Abstract
Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus salivarius are all normal residents of the chicken gastrointestinal tract. Given the interest in using probiotic bacteria in chicken production and the important role of the microbiota in the development and regulation of the host immune system, the objective of the current study was to examine the differential effects of these bacteria on cytokine gene expression profiles of lymphoid tissue cells. Mononuclear cells isolated from cecal tonsils and spleens of chickens were cocultured with one of the three live bacteria, and gene expression was analyzed via real-time quantitative PCR. All three lactobacilli induced significantly more interleukin 1β (IL-1β) expression in spleen cells than in cecal tonsil cells, indicating a more inflammatory response in the spleen than in cecal tonsils. In cecal tonsil cells, substantial differences were found among strains in the capacity to induce IL-12p40, IL-10, IL-18, transforming growth factor β4 (TGF-β4), and gamma interferon (IFN-γ). In conclusion, we demonstrated that L. acidophilus is more effective at inducing T-helper-1 cytokines while L. salivarius induces a more anti-inflammatory response.
The use of probiotics for control of food-borne pathogens, such as Salmonella, and as an alternative to antimicrobial growth promoters (AGP) in poultry production has gained recent interest. Probiotics are defined as live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host through improvements to the intestinal microbial balance (15). The ability of the intestinal microbiota to contribute to the health of the host is well documented; however, the exact mechanisms by which the microbiota exerts its effect remain speculative. There is increasing evidence that through interactions with the innate immune system, probiotic bacteria are capable of modulating immune responses.
The intestinal microbiota is in constant contact with cells of the gut-associated lymphoid tissue (GALT), which includes professional antigen-presenting cells, B cells, T cells, and intestinal epithelial cells. In the case of the chicken, cecal tonsils are the major lymphoid tissues within the GALT. They are located at the proximal region of the cecum (6) and are similar to mammalian jejunal Peyer's patches both morphologically and cellularly (42). In mammals, Peyer's patches play an essential role in controlling the interaction between the host immune system and the intestinal microbiota. Within Peyer's patches, dendritic cells loaded with commensal bacteria induce the majority of IgA-producing plasma B cells (27). Chicken cecal tonsils contain germinal centers and IgA-positive B cells (5) and therefore are assumed to be important to host-commensal bacterium interactions. The interaction of cells from the GALT with commensal bacteria is thought to be critical for establishment and maintenance of intestinal homeostasis.
Lactic acid-producing bacteria (LAB) are normal residents of the small and large intestines of both mammals and birds. Lactobacillus is a genus of Gram-positive facultative anaerobic bacteria that make up the major component of the LAB group. LAB are the most common type of bacteria used as probiotics, and therefore, a great deal of research has focused on host response to these bacteria. Host responses have been shown to occur after in vivo and in vitro exposure to live LAB, various bacterial structural components of LAB, and/or products produced by these bacteria (8, 26, 41, 45). The exact response, however, is dependent on the species and even strain of the bacteria used. In chickens, probiotic LAB have been shown to enhance intestinal mucosal immunity (43), increase the serum antibody response (19, 20), and alter expression of a number of genes associated with the immune response (8).
The immunomodulatory activities of probiotic bacteria may be due to their ability to induce cytokine production, which leads to regulation of innate and adaptive immune responses. In mammals, it is well established that the gastrointestinal microbiota has the capacity to modulate the balance of the different T-helper (Th) cells (Th1, Th2, Th3, and T regulatory [Treg]) and their associated cytokines (13). It has been demonstrated that some species and strains of LAB induce cytokines that promote Th1 effector functions, such as interleukin 12 (IL-12) (12, 22), whereas other strains or species of LAB induce immunoregulatory cytokines, including IL-10 and transforming growth factor β (TGF-β) (31).
Lactobacillus acidophilus, Lactobacillus reuteri, and Lacto bacillus salivarius are normal inhabitants of the chicken intestine (18). Some strains from these three bacterial species have been shown to possess beneficial effects on their host's immune systems and moreover can limit Salmonella colonization (19, 23, 30, 32, 44). In spite of the interest in the use of probiotics in commercial poultry production, to date there is little information available on chicken immune responses to probiotic bacteria. Given the important role of the intestinal microbiota in the development and regulation of the host immune system and the fact that many probiotic effects are mediated through the balance of cytokines, the objective of the current study was to examine the differential cytokine profiles induced by three chicken commensal bacteria, L. acidophilus, L. reuteri, and L. salivarius, in mononuclear cells isolated from the cecal tonsils and spleens of chickens. The long-term objective of this research is to develop rational probiotic cultures that have a superior ability to induce Th1, Th2, or Treg responses in the host.
MATERIALS AND METHODS
Chickens and housing.
