
Values are expressed as means ± SE for n = 6 sheep. [K]Urine: ANG-(1–7) effect in vehicles (F =11.8, P = 0.002) and Beta (F =27.1, P<0.0001). [Na]/[K]urine ratio: Na load effect in control study in both groups (F =11.9, P = 0.0002). ANG-(1–7) effect in vehicles (F =7.2, P = 0.01) and Beta (F =6.5, P = 0.02).
Filtered Na load (FLNa) is shown in Table 4. There were no effects of betamethasone on the FLNa in the males or females, and there was no change during control and ANG-(1–7) infusion studies in the males. There was an increase in the FLNa in the females during control (F = 5.8, P = 0.008) and ANG-(1–7) infusion studies (F = 4.5, P = 0.02).
Table 4.
Sodium-filtered load in 6-mo-old sheep
| Control |
ANG-(1–7) |
|||
|---|---|---|---|---|
| Vehicle | Beta | Vehicle | Beta | |
| Baseline | ||||
| Female | 24.83 ± 1.6 | 23.89 ± 1.2 | 25.89 ± 2.4 | 23.13 ± 1.7 |
| Male | 34.17 ± 4.5 | 29.89 ± 3.0 | 38.99 ± 2.9 | 43.75 ± 4.6 |
| Na load | ||||
| Female | 22.60 ± 3.1 | 20.89 ± 2.2 | 25.26 ± 3.8 | 23.00 ± 3.5 |
| Male | 42.26 ± 5.4 | 38.18 ± 4.3 | 56.77 ± 8.6 | 62.85 ± 6.8 |
| Postload | ||||
| Female | 23.19 ± 3.4 | 22.80 ± 2.8 | 27.62 ± 4.5 | 24.48 ± 3.0 |
| Male | 47.72 ± 5.0 | 43.89 ± 1.8 | 54.42 ± 6.8 | 64.32 ± 10.0 |
Values are expressed as means ± SE for n =5 or 6. Filtered sodium load: (FLNa) (mmol·h−1·kg body wt−1) = GFR × Con Na [plasma]. In females, Na load effect in control study (F = 5.8, P = 0.008) and in ANG-(1–7) study (F = 4.5, P = 0.02).
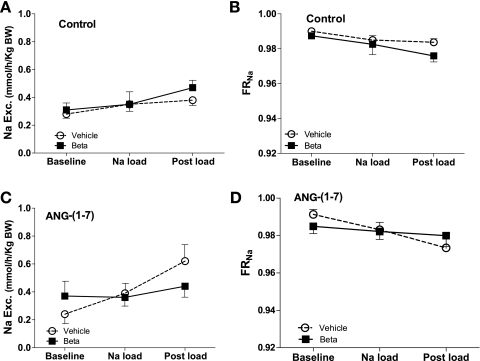
Sodium excretion normalized for body weight in males is shown in Fig. 6, A and C. There was no betamethasone effect on Na excretion in the control experiments. However, in the ANG-(1–7) study, the Na load significantly increased sodium excretion only in vehicle males (F = 4.0, P = 0.03). In addition, infusion of ANG-(1–7) in the vehicle-treated animals resulted in overall enhanced Na excretion compared with the control study (F = 17.3, P = 0.0003). Similar to the filtered load, there was no effect of Beta on fractional reabsorption of sodium (FRNa) (Fig. 6, B and D) in the males in the control study. In the ANG-(1–7) experiments, there was a tendency for the FRNa to decrease in the vehicle-exposed animals (F = 3.6, P = 0.09).
Fig. 6.
Urinary sodium excretion (A and B) and fractional reabsorption of sodium (FRNa) (C and D) during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in male sheep prenatally exposed to vehicle (○, n = 5) or Beta (■, n = 6). There was no effect of Na load on Na excretion (A) and FRNa (B) in the control study. In the ANG-(1–7) study, the Na load increased Na excretion (C) (F = 4.0, P = 0.03), only in the vehicle-treated animals, and there was a tendency for an effect on FRNa (F = 3.6, P = 0.09). Infusion of ANG-(1–7) in the vehicle-treated animals resulted in overall enhanced Na excretion compared with the control study (F = 17.3, P = 0.0003).
