Abstract
An enzyme-linked immunosorbent assay (ELISA) for the detection of IgG antibodies against the pandemic H1N1 2009 influenza A virus, employing a recombinant hemagglutinin protein of the virus, was compared to the hemagglutination inhibition (HI) test using 783 serum samples. The results showed a concordance of 98.4%, suggesting the utility of the ELISA in serosurveillance. Two hundred sixty-nine (100%) serum samples with an HI titer of ≥20 were ELISA reactive.
Influenza viruses are negative-strand RNA viruses that belong to the family Orthomyxoviridae, which includes 4 genera, Influenzavirus A, B, and C and Thogotovirus. Influenza A viruses are widely distributed in nature and can infect a wide variety of mammals and birds. Based on the antigenicity of the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), influenza A viruses have been classified into 16 HA and 9 NA subtypes. Of these, H1, H2, and H3 HA subtypes and N1 and N2 NA subtypes have circulated in human populations. In recent years, H5N1 virus of avian origin was expected to be a pandemic-causing pathogen (4). However, the first pandemic of this century was caused by a novel H1N1 influenza A virus of swine origin that emerged in 2009 (hereinafter called p-H1N1-09) (3, 5).
Due to the circulation of several influenza A virus subtypes, cross-reactivity is a major problem in influenza virus serology. The inhibition of hemagglutination (HI) caused by antibodies to the HA of the virus is routinely used for assessing the prevalence of a specific virus in a community or an animal population.
India was affected by the pandemic H1N1 influenza during the latter half of 2009 (2). In order to understand the degree of exposure of different populations to the virus, an extensive serosurvey was undertaken by the National Institute of Virology, Pune, India. The test of choice, HI, was performed as described earlier (1). Considering the requirement of fresh red blood cells and time and the cumbersome protocol, it was thought important to evaluate the utility of a recombinant HA protein enzyme-linked immunosorbent assay (ELISA) for the detection of p-H1N1-09 IgG antibodies as evidence of exposure to this novel virus.
The HA gene of the p-H1N1-09 influenza virus isolated at the National Institute of Virology (A/India-Blore/NIV310/2009, GenBank accession no. GU292347) was PCR amplified, cloned into the pFastBac1 vector (Invitrogen) within the EcoRI and XhoI restriction sites, and expressed with a baculovirus expression system (Invitrogen) in an insect cell line. The sequence of the cloned HA was identical to that of the original isolate. The HA protein was purified by lentil lectin affinity chromatography (GE Healthcare) and used for ELISA.
An indirect sandwich ELISA was performed. Briefly, a Maxisorb microtiter plate (Nunc) was coated with p-H1N1-09 HA protein, 2 μg/well, and incubated at 37°C for 2 h. The plate was blocked with phosphate-buffered saline (PBS) containing 10% donor calf serum, 0.5% Tween 20, 0.5% gelatin (blocking solution) at 37°C for 30 min. After washing 3 times with the wash solution (PBS containing 0.5% Tween 20), test serum samples and positive and negative controls diluted 1:100 in blocking solution were added to the previously designated wells of the coated assay plates and incubated at 37°C for 30 min. Following 4 washes with the wash solution, horseradish peroxidase-conjugated anti-human IgG (Sigma Chemicals, St. Louis, MO) was added to each well as the detector antibody and allowed to incubate for 30 min. The enzymatic reaction with the substrate (O-phenylenediamine and urea peroxide, 10 min) was stopped by the addition of 4 M H2SO4, and optical density (OD) values were determined at 492 nm. Human serum samples known to be positive and negative for HI antibodies against the pandemic influenza virus were included in every assay plate as controls. The cutoff values for IgG anti-p-H1N1-09 antibodies were calculated as the mean OD values for the results of 3 negative controls in triplicate. Samples with values greater than or equal to the cutoff values were considered antibody positive. Samples showing OD values within 10% of the cutoff value were considered borderline reactive and repeated.
