Abstract
Male sex is associated with higher blood pressure and greater renal injury, perhaps related to greater sensitivity to ANG II. In anesthetized male and female C57BLK/6 mice, we assessed responses of mean arterial pressure (MAP) and renal vascular resistance (RVR; Transonic flow probe) to acute bolus injections of ANG II (0.3–3.0 μg/kg iv) and phenylephrine (PE; 30–300 μg/kg) during low-, normal-, and high-sodium diets. The role of reactive oxygen species was determined by coadministration of tempol. ANG II type 1 and type 2 (AT1 and AT2) receptor and endothelial nitric oxide synthase (NOS3) expression were determined in dissected kidney vessels. While no difference was found on the low-sodium (LS) diet, MAP and RVR responses to ANG II were greater in males during the normal-sodium (NS) and high-sodium (HS) diets (e.g., RVR response at ANG II 3.0 μg/kg during NS: +329 ± 22 vs. +271 ± 28 mmHg·ml−1·min, P = 0.029, effect size = 0.75). Tempol had no effect on the sex-dependent responses on any of the diets. On the LS diet, AT1 and AT2 receptor expression was higher in males. No sex differences were found on the NS diet. On the HS diet, AT1 was higher, and NOS3 expression was lower in males. Acute responses to ANG II are greater in male mice during NS and HS diets, which is, in part, related to differences in AT1, AT2, and NOS3 expression in kidney vessels. Mouse models will be useful to study the role of sex differences in ANG II sensitivity for cardiovascular and renal disease.
Keywords: blood pressure, hypertension, renal, kidney, renal circulation, nitric oxide, reactive oxygen species
in humans, male sex is associated with higher blood pressure (BP) and a more rapid progression of several types of renal diseases (1, 15). There has been an increasing interest in how sex-related differences within the renin-angiotensin-system (RAS) may account for these observations (25). In fact, experimental studies reported sex differences in the expression of various components of the RAS, including a higher expression of the ANG II type 1 (AT1) receptor in tissues of male rats (for review, see Refs. 4 and 23). Furthermore, it has been demonstrated that chronic infusion of ANG II increases BP to a greater extent in male than in female rats (22, 27).
Because of the ample opportunities for gene targeting, mice are increasingly used as models for human conditions, including arterial hypertension and renal disease. Similar to what has been observed in rats, ANG II infusion in mice results in higher BP in males than in females (2, 28, 30). Renal vasoconstriction is a powerful inducer of hypertension (8), and while sex differences in the central response to ANG II have been demonstrated (31–32), the role of the renal circulation has not been investigated. Therefore, we hypothesized that the sensitivity of the BP and renal hemodynamic response to acute ANG II administration would be greater in male compared with female mice.
ANG II-hypertension is exquisitely salt sensitive, and because different levels of sodium intake profoundly modulate the expression of ANG II receptors in the kidney (21), we have examined hemodynamic responses under varying salt-loading conditions. We hypothesized that under conditions of high sodium intake, when AT1 receptor expression has been suggested to be at a maximum (21), sex differences would be particularly prominent. In parallel to the functional responses to ANG II, expression levels of the AT1 and the vasodilatory ANG II type 2 (AT2) receptors were examined in dissected kidney vessels, along with the expression of endothelial nitric oxide synthase (NOS3). NOS3 has been demonstrated to counteract ANG II-induced vasoconstriction in the renal vasculature (24). Finally, reactive oxygen species (ROS) have been shown to contribute to ANG II-dependent responses in the renal circulation (13). We hypothesized that ROS activation may account for potential sex differences in ANG II responsiveness and therefore assessed the impact of free radical scavenging on the acute hemodynamic responses in both males and females.
METHODS
Hemodynamic responses.
Male and female C57BLK/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were studied between 8 and 12 wk of age, housed in our animal facility with a 12:12-h light-dark schedule and fed a regular rodent diet containing 0.29% sodium. One week before the experiments, mice were either continued on the 0.29% normal-sodium (NS) diet, or fed with a low- (0.02%, LS) or high-sodium (4%, HS) diet (Harlan Laboratories, Indianapolis, IN). All experiments were approved by the Medical College of Georgia Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health Guide on the Care and Use of Laboratory Animals.
