Abstract
We present immunogenicity data on the routine vaccination of 103 health care personnel during the 2009 H1N1 national vaccination campaign. The seroprotection rate (percentage of samples with hemagglutination inhibition titers of ≥1:40) was 83.2% at 30 days postvaccination, lower than those obtained in previously published controlled trials. Low baseline antibody levels and an increase in seroprotection in a negative-control cohort suggest that the virus remains prevalent.
The 2009 swine-origin influenza A H1N1 virus pandemic has caused over 15,000 laboratory-confirmed deaths (9). While this is lower than original estimates, transmission continues and the threat of a second wave of infections by a more virulent strain remains. Many countries have already identified severe cases of influenza related to a mutated H1N1 strain (8). Measures for prevention of any further spread of pandemic influenza must be taken.
The most effective measure for prevention of the spread of influenza is mass vaccination. This not only confers primary immunity but also greatly reduces the replication capacity of the virus in the host, thereby decreasing the opportunity for genetic mutation and antigen drift. Health care personnel (HCP) are a high-priority group for vaccination campaigns because of their interaction with patients, who may be sick with the disease or may be particularly susceptible to infection (2). While previous double-blind controlled trials have shown the potential effectiveness of the 2009 H1N1 vaccine, there have been no studies on its routine use and effectiveness (6). Furthermore, there have been no studies on the baseline levels of H1N1 immunity or the immunogenicity of the vaccine in Guangzhou, where the first cases of H1N1 were identified in the People's Republic of China (PRC). We present immunogenicity data on the routine use of the vaccine in a population of HCP at the Guangzhou Center for Disease Control (CDC) in China.
One hundred three HCP presenting for vaccination were enrolled on a rolling and volunteer basis and were administered the vaccine by use of standard procedures (4). Information about previous vaccination with the seasonal vaccine and known influenza-like illness within the last 6 months was recorded. Patients were excluded if they had already received the H1N1 vaccine or if they had received any vaccination in the last 6 weeks. All patients provided written informed consent. The resulting group of participants was composed of 56 males and 47 females, aged 19 to 55 years, all from the Yuexiu district, Guangzhou City, China. Blood samples were collected prior to the vaccination (T0) and at 15 (T15) and 30 (T30) days after the vaccination. A cohort of 145 HCP was enrolled as a negative control, and blood samples were collected before and after the study period. The hemagglutinin inhibition (HI) test was performed on serial 2-fold dilutions of each blood sample (Fig. 1).
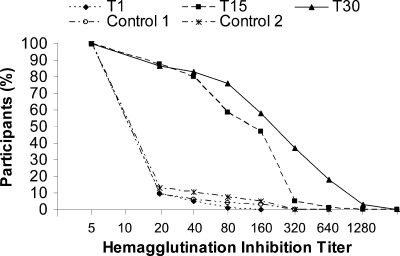
FIG. 1.
Reverse cumulative distribution curves of hemagglutination inhibition titers in both a vaccinated cohort (T0, T15, and T30) and a negative-control cohort (Control 1 and Control 2). Values are expressed as a reciprocal of the dilution. T0, day 0; T15, day 15 after vaccination; T30, day 30 after vaccination; Control 1, negative control before study period; Control 2, negative control after study period.
The influenza A H1N1 monovalent, split-virus, nonadjuvant vaccines were supplied by the Ministry of Health, People's Republic of China, and manufactured by Tianyuan Bio-Pharmaceutical Co., Ltd. (batch number 20090902), through the nationwide vaccination program. Each 0.5-ml dose contained 15 μg hemagglutinin, as prescribed by national guidelines.
Serum HI antibody titers were evaluated by observing the detectable HI titer (≥1:10), seroprotection rate (percentage of samples with HI titers of ≥1:40), and seroconversion rate (percentage of samples with 4-fold increases in HI titer and HI titers of ≥1:40) and the geometric mean titer (GMT) (1). For GMT calculations, antibody levels below the detection limit (<1:10) were assigned the value of 1:5. All values are reported with 95% confidence intervals (CIs). GMT calculations were log transformed prior to statistical tests to account for skewed distribution. All P values are two tailed.