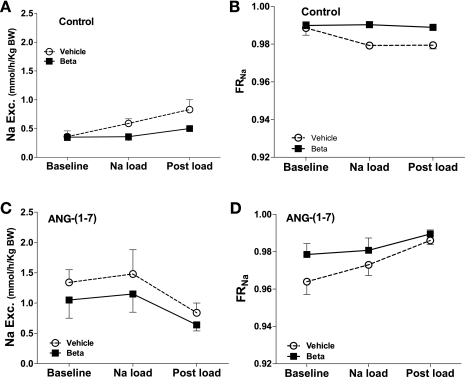
In females, the rate of Na excretion increased with the acute Na load in the control experiment (F = 5.3, P = 0.01), but the rate was lower in the Beta-exposed sheep (F = 5.8, P = 0.02) (Fig. 7, A and C). However, infusion of ANG-(1–7) resulted in overall enhanced Na excretion compared with the control study in vehicle (F = 11.9, P = 0.002) and Beta-exposed animals (F = 12.2, P = 0.002). Significantly higher rates of Na excretion were seen in ANG-(1–7) infusion experiments in females compared with males in both vehicle (F = 10.1, P = 0.006) and Beta (F = 14.6, P = 0.0006) groups, while in the control experiment, only the vehicle-treated females had higher Na excretion than their male counterparts (F = 9.3, P < 0.006).
Fig. 7.
Urinary sodium excretion (A and B) and fractional reabsorption of sodium (FRNa) (C and D) during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1) in conjunction with an acute Na load in female sheep prenatally exposed to vehicle (○, n = 6) or Beta (■, n = 6). There was a significant effect of Beta on Na excretion (A) (F = 5.8, P = 0.02) and FRNa (B) (F =30, P = 0.005) in the control study. The Na load increased Na excretion (F = 5.3, P = 0.01) and decreased FRNa (F = 4.1, P = 0.04) only in the vehicle-treated animals in the control study. There was an effect of ANG-(1–7) on Na excretion (C) and FRNa (D) in vehicle- (Exc.Na: F = 11.9, P = 0.002; FRNa: F = 4.7, P = 0.04) and Beta-exposed animals (Exc.Na: F = 12.2, P = 0.002; FRNa: F = 4.4, P = 0.046) compared with the control study.
In females, FRNa (Fig. 7, B and D) declined following Na load in vehicle-treated animals(F = 4.1, P = 0.04) but not in Beta (F = 30, P = 0.005 difference between groups). ANG-(1–7) infusion significantly decreased FRNa in both female groups (vehicle: F = 4.7, P = 0.040; Beta: F = 4.4, P = 0.046) compared with the control experiment.
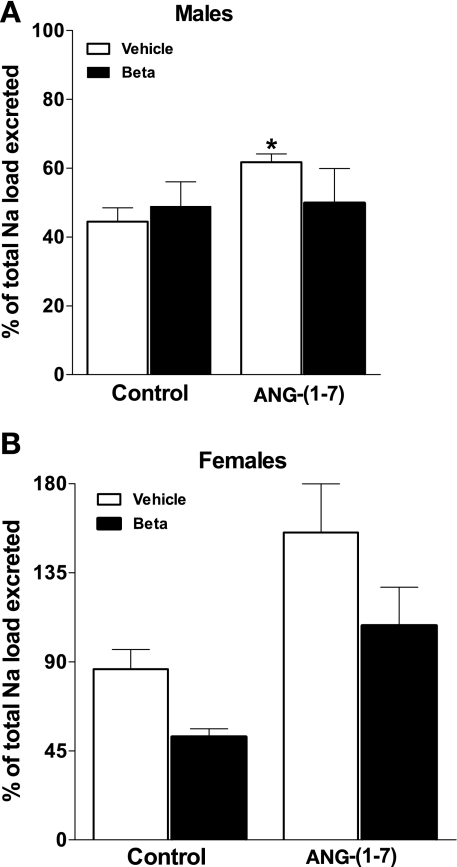
Finally, the percentage of the total Na load excreted during and after hypertonic saline infusion is shown in Fig. 8. Infusion of ANG-(1–7) increased the percentage of the acute Na load excreted in the vehicle-treated males (P = 0.03; Student's t-test) There was no effect of Beta on the percentage of the Na load excreted in the control experiments in males. Infusion of ANG-(1–7) in the females increased the percentage of the administered Na, which was excreted (F = 13.5, P < 0.002) (Fig. 8). However, the Beta females excreted a smaller percentage of the administered Na than did the vehicle females (F = 5.7, P < 0.03). Females excreted a higher percentage of the total Na load than did the males for both treatment groups (vehicle: F = 20.0, P = 0.0005; Beta: F = 6.3, P = 0.02).