Mice were anesthetized with isoflurane and placed on a servo-controlled surgical heating table. The right jugular vein was accessed with a polyethylene catheter (PE-10) for injection of vasoconstrictor agents. The left carotid artery was cannulated with a similar catheter connected to a pressure transducer for direct measurement of mean arterial blood pressure (MAP). Once the mouse was moved into a prone position, the right kidney was accessed, and the renal artery was isolated from the renal vein. Renal blood flow (RBF) was then continuously measured via a 0.5-V Transonic renal flow probe and a TS420 perivascular flowmeter (Transonic Systems, Ithaca, NY). MAP and RBF were recorded and analyzed with a PowerLab data acquisition system (ADInstruments, Boston, MA). Renal vascular resistance (RVR) was calculated as MAP/RBF.
After the surgical procedures and stabilization of hemodynamic parameters, bolus injections of increasing doses of ANG II and phenylephrine (PE) were administered via the right jugular vein. First, PE was injected at 30, 100, and 300 μg/kg and subsequently ANG II at doses of 0.3, 1.0, and 3.0 μg/kg. After an injection, MAP and RBF were allowed to return to preinjection levels before the next dose was given. Each dose of the vasoconstrictors was administered in a volume of 50 μl delivered at a rate of 200 μl/min via an infusion pump. To assess the contribution of superoxide to the hemodynamic responses to ANG II and PE, experiments were conducted in a subset of animals in the presence and absence of tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl), a cell-permeable free radical scavenger. Tempol was infused at 5 mg·kg−1·min−1. According to initial dose-ranging studies, this was the maximum dose at which no direct hemodynamic effects were observed (data not shown).
Kidney vessel harvest.
Mice were euthanized with a lethal dose of pentobarbital sodium (65 mg/kg), and kidneys were removed and immediately placed into a petri dish with cold physiological saline solution (PSS) and added protease inhibitors (final concentrations: 1 μM PMSF, 10 μM leupeptin, 2 μM pepstatin, and 0.0001% aprotinin). After removal of the renal capsule, the kidney was placed between a circle sieve of 70-μm pore size (Biodesign, Carmel, NY). Kidney vessels were immediately isolated by rapid and gentle grating while viewing microscopically. The kidney vessels were subsequently frozen in liquid nitrogen and stored at −80° C until further use.
Western blot analysis.
Kidney vessels were homogenized as previously described for small mesenteric arteries (26) in buffer with added protease inhibitors, and total protein was determined by the Bradford method (Bio-Rad, Hercules, CA). Samples (30 μg) from renal vessels of male and female mice were assessed by standard SDS-PAGE followed by blotting to PVDF membranes, as previously described (5). All blots were incubated overnight with the following primary antibodies: anti-AT1 receptor (Alomone Labs, Jerusalem, Israel), anti-AT2 receptor antibody (Alomone Labs), and anti-endothelial nitric oxide synthase 3 (NOS3) (BD Biosciences, San Jose, CA). Blots were then developed for 1 h using a secondary antibody tagged with infrared dye 680 (AlexaFluor 680 anti-rabbit or anti-mouse IgG; Invitrogen/Molecular Probes, Carlsbad, CA). To normalize all proteins, blots were then double-labeled by overnight incubation with monoclonal anti-β-actin antibody (Sigma, St. Louis, MO) and redeveloped for 1 h with the secondary antibody tagged with infrared dye 800 (Rockland, Gilbertsville, PA). Densitometry was performed on the Odyssey Infrared Imaging System v3.0 (Li-Cor Biosciences, Lincoln, NE).
Statistics.
Baseline data were compared with unpaired t-tests or Mann-Whitney U-tests. The maximum hemodynamic responses to vasoconstrictor agents were analyzed by two-way ANOVA, testing whether responses were dose-dependent (Pdose), whether responses were dependent on sex, independent from dose (Pgroup) or whether there was an effect of sex on the dose response (Pdose·group). Within groups, two-way ANOVAs were carried out to determine whether responses were dependent on diet (Pdiet) and dose (Pdose) and whether there was an effect of the diet on the dose response (Pdiet·dose). A P value <0.05 was considered statistically significant. Where indicated, effect sizes were calculated as Cohen's d = difference between means/pooled standard deviation. Data are expressed as means ± SE.
RESULTS
Acute hemodynamic responses to ANG II and PE in mice on LS, NS, and HS diets.