Newly hatched mixed-sex commercial broiler chicks were obtained from Maple Leaf Hatchery (New Hamburg, Ontario, Canada). Birds were maintained in a floor pen on clean wood shavings at the Arkell Poultry Research Station (University of Guelph, Ontario, Canada). Chicks were provided with free access to food and water. The research complied with University of Guelph Animal Care Committee guidelines.
Experimental design.
Twenty-two mixed-sex commercial broilers were used to determine the effect of various species of lactobacilli on the in vitro cytokine expression profiles of cecal tonsil and spleen mononuclear cells. Chicks were euthanized during week 5 or 6 of age, their cecal tonsils and spleen were removed aseptically, and mononuclear cells from these tissues were isolated. Spleen and cecal tonsil mononuclear cells from 10 and 12 birds, respectively, were isolated and cultured separately. Cells from individual birds were cultured separately for 3, 6, 12, and 18 h. Immediately prior to RNA isolation, cells from two birds were pooled, resulting in 5 biological replicates for the spleen and 6 biological replicates for cecal tonsils.
Preparation of spleen and cecal tonsil mononuclear cells.
Tissues were rinsed three times in 1× Hanks balanced salt solution and then minced with sterile scalpels. The tissue was further disrupted with the flat end of a 10-ml syringe plunger and strained through a 40-μm nylon cell strainer to obtain a single-cell suspension. The suspension was then overlaid onto a Histopaque-1077 (Sigma, Oakville, ON) density gradient, and mononuclear cells at the interface were collected and washed twice in RPMI (Invitrogen, Burlington, Ontario, Canada). Cells were counted by the trypan blue dye exclusion assay before being resuspended in RPMI (Invitrogen) containing 10% fetal bovine serum. For the cecal tonsils cells, 200 U/ml penicillin, 80 μg/ml streptomycin, and 25 mg gentamicin were also included to limit growth of bacteria carried over from the intestine.
Bacterial strains, media, and growth conditions.
In this study, we used L. acidophilus, L. reuteri, L. salivarius, and PT (phage type) 193 nalidixic acid-resistant Salmonella enterica serovar Typhimurium (referred to as S. Typhimurium herein). L. acidophilus was isolated from a commercial probiotic product (Intervet, Whitby, Ontario, Canada), whereas L. reuteri and L. salivarius were isolated in our laboratory from intestinal contents of broiler chickens. Briefly, 250 mg of ileal contents were inoculated into MRS broth (Becton Dickinson, Mississauga, Ontario, Canada), grown at 41°C under anaerobic conditions for 48 h, subcultured twice, and then diluted and plated on MRS plates. Individual colonies were selected, and the bacteria were identified by PCR, amplifying the V3 region of the 16S rRNA gene, sequencing the PCR products, and comparing the sequences directly with nonredundant nucleotides in the GenBank database using BLAST. All strains of lactobacilli were cultured in MRS broth (Becton Dickinson) grown at 41°C under aerobic conditions, while S. Typhimurium was grown in LB broth (Becton Dickinson) at 37°C. All bacteria were harvested by centrifugation (3,000 × g for 15 min) at the beginning of stationary growth phase. Pelleted bacteria were then washed three times in phosphate-buffered saline (PBS) and diluted in RPMI (Invitrogen) containing 10% fetal bovine serum. S. Typhimurium was heat killed prior to use.
In vitro stimulation of spleen and cecal tonsil cells.
For each of the samples, 200 μl of the mononuclear cell suspension (5 × 106 cells/ml) was seeded into 5 wells of a 96-well flat-bottom plate. One well was left unstimulated for each time point, while the other wells were stimulated with 1 × 106 CFU of heat-killed S. Typhimurium, live L. acidophilus, live L. reuteri, or live L. salivarius. The cells were incubated at 41°C in a humidified 5% CO2 environment and harvested for RNA extraction at 3, 6, 12, and 18 h posttreatment.
RNA isolation.
Total RNA was extracted using the TRIzol reagent (Invitrogen) from stimulated and control cells according to the manufacturer's recommendations with the addition of 10 μg glycogen (Invitrogen). Total RNA was then treated with DNase using the DNA-free kit (Ambion, Austin, TX) according to the manufacturer's instructions.
Primers.
