Abstract
Gamma interferon (IFN-γ) is a crucial cytokine for protection against Mycobacterium tuberculosis, but the mechanism of IFN-γ transcription is still unclear. The cyclic AMP (cAMP) responsive element binding (CREB) proteins belong to the bZip (basic leucine zipper) family of transcription factors and are essential for T-cell function and cytokine production. This study focused on the capacity of CREB proteins to regulate IFN-γ transcription in CD3+ T cells obtained from tuberculosis (TB) patients and persons with latent tuberculosis infection (LTBI) in China. The electrophoretic mobility shift assay (EMSA), chromatin immunoprecipitation (ChIP), and Western blotting were used to demonstrate the regulatory role of CREB. EMSA (in vitro) and ChIP (in vivo) experiments suggested CREB could bind to the IFN-γ proximal promoter in persons with LTBI, whereas no binding was detected in TB patients. Western blotting confirmed the expression of CREB proteins, especially serine-133-phosphorylated CREB, was markedly reduced in TB patients compared with persons with LTBI. These results suggested that CREB could promote the transcription and production of IFN-γ through binding with the IFN-γ proximal promoter, but the regulatory role of CREB was decreased in tuberculosis patients owing to diminished expression of CREB proteins, which in turn reduced the IFN-γ production.
Tuberculosis (TB) is a major health problem throughout the world and is responsible for more deaths than any other single infectious disease. In China, an estimated 0.16 million (range, 0.064 to 0.32 million) people died from TB in 2008 (21). TB is a disease mainly caused by Mycobacterium tuberculosis, and host interactions with this bacterium may lead to a cell-mediated protective immune response. Gamma interferon (IFN-γ) functions as a key cytokine in this response by activating infected macrophages and inducing a microbiocidal state. Mice lacking IFN-γ are extremely susceptible to M. tuberculosis, and infection leads to unchecked growth of M. tuberculosis in the organs of these mice (6). In humans, IFN-γ also appears to play a pivotal role because children carrying mutations in the IFN-γ receptor genes and subjects with defects regarding IFN-γ receptor expression exhibit depressed IFN-γ production and increased susceptibility to serious mycobacterial infections (10, 11).
IFN-γ is crucial for protection against M. tuberculosis, but the basis for its selective transcription is still unknown. Within the region between bp −108 and −40 of the IFN-γ promoter are two conserved and essential regulatory elements, which confer activation-specific expression in T cells (14). The distal conserved element (bp −96 to −80) contained a consensus GATA motif and a potential regulatory motif found in the promoter regions of the granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage inflammatory protein (MIP) genes (13). CREB is a 43-kDa transcription factor in the basic leucine zipper (bZIP) family that binds to cyclic AMP responsive element (CRE) via its TGACGTCA sequence and activates the basal transcription machinery (4). The proximal IFN-γ promoter contains CRE-like sequences (ACGT) where CREB binds and regulates IFN-γ transcription. Studies examining the binding of CREB proteins to the IFN-γ proximal promoter yield contradictory results regarding the transcription of IFN-γ. Experiments using Jurkat T cells and transgenic mice suggest that CREB proteins inhibit the transcription of IFN-γ (14, 22); however, these results could not be reproduced using human T cells (15, 16). We performed chromatin immunoprecipitation (ChIP) and the electrophoretic mobility shift assay (EMSA) to investigate whether CREB binds to the IFN-γ proximal promoter and/or functions in IFN-γ secretion in CD3+ T cells obtained from latent TB infection (LTBI) or TB patients. Our results suggest that CREB could promote the transcription and production of IFN-γ by binding with the IFN-γ proximal promoter under conditions of LTBI. However, the regulatory role of CREB is decreased in patients with active TB due to diminished expression of CREB proteins. These results strongly support the idea that CREB is a positive transcriptional regulator of IFN-γ in latent but not active tuberculosis infections.
MATERIALS AND METHODS
Study population.