Baseline hemodynamic data were similar between anesthetized male and female mice on any of the diets (Table 1). Hemodynamic responses to ANG II and PE on the LS diet are shown in Fig. 1, on the NS diet in Fig. 2 and on the HS diet in Fig. 3. On any of the three diets, and in both male and female mice, bolus injections of ANG II and PE led to dose-dependent increases in MAP and RVR along with decreases in RBF (all Pdose < 0.001).
Table 1.
Baseline hemodynamic data
| Diet | Hemodynamic Variable | Male | Female |
|---|---|---|---|
| Low sodium | MAP, mmHg | 89 ± 1 | 87 ± 2 |
| Heart rate, bpm | 585 ± 12 | 566 ± 8 | |
| RBF, ml/min | 0.71 ± 0.05 | 0.73 ± 0.07 | |
| RVR, mmHg·ml−1·min | 118 ± 10 | 126 ± 14 | |
| Normal sodium | MAP, mmHg | 90 ± 2 | 87 ± 4 |
| Heart rate, bpm | 565 ± 19 | 536 ± 18 | |
| RBF, ml/min | 0.88 ± 0.14 | 0.85 ± 0.10 | |
| RVR, mmHg·ml−1·min | 113 ± 19 | 109 ± 12 | |
| High sodium | MAP, mmHg | 90 ± 3 | 88 ± 4 |
| Heart rate, bpm | 546 ± 13 | 538 ± 13 | |
| RBF, ml/min | 0.84 ± 0.19 | 0.84 ± 0.13 | |
| RVR, mmHg·ml−1·min | 120 ± 25 | 114 ± 15 |
Baseline characteristics in male and female mice during normal-sodium, low-sodium, and high-sodium diets. MAP, mean arterial pressure; RBF, renal blood flow; RVR, renal vascular resistance.
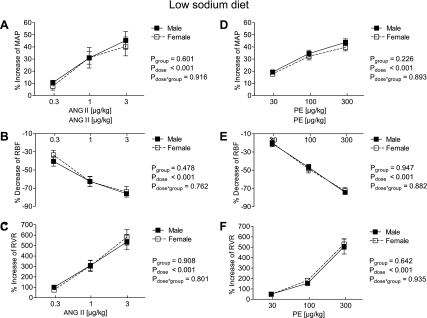
Fig. 1.
Effect of acute graded infusions of ANG II (A–C) and phenylephrine (PE; D–F) on mean arterial pressure (MAP; A and D), renal blood flow (RBF; B and E) and renal vascular resistance (RVR; C and F) in male (■, n = 14) and female mice (□, n = 11) during a low-sodium diet (0.02%).
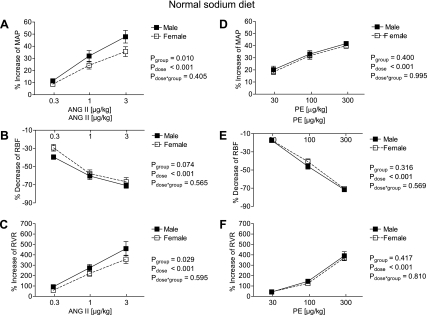
Fig. 2.
Effect of acute graded infusions of ANG II (A–C) and PE (D–F) on MAP (A and D), RBF (B and E) and RVR (C and F) in male (■, n = 13) and female mice (□, n = 15) during a normal sodium diet (0.29%).
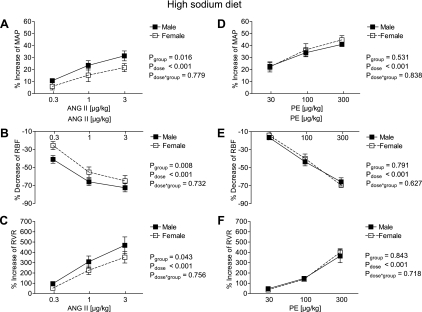
Fig. 3.
Effect of acute graded infusions of ANG II (A–C) and PE (D–F) on MAP (A and D), RBF (B and E), and RVR (C and F) in male (■, n = 10) and female mice (□, n = 11) during a high-sodium diet (4%).
On the LS diet, no differences in the MAP, RBF, and RVR responses to ANG II or PE were detectable between sexes [absolute change in response to 3.0 μg/kg ANG II: MAP: +31 ± 4 vs. +27 ± 2 mmHg in males vs. females, effect size of sex (Cohen's d) = 0.36, RVR: +291 ± 30 vs. +307 ± 34 mmHg·ml−1·min, effect size of sex = −0.17; for responses in % change from baseline: Fig. 1]. Furthermore, heart rate (HR) responses to ANG II and PE were similar between male and female mice on the LS diet [highest dose of ANG II: −1 ± 3% vs. −2 ± 2%, not significant (n.s.); highest dose of PE: −3 ± 2% vs. −5 ± 3%, n.s.].
