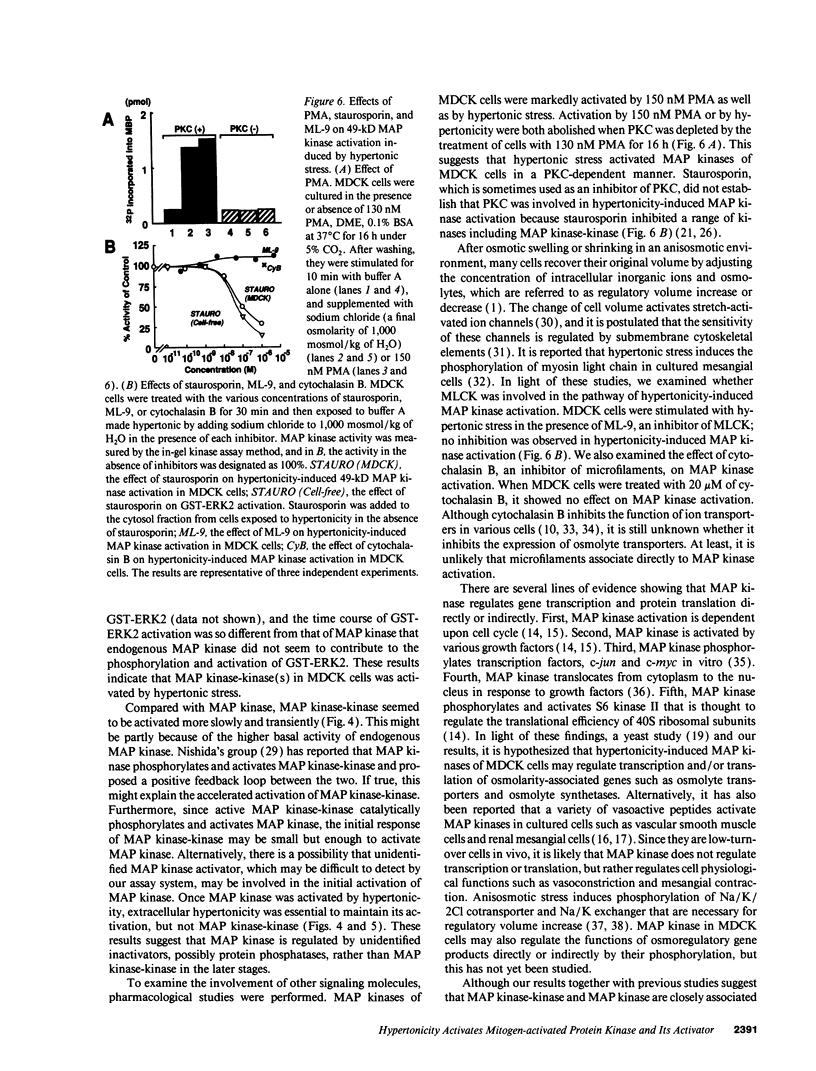
Abstract
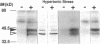
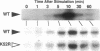
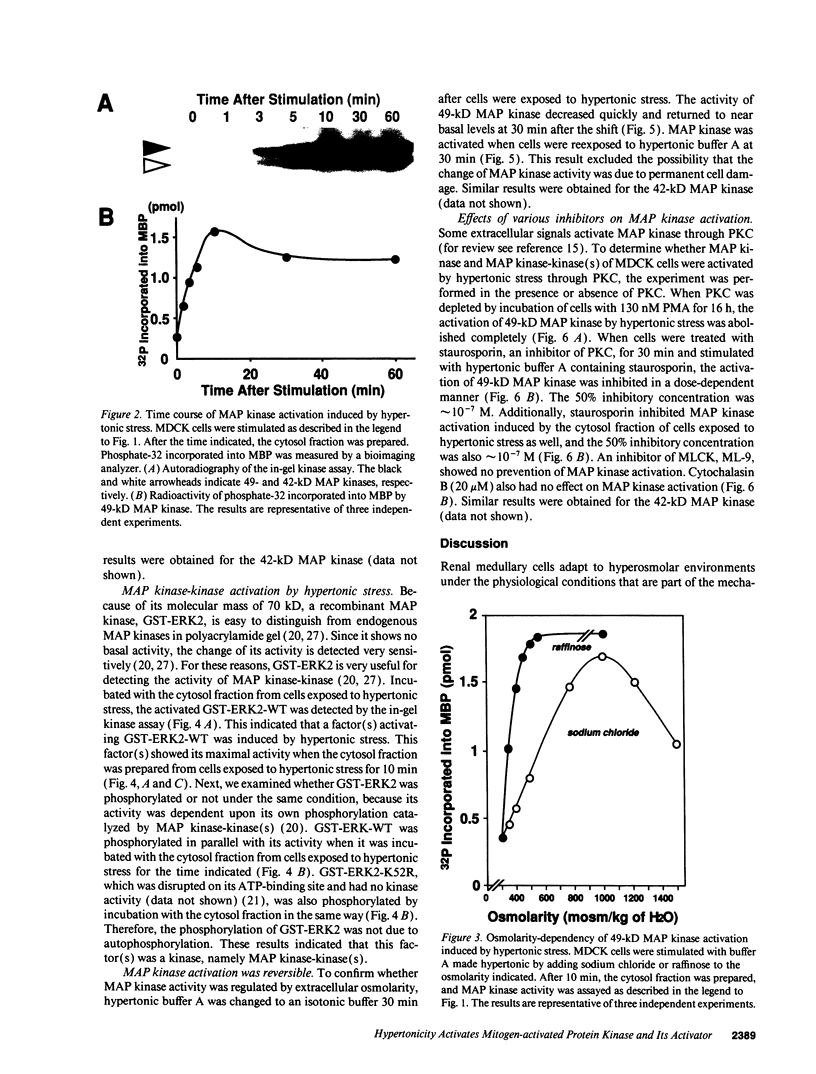
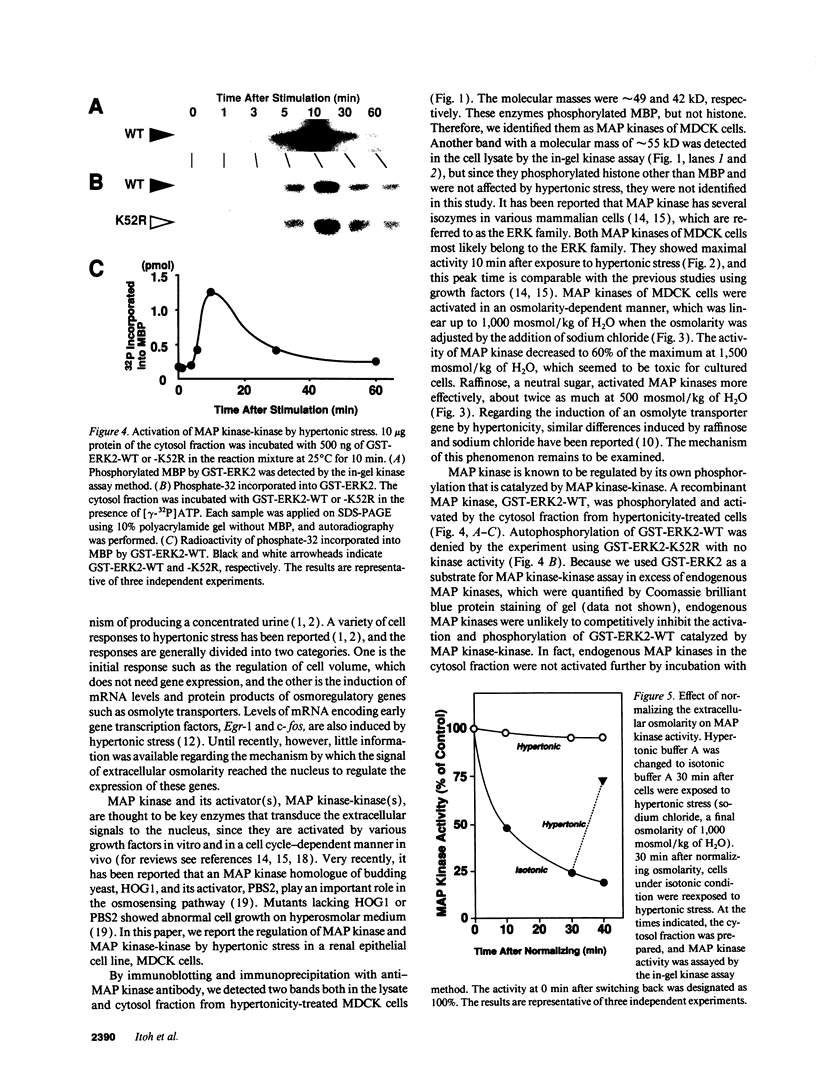
Madin-Darby canine kidney cells behave like the renal medulla and accumulate small organic solutes (osmolytes) in a hypertonic environment. The accumulation of osmolytes is primarily dependent on changes in gene expression of enzymes that synthesize osmolytes (sorbitol) or transporters that uptake them (myo-inositol, betaine, and taurine). The mechanism by which hypertonicity increases the transcription of these genes, however, remains unclear. Recently, it has been reported that yeast mitogen-activated protein (MAP) kinase and its activator, MAP kinase-kinase, are involved in osmosensing signal transduction and that mutants in these kinases fail to accumulate glycerol, a yeast osmolyte. No information is available in mammals regarding the role of MAP kinase in the cellular response to hypertonicity. We have examined whether MAP kinase and MAP kinase-kinase are regulated by extracellular osmolarity in Madin-Darby canine kidney cells. Both kinases were activated by hypertonic stress in a time- and osmolarity-dependent manner and reached their maximal activity within 10 min. Additionally, it was suggested that MAP kinase was activated in a protein kinase C-dependent manner. These results indicate that MAP kinase and MAP kinase-kinase(s) are regulated by extracellular osmolarity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biden T. J., Wollheim C. B. Active transport of myo-inositol in rat pancreatic islets. Biochem J. 1986 Jun 15;236(3):889–893. doi: 10.1042/bj2360889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy C. A., Lightman S. L., Lightman S. L. Developmental and physiological regulation of aldose reductase mRNA expression in renal medulla. Mol Endocrinol. 1989 Sep;3(9):1409–1416. doi: 10.1210/mend-3-9-1409. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989 Aug;257(2 Pt 1):C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. M., Wasserman J. C., Gullans S. R. Immediate early gene and HSP70 expression in hyperosmotic stress in MDCK cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C594–C601. doi: 10.1152/ajpcell.1991.261.4.C594. [DOI] [PubMed] [Google Scholar]