Fig. 8.
Ability to excrete Na in response to an acute Na load as a percentage of the sodium load in sheep prenatally exposed to vehicle (open bars, n = 5 or 6) or Beta (solid bars, n = 6) during saline (control) or ANG-(1–7) infusion (10 pmol·min−1·kg body wt−1). In males, there was an effect of ANG-(1–7), which was confined to the vehicle-treated animals (*P = 0.03, t-test). In females, ANG-(1–7) induced a natriuresis (F = 13.5, P < 0.002), which was attenuated by Beta (F = 5.7, P < 0.03). In addition, females excreted a higher percentage than the males for both groups (vehicle; F = 20.0, P = 0.0005; Beta: F = 6.3, P = 0.02).
DISCUSSION
The effects of prenatal exposure to clinically relevant doses of glucocorticoids on blood pressure and renal function in adolescent animals have not been firmly established. Therefore, this study had two primary goals. One was to establish whether blood pressure would be increased in peripubertal animals by betamethasone (a synthetic glucocorticoid commonly used in patients with premature labor) given at 0.6 gestation (nephrogenic period in sheep and humans). We found resting MAP was elevated to a similar degree in both male and female lambs that were exposed to betamethasone before birth. Thus antenatal steroid-induced elevations in blood pressure are observable around the time of puberty and there is no detectable impact of sex on the response. A second purpose was to determine whether antenatal steroid exposure alters renal function in immature lambs and whether any effects observed demonstrate sex specificity. We postulated that by using animals from the same flock, raised under identical conditions, and studied in the same laboratory, as were the mature rams and ewes that we reported upon earlier (55), we could determine whether the fetal programming effects of antenatal steroid exposure were influenced by age. We observed that renal function was altered in peripubertal female and male lambs by prenatal glucocorticoids, that sex effects were present, and that age appeared to affect the impact of betamethasone.
A glucocorticoid-induced increase in blood pressure has been noted by other investigators using different treatment paradigms in sheep and rats (16, 17, 36, 37). The majority of the studies have been in mature, adult animals, and in most instances, when both sexes were studied, elevations in blood pressure were noted in both (36, 37). In other models of fetal programming, when sex effects on blood pressure responses were observed, they appeared related to the intensity of the programming stimulus or to the age of the offspring at the time of study (4, 21). The elevations in blood pressure in the adolescent male and female lambs may, therefore, reflect the potency of betamethasone as a stimulus. The difference in blood pressure in immature lambs between the steroid- and vehicle-treated groups was similar in magnitude to what we have reported in 1.5-yr-old animals (55), which suggests that any age, programming interactions on blood pressure take longer than 1.5 yr to develop in sheep.
In our experimental conditions, acute Na load produced somewhat inconsistent changes in MAP in vehicle- and betamethasone-treated males and females. At this juncture, the explanation for these changes is unclear, but they could be related to responses of the animals to environmental stimuli, such as people entering or exiting the laboratory during the experiments.
Betamethasone administration during pregnancy did not significantly affect basal RBF or RVR in young male and female offspring. This is in agreement with the reports in young rodent models of prenatally programmed hypertension (36, 37). Therefore, it seems that the magnitude of the reduction in nephrogenesis in these young animals subjected to prenatal glucocorticoids was not sufficient to alter renal hemodynamics.
