Abstract
Background
Women with disabilities (WWD) face significant barriers accessing healthcare, which may affect rates of routine preventive services. We examined the relationship between disability status and routine breast and cervical cancer screening among middle-aged and older unmarried women and the differences in reported quality of the screening experience.
Methods
Data were from a 2003–2005 cross-sectional survey of 630 unmarried women in Rhode Island, 40–75 years of age, stratified by marital status (previously vs. never married) and partner gender (women who partner with men exclusively [WPM] vs. women who partner with women exclusively or with both women and men [WPW]).
Results
WWD were more likely than those without a disability to be older, have a high school education or less, have household incomes <$30,000, be unemployed, and identify as nonwhite. In addition, WWD were less likely to report having the mammogram or Pap test procedure explained and more likely to report that the procedures were difficult to perform. After adjustment for important demographic characteristics, we found no differences in cancer screening behaviors by disability status. However, the quality of the cancer screening experience was consistently and significantly associated with likelihood of routine cancer screening.
Conclusions
Higher quality of cancer screening experience was significantly associated with likelihood of having routine breast and cervical cancer screening. Further studies should explore factors that affect quality of the screening experience, including facility characteristics and interactions with medical staff.
Introduction
Approximately 26 million women in the United States have a disability.1 Disability covers a wide range of impairments, and it can generally be defined as the presence of a limitation in activity or function caused by a biological or psychological condition.2 Because of these limitations, women with disabilities (WWD) may face unique challenges in accessing routine preventive healthcare services, such as cancer screenings. These barriers include attitudes of healthcare providers, communication difficulties with healthcare providers, and lack of provider knowledge about disabilities, lack of transportation, and inaccessible equipment and facilities.3–9 Such barriers may be some of the reasons why WWD are less likely to have routine cancer screenings and be at higher risk of late-stage cancer than women without disabilities.9,10
Cervical cancer screening by Pap tests and breast cancer screenings by mammography are widely acknowledged as effective methods for the early detection of cancer and are recommended as routine screening.11 However, disparities in adherence to recommended cancer screening rates are clearly evident among older women and those with severe disabilities.5,12–14 A report from the U.S. Centers for Disease Control and Prevention (CDC)15 indicated that older women with a disability were less likely to participate in breast cancer screening compared with women in other age groups.
Older women who are unmarried may be particularly at risk for lower rates of cancer screening. The high rates of disability among older unmarried women16 may further exacerbate existing barriers to cancer screening that these women already face because of their age and marital status. According to previous research, older unmarried women are less likely than married women to obtain regular screening tests for cervical cancer,17 although older unmarried women who are sexually active have an increased risk of cervical cancer compared with older married women.18 In addition, older unmarried women are also more likely than their married counterparts to be diagnosed with higher stages of breast cancer and have increased risk for mortality after a diagnosis of breast cancer.19 Similarly, unmarried women are less likely than married women to obtain regular screening for cervical cancer.20 Despite this, no published study has systematically examined how disability status affects routine cancer screening practices specifically in a population of middle-aged and older unmarried women. The underuse of routine breast and cervical cancer screening among this population indicates the need to identify specific barriers to participation.
We examined how breast and cervical cancer screening differed by disability status among a sample of unmarried women aged 40–75 years. Because an individual's decision to continue seeking care21 is influenced by quality of care, we investigated how quality of the screening experience may influence the relationship between disability and likelihood of routine screening. Increasing the routine screening rates in a growing subpopulation of women who have historically been underserved could lead to substantial reductions in cancer morbidity and mortality.11,22–24
Materials and Methods
Study sample
Data for this analysis were from the Cancer Screening Project for Women (CSPW), a 2003–2005 survey examining the cancer screening practices of unmarried women. The sample consisted of women 40–75 years old who received the majority of their healthcare in Rhode Island and had never been diagnosed with cancer other than nonmelanoma skin cancer. The survey was developed in a three-step process: (1) review of existing instruments, (2) focus groups,25 and (3) cognitive-based testing.26 Women were recruited through community events, health fairs, mailings and fliers, personal networks, and print media.27 Participants were randomly assigned to receive a self-administered mailed questionnaire, a computer-assisted telephone interview, or a computer-assisted self-interview.28 Each participant was asked two questions regarding disability: (1) Do you consider yourself to have a disability? and (2) Do others consider you to have a disability? We limited the analyses to participants who answered these two questions (n = 597). A participant was considered to have a disability if she considered herself to have a disability or if she reported others considered her to have a disability. This project was approved by the Brown University Institutional Review Board.
The main outcome variables of interests were self-reported participation in routine cervical cancer screening by Pap tests, routine breast screening by mammograms, and on-schedule screening for breast and cervical cancer using standard definitions from the National Cancer Institute (NCI). Routine cervical cancer screening was defined as having two Pap tests within the last 6 years—one Pap test within the last 3 years and an earlier Pap test within 3 years prior. Routine breast screening was defined as having two mammograms within the last 4 years—one mammogram within the last 2 years and an earlier mammogram within 2 years prior. On-schedule cancer screening was defined as having both routine Pap tests and routine mammograms.