Previously published primers were used for relative quantification of expression of target genes (encoding IL-1β, IL-10, and IL-18) and the β-actin gene, which acted as the reference gene (1, 2, 3). The primers specific for the chicken IL-12p40, gamma interferon (IFN-γ), and TGF-β transcripts were designed, whenever possible, to span exon-exon boundaries (AY262752.1, X99774, and M31160.1 for IL-12p40, IFN-γ, and TGF-β, respectively). The primers were synthesized by Sigma-Aldrich Canada, Ltd. Primer sequences can be found in Table 1. Chicken TGF-β4 is similar to human TGF-β1 in terms of its anti-inflammatory properties (9). Bioactive IL-12p70 is a heterodimer composed of p35 and p40 subunits. IL-12p40 expression is more restricted to antigen-presenting cells, such as macrophages and dendritic cells (DCs), which produce bioactive IL-12, while IL-12p35 is constitutively expressed in many cell types (14). Therefore, in the present study, we measured IL-12 p40 expression. This is despite the potential caveat that p40 is shared with another cytokine, IL-23. However, at this time, there is no published report on sequence or function of chicken IL-23.
TABLE 1.
Sequences and annealing temperatures for real-time PCR primers
| Cytokine | Forward primer (5′-3′) | Reverse primer (5′-3′) | Annealing temp (°C) |
|---|---|---|---|
| IL-1β | GTGAGGCTCAACATTGCGCTGTA | TGTCCAGGCGGTAGAAGATGAAG | 64 |
| IL-10 | AGCAGATCAAGGAGACGTTC | ATCAGCAGGTACTCCTCGAT | 55 |
| IL-12p40 | TTGCCGAAGAGCACCAGCCG | CGGTGTGCTCCAGGTCTTGGG | 64 |
| IL-18 | GAAACGTCAATAGCCAGTTGC | TCCCATGCTCTTTCTCACAACA | 64 |
| IFN-γ | ACACTGACAAGTCAAAGCCGC | AGTCGTTCATCGGGAGCTTG | 60 |
| TGF-β4 | CGGCCGACGATGAGTGGCTC | CGGGGCCCATCTCACAGGGA | 60 |
| β-Actin | CAACACAGTGCTGTCTGGTGGTA | ATCGTACTCCTGCTTGCTGATCC | 58 |
Reverse transcriptase quantitative PCR (RT-qPCR) analyses.
The cDNA synthesis was performed with 1 μg of total RNA using oligo(dT) primers and SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time quantification was performed in a LightCycler 480 instrument (Roche Diagnostics, Laval, Quebec, Canada) using the SYBR green dye. PCR mixtures (final volume of 20 μl) contained 10 μl of the LightCycler 480 SYBR green I master mix (Roche Diagnostics), 5 μl of a 1:5 dilution of the cDNA, and 0.25 mM (each) primers. The cycling conditions included an initial heat-denaturing step at 95°C for 10 min, 55 cycles at 95°C for 10 s, annealing as described in Table 1 for each of the primers, and product elongation and signal acquisition (single mode) at 72°C for 10 s. Following amplification, the melting curves were determined in a three-segment cycle of 95°C for 0 s, 65°C for 15 s, and 95°C for 0 s in the continuous acquisition mode. The temperature transition rates were set at 20°C/s except for segment three of the melting curve analysis, where it was set to 0.1°C/s. Results were analyzed using the RelQuant software program (Roche Diagnostics). Expression levels were normalized to β-actin expression, which was used as an internal housekeeping control. Fold change was determined by dividing results for the treated samples by those for the time-matched untreated controls. Treatments were considered statistically significant at P values of <0.05 using the Wilcoxon matched-pairs test using the GraphPad Prism, version 4.00, software program for Windows (Graphpad Software, San Diego CA), comparing the treated cells to the untreated control cells.
RESULTS
S. Typhimurium and Lactobacillus spp. induce IL-1β.
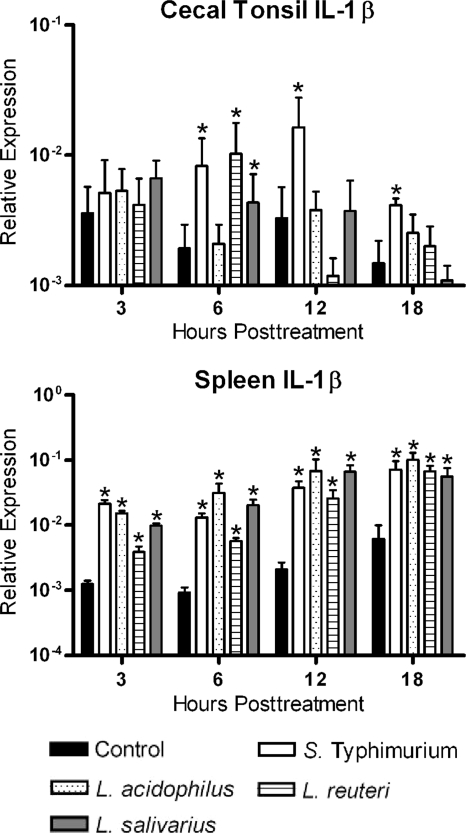
In order to examine the ability of various Lactobacillus species to induce cytokines associated with an inflammatory response, expression of IL-1β was examined in cecal tonsil and spleen mononuclear cells (Fig. 1). Live bacteria (1 × 106 CFU) were added to 1 × 106 cells for a 1:1 ratio, while heat-killed S. Typhimurium was included at the same ratio as a positive control in all experiments. In the case of the spleen mononuclear cells, all bacterial treatments significantly (P < 0.05) increased expression of IL-1β compared to that for the nontreated controls (Fig. 1). In cecal tonsil mononuclear cells, S. Typhimurium consistently increased expression of IL-1β, while L. acidophilus did not increase expression of IL-1β. L. reuteri and L. salivarius increased expression only at 6 h posttreatment (Fig. 1).