- Cowley B. D., Jr, Ferraris J. D., Carper D., Burg M. B. In vivo osmoregulation of aldose reductase mRNA, protein, and sorbitol in renal medulla. Am J Physiol. 1990 Jan;258(1 Pt 2):F154–F161. doi: 10.1152/ajprenal.1990.258.1.F154. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Renal medullary organic osmolytes. Physiol Rev. 1991 Oct;71(4):1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Martin B., Murphy H. R., Uchida S., Murer H., Cowley B. D., Jr, Handler J. S., Burg M. B. Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem. 1989 Oct 5;264(28):16815–16821. [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Goetz-Smith J. D., Stewart D., Beresford B. J., Mellors A. Protein phosphorylation during activation of Na+/H+ exchange by phorbol esters and by osmotic shrinking. Possible relation to cell pH and volume regulation. J Biol Chem. 1986 Jun 15;261(17):8009–8016. [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kaibuchi K., Masuda T., Yamamoto T., Matsuura Y., Maeda A., Shimizu K., Takai Y. A protein factor for ras p21-dependent activation of mitogen-activated protein (MAP) kinase through MAP kinase kinase. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):975–979. doi: 10.1073/pnas.90.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kaibuchi K., Masuda T., Yamamoto T., Matsuura Y., Maeda A., Shimizu K., Takai Y. The post-translational processing of ras p21 is critical for its stimulation of mitogen-activated protein kinase. J Biol Chem. 1993 Feb 15;268(5):3025–3028. [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kwon H. M., Yamauchi A., Uchida S., Preston A. S., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem. 1992 Mar 25;267(9):6297–6301. [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. MAP kinase regulation--the oncogene connection. Trends Cell Biol. 1992 Oct;2(10):283–286. doi: 10.1016/0962-8924(92)90105-v. [DOI] [PubMed] [Google Scholar]