The main exposure variable of interest was quality of the cancer screening experience. We created three different variables to quantify the quality of the mammogram experience, the Pap test experience, and a variable summarizing the mammogram and Pap test screening experiences. Participants who had more than one mammogram were asked how often the procedure was explained and whether their privacy was respected during the procedure. Responses of “All or most of time” were scored as 1, and responses of “None or some of time” were scored as 0. Participants with more than one mammogram were also asked whether the test was difficult to perform “All or most of time” (score = 0) or “None or some of time” (score = 1). Similarly, women who reported more than one Pap test in their lifetime were asked questions about how often their privacy was respected, if the procedure was explained, and if the Pap test was difficult to perform. The item-to-scale correlation was generally high for all three summary scales (0.50–0.74 for the quality of the mammogram, 0.56–0.81 for the quality of the Pap test, and 0.44–0.70 for the quality of the mammogram and the Pap test). A median split was used to create three dichotomous variables from the sum of the scores: quality of mammogram (<3, ≥3), quality of Pap test (<3, ≥3), and quality of mammogram and Pap test (<6, ≥6).
Using the Anderson and Newman framework,29 we selected characteristics considered to be potential determinants of screening behavior. Such factors as poverty, unemployment, lack of health insurance, and lower educational level have consistently been found to pose barriers to cancer screening29,30 and may potentially be associated with disability status. Health behaviors, including physical inactivity, current cigarette consumption, and not having seen a physician within the past year, have also been associated with lack of recent mammogram or Pap test.30 Marital status and partner preference may also be potential confounders in the relationship between disability and cancer screening. Not having a recent mammogram or a recent Pap test is associated with not being currently unmarried.30,31 In addition, women who are sexual minorities have a higher risk for health limitations compared with exclusively heterosexual women32 and may also be less likely to be screened for cancer. These variables were grouped as predisposing characteristics, enabling resources, and personal health practices.
Predisposing characteristics included age (40–49, 50–69, 70–75 years), race (white non-Hispanic vs. nonwhite), history of breast, cervical, or colon cancer in a first-degree relative (yes vs. no), and current employment status (employed vs. not employed). Based on the participants' reported marital status and partner gender status, women were allocated into one of six marital status/partner gender strata: (1) never married women who partner with women (WPW) or with either women or men (hereafter referred to as WPW), (2) previously married WPW, (3) never married women who partner with men (WPM), (4) previously married WPM, (5) never married women with no partner preference (NPP), and (6) previously married NPP. Strata (5) and (6) included women who reported no interest in having a partner and refused to select the gender of a potential future partner. As characteristics of NPP were comparable to those of WPM we combined these two strata with WPM for all analyses. Enabling resources included the following binary variables—income (<$30,000 vs. ≥$30,000) and health insurance (yes vs. no). Moreover, we created a health behavior index to capture personal health practices by summing the number of healthy behaviors (out of 5) reported by participants: never smoke at least 1 cigarette per day or 7 cigarettes per week, has never had a lifetime drinking problem, does strength/flexibility exercise at least once per week, does aerobic activity at least 1 hour per week, and reported going to a primary care physician for a routine checkup once a year or less. A higher score indicated the participant engaged in more health behaviors.
Statistical analyses
Descriptive analysis was conducted to characterize women with and without disabilities. Significant differences between these two groups were reported with the chi-square test. We used logistic regression analysis to examine the association between disability status and likelihood of having routine mammograms, routine Pap tests, and on-schedule testing for Pap test and mammogram. Goodness of fit was assessed for all logistic models with the Hosmer-Lemeshow test. Statistical significance was set at a two-sided 0.05 alpha level. All analyses were performed using Statistical Analysis Software (SAS 9.13, Cary, NC).
Results
Almost one third (28%) of our sample had a disability. WWD were significantly more likely than women with no disabilities (WND) to be a racial minority, to be older, and have less education and less household income (Table 1). WWD were also more likely to report having previously been married and to partner with men or have no partner preference. Similar proportions of women with and without a disability had health insurance (86% vs. 89%, p = 0.43), although a higher proportion of WWD compared with WND reported having a routine physical examination at least once a year (84% vs. 74%, p = 0.006).
Table 1.