On the NS diet, the increase of MAP and RVR to ANG II was greater in the male than in the female mice (for ANG II 3.0 μg/kg: MAP: +37 ± 5 vs. +27 ± 3 mmHg, effect size = 0.76, RVR: +329 ± 22 vs. +271 ± 28 mmHg·ml−1·min, effect size = 0.75; % responses in Fig. 2, A and C), whereas the RBF response failed to reach statistical significance (Fig. 2B). No differences in the MAP, RBF, and RVR responses to PE were detectable between sexes (Fig. 2, D–F). Furthermore, the HR responses to acute ANG II and PE administration were similar between male and female mice (for the highest dose of ANG II: −3 ± 2% vs. −2 ± 3%, n.s.; for the highest dose of PE: −3 ± 2% vs. −3 ± 4%, n.s.).
On the HS diet (% responses in Fig. 3), the increase in MAP and RVR, as well as the decrease in RBF, were greater in the male mice (for ANG II 3.0 μg/kg: MAP: +25 ± 3 vs. +19 ± 2 mmHg, effect size = 0.67, RVR: +292 ± 37 vs. +216 ± 36 mmHg·ml−1·min, effect size = 0.73, Fig. 3, A–C). No differences in the MAP, RBF, and RVR responses to PE were detectable between sexes on the HS diet (Fig. 3, D–F). Furthermore, HR responses to ANG II and PE were similar between male and female mice on the HS (highest dose of ANG II: −1 ± 3% vs. −2 ± 2%, n.s.; highest dose of PE: −3 ± 5% vs. −4 ± 3%, n.s.).
Finally, when comparing the MAP and RVR responses between different sodium diets within each sex, males were not affected by the sodium content of their diet (for MAP response: Pdiet = 0.113, Pdose < 0.001, and Pdiet·dose = 0.646; RVR response: Pdiet = 0.113, Pdose < 0.001, and Pdiet·dose = 0.646 by 2-way ANOVA). In contrast, MAP and RVR responses were modulated by the sodium content in the females (for MAP response: Pdiet < 0.001, Pdose < 0.001, and Pdiet·dose = 0.553; for RVR response: Pdiet = 0.002, Pdose < 0.001, and Pdiet·dose = 0.084 by two-way ANOVA).
Effect of tempol on hemodynamic responses.
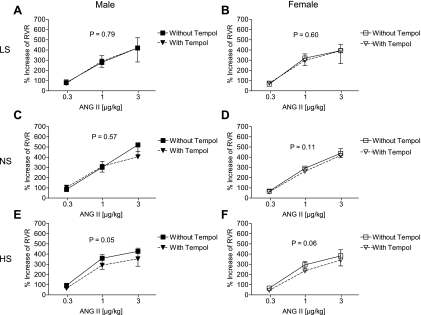
In a subset of animals, hemodynamic responses were examined in the presence and absence of the free radical scavenger, tempol. In mice on the LS diet, tempol had no effect on MAP, HR, RBF, and RVR responses to ANG II (Fig. 4A for the effect of tempol on RVR responses to ANG II in males, Fig. 4B for effect of tempol on RVR in females). Similarly, tempol had no effect on MAP, HR, RBF, and RVR responses to ANG II in mice on the NS diet (Fig. 4C in males, Fig. 4D in females for the effect of tempol on RVR). In mice on the HS diet, tempol had no effect on the MAP or HR response to ANG II. There was a trend toward a reduction in the RBF responses, both in male (P = 0.09) and female mice (P = 0.08). RVR responses were significantly reduced by tempol in male mice on a HS diet with a similar trend in females (Fig. 4, E and F, respectively). Comparing male and female groups, there was no difference in the magnitude of the effect of tempol on the RVR response (P = 0.89). Furthermore, PE responses were not affected on any of the diets (data not shown).
Fig. 4.
Effect of acute graded infusions of ANG II on RVR in the presence and absence of Tempol (5 mg·kg−1·min−1) in male (A, C, E) and female (B, D, F) mice on low- (A and B), normal- (C and D) and high- (E and F) sodium diets (low sodium: male n = 7, female n = 6; normal sodium: male n = 6, female n = 7; high sodium: male n = 5, female n = 6).