FIG. 1.
Relative expression of IL-1β transcripts from cecal tonsil and spleen mononuclear cells. Cecal tonsil and spleen mononuclear cells were cocultured with medium (control), S. Typhimurium, L. acidophilus, L. reuteri, or L. salivarius in a cell-to-bacterium ratio of 1:1. Data are expressed as relative expression of IL-1β normalized to the expression of β-actin. An asterisk indicates a significant difference (P < 0.05) between treated and control cells.
S. Typhimurium and Lactobacillus spp. differentially induce IL-12, IL-18, and IFN-γ.
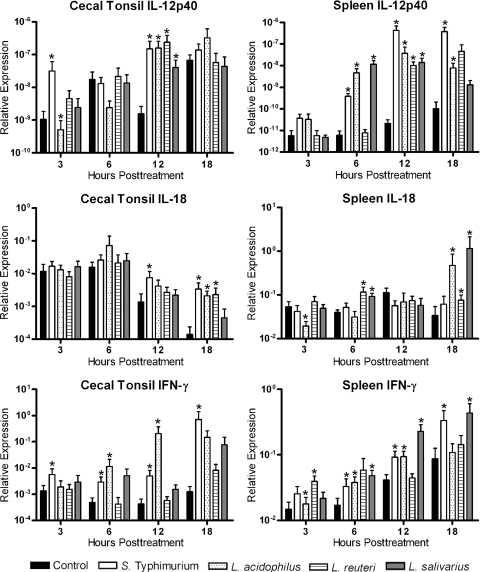
To examine the effect of the three species of Lactobacillus on Th1-type cytokine expression, spleen and cecal tonsil mononuclear cells were stimulated with live bacteria in a 1:1 ratio, and expression of IL-12, IL-18, and IFN-γ was measured at 3, 6, 12, and 18 h posttreatment (Fig. 2). In the cecal tonsil mononuclear cells, expression of IL-12 was significantly increased by all bacterial treatments. IL-18 expression was increased by S. Typhimurium at 12 h posttreatment and by S. Typhimurium, L. acidophilus, and L. reuteri at 18 h posttreatment. IFN-γ was significantly increased by S. Typhimurium and L. acidophilus (Fig. 2). In the spleen mononuclear cells, similar to cecal tonsil cells, IL-12 expression was increased in all bacterial treatments. IFN-γ was consistently and significantly induced by S. Typhimurium and L. acidophilus, while IL-18 expression was increased by all three lactobacilli (Fig. 2).
FIG. 2.
Relative expression of proinflammatory cytokine transcripts (IL-12p40, IL-18, and IFN-γ) from cecal tonsil or spleen mononuclear cells. Cecal tonsil or spleen mononuclear cells were cocultured with medium (control), S. Typhimurium, L. acidophilus, L. reuteri, or L. salivarius in a cell-to-bacterium ratio of 1:1. Data are expressed as the relative expression of cytokine mRNA levels normalized to the expression of β-actin. An asterisk indicates a significant difference (P < 0.05) between treated and control cells.
Lactobacillus spp. differentially induce IL-10 and TGF-β4.
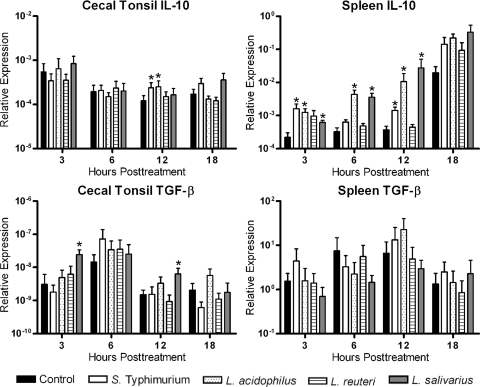
Based on the differential expression of IL-12, IL-18, and IFN-γ, expression of the regulatory cytokines, IL-10 and TGF-β4, was examined (Fig. 3). In cecal tonsil mononuclear cells, L. acidophilus significantly (P < 0.05) increased expression of IL-10. In spleen mononuclear cells, all treatments with the exception of L. reuteri significantly increased expression of IL-10 compared to that for the untreated controls (Fig. 3). Treatment of the cecal tonsil mononuclear cells with various lactobacilli revealed that only L. salivarius significantly (P < 0.05) increased expression of TGF-β4. No significant change (P < 0.05) in expression of TGF-β4 was observed in the spleen cells treated with any of the bacteria (Fig. 3).