- Lohr J. W., Grantham J. J. Isovolumetric regulation of isolated S2 proximal tubules in anisotonic media. J Clin Invest. 1986 Nov;78(5):1165–1172. doi: 10.1172/JCI112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Gotoh Y., Nishida E. Phosphorylation of Xenopus mitogen-activated protein (MAP) kinase kinase by MAP kinase kinase kinase and MAP kinase. J Biol Chem. 1993 Feb 15;268(5):3277–3281. [PubMed] [Google Scholar]
- Oemar B. S., Law N. M., Rosenzweig S. A. Insulin-like growth factor-1 induces tyrosyl phosphorylation of nuclear proteins. J Biol Chem. 1991 Dec 25;266(36):24241–24244. [PubMed] [Google Scholar]
- Pewitt E. B., Hegde R. S., Haas M., Palfrey H. C. The regulation of Na/K/2Cl cotransport and bumetanide binding in avian erythrocytes by protein phosphorylation and dephosphorylation. Effects of kinase inhibitors and okadaic acid. J Biol Chem. 1990 Dec 5;265(34):20747–20756. [PubMed] [Google Scholar]
- Pomerance M., Schweighoffer F., Tocque B., Pierre M. Stimulation of mitogen-activated protein kinase by oncogenic Ras p21 in Xenopus oocytes. Requirement for Ras p21-GTPase-activating protein interaction. J Biol Chem. 1992 Aug 15;267(23):16155–16160. [PubMed] [Google Scholar]
- Rossomando A., Wu J., Weber M. J., Sturgill T. W. The phorbol ester-dependent activator of the mitogen-activated protein kinase p42mapk is a kinase with specificity for the threonine and tyrosine regulatory sites. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5221–5225. doi: 10.1073/pnas.89.12.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Hwang S. M., Stern J., Pleasure D. Inositol uptake by cultured isolated rat Schwann cells. Biochem Biophys Res Commun. 1984 Apr 30;120(2):486–492. doi: 10.1016/0006-291x(84)91280-4. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Wang Y., Jones J. M., Dunn M. J. Protein kinase C regulates activation of mitogen-activated protein kinase and induction of proto-oncogene c-fos by endothelin-1. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S29–S32. doi: 10.1097/00005344-199204002-00010. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Takeda M., Homma T., Breyer M. D., Horiba N., Hoover R. L., Kawamoto S., Ichikawa I., Kon V. Volume and agonist-induced regulation of myosin light-chain phosphorylation in glomerular mesangial cells. Am J Physiol. 1993 Mar;264(3 Pt 2):F421–F426. doi: 10.1152/ajprenal.1993.264.3.F421. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kawahara Y., Ishida Y., Koide M., Shii K., Yokoyama M. Angiotensin II stimulates two myelin basic protein/microtubule-associated protein 2 kinases in cultured vascular smooth muscle cells. Circ Res. 1992 Sep;71(3):620–630. doi: 10.1161/01.res.71.3.620. [DOI] [PubMed] [Google Scholar]
- Uchida S., Kwon H. M., Yamauchi A., Preston A. S., Marumo F., Handler J. S. Molecular cloning of the cDNA for an MDCK cell Na(+)- and Cl(-)-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Yamauchi A., Preston A. S., Kwon H. M., Handler J. S. Medium tonicity regulates expression of the Na(+)- and Cl(-)-dependent betaine transporter in Madin-Darby canine kidney cells by increasing transcription of the transporter gene. J Clin Invest. 1993 Apr;91(4):1604–1607. doi: 10.1172/JCI116367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Rossomando A. J., Her J. H., Del Vecchio R., Weber M. J., Sturgill T. W. Autophosphorylation in vitro of recombinant 42-kilodalton mitogen-activated protein kinase on tyrosine. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9508–9512. doi: 10.1073/pnas.88.21.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Kwon H. M., Preston A. S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992 Jan 5;267(1):649–652. [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Preston A. S., Kwon H. M., Handler J. S. Hypertonicity stimulates transcription of gene for Na(+)-myo-inositol cotransporter in MDCK cells. Am J Physiol. 1993 Jan;264(1 Pt 2):F20–F23. doi: 10.1152/ajprenal.1993.264.1.F20. [DOI] [PubMed] [Google Scholar]
- Yordy M. R., Bowen J. W. Na,K-ATPase expression and cell volume during hypertonic stress in human renal cells. Kidney Int. 1993 Apr;43(4):940–948. doi: 10.1038/ki.1993.132. [DOI] [PubMed] [Google Scholar]