Expression of AT1 and AT2 receptors and NOS3 in kidney vessels.
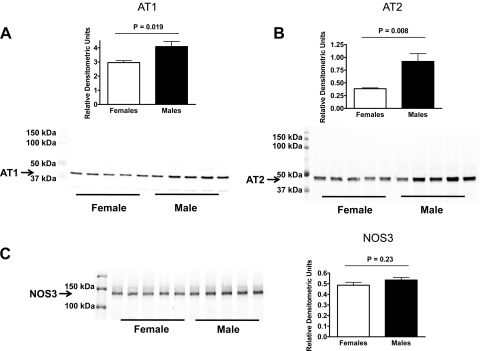
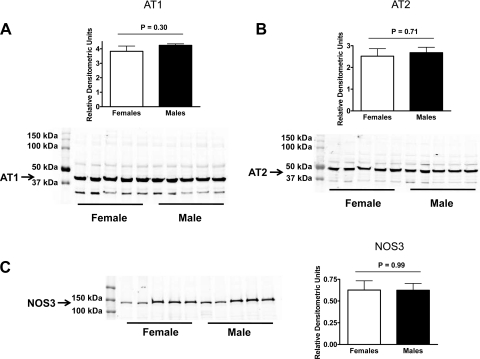
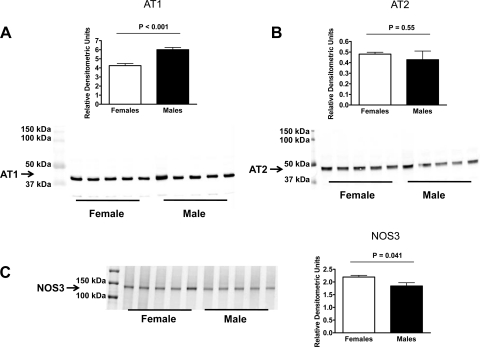
In kidney vessel homogenates from mice on a LS diet, AT1, and AT2 receptor expression was higher in male than in female mice (Fig. 5, A and B), while no difference was found for NOS3 expression (Fig. 5C). In vessels from mice on a NS diet, no differences were found between sexes for AT1 and AT2 receptor and NOS3 expression (Fig. 6, A–C). On the HS diet, AT1 receptor expression was higher in male than in female mice (Fig. 7A), with no difference in AT2 receptor expression (Fig. 7B). Furthermore, NOS3 expression was higher in vessel homogenates from female than from male mice on the HS diet (Fig. 7C).
Fig. 5.
Expression of the AT1 receptor (A), AT2 receptor (B), and NOS3 (C), in kidney vessels from male and female mice on a low-sodium diet (all n = 5).
Fig. 6.
Expression of the AT1 receptor (A), AT2 receptor (B), and NOS3 (C) in kidney vessels from male and female mice on a normal sodium diet (all n = 5).
Fig. 7.
Expression of the AT1 receptor (A), AT2 receptor (B), and NOS3 (C) in kidney vessels from male and female mice on a high-sodium diet (all n = 5).
DISCUSSION
The main finding of the present study was that, male mice have a greater MAP and renal hemodynamic response to ANG II when maintained on a NS or HS diet compared with female mice, while no sex difference was detectable during the LS diet. These sex differences in ANG II responses were not due to differences in free radical production, but they were, in part, related to differences in the expression of AT1 and AT2 receptor and/or NOS3 in kidney vessels.
In humans, only a few studies have examined the role of sex on ANG II-mediated pressor and renal hemodynamic responses. Gandhi et al. (7) demonstrated a longer duration of the MAP response to acute graded infusions of ANG II in males compared with females, albeit the maximum pressor responses were similar between sexes. Interestingly, the decrease of renal perfusion was greater in females compared with males (7). In a separate study by Miller at al. (14), glomerular filtration rate (GFR) was maintained in males during acute infusion of ANG II, while GFR decreased in females. Renal plasma flow declined similarly in both groups. This could be interpreted as relatively more postglomerular than preglomerular vasoconstriction in male compared with female subjects. Subsequent studies from the same group showed that renal hemodynamic responses to ANG II in humans were highly dependent on gene polymorphisms of the ANG II type 1 receptor and of NOS3, complicating this issue in humans (17, 19).
