Abstract
To date, the most promising vaccination strategies for the control of bovine tuberculosis (TB) focus on improving the efficacy of Mycobacterium bovis bacillus Calmette-Guérin (BCG). However, vaccination with BCG results in sensitization of animals to bovine tuberculin and compromises tests currently used for diagnosis of bovine TB infection. Thus, the development of specific diagnostic reagents capable of discriminating between infected and uninfected vaccinated animals (DIVA) is of high priority. To test the hypothesis that M. bovis-secreted proteins are likely to contain immunogenic antigens that can be used to increase the specificity of diagnostic tests, we screened 379 pools of overlapping peptides representing 119 antigens for their ability to stimulate a gamma inferferon (IFN-γ) response in vitro using whole blood from both TB reactor and BCG-vaccinated animals. Peptide pools representing antigens Rv3020c and Rv2346c induced responses in 61% and 57% of the TB reactor animals, respectively, without inducing responses in any BCG-vaccinated animal studied. Furthermore, individual peptides contained within pools recognized by BCG vaccinates were identified that were specific and induced IFN-γ responses in TB reactor animals. From these results, we constructed a cocktail of nine peptides representing multiple antigen targets that was recognized by 54% of TB reactor animals but also failed to induce responses in any BCG-vaccinated animal studied. In summary, we have identified three peptide cocktails for prioritization in larger trials to discriminate between M. bovis infection and BCG vaccination.
Despite the current “test and slaughter” control policy, the incidence of bovine tuberculosis (BTB), a zoonotic infection in cattle caused by Mycobacterium bovis, has been steadily rising in Great Britain over the last 20 years (10). Thus, the British government has acknowledged the urgent need for an effective cattle vaccine. To date, the only available vaccine for bovine tuberculosis is M. bovis bacillus Calmette-Guérin (BCG), an attenuated strain of M. bovis which has shown various levels of efficacy in cattle (4, 6, 23). More recent studies have shown that by utilizing a heterologous prime-boost approach, the efficacy of BCG vaccination can be significantly improved following boosting with DNA (17), protein (24), or viral (19-21) subunit vaccines. However, vaccination with BCG results in sensitization of animals to bovine tuberculin and compromises the single intradermal comparative tuberculin test (SICCT) currently used for diagnosis of bovine TB infection. Thus, to ensure the continuation of testing and slaughter-based control strategies, it is imperative that a complementary diagnostic test capable of discriminating between infected and uninfected vaccinated animals (DIVA) is developed in parallel with vaccine initiatives.
Different but complementary approaches have been used to identify antigens showing potential as a DIVA reagent. Genetic analysis has revealed regions of difference (RDs) deleted during the evolution of BCG (3, 5, 11), and several of the antigens (including ESAT-6 [Rv3875] and CFP-10 [Rv3874]) located in these RDs have been shown to possess outstanding diagnostic potential for the detection of mycobacterial infection in both cattle and humans (1, 2, 7, 13, 22). Furthermore, some of these antigens (e.g., Rv3875, Rv3874, Rv1986, Rv3872, and Rv3878) are differentially recognized by M. bovis-infected cattle compared to BCG-vaccinated animals (8, 22). Alternatively, microarray analysis that quantified the level of M. bovis gene expression revealed the “abundant invariome,” a population of gene products that were consistently expressed at high levels under a variety of different culture conditions (16). One member of the abundant invariome, Rv3615c, stimulated gamma interferon (IFN-γ) responses in M. bovis-infected cattle but not in BCG-vaccinated animals (15). In addition, Rv3615c induced responses in a proportion of animals that failed to recognize an ESAT-6-CFP-10 peptide cocktail, suggesting that target antigens identified through different approaches have the potential to complement each other in the detection of M. bovis-infected cattle.
In tuberculosis research, it has long been held that active secretion of antigenic proteins by mycobacteria induces strong cellular immune responses in the host. Indeed, we have often observed that, in general, secreted proteins are among the most frequently recognized antigens in M. bovis-infected cattle. Thus, the objective of this present study was to screen a panel of potential M. bovis secreted antigens in order to identify immunogenic targets and to formulate peptide cocktails that distinguish between M. bovis-infected and BCG-vaccinated animals in blood-based screening assays.