Peripheral blood was obtained from 25 active pulmonary TB patients and 18 persons with LTBI. Patients were selected from the Beijing Chest Hospital from January 2007 through December 2007 and had received <4 weeks of anti-TB therapy. The diagnosis of TB was established based on clinical and radiological data together with the positive identification of acid-fast bacilli in sputum.
Persons considered “healthy” worked in the TB clinic of the Beijing Chest Hospital, had a history of close contact with active TB patients, and were positive for the purified protein derivative (PPD) tuberculin skin test (TST) (induration, >10 mm).
Based on the sensitivity and specificity of the PPD TST and the high prevalence of TB in clinical settings, which increases the risk for progression to active TB, induration of >10 mm is considered positive for LTBI (1).
This work was approved by the institutional review boards of the Beijing Tuberculosis and Thoracic Tumor Research Institute. Informed consent was signed by everyone involved in the study.
Antigens.
Cells of the Mycobacterium tuberculosis strain H37Rv were harvested in the mid-exponential phase of mycobacterial growth and boiled in an 80°C water bath for 20 min. After being centrifuged at 10,000 × g for 10 min, the supernatant was used as antigens (Ags) for incubation with and stimulation of CD3+ T cells for 48 or 72 h.
Preparation of T cells and nuclear proteins.
Peripheral blood mononuclear cells (PBMCs) were isolated by differential centrifugation through Ficoll-Paque. Freshly separated PBMCs were incubated with magnetic beads conjugated to monoclonal anti-CD3+ antibodies (Abs; Miltenyi Biotec, Germany), and the autoMACS magnetic cell separator (Miltenyi Biotec, Germany) was used to positively select CD3+ cells. The purity of CD3+ cells was 93.2% by flow cytometry analysis with FACSCalibur (BD Biosciences, United States).
After incubation of CD3+ cells stimulated by heat-killed M. tuberculosis Ag, the cellular pellets of CD3+ cells were suspended in buffer A containing 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and 5 μl of 10 μg/μl each aprotinin, leupeptin, and chymostatin (Sangon Biotech, China). After incubation on ice for 10 min, cells were centrifuged at 12,000 × g for 3 min at 4°C, and supernatants were discarded. Pellets were resuspended in buffer B containing 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 10% glycerol, 1 mM DTT, 0.5 mM PMSF, and 5 μl of 10 μg/μl each aprotinin, leupeptin, and chymostatin. The samples were frozen and thawed twice using liquid nitrogen and a 37°C water bath, shaken vigorously at 4°C for 30 min, and centrifuged at 14,000 × g for 10 min. The supernatants were collected and quantified using the Bradford method and were aliquoted and stored at −70°C.
EMSA.
The target probe contained the IFN-γ proximal promoter sequences (bp −71 to −40). The wild-type and mutated CREB consensus binding site probes and the NF-κB binding site probe were used as competitors for the IFN-γ proximal promoter probe. The probe sequences were as follows: IFN-γ, −71 to −40, 5′-AAAACTTGTGAAAATACGTAATCCTCAGGAGA-3′; CREB consensus binding site (wild type), 5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′; CREB consensus binding site (mutated), AGAGATTGCCTGTGGTCAGAGAGCTAG-3′; and NF-κB binding site, 5′-AGTTGAGGGGACTTTCCCAGGC-3′. The underlined nucleotides on the probes are essential for binding to CREB. The 5′ ends of all single-stranded probes were digoxigenin (DIG) labeled (Sangon Biotech, China).
Nuclear proteins (8 μg) were incubated in 2 mM DTT at 37°C for 10 min. After a 5-min equilibration to 25°C, 5 μl of nuclear proteins was added to 2 μl of the DIG-labeled probes. The 25-μl reaction mixture contained 1 μg poly(dI-dC) (Roche; catalog no. 108812), 2 mM MgCl2, 25 mM HEPES (pH 7.5), and 100 mM NaCl. After hybridization in 25°C for 1 h, the complexes were resolved by electrophoresis on 5% nondenaturing polyacrylamide gels in 0.5× TBE (Tris-borate-EDTA) buffer at 10 V/cm for 1 h. The gel was then transferred to a positively charged nylon membrane using a semidry transfer cell. DNA was detected using the DIG High Prime DNA labeling and detection starter kit I according to the manufacturer's instructions (Roche; catalog no. 11745832910).