Sociodemographic Characteristics of Cancer Screening Project for Women Participants by Disability Status
| WWD n (%) | WND n (%) | p valueb | |
|---|---|---|---|
| Total | 166 (27.8) | 431 (72.2) | |
| Age | |||
| 40–49 | 55 (33.1) | 197 (45.7) | 0.002 |
| 50–69 | 92 (55.4) | 212 (49.2) | |
| 70–75 | 19 (11.5) | 22 (5.1) | |
| Level of formal education | |||
| High school or less | 55 (33.1) | 37 (8.6) | <0.0001 |
| Some college or technical training | 52 (31.3) | 108 (25.1) | |
| College degree or more | 58 (34.9) | 286 (66.4) | |
| Refused/missing | 1 (<1.0) | 0 (0) | |
| Race/ethnicity | |||
| White nonHispanic | 91 (54.8) | 349 (81.0) | <0.0001 |
| Nonwhite | 73 (44.0) | 80 (18.6) | |
| Refused/missing | 2 (1.2) | 2 (<1%) | |
| Marital status/partner preference | |||
| Never married WPW | 19 (11.5) | 120 (27.8) | <0.0001 |
| Previously married WPW | 17 (10.2) | 49 (11.4) | |
| Never married WPW/NPP | 48 (28.9) | 117 (27.2) | |
| Previously married WPW/NPP | 82 (49.4) | 145 (33.6) | |
| Working full-time or part-time | |||
| Yes | 49 (29.5) | 370 (85.9) | <0.0001 |
| No | 115 (69.3) | 60 (13.9) | |
| Refused/missing | 2 (1.2) | 1 (<1.0) | |
| Household incomeb | |||
| <$30,000 | 122 (73.5) | 110 (25.5) | <0.0001 |
| ≥$30,000 | 25 (15.1) | 199 (46.2) | |
| Refused/missing | 8 (4.8) | 20 (4.6) | |
| Currently has health insurance | |||
| Yes | 143 (86.1) | 385 (89.3) | 0.43 |
| No | 21 (12.7) | 44 (10.2) | |
| Refused/missing | 2 (1.2) | 2 (<1%) | |
| Routine physical examination once a year | |||
| Yes | 140 (84.3) | 318 (73.8) | 0.006 |
| No | 113 (26.2) | 26 (15.7) | |
WWD, women with disabilities; WND, women with no disabilities.
Wald chi-square test comparing WWD with WND.
Frequency of breast and cervical cancer screening
Approximately 94% of the women in our sample reported ever having a mammogram, and 85% had a recent mammogram. This exceeds the Healthy People 2010 goals of 80% and 70%, respectively.22 In addition, >60% of the participants reported a routine mammogram. Similarly, the proportion of women who ever had a Pap test and had a recent Pap test approximated the Healthy People 2010 goal of 97% and 90%, respectively.
Despite the overall high rates of breast and cervical cancer screening, there were important differences in Pap test use by disability status. WND were significantly more likely than WWD to report having a recent Pap test (OR = 2.70, 95% CI = 1.68-4.35) and a routine Pap test (OR = 2.12, 95% CI = 1.43-3.13). In addition, we found that WWD were more likely to be off-schedule for both of these cancer screenings compared with WND (OR = 1.95, 95% CI = 1.15-3.30) (Table 2). However, the differences in breast and cervical cancer screening between women with and without disability were no longer significant after adjusting for demographics and health behavior characteristics (adjOR for routine mammogram = 1.28, 95% CI = 0.80-2.06; adjOR for routine Pap test = 1.45, 95% CI = 0.78-2.69, respectively).
Table 2.
Cancer Screening Rates among CSPW Participants
| |
|
CSPW participants |
|||
|---|---|---|---|---|---|
| HP 2010 goals (%) | Total n (%) | WWD n (%) | WND n (%) | Unadjusted OR (95% CI) | |
| Ever mammogram | |||||
| Yes | 80 | 561 (94) | 155 (93) | 406 (94.2) | Reference |
| No | 33 (6) | 10 (6) | 23 (5.4) | 1.14 (0.53, 2.45) | |
| Recent mammogram | |||||
| Yes | 70 | 509 (85) | 136 (82) | 373 (87) | Reference |
| No | 88 (15) | 30 (18) | 58 (13) | 1.42 (0.88, 2.30) | |
| Routine mammogram | |||||
| Yes | — | 388 (65) | 102 (61) | 286 (66) | Reference |
| No | — | 209 (35) | 64 (39) | 145 (34) | 1.24 (0.85, 1.79) |
| Ever Pap testa | |||||
| Yes | 97 | 587 (98) | 160 (96) | 427 (99) | Reference |
| No | 6 (1) | 4 (2) | 2 (1%) | 5.34 (0.97, 29.42) | |
| Don't know/Refuse | 4 (1) | 2 (1) | 2 (1%) | 5.34 (0.48, 59.26) | |
| Recent Pap testa | |||||
| Yes | 90 | 514 (86) | 127 (77) | 387 (90) | Reference |
| No | — | 83 (14) | 39 (23) | 44 (10) | 2.70 (1.68, 4.35) |
| Routine Pap test | |||||
| Yes | — | 446 (75) | 106 (64) | 340 (79) | Reference |
| No | — | 151 (25) | 60 (36) | 91 (21) | 2.12 (1.43, 3.13) |
| On-schedule for breast and cervical screening (n = 597) | |||||
| Both on-schedule | 356 (60) | 90 (54) | 266 (62) | Reference | |
| Either one off-schedule | 168 (28) | 47 (28) | 121 (28) | 1.15 (0.76, 1.74) | |
| Both off-schedule | 73 (12) | 29 (17) | 44 (10) | 1.95 (1.15, 3.30) | |
| On-schedule for breast, cervical, and colorectal screening (n = 345) | |||||
| All on-schedule | 272 (46) | 42 (38) | 96 (41) | Reference | |
| At least one off-schedule | 300 (50) | 58 (52) | 124 (53) | 1.07 (0.66, 1.73) | |
| All off-schedule | 25 (4) | 11 (10) | 14 (6) | 1.80 (0.75, 4.28) | |
Includes women without a uterine cervix.