During ANG-(1–7) infusion, RBF increased and RVR decreased in young male vehicle-treated animals, which agrees with data from Sampaio et al. (43), who showed that a low dose of ANG-(1–7) infusion (110 fmol/min) for 10 min significantly increased RBF in male rats. The observations are also consistent with in vitro studies demonstrating vasodilator effects on renal arterioles isolated from young male rabbits (41). These results suggest that ANG-(1–7) participates in the control of renal hemodynamics by decreasing RVR. The modulation of renal vascular tone by ANG-(1–7) is possibly associated with the activation of bradykinin (54) and increased nitric oxide production (41) or a decrement in ANG II-induced vasoconstriction (31).
The prenatal betamethasone exposure decreased the renal vascular response to ANG-(1–7) in young male offspring. In our previous study on 1.5-yr-old animals (55), there was no evidence of any RBF or RVR responses to ANG-(1–7) in either the vehicle- or steroid-exposed animals. This suggests that in normal male sheep, there is a loss of the renal vascular response to ANG-(1–7) with age. Such changes may represent age-induced impairment of endothelial vasodilation (9, 32), possibly related to nitric oxide deficiency in the males (5). The prenatal betamethasone exposure in males may hasten the appearance the age-related loss of the renal vasodilatory response to ANG-(1–7).
Elevations in RBF and decreases in RVR can be induced by high sodium intake, and these effects are blunted in hypertensive men and experimental male animals (25, 40). We did not find any difference in the RBF and RVR changes caused by an acute sodium load between young vehicle and steroid-exposed male offspring, even though the blood pressure was different in the two groups. However, there was a loss of response to the Na load during the ANG-(1–7) infusions. This suggests that the mechanism mediating the increase in RBF and RVR caused by Na load or ANG-(1–7) share a similar pathway and that the dose of ANG-(1–7) elicited the maximal response in the vehicle-treated animals. This response could involve vasodilator mechanisms that include enhanced contributions of prostaglandins and nitric oxide (19, 38). The lack of change in effective RBF and RVR in response to ANG-(1–7) in the young female lambs implies a sex difference in renal vascular bed sensitivity to the peptide. This lack of a response in females also suggests that ANG-(1–7) plays little role in regulating renal blood flow in immature or mature ewes.
An important finding of this study was that there was no difference in basal GFR adjusted for body weight in vehicle and betamethasone-exposed offspring at the young age. In contrast, basal GFR was significantly decreased in mature rams but not adult ewes prenatally exposed to betamethasone (55). These results showing that prenatal steroid-exposed males have an age-dependent fall in GFR are consistent with those reported by other groups (36, 60) and in other models of hypertension induced by an AT1 receptor antagonist during the nephrogenic period (30, 42). The steroid-induced changes may have important implications for the sex differences noted in the age-related progression of renal dysfunction (7, 50).
There was an overall increase in GFR in young female vehicle and betamethasone exposed offspring following the sodium load. This is similar to what we have observed in the mature females (55). Also, this finding agrees with other studies showing an increase in GFR following hypertonic Na loading at a dose of 0.03 meq·kg−1·min−1 for 90 min in normal Na-replete female dogs (45).
The results of our study demonstrate that higher glomerular FF in prenatal steroid exposed males is associated with ANG-(1–7) infusion. An elevated glomerular FF could reflect glomerular hyperfiltration and increased glomerular capillary pressure. In females, acute Na load following ANG-(1–7) infusion tended to increase glomerular FF, suggesting an interaction between the effects of the peptide and high salt on renal hemodynamics.
Plasma Na+ and K+ levels did not change significantly in the groups during the experiment. Urinary Na+ and K+ levels were relatively consistent in the males; thus, the [Na]/[K]urine ratio, a possible marker for mineralosteroid activity (12), showed no change, which agrees with others' reports that ANG-(1–7) has no apparent aldosterone-stimulating effects (26). In contrast, the ratio increased in both female groups during ANG-(1–7), which may represent suppression of mineralocorticoid activity by the peptide. The sex difference in [Na]/[K]urine ratio response to the peptide possibly reflects that sex is a strong determinant of the aldosterone response.