MATERIALS AND METHODS
Cattle.
All animals were housed at the Veterinary Laboratories Agency at the time of blood sampling, and procedures were conducted within the limits of a United Kingdom Home Office License under the Animal (Scientific Procedures) Act 1986, which were approved by the local ethical review committee. The following groups of animals were used in this study.
(i) TB reactors.
Heparinized blood samples were obtained from naturally infected, SICTT-positive reactors from herds known to have bovine tuberculosis (BTB) as determined by the Animal Health Agency (Animal Health). Heparinized blood samples were also obtained from four animals who were experimentally infected ca. 6 months with an M. bovis field strain from Great Britain (AF 2122/97) by intratracheal instillation of 1 × 103 CFU as previously described (9). A detailed postmortem examination of the TB reactor animals revealed visible TB lesions in all but four animals, confirming the presence of active disease. These lesions were present in lung tissue and in individual lymph nodes of the upper and/or lower respiratory tract, consistent with the disease profile commonly associated with M. bovis infection in United Kingdom cattle.
(ii) BCG vaccinated.
Heparinized blood samples were obtained from animals vaccinated with BCG as previously described (18). Briefly, calves (ca. 6 months of age) from BTB-free herds were vaccinated with BCG Danish (Statens Serum Institutet, Copenhagen, Denmark) by subcutaneous injection of 1 × 106 CFU into the side of the neck.
Production and preparation of peptides and antigens.
A total of 119 secreted, or potentially secreted, proteins were selected for antigen screening as previously described (12). Briefly, candidate proteins were chosen based on (i) the presence of signal sequences (e.g., Rv0192A, Rv0559c, etc), (ii) linkage to ESX loci (e.g., Rv3449, Rv3883c, etc), (iii) whether they were members of the ESX family (e.g., Rv1197, Rv1198, etc), or (iv) prior evidence for secretion from the literature (e.g., Rv1435c and Rv0867c). Peptides were synthesized (JPT Peptide Technologies GmbH, Berlin, Germany) in pools of 20-mers overlapping by 12 amino acids for each of the genes of interest. In total, 379 peptide pools containing a total of 4,129 peptides were evaluated. These peptide pools were dissolved in RPMI 1640 (Gibco, United Kingdom) containing 20% dimethyl sulfoxide (DMSO) to obtain a concentration of 1 mg/ml/peptide, and the peptide pools were used to stimulate whole blood at a final concentration of 5 μg/ml/peptide. Peptides that comprised the pools for some antigens were synthesized individually (Mimotopes, Pty., Ltd., Clayton, Australia), dissolved in RPMI 1640 containing 20% DMSO to obtain a concentration of 5 mg/ml, and used individually to stimulate whole blood at a final concentration of 10 μg/ml, or formulated into additional peptide pools at a concentration of 10 μg/ml/peptide. Peptides from ESAT-6 and CFP-10 were formulated to obtain a peptide cocktail as previously described (22) and were used at a final concentration of 5 μg/ml/peptide. This peptide cocktail was used as a “gold standard” with which to compare the immunogenicities of the other antigens.
Bovine tuberculin (purified protein derivate [PPD]-B) was supplied by the Tuberculin Production Unit at the Veterinary Laboratories Agency, Weybridge, Surrey, United Kingdom, and was used at a final concentration of 10 μg/ml. Staphylococcal enterotoxin B (SEB; Sigma-Aldrich, United Kingdom) was included as a positive control at a final concentration of 1 μg/ml, while whole blood was incubated with RPMI 1640 alone as a negative control.
IFN-γ ELISA.
Whole-blood aliquots (250 μl) were added in duplicate to antigen in 96-well plates and incubated at 37°C in the presence of 5% CO2 for 24 h, following which plasma supernatants were harvested and stored at −80°C until required. Quantification of IFN-γ in the plasma supernatants was determined using the Bovigam enzyme-linked immunosorbent assay (ELISA) kit (Prionics AG, Switzerland). A result was considered positive if the optical density at 450 nm (OD450) with antigen minus the OD450 without antigen (ΔOD450) was ≥0.1 in both of the duplicate wells.