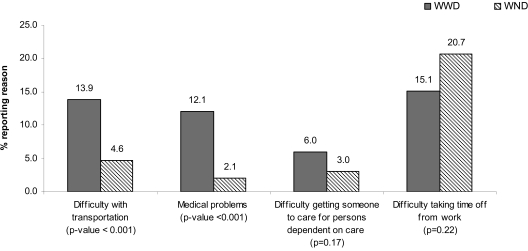
There is some evidence that WWD and WND face different types of barriers to care, based on the different reasons given for putting off or avoiding cancer screening (Fig. 1). A higher proportion of WWD compared with WND reported transportation (14% vs. 5%, p < 0.001) and medical problems (12% vs. 2%, p < 0.001) as reasons for putting off or avoiding cancer screening, by self-reported disability status. There was no statistically significant difference between the proportions in the two groups reporting having difficulty getting someone to care for persons dependent on their care or in difficulty taking time off from work.
FIG. 1.
Reasons for putting off or avoiding cancer screening by self-reported disability status, Cancer Screening Project for Women, 2003–2005.
Quality of screening experience
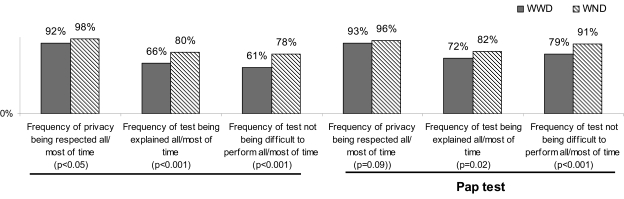
Among those who had more than one mammogram, a higher proportion of WND compared with WWD reported having their privacy respected and having the procedure explained all or most of the time. Similarly, a higher proportion of WND compared with WWD reported having their privacy respected and having the Pap test explained all or most of the time. In addition, WWD reported more difficulty with the mammogram and Pap test. Approximately 40% of WWD described the mammogram and >25% described the Pap test as being difficult to perform all or most of the time (Fig. 2).
FIG. 2.
Proportion of women reporting specific indicators of quality of cancer screening experience, by disability status and type of screening, Cancer Screening Project for Women, 2003–2005.
Positive quality of the cancer screening experience was associated with increased likelihood of engaging in routine Pap test (adjOR = 1.89, 95% CI = 1.18-3.03) and being on-schedule for both breast and cervical cancer screening (adjOR = 1.82, 95% CI = 1.18-2.81). In addition, age was associated with routine breast and cervical cancer screening but in different directions. Women who were older were more likely to report routine mammograms but less likely to report routine Pap test. Although tests for interaction between quality of cancer screening experience and disability status were not significant (p = 0.49 for routine Pap test, p = 0.53 for mammogram, p = 0.43 for on-schedule for both tests), there is some evidence that associations between cancer screening and disability status are stronger for those with high-quality cancer screening experiences (Table 3). Among the participants with high-quality cancer screening experience, the skewed confidence interval suggests that the odds of WND having routine mammogram, routine Pap test, and being on-schedule for both tests were higher than those of WWD.
Table 3.
Adjusted Odds for Routine and On-Schedule Breast, Cervical, and Colorectal Cancer Screening
| |
Routine screening |
On-schedule screening |
|
|---|---|---|---|
| Mammogram | Pap test | Mammogram and Pap test | |
| Total | 471 | 531 | 456 |
| Health behavior index | 1.06 (0.87, 1.30) | 1.25 (1.03, 1.53)* | 1.08 (0.90, 1.30) |
| Quality of cancer screening experience | |||
| High | 1.28 (0.80, 2.06) | 1.89 (1.18, 3.03) | 1.82 (1.18, 2.81) |
| Low | Reference | Reference* | Reference* |
| Has a disability | |||
| Yes | Reference | Reference | Reference |
| No | 0.78 (0.40, 1.52) | 1.45 (0.78, 2.69) | 1.19 (0.67, 2.13) |
| Age, years | |||
| 40–49 | Reference* | Reference* | Reference* |
| 50–69 | 2.74 (1.65, 4.54) | 0.85 (0.51, 1.42) | 1.64 (1.03, 2.61) |
| 70–75 | 2.63 (0.99, 6.96) | 0.24 (0.10, 0.55) | 0.55 (0.24, 1.26) |
| Level of formal education | |||
| High school diploma or less | Reference | Reference | Reference |
| Some college or technical training | 1.35 (0.65, 2.81) | 1.73 (0.88, 3.41) | 1.40 (0.70, 2.80) |
| College degree or more | 1.45 (0.70, 2.98) | 2.05 (1.04, 4.04) | 1.58 (0.80, 3.10) |
| Race/ethnicity | |||
| White Non-Hispanic | 2.13 (1.24, 3.65) | 1.03 (0.59, 1.78) | 1.46 (0.87, 2.46) |
| Nonwhite | Reference* | Reference | Reference |
| Marital status/partner preference | |||
| Never married WPW | Reference | Reference | Reference |
| Previously married WPW | 1.19 (0.50, 2.83) | 1.29 (0.52, 3.18) | 1.57 (0.70, 3.53) |
| Never married WPM/NPP | 1.34 (0.66, 2.73) | 0.96 (0.47, 1.96) | 1.16 (0.61, 2.20) |
| Previously married WPM/NPP | 1.02 (0.53, 1.97) | 1.02 (0.51, 2.04) | 1.16 (0.63, 2.13) |
| Working full-time or part-time | |||
| Yes | 1.15 (0.60, 2.23) | 0.46 (0.24, 0.88) | 0.68 (0.37, 1.24) |