FIG. 3.
Relative expression of anti-inflammatory cytokine transcripts (IL-10 and TGF-β4) from cecal tonsil and spleen mononuclear cells. Cecal tonsil and spleen mononuclear cells were cocultured with medium (control), S. Typhimurium, L. acidophilus, L. reuteri, or L. salivarius in a cell-to-bacterium ratio of 1:1. Data are expressed as the relative expression of cytokine mRNA levels normalized to the expression of β-actin. An asterisk indicates a significant difference (P < 0.05) between treated and control cells.
DISCUSSION
Probiotic bacteria, including lactobacilli, are thought to have beneficial effects for the host. Among these benefits, the immunomodulatory activities of these bacteria are of note. Despite the evidence for the ability of lactobacilli to induce or regulate immune responses in chickens, the underlying mechanisms of this phenomenon are unknown. Moreover, to design rational probiotics with a differential ability to regulate immune responses, it is important to gain an in-depth understanding of the immune response profiles associated with cells exposed to different species or strains of lactobacilli. In the present study, we demonstrated that live lactobacilli commonly used as probiotics and isolated from the chicken gastrointestinal tract induced expression of IL-1β, IL-12p40, IFN-γ, IL-18, IL-10, and TGF-β4 in chicken mononuclear cells cultured in vitro. Expression of these genes was dependent on both the location from which the cells were isolated and the bacterial isolates used.
Given the known effects of Salmonella on cytokine expression both in vitro and in vivo, S. Typhimurium was included as a positive control. Our results correspond to previously published literature (7, 42) in that treatment of the cecal tonsil mononuclear cells with S. Typhimurium significantly increased expression of proinflammatory and Th1-like cytokines, including, IL-1β, IL-12p40, IL-18, and IFN-γ, compared to that for control cells.
When the cytokine responses of cells isolated from the spleen and cecal tonsil tissues are compared, similar patterns of expression were observed, with one notable exception. The most prominent difference was expression of IL-1β, a proinflammatory cytokine. IL-1β is involved in the initiation and amplification of the inflammatory response, and there is a correlation between the levels of IL-1β and the amount of intestinal inflammation (33). Splenocytes responded to all bacteria with an increase in IL-1β expression, while the cecal tonsil mononuclear cells had a limited IL-1β response. Interestingly, the greatest response of the cecal tonsil cells was to the S. Typhimurium treatment, indicating that these cells still have the capacity to mount an inflammatory response to invasive and potentially pathogenic bacteria while remaining minimally responsive to commensal bacteria. The lower inflammatory response to commensal bacteria within the GALT has been found previously in mammals. The described mechanisms include the presence of Toll-like receptors (TLRs) on regulatory T cells that exert immunosuppressive activity (10) and the interference with inflammatory signaling pathways and effector functions through the inhibition of IκB ubiquitination (29). It has also been speculated that commensal bacteria have the ability to produce proteins with immunosuppressive effects (39). The presence of any of these mechanisms in avian species has yet to be determined; however, based on our results, it is conceivable that some of the above mechanisms are conserved and may be present in the chicken.
Given the importance of the mucosal immune system when it comes to commensal bacteria, the response mounted by cecal tonsil mononuclear cells is of interest. Coculture of cecal tonsil mononuclear cells with the three species of lactobacilli resulted in different cytokine profiles. L. acidophilus increased expression of the Th1-associated cytokines, IL-12p40, IL-18, and IFN-γ. As expected given the synergistic effect of IL-12 and IL-18 (28), L. acidophilus, the bacterial isolate that induced the highest expression of both cytokines, also had the largest increase in expression of IFN-γ. The observed increase in IFN-γ expression is in agreement with findings in our previous study examining cytokine expression in cecal tonsil mononuclear cells after treatment with L. acidophilus components, where it was demonstrated that cecal tonsil cells responded to L. acidophilus DNA with increased expression of a number of genes, including those encoding IL-18 and IFN-γ (8). In addition, we have demonstrated that a commercial probiotic product containing L. acidophilus significantly decreased Salmonella counts recovered from chickens (21). Given that clearance of Salmonella in chickens is primarily associated with a Th1-dominated response marked by high levels of IFN-γ (11, 38, 40), it is conceivable that the induced expression of proinflammatory cytokines by L. acidophilus may be associated with its ability to reduce intestinal colonization with Salmonella. Although studies performed in mammals have demonstrated that this may indeed be the case (4, 37), we previously did not find an association between proinflammatory cytokine expression and probiotic-conferred protection against colonization with Salmonella (19). The lack of IL-12 and IFN-γ expression in our previous study compared to findings in the present study, however, may be due to the fact that a combination of bacteria was used as a probiotic rather than L. acidophilus alone.