Statistical analysis.
Kruskal-Wallis test with Dunn's multiple comparison test and receiver operator characteristic (ROC) curve analysis were performed using GraphPad Prism 5 software, while Fisher's exact test was performed using GraphPad Instat 3 software (both GraphPad Software, Inc.).
RESULTS
To test the hypothesis that M. bovis secreted proteins are likely to contain immunogenic antigens that can be used to increase the specificity of diagnostic tests, we screened 379 pools of overlapping peptides (4,129 peptides in total, representing 119 antigens) for their ability to stimulate an IFN-γ response in vitro using whole blood from both TB reactor (n = 23) and BCG-vaccinated (n = 8) animals. As expected, all TB reactor and BCG-vaccinated animals responded to PPD-B and to the positive control antigen SEB, while 22 TB reactor animals (96%) and 2 BCG-vaccinated animals responded to the ESAT-6-CFP-10 peptide cocktail (data not shown). Of the 379 peptide pools, approximately half (n = 184) failed to induce IFN-γ in any of the TB reactor or BCG-vaccinated animals. For the remaining 195 peptide pools, 163 and 77 were recognized by TB reactor and BCG-vaccinated animals, respectively, with 45 being recognized by both groups of animals (Table 1). Encouragingly, with regard to differential diagnostic reagents, 118 different peptide pools were recognized by TB reactor animals but failed to induce an IFN-γ response in any of the BCG-vaccinated animals studied.
TABLE 1.
Recognition of the secreted antigen peptide pools
| Peptide pool group (total) | No. (%) of peptide pools recognized |
|
|---|---|---|
| TB reactors | BCG vaccinated | |
| All pools (379) | 163 (43) | 77 (20) |
| TB reactor pools (163) | 163 (100) | 45 (28) |
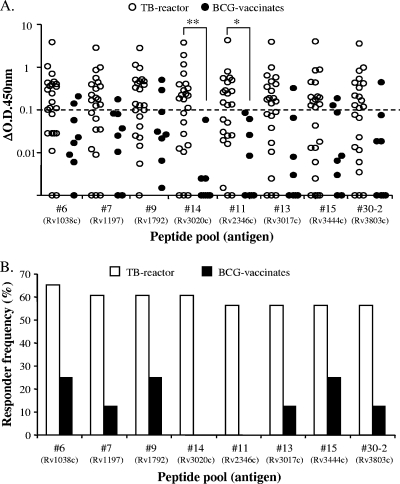
A hierarchy of responses to the different peptide pools was noted, with responder frequencies ranging from 4% to 65% in the TB reactor animals and 13% to 38% in the BCG-vaccinated animals (see Table S1 in the supplemental material). Figure 1 details the IFN-γ response to the top eight most frequently recognized peptide pools: i.e., those that induced an IFN-γ response in more than half of the TB reactor animals studied. Strikingly, all but one peptide pool (30-2) represented antigens belonging to the ESAT-6 protein family. Peptide pools 14 and 11 (representing antigens Rv3020c and Rv2346c, respectively) induced significantly greater levels of IFN-γ production in TB reactor animals compared to BCG vaccinates (Fig. 1A). Responder frequencies to the peptide pools were calculated using a cutoff value of ≥0.1 ΔOD450 for a positive response, and these results are shown in Fig. 1B. Of note, Rv3020c and Rv2346c were not recognized by any of the BCG-vaccinated animals (Fig. 1B), suggesting that they may contain peptides with potential application as DIVA reagents.
FIG. 1.
Identification of potential antigens for differential diagnosis. (A) IFN-γ production in whole blood from TB reactor (open circles) and BCG-vaccinated (closed circles) animals. Each symbol represents an individual animal. The dashed horizontal line represents the cutoff for a positive response. *, P < 0.05; **, P < 0.01 (Kruskal-Wallis test with Dunn's multiple comparison test). (B) The responder frequencies of 23 TB reactor (TB) and 8 BCG-vaccinated (BCG) animals to the most frequently recognized secretome peptide pools antigens are shown.