| No | Reference | Reference* | Reference |
| Household income | |||
| <$30,000 | Reference | Reference* | Reference |
| ≥$30,000 | 1.36 (0.72, 2.55) | 2.24 (1.24, 4.05) | 1.65 (0.94, 2.87) |
| Currently has health insurance | |||
| Yes | 1.34 (0.60, 2.96) | 1.41 (0.71, 2.79) | 1.32 (0.64, 2.72) |
| No | Reference | Reference | Reference |
| First-degree relative with breast cancer | |||
| Yes | 1.42 (0.76, 2.65) | NA | 1.28 (0.73, 2.23) |
| No | Reference | Reference | Reference |
| Don't know | a | NA | a |
| First-degree relative with cervical cancer | |||
| Yes | NA | 1.54 (0.65, 3.66) | 0.71 (0.35, 1.47) |
| No | Reference | Reference | Reference |
| Don't know | NA | a | a |
p value for Wald chi-square test, < 0.05.
Not computed because of small sample size.
We conducted a sensitivity analysis to see if the results for the model with the routine Pap test outcome and the model with on-schedule screening for breast and cervical cancer would differ if the sample was restricted to women with no hysterectomy. Similarly, we assessed whether modes for recruitment or modes of survey completion affected parameter estimates in all three models. No significant changes in the parameter estimates (<10%) were noted in any of the models in the sensitivity analysis. For these reasons, the final models do not include mode of recruitment or mode of survey completion.
Discussion
Since 1995, the Rhode Island Women's Cancer Screening Program has provided no-cost Pap tests and mammograms to eligible women in the state.33 This has undoubtedly contributed to Rhode Island's having the highest percentage of women in the nation getting screening mammograms.33 Although WWD still had lower rates of routine mammogram and Pap tests in our unadjusted analyses, these differences were not noted once we adjusted for demographic characteristics. This indicates that the differences in routine mammogram and Pap tests rates between these two groups can be explained by the confounding influence of sociodemographic and health behavior characteristics. Consistent with published reports, disability was associated with indicators of socioeconomic disadvantage, such as unemployment, lower education, and lower income.34,35
Whereas most studies have suggested WWD have lower cancer screening rates compared with WND,36–40 several have found no overall differences between the two groups.34,41,42 We found that type of cancer screening mattered. WWD and WND had comparable breast cancer screening. The proportion of middle-aged and older unmarried women in our sample who received mammograms at the recommended intervals was higher than previously reported in the literature8,43 and exceeded the Healthy People 2010 national objectives.44 This differs from other studies that reported that women with mental health or cognitive problems were less likely to attend breast screening.45,46 In contrast, WWD in our study had lower rates for all measures of cervical cancer screening compared with WND, similar to previously reported results.40 In particular, the gap between the desired and actual proportion of WWD who reported a Pap test within the last 3 years was significant, falling below the Healthy People 2010 goal.44
It is crucial to understand the series of steps that contribute to successful participation in routine and on-schedule cancer screening. These include receiving and acting on appropriate information about cancer screening, adequate transportation and assistance to the cancer screening facility, and having the healthcare professional perform the examination with minimal pain or patient discomfort. In addition, similar to other studies, we found that reasons for putting off or delaying a routine cancer screening included transportation and taking time off from work.47,48 Previous research has documented how such entry barriers lead to limited uptake of preventive care services among WWD.5,49 For example, healthcare clinicians may erroneously assume that disability limits a woman's sexual activity and not provide adequate patient education about cancer screening.5 A previous study found that WWD did not have routine gynecological cancer screening services despite having seen a general healthcare provider within the last 6 months.50 Similarly, a high proportion of women in our sample had a current primary care provider (91%, data not shown), but the proportion of our sample reporting routine Pap tests was still below the national objectives.