For the competitive EMSA, a 100-fold molar excess of unlabeled probe (IFN-γ proximal promoter probe, CREB consensus binding site wild-type or mutated probe, or NF-κB binding site probe) was added, and the mixture was incubated on ice for 25 min before adding the labeled probe.
ChIP.
Freshly purified CD3+ T cells were cultured in 1 ml of RPMI with 10% human serum and 100 μg/ml penicillin in 24-well plates at 2 × 106 cells/well and were incubated with 8 μg/ml heat-killed M. tuberculosis Ags at 37°C in a 5% CO2 incubator for 24 h. The ChIP assay kit was used according to the manufacturer's specifications (Millipore, catalog no. 17-295). The primers used in PCR amplification were specific to the IFN-γ proximal promoter: 5′-TCTTCTAATAGCTGATCT-3′ and 5′-AAGGAAACTCTAACTACA-3′. PCR amplification yielded a 204-bp product.
Western blotting.
Eight micrograms of nuclear proteins was used in each sample. After electroblotting, a nitrocellulose membrane was blocked with 2% fat-free milk in Tris-buffered saline (TBS) buffer for 2 h at room temperature. The membrane was exposed to anti-CREB primary Abs (rabbit polyclonal IgG, 1:2,000; 100 ng/ml) in TBS with 5% bovine serum albumin (BSA) and 0.05% Tween overnight at 4°C. The membrane was washed three times with TBS-Tween and incubated with secondary Ab (mouse anti-rabbit IgG, 1:1,000; 1 μg/ml) in blocking buffer for 1 h at room temperature. After being washed three times in TBS-Tween and once in TBS, the membrane was drained briefly and Ab binding was detected by enhanced chemiluminescence.
In some Western blotting experiments, anti-serine-133-phosphorylated CREB Abs and anti-BCL-2 Abs were used instead of anti-CREB primary Abs. All primary Abs were obtained from Santa Cruz Biotechnology.
Statistical analysis.
The chi-square test was used to compare data from persons with LTBI and active TB patients. A P value of <0.05 was considered statistically significant.
RESULTS
CREB binding to the IFN-γ proximal promoter in vitro.
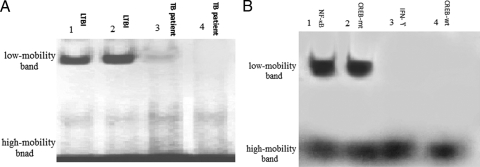
EMSA was performed with the DIG-labeled IFN-γ proximal promoter probe. Nuclear proteins were obtained from purified CD3+ T cells of 18 persons with LTBI and 25 TB patients. EMSA results were comparable for all the 18 persons with LTBI such that one low-mobility band and one high-mobility band were observed after the CD3+ T cells were stimulated by M. tuberculosis Ags (Fig. 1). However, in 25 TB patients, the low-mobility band was either undetectable (18 patients; P < 0.05, chi-square test) or weakly detectable (7 patients), suggesting that in TB patients, proteins binding to the IFN-γ proximal promoter were absent or reduced.
FIG. 1.
EMSA (A) and competitive EMSA (B). (A) Nuclear proteins from CD3+ T cells from persons with LTBI in lanes 1 and 2 or from TB patients (lanes 3 and 4); the low-mobility band was weakly detectable (lane 3) or undetectable (lane 4). (B) Before the labeled IFN-γ proximal promoter probe was added, an excess of the unlabeled NF-κB probe (NF-κB), the mutated CREB probe (CREB-mt), the IFN-γ proximal promoter probe (IFN-γ), or the wild-type CREB probe (CREB-wt) was added to the nuclear proteins from LTBI subjects. The low-mobility band could be abrogated by the unlabeled IFN-γ proximal promoter probe and the wild-type CREB probe but not by the NF-κB probe or the mutated CREB probe.