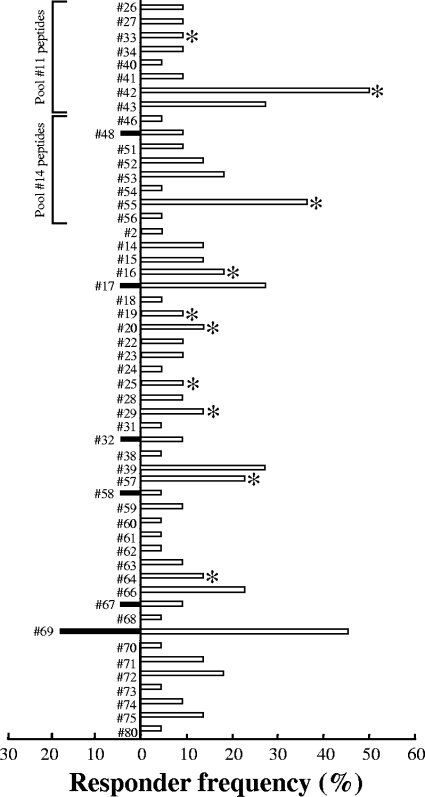
Although six out of the top eight most frequently recognized peptide pools induced IFN-γ responses in some BCG-vaccinated animals, we next reasoned that a fine-detail investigation of the immunogenicity of the components of these pools may reveal additional individual peptides with potential use as DIVA reagents. To this end, overlapping peptides contained within these peptide pools were screened individually for their ability to induce IFN-γ production in both TB reactor (n = 22) and BCG-vaccinated (n = 23) animals. In these experiments, 19 TB reactor animals (86%) but no BCG-vaccinated animals responded to the ESAT-6-CFP-10 peptide cocktail (data not shown). Fifty-three individual peptides were identified as immunogenic in TB reactor animals, with responder frequencies ranging from 5% to 50% (Fig. 2). Of these peptides, six (peptides 17, 32, 48, 58, 67, and 69) also induced IFN-γ responses in BCG-vaccinated animals, with responder frequencies ranging from 4% to 17% (Fig. 2).
FIG. 2.
Immunogenicity of individual secretome peptides. Shown are the responder frequencies of 22 TB reactor animals (open bars) and 23 BCG-vaccinated animals (filled bars) to individual secretome peptides. Asterisks indicate peptides selected for inclusion in the Sec 1 peptide pool (see the text for the rationale). Also noted are the immunogenic individual peptides for pools 11 and 14.
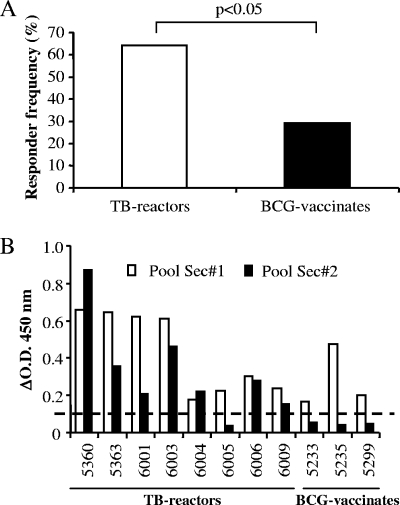
Although we identified two peptide pools (representing Rv3020c and Rv2346c) as potential DIVA reagents, these pools were focused on single antigens and may represent a limited repertoire of immunogenic epitopes recognized by a subset of TB reactor animals. Thus, we hypothesized that a peptide cocktail derived from multiple antigens with different epitope specificities may enhance the likelihood of detecting TB reactor animals. To test this hypothesis, a peptide pool (Sec 1) consisting of 10 peptides (indicated in Fig. 2) was constructed. First, peptides 42 and 55 were selected as they were the two most frequently recognized peptides (responder frequencies of 50% and 36%, respectively) and also because they belonged to peptide pools not recognized by BCG-vaccinated animals (pools 11 and 14, respectively) (Fig. 1). A further four peptides (peptides 20, 29, 33, and 64) were next selected as they were recognized in TB reactor animals that failed to respond to peptide 42 or 55 (data not shown). Finally, a further four peptides (peptides 16, 19, 25, and 57) were included due to their location in regions of homology between multiple ESAT-6 proteins (see reference 12 and Table S2 in the supplemental material). In total, this peptide cocktail represented epitopes from 12 different antigens (Table S2). As shown in Fig. 3A, the responder frequency to the Sec 1 peptide pool was significantly greater in TB reactor animals (P < 0.05; Fisher's exact test), with 14 out of 22 (64%) TB reactor animals recognizing the peptide pool compared with 6 out of 21 (29%) BCG-vaccinated animals. In order to optimize the peptide pool for use as a DIVA reagent, the individual peptide components of the Sec 1 peptide pool were rescreened for their