Beyond entry barriers, all WWD may experience other difficulties with the procedure and the cancer screening experience itself. Communication difficulties, difficulties with the screening experience, and staff attitudes contribute to the quality of the cancer screening experience, especially for WWD. Factors that contribute to the quality of the experience, such as privacy and respect, can be grouped as secondary access factors, which impact the ability of an individual to continue care after she is in the healthcare system.20
Previous studies found that having additional reassurance and privacy50 and clear patient-doctor communication were associated with regular participation in mammography.51 In addition, an earlier study found that women who rated the overall quality of their healthcare as excellent had higher odds of receiving an annual Pap smear.52 Our study is one of the first studies to specifically examine how quality of the cancer screening experience and disability status are associated with receiving routine breast and cervical cancer screening among an older, unmarried female population. Although there have been recent gains in eliminating disparities in cancer screenings rates, it is imperative to also ensure the quality of the cancer screening experience for all women. Our results support the hypothesis that quality of the screening experience affects routine mammogram and Pap test rates, even after controlling for important individual characteristics and primary healthcare access factors. Consequently, there is a need to identify appropriate strategies that will ensure an optimal cancer screening experience for all women. These strategies should explore how best to provide information and equitable access. In addition, they should address the need to specifically tailor interventions to specific types of disabilities as needed. Our findings suggest that public health strategies for increasing the proportion of older WWD who obtain routine cancer screening should focus on improvement of quality of the screening experience instead of focusing only on individual patient behavior.
Study limitations and strengths
This study had several limitations. Disabilities encompass a wide range of limitations, including cognitive or psychological problems. WWD are a heterogeneous group with enormous diversity in limitations, underlying conditions, and duration of disability. Previous studies found differences in cancer screening rates according to disability severity, so using a general self-report of disability might dilute any association between disability and cancer screening.14,43 Second, this study was based on self-reported behaviors, which may underestimate53 or overestimate54 cancer screening prevalence. Third, nonprobability-based sampling procedures may lead to limited generalizability of our results. Generalizability may also be limited because our sample focused on women who received most of their medical care in Rhode Island. Finally, we were unable to interpret the causal relationship between individual cancer adherence and the other variables because of the cross-sectional nature of the study design.
Nevertheless, this study extends previous knowledge by taking initial steps to assess whether there are differences in quality of the cancer screening experience in a group of middle-aged and older unmarried women with overall high rates of cancer screening. Information on this subpopulation of older unmarried women is not readily available. One of the strengths of the CSPW is its focus on unmarried women between the ages of 40 and 75, a subpopulation of women for whom large sample sizes have not been previously available. Further research efforts will include refining our quality of cancer screening experience variable. Although the quality of cancer screening experience variable in this current study is rudimentary, it does provide some evidence that less tangible barriers may be affecting cancer screening practices. In addition to including more information about type and severity of disability, future research directions should explore barriers to quality and the relationship between consumer satisfaction and cancer screening.
Acknowledgment
Support for this research was provided by the National Cancer Institute, K07-CA87070, to M.A.C.
Disclosure Statement
No competing financial interests exist.
References
- 1.Waldrop J. Stern SM. Disability status: 2000. Census 2000 brief. U.S. Department of Commerce Economics and Statistics Administration U.S. Census Bureau. 2003 [Google Scholar]
- 2.Verbrugge LM. Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 3.Nosek MA. Young ME. Rintala DH. Howland BA. Foley CC. Bennett JL. Barriers to reproductive health maintenance among women with physical disabilities. J Womens Health. 1995;4:505–518. [Google Scholar]
- 4.Becker H. Stuifbergen A. Tinkle M. Reproductive health care experiences of women with physical disabilities: A qualitative study. Arch Phys Med Rehabil. 1997;78(Suppl):S-26. doi: 10.1016/s0003-9993(97)90218-5. [DOI] [PubMed] [Google Scholar]
- 5.Nosek MA. Howland CA. Breast and cervical cancer screening among women with physical disabilities. Arch Phys Med Rehabil. 1997;78((Suppl)):S-39. doi: 10.1016/s0003-9993(97)90220-3. [DOI] [PubMed] [Google Scholar]