Specificity of protein binding to the IFN-γ proximal promoter.
Competitive EMSA was performed with an excess of unlabeled IFN-γ proximal promoter probe, which was added to the nuclear extracts derived from the T cells of persons with LTBI before incubation with labeled IFN-γ proximal promoter probe. The low-mobility complex was completely outcompeted by the excess unlabeled probe (Fig. 1B, lane 3), demonstrating the specificity of the DNA-binding proteins for the IFN-γ proximal promoter.
For identification of CREB in the binding complex, an excess of unlabeled CREB wild-type or mutated probe or the NF-κB binding site probe was added in the competitive EMSA experiments. Figure 1B shows that the low-mobility complex binding to the IFN-γ proximal promoter was eliminated by the excess of unlabeled wild-type CREB probe (lane 4) but not by the unlabeled mutated CREB probe (lane 2) or the NF-κB probe (lane 1). These results suggest that the protein complex binding to the IFN-γ proximal promoter includes CREB.
In vivo CREB binding to the IFN-γ proximal promoter.
ChIP was performed using CD3+ T cells that were freshly obtained from 12 TB patients and 10 persons with LTBI. Briefly, DNA-binding proteins were cross-linked to their target DNA sequences with formaldehyde, and specific anti-CREB Abs were used in immunoprecipitation after cross-linkages were reversed. PCR with primers specific to the IFN-γ proximal promoter sequence was used to test whether CREB proteins bound to the promoter. A 204-bp product was amplified from the T cells of all persons with LTBI, but no amplicon was observed when T cells from 12 TB patients were used (P < 0.01; chi-square test), despite extending the incubation time from 48 h to 72 h (Fig. 2). The ChIP results suggest that stimulation by M. tuberculosis Ags results in the production of CREB proteins in CD3+ T cells from persons with LTBI but not from TB patients. CREB proteins could bind to the IFN-γ proximal promoter in vivo.
FIG. 2.
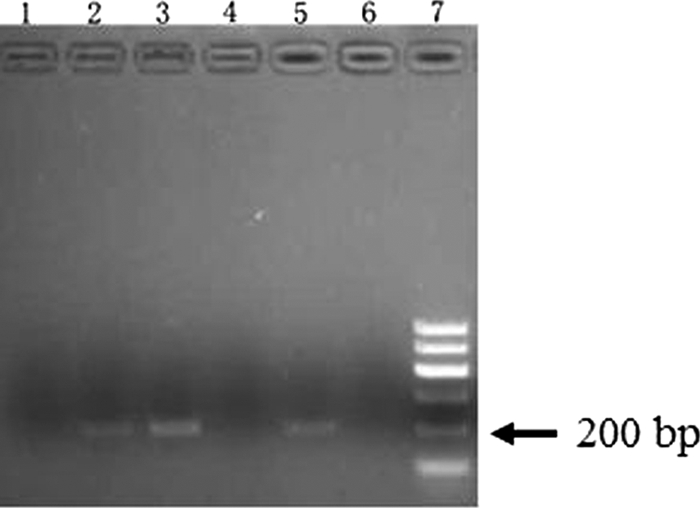
ChIP. PCR was performed with primers specific for the IFN-γ proximal promoter probe. In lanes 1, 4, and 6, the CD 3+ T cells were extracted from a TB patient. Lane 1 was incubated without M. tuberculosis Ag stimulation; lanes 4 and 6 were incubated with M. tuberculosis Ags (8 μg/ml) for 48 h (lane 4) or 72 h (lane 6), and then 1% formaldehyde was added to the cells to cross-link DNA to protein, and immunoprecipitation was performed with anti-CREB, followed by PCR amplification. The positive control is in lanes 2 and 3. DNA was added to PCR after sonication (lane 2). Lane 3 is genomic DNA was used as template in PCR; the CD 3+ T cells extracted from persons with LTBI were incubated with M. tuberculosis Ags for 24 h (lane 5).