A total of 783 serum samples previously screened by the HI test for the presence of p-H1N1-09 antibodies were retested in the ELISA. As evident from the results (Table 1), the ELISA emerged as an excellent assay for the detection of virus-specific antibodies. Of the 397 HI-negative samples, 389 were scored negative in the ELISA, giving 98% specificity in the comparison to the gold-standard HI test. Importantly, all samples with HI titers of >20 (n = 269) were positive in the ELISA, documenting 100% sensitivity of the ELISA. A large number of samples (n = 117) exhibited low HI titers (1:10). Usually, reactivity at this dilution is not considered specific. Of these, only 4 were recorded as reactive in the ELISA. When HI-negative samples with titers of 10 were considered antibody negative, the ELISA specificity was 97.7% and the concordance between the HI and ELISA results was 98.5%. To examine the relationship of the HI and ELISA titers, 2-fold dilutions of the test samples were tested in the ELISA. The reciprocal of the highest dilution at which the OD value was greater than or equal to the cutoff value was considered the IgG anti-p-H1N1-09 HA titer of the serum. All HI-positive sera with titers ≥20 were titrated in the ELISA. When the HI and ELISA titers were compared, the Spearman's rank correlation coefficient was estimated to be 0.864. Thus, a good correlation between the HI and ELISA titers was apparent. In addition, a linear relationship was noted when the log HI and ELISA titers were compared (Fig. 1).
TABLE 1.
Relationship of HI titers and IgG positivity in ELISA against p-H1N1-09 virus
| HI titer | No. of samples ELISA positive/no. tested (%) |
|---|---|
| 0 (negative) | 8/397 (2) |
| 1:10 | 4/117 (3.5) |
| 1:20 | 101/101 (100) |
| 1:40 | 77/77 (100) |
| 1:80 | 44/44 (100) |
| 1:160 | 26/26 (100) |
| 1:320 | 13/13 (100) |
| 1:640 | 6/6 (100) |
| 1:1,280 | 2/2 (100) |
FIG. 1.
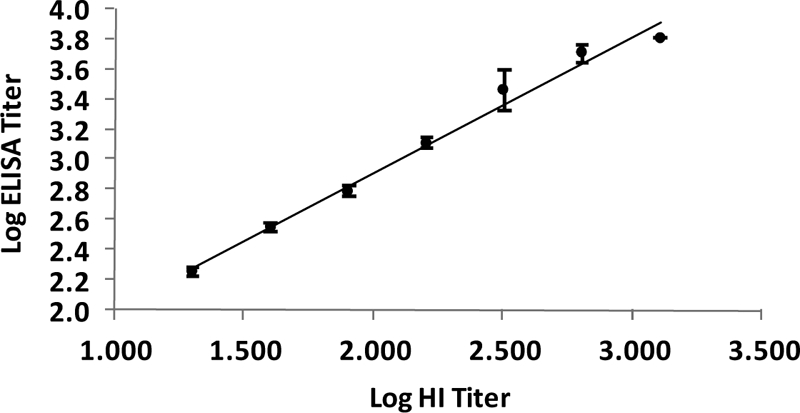
Relationship of log HI and ELISA titers. Each point represents the mean positive ELISA titer for a given HI titer value. Error bars represent standard errors of the means.
The data in Table 2 show the relationship of ELISA reactivities of the 397 serum samples that were negative for p-H1N1-09 in the HI test (Table 1), exhibiting various HI titers with respect to seasonal influenza viruses. Of these, 82 samples were negative for seasonal influenza antibodies, while high HI titers against individual strains, as well as reactivity to multiple seasonal viruses, were noted in a large number of the samples. Thus, the ELISA is highly specific in detecting IgG anti-p-H1N1-09. Of the 8 ELISA-reactive samples from this category, one was positive for both H3 (1:40) and B/Yamagata/1688 (1:80) HI antibodies. Thus, 7/8 ELISA-positive samples were nonreactive for seasonal influenza HI antibodies, negating cross-reactivity with these viruses as being responsible for the positivity recorded in the ELISA. These results clearly document that the ELISA described here is highly specific and sensitive.
TABLE 2.