In spite of the induction of IL-12 expression similar to that seen with L. acidophilus, L. reuteri did not induce significant IFN-γ expression. The reason for this is unknown at this time, but this is not due to an increase in IL-10 or TGF-β. The inability of L. reuteri to increase expression of IL-10 corresponds to the results of others, who demonstrated a reduced capacity of L. reuteri to induce IL-10 (12, 26). In mammals, the molecular mechanisms that L. reuteri uses to inhibit proinflammatory cytokine production include inhibition of nuclear translocation of nuclear factor-κB (NF-κB) (26) and inhibition of tumor necrosis factor (TNF) production by preventing activation of c-Jun and the mitogen-activated protein (MAP) kinase-regulated transcription factor activator protein 1 (AP-1) (25). Further studies are needed to examine the molecular mechanisms that this L. reuteri strain utilizes to limit IFN-γ expression. Given that this phenomenon appears to be independent of IL-10 expression, it is possible that, similar to other strains of the species, this strain limits IFN-γ expression by interfering with the NF-κB and MAP kinase pathways.
Cecal tonsil mononuclear cells cocultured with L. salivarius responded very differently than did cells cocultured with L. acidophilus or L. reuteri. L. salivarius did not induce IL-18 or IFN-γ expression and induced less IL-12 than both of the other lactobacilli. It was also the only bacterium to increase expression of TGF-β4. Our results are in agreement with those of Sheil and colleagues (36), who demonstrated that the probiotic ability of L. salivarius is due to a reduced production of proinflammatory cytokines, such as IL-12, and increased expression of TGF-β. TGF-β is expressed in a wide range of cells and tissues and functions to downregulate the expression of many cytokines and cytokine-induced effects. Considering the established immunomodulatory ability of L. salivarius (30) and the importance of TGF-β in maintaining intestinal homeostasis (35), it is tempting to speculate that one of the molecular mechanisms employed by L. salivarius works through the induction of TGF-β.
The mechanisms by which Lactobacillus bacteria differentially stimulate chicken cells are largely unknown. Bacterial cell wall components are in direct contact with several immune cell types and can bind various pattern recognition receptors, including TLRs. Studies performed in mammals demonstrate that the differential immunostimulatory effects of Gram-positive bacteria may be due to differences in lipoteichoic acids (34). Regardless of the mechanisms, the comparison of the ability of the three Lactobacillus spp. to induce cytokine expression has highlighted some important similarities and differences that are consistent with findings of other in vitro studies performed in mammals (12, 16, 17). L. acidophilus induced more IFN-γ, IL-12, and IL-1β expression and therefore has a greater capacity to induce a putative Th1 response than L. reuteri and L. salivarius. L. salivarius limited proinflammatory cytokine expression and had the unique ability to induce TGF-β, indicating that these bacteria may stimulate an immunoregulatory response.
In conclusion, we report that three isolates of Lactobacillus spp. are capable of stimulating spleen and cecal tonsil cells in vitro. The stimulation was dependent on both the location from which the cells were isolated and, more importantly, the bacteria themselves, since the different bacterial isolates exerted different cytokine expression patterns. Further studies are needed to elucidate the molecular mechanisms responsible for the variable effects observed for these lactobacilli and to determine if the observed ability of these strains of lactobacilli to alter cellular cytokine profiles can be correlated to their in vivo effect on immune response. Understanding the molecular mode of action of bacteria allows for more-accurate selection of probiotic bacteria for use in poultry production.
Acknowledgments
Jennifer Brisbin is a recipient of a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC). This study was supported by the Canadian Poultry Research Council, Poultry Industry Council, NSERC, and Agriculture and Agri-Food Canada.