- 6.Shabas D. Weinreb H. Preventive health care in women with multiple sclerosis. J Womens Health Gend Based Med. 2000;9:389–395. doi: 10.1089/15246090050020709. [DOI] [PubMed] [Google Scholar]
- 7.DeJong G. Pablsbo S. Beatty P. Jones G. Kroll T. Neri M. The organization and financing of services for persons with disabilities. Milbank Q. 2002;80:261–301. doi: 10.1111/1468-0009.t01-1-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker H. Stuifbergen A. What makes it so hard? Barriers to health promotion experienced by people with multiple sclerosis and polio. Fam Community Health. 2005;27:75–85. doi: 10.1097/00003727-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Verger P. Aulagnier M. Souville M, et al. Women with disabilities: General practitioners and breast cancer screening. Am J Prev Med. 2005;28:215–220. doi: 10.1016/j.amepre.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Welner S. Screening issues in gynecologic malignancies for women with disabilities critical considerations. J Womens Health. 1998;7:281–285. doi: 10.1089/jwh.1998.7.281. [DOI] [PubMed] [Google Scholar]
- 11.Duffy SW. Tabár L. Smith RA. The Mammographic Screening Trials: Commentary on the recent work by Olsen and Gøtzsche. CA Cancer J Clin. 2002;52:68–71. doi: 10.3322/canjclin.52.2.68. [DOI] [PubMed] [Google Scholar]
- 12.Blustein J. Weiss LJ. The use of mammography by women aged 75 and older: Factors related to health, functioning and age. J Am Geriatr Soc. 1998;46:941–946. doi: 10.1111/j.1532-5415.1998.tb02746.x. [DOI] [PubMed] [Google Scholar]
- 13.Burack RC. Gurney JG. McDaniel AM. Health status and mammography use among older women. J Gen Intern Med. 1998;13:366–372. doi: 10.1046/j.1525-1497.1998.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng E. Myers E. Wolf L. Mobility impairments and use of preventive services in women with multiple sclerosis: Observational study. BMJ. 2001;323:986–989. doi: 10.1136/bmj.323.7319.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control Prevention. Use of cervical and breast cancer screening among women with and without functional limitations—United States, 1994–1995. MMWR. 1998;47:853–856. [PubMed] [Google Scholar]
- 16.Schoeni RF. Freedman VA. Martin LG. Socioeconomic and demographic disparities in trends in old-age disability. TRENDS Working Paper 05-1. 2005 [Google Scholar]
- 17.Hewitt M. Devesa SS. Breen N. Cervical cancer screening among U.S. women: Analyses of the 2000 National Health Interview Survey. Prev Med. 2004;39:270–278. doi: 10.1016/j.ypmed.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Yasmeen S. Romano PS. Pettinger M, et al. Incidence of cervical cytological abnormalities with aging in the Women's Health Initiative: A randomized controlled trial. Obstet Gynecol. 2006;108:410–419. doi: 10.1097/01.AOG.0000225976.69396.fb. [DOI] [PubMed] [Google Scholar]
- 19.Osborne C. Ostir GV. Du X. Peek MK. Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 20.Martin LM. Wingo PA. Calle EE. Heath CW., Jr Comparisons of mammography and Pap test use from the 1987 and 1992 National Health Interview Surveys: Are we closing the gaps? Am J Prev Med. 1996;12:82–90. [PubMed] [Google Scholar]
- 21.Lurie N. Studying access to care in managed care environments. Health Serv Res. 1997;32:691–707. [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Update: National breast and cervical cancer early detection program. MMWR. 1996;45:484–487. [PubMed] [Google Scholar]
- 23.U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 24.Nyström L. Andersson I. Bjurstam N. Frisell J. Nordenskjöld B. Rutqvist LE. Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet. 2002;359:909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 25.Clark MA. Bonacore L. Wright W. Rakowski W. The Cancer Screening Project for Women: Experiences with cancer screening among women who partner with men and women who partner with women. Women Health. 2003;38:19–33. doi: 10.1300/J013v38n02_02. [DOI] [PubMed] [Google Scholar]
- 26.Clark MA. Armstrong G. Bonacore L. Measuring sexual orientation and gender expression among middle-aged and older women in a cancer screening study. J Cancer Educ. 2005;20:108–112. doi: 10.1207/s15430154jce2002_12. [DOI] [PubMed] [Google Scholar]
- 27.Clark MA. Neighbors C. Wasserman M, et al. Strategies and cost of recruitment of middle-aged and older unmarried women in a cancer screening study. Cancer Epidemiol Biomarkers Prev. 2007;16:2605–2614. doi: 10.1158/1055-9965.EPI-07-0157. [DOI] [PubMed] [Google Scholar]
- 28.Clark MA. Rogers ML. Armstrong GF. Rakowski WR. Kviz FJ. Differential response effects of data collection mode in a cancer screening study of unmarried women ages 40–75 years. A randomized trial. BMC Med Res Methodol. 2008;8:1–11. doi: 10.1186/1471-2288-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson RM. Revisiting the behavioral model and access to medical care: Does it matter. J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 30.Hsia J. Kemper E. Kiefe C, et al. The importance of health insurance as a determinant of cancer screening: Evidence from the Women's Health Initiative. Prev Med. 2000;31:261–270. doi: 10.1006/pmed.2000.0697. [DOI] [PubMed] [Google Scholar]
- 31.Coughlin SS. Uhler RJ. Hall HI. Briss PA. Nonadherence to breast and cervical cancer screening: What are the linkages to chronic disease risk? Prev Chronic Dis. 2004;1:1–15. [PMC free article] [PubMed] [Google Scholar]