The fractional reabsorption of sodium (FRNa) was decreased by Na load (Figs. 6 and 7) in the vehicle-treated females. These results imply that elevations in glomerular filtration combined with reductions in Na reabsorption are involved in the higher Na excretion in the vehicle females compared with the vehicle males (Figs. 6–8). The fact that the FLNa is equivalent in the vehicle and Beta-exposed females, while the FRNa is reduced by Beta is consistent with altered tubular function induced by steroid exposure.
In the present study, there was no effect of prenatal treatment with a clinically relevant dose of betamethasone on sodium excretion following an acute sodium load in the young male offspring. This is also true when the data are expressed as a percentage of the sodium load excreted during the experiment. This contrasts with the impairment in sodium excretion noted in the mature male offspring following an identical hypertonic saline infusion (55). As mentioned above, GFR was not reduced by antenatal glucocorticoid treatment in the young males, while in the older animals, there was a reduction in GFR associated with steroid exposure (55). It is possible that the fall in GFR in the older animals is part of the explanation of this age-dependent decline in the ability to excrete a sodium load in the steroid-treated males. Alternatively, if the steroid treatment alters sodium transporters in sheep as it does in rats (13, 57), it may be that the consequences of changes in transporter activity are not as robust in immature as in mature animals.
We hypothesized that ANG-(1–7) infusion would enhance excretion of the sodium load and that the effect would be compromised by prenatal betamethasone exposure. The natriuretic responses we found in the vehicle-treated animals are similar to those reported by other groups in dogs (28) and rats (56) and suggest that ANG-(1–7) inhibits sodium reabsorption. Prenatal betamethasone exposure blocked the natriuretic responses to ANG-(1–7) in the young males. The mechanisms by which prenatal steroid exposure altered responses to ANG-(1–7) are not fully understood but may be related to increased levels of ACE activity in the blood of betamethasone-exposed animals (48). ACE rapidly catabolizes ANG-(1–7), and elevated enzyme levels could thereby reduce responses to infusions of the peptide (49). Another potential explanation for the lack of response to ANG-(1–7) is a steroid-induced reduction in the receptor for the peptide in the kidney. Evidence suggests that ANG-(1–7) is a ligand for the G protein-coupled receptor, Mas, which is found in the kidney (15, 44), and studies in mice lacking the receptor indicate it is important for maintaining fractional sodium excretion (39). There is also precedent for this possibility in that antenatal betamethasone alters the proportion of the type 1 and type 2 receptors for angiotensin found in proximal tubules from adult offspring exposed to steroid compared with controls (23).
The significant response to ANG-(1–7) in the vehicle-treated young males compared with the lack of a response in the older males (55) indicates that the natriuresis induced by ANG-(1–7) after a Na load is influenced by age. Accordingly, it is tempting to speculate that one of the effects of antenatal steroid exposure is to accelerate the age-related loss of this natriuretic effect of ANG-(1–7) in males. Early maturational changes in other organ systems have been noted in response to stimuli associated with fetal programming (8) and features common to age-related changes in the kidney are also found in models of fetal programming (3).
We found an inhibitory effect of steroid exposure on the capacity to excrete sodium following a nonpressor sodium load in the prepubertal ewes. This was somewhat surprising because this effect was absent in mature ewes (55). It is possible that sexual maturation and the associated changes in sex steroids in the females provide them with resistance to some of the detrimental effects of prenatal glucocorticoid exposure on sodium excretion. Gonadal steroid receptors are present in the kidney (24, 52), and estrogen alters some of the factors that influence sodium excretion. For example, ACE and AT1 receptors are downregulated by estrogen, while upregulation of renal AT2 receptors appears dependent upon estradiol (1, 27). Such changes, while favoring sodium excretion and perhaps overcoming the effects of antenatal glucocorticoid in adult females, may be absent in immature animals.
Finally, there was a marked difference in sodium excretion in response to ANG-(1–7) in the betamethasone-exposed immature males compared with females (F = 14.6, P = 0.0006). This was also true when the data were expressed as a percentage of the sodium load excreted (F = 6.3, P = 0.02). This difference was found in the older animals as well (55) and suggests some interaction between the fetal programming effects of antenatal betamethasone exposure and sex.