In the rat, studies have shown that the BP increase in response to chronic infusion of ANG II is greater in males compared with females (22, 27). In fact, when a sufficiently low dose of ANG II is administered, female rats even demonstrate a reduction of BP, which is associated with greater renal and cardiac AT2 receptor and renal angiotensin-converting enzyme 2 (ACE2) expression (22). ACE2 favors the generation of ANG 1–7, which has vasodilatory effects in the vasculature (3). Furthermore, glomerular AT1 receptor binding is greater in male than in female rats (20).
To our knowledge, three studies have been performed in the mouse, all demonstrating that chronic infusion of ANG II leads to greater increases of BP in males compared with females (2, 28, 30). Xue at al. (30) used a 7-day chronic infusion of ANG II at a dose of 800 ng·kg−1·min−1, which is considered a relatively high dose in mice. BP and HR were measured in chronically instrumented, conscious animals. Furthermore, baroreflex function was assessed before and after the 7-day ANG II infusion by evoking HR changes via acute graded infusions to PE and sodium nitroprusside. The BP increase to ANG II was greater in male mice from day 1 of the infusion and remained greater during the entire 7-day infusion period. Baseline HR was higher in the females, but interestingly, the decrease of HR during ANG II infusion was blunted in the male mice, associated with reduced baroreflex sensitivity and a greater response of BP to ganglionic blockade with hexamethonium. These data suggest that increased sympathetic nerve activity and baroreflex dysfunction contribute to the higher BP response to chronic ANG II in males.
The second study by Ebrahimian et al. (2) demonstrated that the greater increase of BP to ANG II at a dose of 400 ng/kg in the males was associated with greater plasma levels of thiobarbituric-acid reducing substances, i.e., increased oxidative stress. Recently, a third study has demonstrated that the sex difference in the BP response to infusion of ANG II persists when the endogenous RAS is blocked by angiotensin-converting enzyme inhibition (28). This study also showed that while urinary isoprostane excretion is higher in females, this was not affected by ANG II infusion. Therefore, the role of oxidative stress in mediating sex differences in ANG II hypertension is contentious.
In the present study, we examined sex differences in the acute responses of BP and, in particular, of RBF, considering the key role of renal hemodynamics for long-term BP control (8). Sodium intake profoundly modulates the expression of the AT1 receptor in the kidney, with previous studies suggesting that maximum expression is induced by a high-sodium diet (21). We, therefore, hypothesized that sex differences might be revealed, in particular, under conditions of high sodium intake. In fact, MAP and RVR responses to ANG II were greater in males than in females during the NS and HS diets. Similar effect sizes of sex were observed on these two diets, while there was no sex difference when mice were on the LS diet. Responses to PE did not differ between sexes on any of the diets. Further, sex differences were not detectable in the ANG II- and PE-induced decreases in HR, suggesting that alterations in baroreflex function do not contribute to the differences in MAP and renal vascular responses in an acute setting.
The increase of MAP and renal vascular resistance in response to chronic ANG II infusion has been shown to depend on ROS generation in rats and mice (11, 16), and interestingly, sensitivity to ANG II increases in the rat renal circulation under these conditions (9). Of note, superoxide appears to contribute to acute hemodynamic responses to ANG II (13). Therefore, we examined whether differences in sensitivity to free radical scavenging could account for the observed sex differences. We found that tempol had no effect on any of the responses to ANG II in mice on the LS and NS diets. On the HS diet, tempol reduced the renal responses to ANG II, but there was no sex difference. Thus, ROS production does not account for the sex differences in the ANG II response on any of the diets, suggesting that the sex differences are most likely due to variations in the ANG II receptors and/or signaling pathways.
We then examined the expression of the AT1 and AT2 receptors and NOS3 in dissected kidney vessels. On the LS diet, AT1 receptor expression was higher in males. However, AT2 receptor expression was also increased in males, and this may have masked the sex difference in the ANG II response on the LS diet. No sex differences were found on the NS diet. On the HS diet, AT1 was higher and NOS3 expression lower in males, which corresponds to the observed greater ANG II response in males on the HS diet. To our knowledge, data in mice were thus far limited to reports on higher plasma ACE1 levels and higher cardiac expression of ACE1 in males compared with female animals (6, 12). On the LS and HS diets, the functional responses and expressional data correlate well. While on the NS diet, the expression data do not fully explain the hemodynamic responses. It has to be acknowledged, however, that we have measured total cellular, i.e., cytosolic and membrane AT1, AT2, and NOS3 abundance. A previous study has demonstrated significant sex differences in the subcellular localization of neuronal AT1 receptors (29). Only the AT1 and AT2 receptors on the cell membrane will be exposed to extracellular ANG II, and sex differences in subcellular localization of these receptors may play a role in kidney vessels. Of note, nitric oxide production by NOS3 also depends on subcellular localization (18). Another possibility is that there could be differences in endothelium or smooth muscle expression or activity not accounted for in the vascular preparation used in the current study. Finally, sex differences in postreceptor signaling of AT1 and AT2 receptors are clearly a possibility, and this needs to be addressed in future studies.