ability to induce an IFN-γ response in BCG-vaccinated animals. These experiments identified only a single peptide (peptide 64) as being immunogenic in some BCG-vaccinated animals (data not shown). Thus, we formulated a second peptide pool (Sec 2) that lacked this peptide and compared the ability of both Sec 1 and Sec 2 to induce IFN-γ in both TB reactor animals (n = 8) and BCG-vaccinated animals (n = 3) previously demonstrated to recognize the former peptide pool. Omitting peptide 64 from the pool had little effect on the responder frequency for TB reactor animals, with seven of the eight animals still producing IFN-γ above the cutoff (Fig. 3B). Overall, 7 out of 13 (54%) TB reactor animals produced IFN-γ in response to Sec 2 (data not shown). In contrast, removal of peptide 64 completely abrogated the response in all BCG-vaccinated animals tested (Fig. 3B).
FIG. 3.
Optimization of a secretome peptide pool that is preferentially recognized by TB reactor animals. (A) Responder frequency of 22 TB reactor and 21 BCG-vaccinated animals to the Sec 1 peptide pool (P < 0.05; Fisher's exact test); (B) IFN-γ responses (ΔOD450) from eight TB-reactor and three BCG-vaccinated animals to both the Sec 1 and Sec 2 peptide pools. The dashed horizontal line represents the cutoff for a positive response. Details of the peptide components of Sec 1 and Sec 2 are given in Table S2 in the supplemental material.
DISCUSSION
The results presented herein have significant importance with regard to the development of DIVA reagents. Screening of 119 secreted, or potentially secreted, proteins revealed three unique peptide pools that were frequently recognized by M. bovis-infected cattle but failed to induce an IFN-γ response in any BCG-vaccinated animals studied. Two of these peptide pools consisted of overlapping peptides that represented the full amino acid sequence for two individual antigens, Rv2346c and Rv3020c, while the third (Sec 2) consisted of a cocktail of nine peptides derived from multiple antigens.
The underlying mechanism for the differential recognition of Rv2346c and Rv3020c remains unclear. Both genes are located in the genomes of M. bovis and BCG, and genome analysis revealed identical amino acid sequences between the two strains. Thus, the lack of immune responses to Rv2346c and Rv3020c seen in BCG-vaccinated animals is unlikely to be explained by deletions or amino acid sequence alterations within these two proteins in the BCG used for vaccination. Alternatively, it is possible that the immune response to a given mycobacterial antigen may be closely related to its level of gene transcription (14). To this end, it is interesting to note that Rv2346c was identified as one of the top 50 most highly expressed genes of M. tuberculosis when grown in vitro (16). However, our own studies revealed no difference in the level of gene expression for either Rv2346c or Rv3020c when macrophages were infected with either M. bovis or BCG (P. Golby, personal communication). It should be noted that these were in vitro infection experiments, and the comparative level of gene expression for Rv2346c or Rv3020c in M. bovis-infected versus BCG-vaccinated animals in vivo remains unknown.