The EMSA and ChIP data suggest that the capacity of CREB protein to bind to the IFN-γ proximal promoter was reduced in TB patients compared with that in persons with LTBI. To ascertain whether the reduction was due to the decreased levels of CREB proteins in TB patients, Western blotting was performed using an anti-CREB Ab. Of 25 TB patients, CREB was not detected in 20 TB patients (Fig. 3, lanes 3 and 4); however, CREB was detected in all 18 persons with LTBI (Fig. 3, lanes 1 and 2). Anti-serine-133-phosphorylated CREB Ab also was used in Western blotting. Phosphorylated CREB was detected in all 18 persons with LTBI but in none of the 25 TB patients (Fig. 3). β-Actin Ab was used as control in Western blotting and was found in TB patients and persons with LTBI. These results indicated that the level of CREB protein, and specifically serine-133-phosphorylated CREB protein, was markedly decreased in TB patients compared with that in persons with LTBI.
FIG. 3.
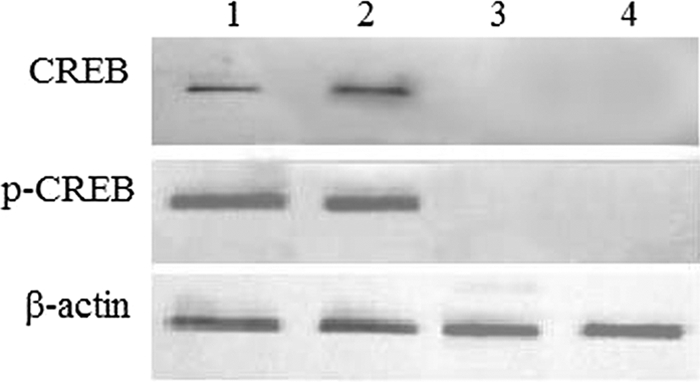
CREB and phosphorylated-CREB (p-CREB) proteins were diminished in TB patients compared to persons with LTBI. Specific anti-CREB Ab, anti-serine-133-phosphorylated CREB Ab, and anti β-actin Ab were used in Western blotting. In lanes 1 and 2, samples were from persons with LTBI, and in lanes 3 and 4, samples were from TB patients.
DISCUSSION
IFN-γ-producing T cells protect against acute infection by M. tuberculosis, and these mechanisms may also be operative during LTBI (20). IFN-γ has been considered a key macrophage-activating cytokine during TB, results in altered trafficking of intracellularly residing mycobacteria that induce phagosome-lysosome fusion, and may be critical to containment of M. tuberculosis (7). The transcription of IFN-γ is controlled by regulatory regions in its distal (bp −96 to −80) and proximal (bp −73 to −40) promoters (13). The proximal promoter plays an important role in gene regulation and may contribute to the differential expression of IFN-γ. Proteins binding to the proximal promoter markedly affect the activity of the IFN-γ promoter and the transcription of IFN-γ (14).
CREB protein is an essential transcription factor for T-cell differentiation and IFN-γ production. Deletion of CREB proteins induces defective T-cell differentiation and IFN-γ production in transgenic mice (2). To assess whether these events occur in human T cells, we performed experiments with CD3+ T cells from TB patients and persons with LTBI.
EMSA is a powerful technique used to quantitatively analyze sequence-specific DNA-binding proteins in vitro. Traditionally a radiolabeled probe is used, and when the probe and proteins form a complex, it is retarded by the gel and appears as a mobility-shifted band in comparison to the free probe. Because safety concerns are associated with the proper storage, usage, and disposal of radioactive substances and nonradioactive EMSA is sensitive, efficient, and safer (8), a DIG-labeled probe was used in the current research. ChIP is a useful technique for fine-mapping the location of transcriptional factors in vivo when the proteins interacting with the DNA target are known (19). Our in vitro (EMSA) and in vivo (ChIP) DNA binding results showed good agreement in CREB protein binding to the IFN-γ proximal promoter. However, EMSA results indicated that in 72% (18/25) of samples from TB patients, no CREB protein bound to the promoter. In comparison, ChIP results indicated no CREB protein binding in 100% (12/12) of samples from TB patients. This is probably because many different factors that can affect binding are included in the complex biological environment in vivo but are not present under the defined in vitro binding conditions used for the EMSA experiments (3).