Of the 103 participants, 7 failed to return on day 15 and 8 failed to return on day 30. Therefore, 103, 96, and 95 patient samples were received for each time point (T0, T15, and T30). No adverse side effects or flu-like symptoms were reported for these eight patients.
Pre- and postimmunization HI titers are shown in Table 1. At the baseline (T0), 10 patients (9.7%) had detectable titers of antibody against the pandemic H1N1 vaccine strain. Five patients (4.9%) had antibody titers that were greater than 1:40.
TABLE 1.
Reciprocal HI titers, seropositive rates, seroprotection rates, and seroconversion rates at the baseline and at days 15 and 30 in healthy HCP immunized with the 2009 pandemic influenza A H1N1 vaccinea
| Time point | Participant | GMT (95% CI) | % with titers of ≥1:10 (95% CI) | % with titers of ≥1:40 (95% CI) | Seroconversion rate (%) (95% CI) |
|---|---|---|---|---|---|
| T0 | 103 | 5.96 (5.34-6.64) | 9.7 (3.9-15.5) | 4.8 (0.6-9.1) | |
| T15 | 96 | 63.50 (49.57-81.34)* | 87.5 (80.8-94.2)* | 80.2 (72.1-88.3)* | 79.2 (70.9-87.4) |
| T30 | 95 | 111.09 (80.97-152.42)* | 86.4 (79.3-93.4) | 83.2 (75.5-90.8) | 82.1 (74.3-90.0) |
Tn, day n; *, based on a test of significance between time points T0 and T15 and between time points T15 and T30(P < 0.05).
At day 15 (T15) after vaccination, 84 of 96 participants had detectable antibody titers and 77 (80.2%) had titers above 1:40, with 76 (79.2%) representing a 4-fold increase from the baseline. At day 30 (T30), 80 of 95 (83.2%) participants had titers above 1:40, representing a seroconversion rate of 83% (79/95) from the baseline. Similar results were found for the GMT and the geometric mean increase from the baseline. A significant increase in GMT was found between T15 and T30. A slight increase in immunogenicity endpoints was found in the negative-control group during the study period (Table 2).
TABLE 2.
Reciprocal HI titers, seropositive rates, and seroprotection rates observed before and after the study period in an unvaccinated negative-control cohort of healthy HCPa
| Time point relative to study period | Participant | GMT (95% CI) | % with titers of ≥1:10 (95% CI) | % with titers of ≥1:40 (95% CI) |
|---|---|---|---|---|
| Before | 145 | 6.26 (5.54-7.07) | 9.7 (4.8-14.5) | 6.2 (2.2-10.2) |
| After | 145 | 7.02 (6.03-8.17) | 13.1 (7.5-18.7) | 10.3 (5.3-15.4) |
The seroconversion rate observed after the study period was 4.8%(95% CI, 1.3 to 8.4%).
The seroprotection rate in this study (83%) was lower than the rates reported for adults under the age of 64 years in previous randomized controlled trials in China (94.3 to 97.1%), the United States (98%), and Australia (95%) (3, 5, 7, 10). The current study focused on the routine use of the vaccine in a group of HCP as part of the national immunization campaign. There is no evidence suggesting that HCP would have an immune response different from that of the general population. Therefore, it is possible that the lower level of immunogenicity found in this study is due to implementation differences associated with the routine use of the vaccine in an immunization program.
This study was performed in October, after a wave of H1N1 infections in Guangzhou. Despite this, the baseline seroprotection rate (4.8 to 6.2%) was consistent with multicenter controlled trials started in July 2009 in China (3.8 to 6.9%) (5, 10). The low baseline antibody levels suggest that very few infections occurred among HCP prior to October 2009. Additionally, the 14 subjects that reported a fever in the 6 months prior to the study did not have significantly higher baseline titers (P = 0.53 by Fisher's exact test) or postvaccination seroconversion rates (P = 0.26). Interestingly, the seroconversion rate in the negative-control group was 4.2%. While this was significantly lower than the 82.3% observed in the vaccine group, the H1N1 virus may still be circulating in Guangzhou. This reiterates the importance of continued H1N1 vaccination, particularly among high-priority groups, like HCP.