It is unlikely that IFN-γ responses to a single protein antigen will be sufficient for the detection of M. bovis infection of cattle. Indeed, the QuantiFERON-TB Gold test for human M. tuberculosis infection utilizes synthetic peptides from two different M. tuberculosis antigens (ESAT-6 and CFP-10). With this in mind, we formulated a peptide cocktail (Sec 1) that contained individual peptides isolated from various peptide pools representing the most frequently recognized antigens. Test cutoff values for the Sec 1 cocktail were determined by ROC curve analysis of the Sec 1-specific IFN-γ responses of TB reactor and BCG-vaccinated animals. At cutoff values calculated for predetermined specificities set between 95 and 100%, the relative sensitivity of the Sec 1 peptide cocktail in detecting M. bovis-infected animals was 41% (data not shown), a drop of over 20% in sensitivity when compared to the responder frequency for Sec 1 in TB reactor animals (Fig. 3A). In contrast, removal of peptide 64 to produce the peptide cocktail Sec 2 resulted in only a 10% drop in assay sensitivity. Thus, to maintain assay sensitivity, we have focused on the Sec 2 peptide cocktail, using the standard cutoff value (ΔOD450) for positive IFN-γ responses.
The Sec 2 cocktail contained several immunodominant peptides with restricted expression among the ESAT-6 proteins: e.g., peptide 55 is located only within Rv3020c, while peptide 42 is located in Rv2346c and Rv1793 (see Table S2 in the supplemental material). However, given the high degree of amino acid similarity between the members of the ESAT-6 protein family, several of these peptide sequences represented multiple antigens. For example, peptides 16 and 20 are located in Rv1038c, Rv1197, Rv1792, Rv2347c, and Rv3620c, while peptide 33 is located in Rv1198, Rv2346c, Rv3619c, Rv1037c, and Rv1793. Thus, targeting these shared sequences not only reduces the number of different components within the DIVA reagent but may also exploit a potentially greater antigenic load for these regions.
The ESAT-6-CFP-10 peptide cocktail used in the studies presented herein has been developed as a DIVA reagent in cattle, with reported sensitivities of approximately 78% in M. bovis-infected animals (15, 22). Thus, one area of research of high importance is the identification of reagents that may complement the ESAT-6-CFP-10 peptide cocktail in the diagnosis of bovine TB. Recently, we have demonstrated that 4 out of 7 (57%) M. bovis-infected animals that failed to recognize the ESAT-6-CFP-10 peptide cocktail did mount an IFN-γ response to the antigen Rv3615c, theoretically increasing diagnostic sensitivity to 91% without compromising specificity in BCG-vaccinated animals (15). In our current study, 5 out of 13 (38%) TB-reactor animals recognized Rv3615c (data not shown), results similar to those previously reported (15). All five of these animals recognized the Sec 2 peptide cocktail, which also induced responses in a further two animals (overall responder frequency of 54%), allowing us to speculate that the Sec 2 peptide cocktail may be as good, if not better, at complementing ESAT-6-CFP-10 in the diagnosis of bovine TB without compromising specificity in BCG-vaccinated animals. Of the 57 individual TB reactor animals that have been used in the various immunogenicity screens described in this study, only 5 failed to produce IFN-γ in response to stimulation with the ESAT-6-CFP-10 peptide cocktail (data not shown). Unfortunately, neither the peptide pools representing Rv2346c and Rv3020c nor any of the individual secretome peptides or the Sec 2 peptide cocktail was recognized by these animals (data not shown). Thus, a larger field trial is required to evaluate whether any of the peptide reagents described herein may have the potential to increase diagnostic sensitivity of bovine TB without compromising specificity in BCG-vaccinated animals when used in combination with ESAT-6-CFP-10. Furthermore, field trials of these peptide reagents in cattle infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium kansasii would also evaluate their ability to differentially diagnose M. bovis infection with respect to other mycobacterial infections of cattle.
In summary, the results of this study demonstrate that cocktails of synthetic peptides derived from secreted or potentially secreted antigens have the capacity to distinguish between M. bovis-infected and BCG-vaccinated animals in blood-based screening assays.