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Abdul-Careem, M. F., B. D. Hunter, A. J. Sarson, A. Mayameei, H. Zhou, and S. Sharif. 2006. Marek's disease virus-induced transient paralysis is associated with cytokine gene expression in the nervous system. Viral Immunol. 19:167-176. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Careem, M. F., B. D. Hunter, P. Parvizi, H. R. Haghighi, N. Thanthrige-Don, and S. Sharif. 2007. Cytokine gene expression patterns associated with immunization against Marek's disease in chickens. Vaccine 25:424-432. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Careem, M. F., K. Haq, S. Shanmuganathan, L. R. Read, K. A. Schat, M. Heidari, and S. Sharif. 2009. Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek's disease virus. Virology 393:250-257. [DOI] [PubMed] [Google Scholar]
- 4.Bao, S., K. W. Beagley, M. P. France, J. Shen, and A. J. Husband. 2000. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Shira, E., D. Sklan, and A. Friedman. 2003. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 27:147-157. [DOI] [PubMed] [Google Scholar]
- 6.Bar Shira, E., D. Sklan, and A. Friedman. 2005. Impaired immune responses in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 105:33-45. [DOI] [PubMed] [Google Scholar]
- 7.Berndt, A., A. Wilhelm, C. Jugert, J. Pieper, K. Sachse, and U. Methner. 2007. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 75:5993-6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisbin, J. T., H. Zhou, J. Gong, P. Sabour, M. R. Akbari, H. R. Haghighi, H. Yu, A. Clarke, A. J. Sarson, and S. Sharif. 2008. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev. Comp. Immunol. 32:563-574. [DOI] [PubMed] [Google Scholar]
- 9.Burt, D. W., and A. S. Law. 1994. Evolution of the transforming growth factor-beta superfamily. Prog. Growth Factor Res. 5:99-118. [DOI] [PubMed] [Google Scholar]
- 10.Caramalho, I., T. Lopes-Carvalho, D. Ostler, S. Zelenay, M. Haury, and J. Demengeot. 2003. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell, L., P. Kaiser, P. Barrow, M. A. Jones, C. Johnston, and P. Wigley. 2009. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 128:53-59. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, H. R., H. Frokiar, and J. J. Pestka. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171-178. [DOI] [PubMed] [Google Scholar]
- 13.Corthesy, B., H. R. Gaskins, and A. Mercenier. 2007. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 137:781S-790S. [DOI] [PubMed] [Google Scholar]
- 14.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, E. Nickbarg, R. Chizzonite, S. F. Wolf, and G. Trinchieri. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Agriculture Organization of the United Nations and WHO. 2002. Guidelines for the evaluation of probiotics in Food. Food and Agriculture Organization of the United Nations, Rome, Italy.
- 16.Fink, L. N., and H. Frøkiær. 2008. Dendritic cells from Peyer's patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to symbiotic gut bacteria. Scand. J. Immunol. 68:270-279. [DOI] [PubMed] [Google Scholar]
- 17.Gackowska, L., J. Michalkiewicz, M. Krotkiewski, A. Helmin-Basa, I. Kubiszewska, and D. Dzierzanowska. 2006. Combined effect of different lactic acid bacteria strains on the mode of cytokines pattern expression in human peripheral blood mononuclear cells. J. Physiol. Pharmacol. 57:13-21. [PubMed] [Google Scholar]
- 18.Gong, J., W. Si, R. J. Forster, R. Huang, H. Yu, Y. Yin, C. Yang, and Y. Han. 2007. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol. Ecol. 59:147-157. [DOI] [PubMed] [Google Scholar]
- 19.Haghighi, H. R., J. H. Gong, C. L. Gyles, M. A. Hayes, B. Sanei, P. Parvizi, H. Gisavi, J. R. Chambers, and S. Sharif. 2005. Modulation of antibody-mediated immune response by probiotics in chickens. Clin. Diagn. Lab. Immunol. 12:1387-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haghighi, H. R., J. H. Gong, C. L. Gyles, M. A. Hayes, H. J. Zhou, B. Sanei, J. R. Chambers, and S. Sharif. 2006. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 13:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haghighi, H. R., M. F. Abdul-Careem, R. A. Dara, J. R. Chambers, and S. Sharif. 2008. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 126:225-233. [DOI] [PubMed] [Google Scholar]
- 22.Hessle, C., B. Andersson, and A. E. Wold. 2000. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 68:3581-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizerwetter-Swida, M., and M. Binek. 2009. Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Pol. J. Vet. Sci. 12:15-20. [PubMed] [Google Scholar]
- 24.Lebman, D. A., and J. S. Edmiston. 1999. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 15:1297-1304. [DOI] [PubMed] [Google Scholar]