Perspectives and Significance
The findings from this study extend previous observations of greater BP responses in male mice during chronic ANG II infusion in an acute setting. Furthermore, we showed that the renal response is exaggerated in males on NS and HS diets, which is associated with sex differences in the renal vascular expression of the AT1 and AT2 receptors and NOS3. Future studies need to clarify the role of hormones in mediating these sex differences. Beyond hormonal effects, recent studies have demonstrated important direct influences of sex chromosomes on ANG II responses (10), and this will be an exciting area of future research. Because sex differences in the RAS are strikingly conserved across species, rodent models in combination with gene-targeting technology will provide powerful tools to unravel the mechanisms and the impact of sex-related differences in the RAS for cardiovascular and renal diseases.
GRANTS
This study was supported by a fellowship from the Deutsche Forschungsgemeinschaft (to M. P. Schneider) and by Established Investigator Awards from the American Heart Association (to D. M. Pollock and J. S. Pollock). The authors also gratefully acknowledge funding from the National Institutes of Health (HL60653 to J. S. Pollock; HL69999 to J. S. Pollock and D. M. Pollock).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Ashlee Tipton with the Western blots in this study.
REFERENCES
- 1.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens 25: 1263–1271, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7). Hypertension 30: 535–541, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol 297: F471–F480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freshour JR, Chase SE, Vikstrom KL. Gender differences in cardiac ACE expression are normalized in androgen-deprived male mice. Am J Physiol Heart Circ Physiol 283: H1997–H2003, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension 31: 90–96, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl 55: S35–S41, 1996 [PubMed] [Google Scholar]
- 9.Imig JD. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am J Hypertens 13: 810–818, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 55: 1275–1282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Lim YK, Retnam L, Bhagavath B, Sethi SK, bin Ali A, Lim SK. Gonadal effects on plasma ACE activity in mice. Atherosclerosis 160: 311–316, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int 55: 278–285, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 11: 319–329, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to Tempol in angiotensin II-Infused hypertensive rats. Hypertension 37: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Page A, Reich H, Zhou J, Lai V, Cattran DC, Scholey JW, Miller JA. Endothelial nitric oxide synthase gene/gender interactions and the renal hemodynamic response to angiotensin II. J Am Soc Nephrol 16: 3053–3060, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 88: 10480–10484, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich H, Duncan JA, Weinstein J, Cattran DC, Scholey JW, Miller JA. Interactions between gender and the angiotensin type 1 receptor gene polymorphism. Kidney Int 63: 1443–1449, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol 292: R794–R799, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ruan X, Wagner C, Chatziantoniou C, Kurtz A, Arendshorst WJ. Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. J Clin Invest 99: 1072–1081, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Sandberg K, Ji H. Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther 10: 15–23, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Stegbauer J, Kuczka Y, Vonend O, Quack I, Sellin L, Patzak A, Steege A, Langnaese K, Rump LC. Endothelial nitric oxide synthase is predominantly involved in angiotensin II modulation of renal vascular resistance and norepinephrine release. Am J Physiol Regul Integr Comp Physiol 294: R421–R428, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension 39: 597–602, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol 83: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Venegas-Pont M, Sartori-Valinotti JC, Glover PH, Reckelhoff JF, Ryan MJ. Sexual dimorphism in the blood pressure response to angiotensin II in mice after angiotensin-converting enzyme blockade. Am J Hypertens 23: 92–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Milner TA, Speth RC, Gore AC, Wu D, Iadecola C, Pierce JP. Sex differences in angiotensin signaling in bulbospinal neurons in the rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 295: R1149–R1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: the role of central nitric oxide. Am J Physiol Heart Circ Physiol 297: H1638–H1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]