Some studies have reported that IFN-γ levels are depressed in patients with active TB (9, 23), especially in malnourished patients with moderately advanced or far-advanced pulmonary disease (18). In the current research, based on Western blotting, the expression of CREB, and especially serine-133-phosphorylated CREB, was markedly reduced in TB patients. These data suggest that the reduced expression of CREB protein was one of the reasons for depressed IFN-γ levels in patients with active TB.
To our knowledge, this study was the first to analyze the function of CREB on IFN-γ transcription in Chinese individuals with LTBI or TB. Our EMSA experiments indicated that 72% of samples from TB patients lacked the low-mobility complex bound to the IFN-γ proximal promoter. Competitive EMSA demonstrated that the binding complex included CREB. ChIP results suggested that regardless of incubation time with heat-killed M. tuberculosis Ags (48 h or 72 h), negative results were obtained from CD3+ T cells from TB patients compared with positive results from individuals with LTBI. Western blotting indicated that the expression of CREB was markedly reduced in TB patients. Taken together, these results suggest that CD3+ T cells extracted from TB patients produce little or no CREB when stimulated by M. tuberculosis Ags, and the expression of CREB proteins was notably reduced in TB patients compared with persons with LTBI. These finding strongly support that CREB proteins play a positive regulatory role in transcription and production of IFN-γ in individuals with LTBI.
China is one of the 22 countries with a high burden of TB, and the population of TB patients in China is surpassed only by India. With the prevalence of HIV and multidrug-resistant TB and the potential of persons with LTBI to develop active TB, the control and treatment of TB are great challenges (5). Understanding the immune mechanisms of LTBI is very important in the control of TB. IFN-γ is a key cytokine in the control of M. tuberculosis infection. Humans defective in genes for IFN-γ or the IFN-γ receptor are prone to serious mycobacterial infections, including M. tuberculosis. The regulation of IFN-γ transcription is complex, involving multiple enhancer and repressor elements in response to mycobacterial Ags (12, 17). Further work should continue to focus on the mechanism for decreased IFN-γ production in TB patients, which will enrich our knowledge of protective immunity against M. tuberculosis and contribute to finding new strategies for the control and treatment of TB.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30771916) and National S65120T Major Project of China (no. 2008ZX10003-005).
We have no conflicts of interest to disclose.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.American Thoracic Society. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 161:S221-S247. [DOI] [PubMed] [Google Scholar]
- 2.Aune, T. M. 2001. Transcriptional reprogramming during T helper cell differentiation. Immunol. Res. 23:193-204. [DOI] [PubMed] [Google Scholar]
- 3.Bai, G., M. A. Gazdik, D. D. Schaak, and K. A. McDonough. 2007. The Mycobacterium bovis BCG cyclic AMP receptor-like protein is a functional DNA binding protein in vitro and in vivo, but its activity differs from that of its M. tuberculosis ortholog, Rv3676. Infect. Immun. 75:5509-5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, W., Y. L. Yu, and S. F. Lee. 2001. CREB is one component of the binding complex of the Ces-2/E2A-HLF binding element and is an integral part of the interleukin-3 survival signal. Mol. Cell. Biol. 21:4636-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley, C. L. 2010. Update in tuberculosis 2009. Am. J. Respir. Crit. Care Med. 181:550-555. [DOI] [PubMed] [Google Scholar]