Subjects who received the seasonal vaccine (n = 40) did not have significantly higher seroprotection (P = 0.37) or seroconversion (P = 0.29) rates, suggesting that the seasonal vaccine confers no immunity to the H1N1 strain.
Although previous studies have shown that the 7.5-μg formulation is sufficiently effective and elicits less adverse reactions than higher concentrations, only the 15-μg formulation supplied by the Department of Health was used in this study. Our study showed a significantly lower immune response than previous studies, including those testing the 7.5-μg formulation. The multicenter trial in China found an 89.5% seroprotection rate in adults aged 18 to 60 with the use of the 7.5-μg formulation (5).
One major limitation of the study was the sample size (n = 103). This limited the statistical significance of confounding variables such as previous flu-like illness or seasonal flu vaccine contradiction. Despite this, the lower immune response found in our study suggests that there could be a difference in vaccine effectiveness with the use of the vaccine in a routine setting compared to the level obtained in a double-blind controlled trial. This is the first such study and is the only study of vaccine effectiveness performed in Guangzhou, where the first outbreaks of H1N1 in China occurred. Continued routine-use studies enrolling larger populations of patients should be performed.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.European Committee for Proprietary Medicinal Products. 1997. Note for guidance on harmonization of requirements for influenza vaccines, March 1997 (CPMP/BWP/214/96). European Agency for the Evaluation of Medicinal Products, London, United Kingdom.
- 2.Fiore, A. E., D. K. Shay, K. Broder, J. K. Iskander, T. M. Uyeki, G. Mootrey, J. S. Bresee, and N. S. Cox. 2008. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recommend. Rep. 57:1-60. [PubMed] [Google Scholar]
- 3.Greenberg, M. E., M. H. Lai, G. F. Hartel, C. H. Wichems, C. Gittleson, J. Bennet, G. Dawson, W. Hu, C. Leggio, D. Washington, and R. L. Basser. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361:2405-2413. [DOI] [PubMed] [Google Scholar]
- 4.Kendal, A. P., M. S. Pereira, and J. J. Skehel. 1982. Concepts and procedures from laboratory-based influenza surveillance. Centers for Disease Control and Prevention, Atlanta, GA.
- 5.Liang, X. F., H. Q. Wang, J. Z. Wang, H. H. Fang, J. Wu, F. C. Zhu, R. C. Li, S. L. Xia, Y. L. Zhao, F. J. Li, S. H. Yan, W. D. Yin, K. An, D. J. Feng, X. L. Cui, F. C. Qi, C. J. Ju, Y. H. Zhang, Z. J. Guo, P. Y. Chen, Z. Chen, K. M. Yan, and Y. Wang. 2010. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375:56-66. [DOI] [PubMed] [Google Scholar]
- 6.McConnell, J. 2009. Influenza in the Asia-Pacific. Lancet Infect. Dis. 9:590-591. [DOI] [PubMed] [Google Scholar]
- 7.Plennevaux, E., E. Sheldon, M. Blatter, M. K. Reeves-Hoché, and M. Denis. 2010. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 375:41-48. [DOI] [PubMed] [Google Scholar]
- 8.Shetty, P. 2010. H1N1 vaccine access and excess. Lancet Infect. Dis. 10:75. [Google Scholar]
- 9.WHO. 2010. Pandemic (H1N1) 2009—update 89. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/don/2010_02_26/en/index.html.
- 10.Zhu, F.-C., H. Wang, H.-H. Fang, J. G. Yang, X. J. Lin, X.-F. Liang, X.-F. Zhang, H.-X. Pan, F.-Y. Meng, Y. M. Hu, W.-D. Liu, C.-G. Li, W. Li, X. Zhang, J. M. Hu, W. B. Peng, B. P. Yang, P. Xi, H.-Q. Wang, and J.-S. Zheng. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361:2414-2423. [DOI] [PubMed] [Google Scholar]