Perspectives and Significance
These experiments in sheep demonstrate that prenatal exposure to a clinically relevant dose of betamethasone at a stage in gestation when it is frequently given to pregnant women is associated with renal dysfunction in peripubertal offspring. Overall, the effects tend to be inhibitory relative to sodium excretion and renal blood flow responses to ANG-(1–7). The picture resembles a situation of greater ANG II and less ANG-(1–7) “tone” in the kidney and is consistent with our observations of an increase in the ACE to ACE2 ratio in the kidney (48). Considering that the females in this study were only about 24 wk old, and puberty is about 30 wk in ewes (59), it is somewhat surprising that the effects of antenatal steroids on the kidney show sex specificity. There is evidence that sex does influence the renal responses to ANG II after puberty (33) in people, but little is known about the peripubertal effects of the angiotensin peptides. When considered in light of the alterations in the intrarenal RAS that we have reported, these effects may represent markers of greater susceptibility to kidney damage from renal insults acquired as aging progresses (55).
GRANTS
The work described in the paper was supported by National Institutes of Health Grants HD 47584 and HD 17644.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank David Jones and Eric Lesane for the help with the animals.
REFERENCES
- 1.Armando I, Jezova M, Juorio AV, Terron JA, Falcon-Neri A, Semino-Mora C, Imboden H, Saavedra JM. Estrogen upregulates renal angiotensin II AT(2) receptors. Am J Physiol Renal Physiol 283: F934–F943, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJP, Fall CHD. Fetal and infant origins of cardiovascular disease. Arch Dis Child 68: 797–799, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol 298: F235–F247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baylis C. Sexual dimorphism, the aging kidney, and involvement of nitric oxide deficiency. Semin Nephrol 29: 569–578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol 9: 699–709, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Baylis C. Sexual dimorphism of the aging kidney: role of nitric oxide deficiency. Physiology 23: 142–150, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield FH, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, Harding JE, Challis JR. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology 145: 4278–4285, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Brandon AE, Boyce AC, Lumbers ER, Zimanyi MA, Bertram JF, Gibson KJ. Glomerular hypertrophy in offspring of subtotally nephrectomized ewes. Anat Rec (Hoboken) 291: 318–324, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 12.Chacko M, Fordtran JS, Emmett M. Effect of mineralocorticoid activity on transtubular potassium gradient, urinary [K]/[Na] ratio, and fractional excretion of potassium. Am J Kidney Dis 32: 47–51, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson WD, Sackner MA. Simplification of the anthrone method for the deternination of inulin in clearance studies. J Lab Clin Med 62: 351–356, 1963 [PubMed] [Google Scholar]
- 15.Dilauro M, Burns KD. Angiotensin-(1–7) and its effects in the kidney. Sci World J 9: 522–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 98: 137–142, 2000 [PubMed] [Google Scholar]
- 19.Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J Am Soc Nephrol 9: 1716–1722, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol 295: R1941–R1952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gender Med 5: S121–S132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwathmey-Williams T, Shaltout HA, Diz DI, Figueroa JP, Rose JC, Chappell MC. Steroid-induced fetal programming alters angiotensin II receptor subtype expression in the sheep kidney. FASEB J 22: 735–735.15, 2008 [Google Scholar]
- 24.Hagenfeldt Y, Eriksson HA. The estrogen receptor in the rat kidney. Ontogeny, properties and effects of gonadectomy on its concentration. J Steroid Biochem 31: 49–56, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Hall JE, Guyton AC, Smith MJ, Jr., Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol Renal Fluid Electrolyte Physiol 239: F271–F280, 1980 [DOI] [PubMed] [Google Scholar]
- 26.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol Renal Fluid Electrolyte Physiol 270: F141–F147, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension 42: 1157–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V. Effect of intrarenal infusion of angiotensin-(1–7) in the dog. Kidney Blood Press Res 23: 89–94, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Jung K, Klotzek S, Schulze BD. Refinements of assays for low concentrations of inulin in serum. Nephron 54: 360–361, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Sex and age differences of renal function in rats with reduced ANG II activity during the nephrogenic period. Am J Physiol Renal Physiol 293: F506–F510, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Mahon JM, Carr RD, Nicol AK, Henderson IW. Angiotensin(1–7) is an antagonist at the type 1 angiotensin II receptor. J Hypertens 12: 1377–1381, 1994 [PubMed] [Google Scholar]