- 6.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatfield, J., and J. Pieters. 2003. Molecular mechanisms of host-pathogen interaction: entry and survival of mycobacteria in macrophages. Adv. Immunol. 81:45-96. [DOI] [PubMed] [Google Scholar]
- 8.Kass, J., R. Artero, and M. K. Baylies. 2000. Non-radioactive electrophoretic mobility shift assay using digoxigenin-ddUTP labeled probes. Dros. Infect. Serv. 83:185-188. [Google Scholar]
- 9.Lin, Y., M. Zhang, F. M. Hofman, J. Gong, and P. F. Barnes. 1996. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect. Immun. 64:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 11.Ottenhoff, T. H., D. Kumararatne, and J. L. Casanova. 1998. Novel human immune-deficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 12.Pasquinelli, V., J. C. Townsend, J. O. Jurado, I. B. Alvarez, M. F. Quiroga, P. F. Barnes, B. Samten, and V. E. García. 2009. IFN-gamma production during active tuberculosis is regulated by mechanisms that involve IL-17, SLAM, and CREB. J. Infect. Dis. 199:661-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penix, L., W. M. Weaver, Y. Pang, H. A. Young, and C. B. Wilson. 1993. Two essential regulatory elements in the human interferon gamma promoter confer activation specific expression in T cells. J. Exp. Med. 178:1483-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penix, L. A., M. T. Sweetser, W. M. Weaver, J. P. Hoeffler, T. K. Kerppola, and C. B. Wilson. 1996. The proximal regulatory element of the interferon-gamma promoter mediates selective expression in T cells. J. Biol. Chem. 271:31964-31972. [DOI] [PubMed] [Google Scholar]
- 15.Samten, B., P. Ghosh, A. K. Yi, S. E. Weis, D. L. Lakey, R. Gonsky, U. Pendurthi, B. Wizel, Y. Zhang, M. Zhang, J. Gong, M. Fernandez, H. Safi, R. Vankayalapati, H. A. Young, and P. F. Barnes. 2002. Reduced expression of nuclear cyclic adenosine 5′-monophosphate response element-binding proteins and IFN-gamma promoter function in disease due to an intracellular pathogen. J. Immunol. 168:3520-3526. [DOI] [PubMed] [Google Scholar]
- 16.Samten, B., S. T. Howard, S. E. Weis, S. Wu, H. Shams, J. C. Townsend, H. Safi, and P. F. Barnes. 2005. Cyclic AMP response element-binding protein positively regulates production of IFN-gamma by T cells in response to a microbial pathogen. J. Immunol. 174:6357-6363. [DOI] [PubMed] [Google Scholar]
- 17.Samten, B., J. C. Townsend, S. E. Weis, A. Bhoumik, P. Klucar, H. Shams, and P. F. Barnes. 2008. CREB, ATF, and AP-1 transcription factors regulate IFN-gamma secretion by human T cells in response to mycobacterial antigen. J. Immunol. 181:2056-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swaminathan, S., J. Gong, M. Zhang, B. Samten, L. E. Hanna, P. R. Narayanan, and P. F. Barnes. 1999. Cytokine production in children with tuberculous infection and disease. Clin. Infect. Dis. 28:1290-1293. [DOI] [PubMed] [Google Scholar]
- 19.Taverner, N. V., J. C. Smith, and F. C. Wardle. 2004. Identifying transcriptional targets. Genome Biol. 5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson, K. A., O. M. Kon, S. M. Newton, G. Meintjes, R. N. Davidson, G. Pasvol, and R. J. Wilkinson. 2006. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J. Infect. Dis. 193:354-359. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2009. Global tuberculosis control: a short update to the 2009 report. WHO report 2009. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_report/2009/update/en/index.html.
- 22.Zhang, F., D. Z. Wang, M. Boothby, L. Penix, R. A. Flavell, and T. M. Aune. 1998. Regulation of the activity of IFN-gamma promoter elements during Th cell differentiation. J. Immunol. 161:6105-6112. [PubMed] [Google Scholar]
- 23.Zhang, M., Y. Lin, D. V. Iyer, J. Gong, J. S. Abrams, and P. F. Barnes. 1995. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect. Immun. 63:3231-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]