- 32.Cochran SD. Mays VM. Bowen D, et al. Cancer-related risk indicators and preventive screening behaviors among lesbians and bisexual women. Am J Public Health. 2001;91:591–597. doi: 10.2105/ajph.91.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhode Island Department of Health, Cancer Control Program. Women's Cancer Screening Program. www.health.ri.gov/disease/cancer/women-screening.php www.health.ri.gov/disease/cancer/women-screening.php
- 34.Iezzoni LI. McCarthy EP. Davis RB. Harris-David L. O'Day B. Use of screening and preventive services among women with disabilities. Am J Med Qual. 2001;16:135–144. doi: 10.1177/106286060101600405. [DOI] [PubMed] [Google Scholar]
- 35.Balluz L. Ahluwalia IB. Murphy W. Mokdad A. Giles W. Harris VB. Surveillance for certain health behaviors among selected local areas—United States, Behavioral Risk Factor Surveillance System, 2002. MMWR Surveill Summ. 2004;53(SS05):1–100. [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services. Understanding and improving health and objectives for improving health. 2nd. Washington, DC: U.S. Government Printing Office; 2000. Healthy people 2010. [Google Scholar]
- 37.U.S. Department of Health and Human Services. Healthy people 2000, progress review, people with disabilities. Washington, DC: U.S. Government Printing Office; 1997. pp. 1–3. [Google Scholar]
- 38.Thierry JM. Increasing breast and cervical cancer screening among women with disabilities. J Womens Health Gend Based Med. 2000;9:9–12. doi: 10.1089/152460900318894. [DOI] [PubMed] [Google Scholar]
- 39.Iezzoni LI. McCarthy EP. Davis RB. Siebens H. Mobility impairments and use of screening and preventive services. Am J Public Health. 2000;90:955–961. doi: 10.2105/ajph.90.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schootman M. Jeffe DB. Identifying factors associated with disability-related differences in breast cancer. Cancer Causes Control. 2003;14:97–107. doi: 10.1023/a:1023091308625. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez A. Farmer GS. Grant D. Papachristou T. Disability and preventive cancer screening: Results from the 2001 California Health Interview Survey. Am J Public Health. 2005;95:2057–2063. doi: 10.2105/AJPH.2005.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bechmann CRB. Gittler M. Barzansky BM. Bechmann CA. Gynecologic health care of women with disabilities. Obstet Gynecol. 1989;74:75–79. [PubMed] [Google Scholar]
- 43.Smeltzer SC. Preventive health screening for breast and cervical cancer and osteoporosis in women with physical disabilities. Fam Community Health. 2006;S1-29(1S):355–435. doi: 10.1097/00003727-200601001-00007. [DOI] [PubMed] [Google Scholar]
- 44.Cochran SD. Mays VM. Physical health complaints among lesbians, gay men, and bisexual and homosexually experienced heterosexual individuals: Results from the California Quality of Life Survey. Am J Public Health. 2007;97:2048–2055. doi: 10.2105/AJPH.2006.087254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan G. Glasson EJ. Hussain R. Breast cancer and the uptake of mammography screening services by women with intellectual disabilities. Prev Med. 2003;37:507–512. doi: 10.1016/s0091-7435(03)00177-4. [DOI] [PubMed] [Google Scholar]
- 46.Werneke U. Horn O. Maryon-Davis A. Wessely S. Donnan S. McPherson K. Uptake of screening for breast cancer in patients with mental health problems. J Epidemiol Community Health. 2006;60:600–605. doi: 10.1136/jech.2005.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peipins L. Shapiro J. Bobo J. Berkowitz Z. Impact of women's experiences during mammography on adherence to rescreening (United States) Cancer Causes Control. 2006;17:439–447. doi: 10.1007/s10552-005-0447-7. [DOI] [PubMed] [Google Scholar]
- 48.Lauver D. Henriques J. Settersten L. Bumann M. Psychosocial variables, external barriers, and stage of mammography adoption. Health Psychol. 2003;22:649–653. doi: 10.1037/0278-6133.22.6.649. [DOI] [PubMed] [Google Scholar]
- 49.Legg JS. Clement DG. White KR. Are women with self-reported cognitive limitation at risk for underutilization of mammography? J Health Care Poor Underserved. 2004;15:688–702. doi: 10.1353/hpu.2004.0066. [DOI] [PubMed] [Google Scholar]
- 50.Klingbeil H. Baer HR. Wilson PE. Aging with a disability. Arch Phys Med Rehabil. 2004;85:68–73. doi: 10.1016/j.apmr.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Elwood M. McNoe B. Smith T. Bandaranayake M. Doyle TC. Once is enough—Why some women do not continue to participate in a breast cancer screening program. NZ Med J. 1998;111:180–183. [PubMed] [Google Scholar]
- 52.Otero-Sabogal R. Owens D. Canchola J. Golding JM. Tabnak F. Fox P. Mammography rescreening among women of diverse ethnicities: Patient, provider, and health care system factors. J Health Care Poor Underserved. 2004;15:390–412. doi: 10.1353/hpu.2004.0048. [DOI] [PubMed] [Google Scholar]
- 53.Borders TF. Warner RD. Sutkin G. Satisfaction with health care and cancer screening practices among women in a largely rural region of West Texas. Prev Med. 2003;36:652–658. doi: 10.1016/s0091-7435(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 54.Kagay CR. Quale C. Smith-Bindman R. Screening mammography in the American elderly. Am J Prev Med. 2006;31:142–149. doi: 10.1016/j.amepre.2006.03.029. [DOI] [PubMed] [Google Scholar]