- 25.Lin, Y. P., C. H. Thibodeaux, J. A. Peña, G. D. Ferry, and J. Versalovic. 2008. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm. Bowel Dis. 14:1068-1083. [DOI] [PubMed] [Google Scholar]
- 26.Ma, D. L., P. Forsythe, and J. Bienenstock. 2004. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect. Immun. 72:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massacand, J. C., P. Kaiser, B. Ernst, A. Tardivel, K. Bürki, P. Schneider, and N. L. Harris. 2008. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS One 3:e2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahira, M., H. J. Ahn, W. R. Park, P. Gao, M. Tomura, C. S. Park, T. Hamaoka, T. Ohta, M. Kurimoto, and H. Fujiwara. 2002. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J. Immunol. 168:1146-1153. [DOI] [PubMed] [Google Scholar]
- 29.Neish, A. S. 2009. Microbes in gastrointestinal health and disease. Gastroenterology 136:65-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hara, A. M., P. O'Regan, A. Fanning, C. O'Mahony, J. Macsharry, A. Lyons, J. Bienenstock, L. O'Mahony, and F. Shanahan. 2006. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 118:202-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Mahony, L., L. O'Callaghan, J. McCarthy, D. Shilling, P. Scully, S. Sibartie, E. Kavanagh, W. O. Kirwan, H. P. Redmond, J. K. Collins, and F. Shanahan. 2006. Differential cytokine response from dendritic cells to symbiotic and pathogenic bacteria in different lymphoid compartments in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G839-G845. [DOI] [PubMed] [Google Scholar]
- 32.Pascual, M., M. Hugas, J. I. Badiola, J. M. Monfort, and M. Garriga. 1999. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microbiol. 65:4981-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinecker, H. C., M. Steffen, C. Doehn, J. Petersen, I. Pflüger, A. Voss, and A. Raedler. 1991. Proinflammatory cytokines in intestinal mucosa. Immunol. Res. 10:247-248. [DOI] [PubMed] [Google Scholar]
- 34.Ryu, T. H., J. E. Baik, J. S. Yang, S. Kang, J. Im, C. Yun, D. W. Kim, K. Lee, D. K. Chung, H. R. Ju, and S. H. Han. 2009. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int. Immunopharmacol. 9:127-133. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Munoz, F., A. Dominguez-Lopez, and J. K. Yamamoto-Furusho. 2008. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 14:4280-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheil, B., J. McCarthy, L. O'Mahony, M. W. Bennett, P. Ryan, J. J. Fitzgibbon, B. Kiely, J. K. Collins, and F. Shanahan. 2004. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut 53:694-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoycheva, M., and M. Murdjeva. 2005. Serum levels of interferon-gamma, interleukin-12, tumour necrosis factor-alpha, and interleukin-10, and bacterial clearance in patients with gastroenteric Salmonella infection. Scand. J. Infect. Dis. 37:11-14. [DOI] [PubMed] [Google Scholar]
- 38.Swaggerty, C. L., I. Y. Pevzner, H. He, K. J. Genovese, D. J. Nisbet, P. Kaiser, and M. H. Kogut. 2009. Selection of broilers with improved innate immune responsiveness to reduce on-farm infection by foodborne pathogens. Foodborne Pathog. Dis. 6:777-783. [DOI] [PubMed] [Google Scholar]
- 39.Tlaskalová-Hogenová, H., R. Stepánková, T. Hudcovic, L. Tucková, B. Cukrowska, R. Lodinová-Zádníková, H. Kozáková, P. Rossmann, J. Bártová, D. Sokol, D. P. Funda, D. Borovská, Z. Reháková, J. Sinkora, J. Hofman, P. Drastich, and A. Kokesová. 2004. Symbiotic bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 93:97-108. [DOI] [PubMed] [Google Scholar]
- 40.Withanage, G. S. K., P. Wigley, P. Kaiser, P. Mastroeni, H. Brooks, C. Powers, R. Beal, P. Barrow, D. Maskell, and I. McConnell. 2005. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 73:5173-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao, S. D., D. Z. Zhang, H. Lu, S. H. Jiang, H. Y. Liu, G. S. Wang, G. M. Xu, Z. B. Zhang, G. J. Lin, and G. L. Wang. 2003. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv. Ther. 20:253-260. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda, M., S. Tanaka, H. Arakawa, Y. Taura, Y. Yokomizo, and S. Ekino. 2002. A comparative study of gut-associated lymphoid tissue in calf and chicken. Anat. Rec. 266:207-217. [DOI] [PubMed] [Google Scholar]
- 43.Yurong, Y., S. Ruiping, Z. Shimin, and J. Yibao. 2005. Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens. Arch. Anim. Nutr. 59:237-246. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, G., L. Ma, and M. P. Doyle. 2007. Potential competitive exclusion bacteria from poultry inhibitory to Campylobacter jejuni and Salmonella. J. Food Prot. 70:867-873. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, L., N. Li, R. Caicedo, and J. Neu. 2005. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α-induced interleukin-8 production in caco-2 cells. J. Nutr. 135:1752-1757. [DOI] [PubMed] [Google Scholar]