Acknowledgments
This study was funded by the Department for Environment, Food and Rural affairs (DEFRA), United Kingdom.
We are indebted to Animal Health for identifying naturally M. bovis-infected tuberculin-positive cattle and to the staff of the Animal Service Unit for their dedication to the welfare of the animals housed at VLA.
Footnotes
Published ahead of print on 28 July 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Aagaard, C., M. Govaerts, V. Meikle, A. J. Vallecillo, J. A. Gutierrez-Pabello, F. Suarez-Guemes, J. McNair, A. Cataldi, C. Espitia, P. Andersen, and J. M. Pollock. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard, C., M. Govaerts, L. Meng Okkels, P. Andersen, and J. M. Pollock. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 4.Berggren, S. A. 1981. Field experiment with BCG vaccine in Malawi. Br. Vet. J. 137:88-96. [DOI] [PubMed] [Google Scholar]
- 5.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. U. S. A. 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. De Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 8.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean, G. S., S. G. Rhodes, M. Coad, A. O. Whelan, P. J. Cockle, D. J. Clifford, R. G. Hewinson, and H. M. Vordermeier. 2005. Minimum infective dose of Mycobacterium bovis in cattle. Infect. Immun. 73:6467-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Rua-Domenech, R. 2006. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb.) 86:77-109. [DOI] [PubMed] [Google Scholar]
- 11.Gordon, S. V., R. Brosch, A. Billault, T. Garnier, K. Eiglmeier, and S. T. Cole. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32:643-655. [DOI] [PubMed] [Google Scholar]
- 12.Jones, G. J., S. V. Gordon, R. G. Hewinson, and H. M. Vordermeier. 2010. Screening of predicted secreted antigens from Mycobacterium bovis reveals the immunodominance of the ESAT-6 protein family. Infect. Immun. 78:1326-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalvani, A., P. Nagvenkar, Z. Udwadia, A. A. Pathan, K. A. Wilkinson, J. S. Shastri, K. Ewer, A. V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469-477. [DOI] [PubMed] [Google Scholar]
- 14.Rogerson, B. J., Y. J. Jung, R. LaCourse, L. Ryan, N. Enright, and R. J. North. 2006. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology 118:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidders, B., C. Pirson, P. J. Hogarth, R. G. Hewinson, N. G. Stoker, H. M. Vordermeier, and K. Ewer. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidders, B., M. Withers, S. L. Kendall, J. Bacon, S. J. Waddell, J. Hinds, P. Golby, F. Movahedzadeh, R. A. Cox, R. Frita, A. M. Ten Bokum, L. Wernisch, and N. G. Stoker. 2007. Quantification of global transcription patterns in prokaryotes using spotted microarrays. Genome Biol. 8:R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner, M. A., D. N. Wedlock, G. W. de Lisle, M. M. Cooke, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2005. The order of prime-boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins Hsp65, Hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect. Immun. 73:4441-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vordermeier, H. M., K. Huygen, M. Singh, R. G. Hewinson, and Z. Xing. 2006. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vordermeier, H. M., S. G. Rhodes, G. Dean, N. Goonetilleke, K. Huygen, A. V. Hill, R. G. Hewinson, and S. C. Gilbert. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vordermeier, H. M., B. Villarreal-Ramos, P. J. Cockle, M. McAulay, S. G. Rhodes, T. Thacker, S. C. Gilbert, H. McShane, A. V. Hill, Z. Xing, and R. G. Hewinson. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waddington, F. G., and D. C. Ellwood. 1972. An experiment to challenge the resistance to tuberculosis in B.C.G. vaccinated cattle in Malawi. Br. Vet. J. 128:541-552. [DOI] [PubMed] [Google Scholar]
- 24.Wedlock, D. N., M. Denis, M. A. Skinner, J. Koach, G. W. de Lisle, H. M. Vordermeier, R. G. Hewinson, S. van Drunen Littel-van den Hurk, L. A. Babiuk, R. Hecker, and B. M. Buddle. 2005. Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone. Infect. Immun. 73:3540-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]