- 32.Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction : potential implications for pharmacotherapy. Drugs Aging 20: 527–550, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int 55: 278–285, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Moritz KM, Dodic M, Wintour EM. Kidney development and the fetal programming of adult disease. Bioessays 25: 212–220, 2003 [DOI] [PubMed] [Google Scholar]
- 34a.NIH Consensus Development Panel NIH Consensus Development Conference 1995 Effects of corticosteroid for fetal maturation on perinatal outcomes. JAMA 273: 413–418, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Orlando R, Floreani M, Padrini R, Palatini P. Determination of inulin clearance by bolus intravenous injection in healthy subjects and ascitic patients: equivalence of systemic and renal clearances as glomerular filtration markers. Br J Clin Pharmacol 46: 605–609, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osei SY, Ahima RS, Minkes RK, Weaver JP, Khosla MC, Kadowitz PJ. Differential responses to angiotensin-(1–7) in the feline mesenteric and hindquarters vascular beds. Eur J Pharmacol 234: 35–42, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int 75: 1184–1193, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Redgrave J, Rabinowe S, Hollenberg NK, Williams GH. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J Clin Invest 75: 1285–1290, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Salazar F, Reverte V, Saez F, Loria A, Llinas MT, Salazar FJ. Age- and sodium-sensitive hypertension and sex-dependent renal changes in rats with a reduced nephron number. Hypertension 51: 1184–1189, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol 284: H1985–H1994, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Sampaio WO, Souza Dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sandgaard NC, Andersen JL, Bie P. Hormonal regulation of renal sodium and water excretion during normotensive sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R11–R18, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Schnurr E, Lahme W, Kuppers H. Measurement of renal clearance of inulin and PAH in the steady state without urine collection. Clin Nephrol 13: 26–29, 1980 [PubMed] [Google Scholar]
- 47.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Deleterious cardiovascular effects of antenatal betamethasone exposure in young and adult sheep. FASEB J 22: 1129.16, 2008 [Google Scholar]
- 48.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 53: 404–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gender Med 5: S3–S10, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Singh RR, Denton KM, Bertram JF, Jefferies AJ, Head GA, Lombardo P, Schneider-Kolsky M, Moritz KM. Development of cardiovascular disease due to renal insufficiency in male sheep following fetal unilateral nephrectomy. J Hypertens 27: 386–396, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Sion-Vardi N, Kaneti J, Segal-Abramson T, Giat J, Levy J, Sharoni Y. Gonadotropin-releasing hormone specific binding sites in normal and malignant renal tissue. J Urol 148: 1568–1570, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Smith J, Nakahara S, Westwood B, Figueroa J, Chappell M, Rose JC. Prenatal betamethasone exposure alters renin angiotensin system enzyme expression in adult male sheep. Reprod Sci 15: 274A, 2008. 18421022 [Google Scholar]
- 54.Souza Dos Santos RA, Passaglio KT, Pesquero JB, Bader M, Simoes e Silva AC. Interactions between angiotensin-(1–7), kinins, and angiotensin II in kidney and blood vessels. Hypertension 38: 660–664, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallon V, Richter K, Heyne N, Osswald H. Effect of intratubular application of angiotensin 1–7 on nephron function. Kidney Blood Press Res 20: 233–239, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Velazquez H, Bartiss A, Bernstein P, Ellison DH. Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 270: F211–F219, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod 3: 130–140, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Massman GA, Rose JC, Figueroa JP. Differential effects of a clinical dose of betamethasone on nephron endowment and glomerular filtration in adult sheep. Reprod Sci 17: 186–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]