HI titers against seasonal influenza viruses for 397 samples that were HI negative for p-H1N1-09 antibodiesa
| No. of samples HI negative |
||||
|---|---|---|---|---|
| Titer | H1 | H3 | B/Yamagata/1688 | B/Victoria/287 |
| 10 | 0 | 1 | 2 | 0 |
| 20 | 44 | 26 | 56 | 43 |
| 40 | 27 | 46 | 60 | 30 |
| 80 | 7 | 31 | 47 | 23 |
| 160 | 1 | 26 | 34 | 5 |
| 320 | 1 | 20 | 13 | 1 |
| 640 | 1 | 22 | 9 | 2 |
| ≥1,280 | 1 | 9 | 4 | 0 |
| Total | 82 | 181 | 225 | 104 |
Eight of 397 samples were positive in ELISA. Several samples were reactive for multiple seasonal influenza viruses.
The ELISA reactivity pattern strongly suggests that the cutoff for positivity in the HI test should be 20 for the population under surveillance. This ELISA was further used to screen 204 samples collected in early 2009 from the general population of a semiurban area, i.e., before the pandemic activity in India, and all were scored negative, confirming no exposure of the population to the novel pandemic or a closely related virus. Interestingly, after the establishment of the pandemic, 6.5% (6/92) of the blood donors were reactive.
When we compared the time-tested HI test with the newly developed ELISA, the following points emerged. (i) The tests were comparable in detecting virus-specific antibodies, and (ii) a good correlation was observed for quantitation (Spearman's rank correlation coefficient, 0.864). Clearly, for some samples, the HI and ELISA titers did not match. The HI test has been in use for several decades, and protective antibodies against a strain of influenza virus for a given community are determined on the basis of HI titers. The ELISA for the novel pandemic virus compared well with the HI test, suggesting its utility even for quantitative applications. Unless the newly developed method is tried in the field, its true suitability cannot be ascertained. (iii) A cost analysis based on 10,000 tests showed that the cost per sample for the HI test is Rs 75 (Indian; United States, $1.60), while the cost for the ELISA is Rs 100 (United States, $2.10). Thus, the ELISA is equally affordable. (iv) The ELISA is useful for the novel pandemic virus, and similar ELISAs for seasonal influenza viruses may not be possible on account of cross-reactivity. Whether the HA protein used will be able to identify infections with drifted strains of the virus in the future remains to be seen.
The results strongly suggest that the recombinant HA protein-based ELISA is an excellent alternative to the HI test to understand the exposure of a population to the pandemic virus. The ELISA is rapid (3.5 h), allows the handling of a large number of samples, and obviates the requirement of red blood cells. The technique is routinely used in all laboratories and, hence, easily adaptable. With the use of species-specific anti-IgG conjugates, the same protocol can be extended to various animal species to understand the exposure of various animals to the virus. The utility of this ELISA in the assessment of immune response to vaccines prepared employing different methodologies needs to be carefully evaluated.
Acknowledgments
We thank the Director, NIV, for support. Thanks are due to M. S. Chadha, group leader, Human Influenza Group, for the p-H1N1-09 isolate. Timely help from K. S. Lole and V. Verma is gratefully acknowledged.
The Indian Council of Medical research provided financial assistance for conducting the study.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Gurav, Y. K., S. D. Pawar, M. S. Chadha, V. A. Potdar, A. S. Deshpande, S. S. Koratkar, A. H. Hosmani, and A. C. Mishra. Pandemic influenza A (H1N1) 2009 outbreak in a residential school at Panchgani, Maharashtra, India. Indian J. Med. Res., in press. [PubMed]
- 2.John, T. J., and M. Moorthy. 2010. 2009 pandemic influenza in India. Indian Pediatr. 47:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, F. S. Dawood, S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. [DOI] [PubMed] [Google Scholar]
- 4.Peiris, J. S. M., M. D. de Jong, and Y. Guan. 2007. avian influenza virus (H5N1): a threat to human health. Clin. Microbiol. Rev. 20:243-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Padilla, R., D. de la Rosa-Zamboni, S. Ponce de Leon, M. Hernandez, F. Quinones-Falconi, E. Bautista, A. Ramirez-Venegas, J. Rojas-Serrano, C. E. Ormsby, A. Corrales, A. Higuera, E. Mondragon, J. A. Cordova-Villalobos, and the International Working Group on Influenza. 2009. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N. Engl. J. Med. 361:680-689. [DOI] [PubMed] [Google Scholar]


