Abstract
Toxoplasma gondii is an intracellular parasite that shows a unique capacity to infect a variety of cell types in warm-blooded animals. It can invade and survive well inside immune cells, such as macrophages, that disseminate the parasite around the body because of their migratory properties. The aim of the present study was to evaluate the role of T. gondii cyclophilin 18 (TgCyp18) in the proliferation and migration of macrophages and spleen cells (mainly T lymphocytes) in order to understand the effects of TgCyp18 on the dynamics of the infection. A high dose of TgCyp18 enhanced the proliferation of macrophages and spleen cells in a cysteine-cysteine chemokine receptor 5 (CCR5)-independent way. In contrast, TgCyp18 controlled the migration of macrophages and spleen cells in dose- and CCR5-dependent manners. Our data suggest that TgCyp18 recruits cells and enhances the growth of host cells at the site of infection for maintenance of the interaction between the parasite and host.
Toxoplasma gondii is an intracellular parasite that shows a unique capacity to infect a variety of cell types in warm-blooded animals (20). It can invade and survive well inside immune cells, such as macrophages and dendritic cells (DCs), which disseminate the parasite around the body through their migratory properties (9, 12, 27). In the host, macrophages and lymphocytes play essential roles against pathogens. Macrophages are crucial in the initiation and regulation of T-lymphocyte responses during Toxoplasma infection (9, 18, 30). Toxoplasma-infected macrophages, DCs, and neutrophils are important sources of interleukin-12 (IL-12) early in infection (4, 7, 8, 23, 34). IL-12 acts on both natural killer (NK) cells and T cells to promote the production of interferon gamma (IFN-γ) and create a positive feedback loop whereby IFN-γ promotes classical activation of macrophages (30). Moreover, CD4+ and CD8+ T cells have been demonstrated to be important in controlling T. gondii infection (1, 16, 17).
Interestingly, T. gondii stimulates IL-12 production through a unique pathway that involves the triggering of cysteine-cysteine chemokine receptor 5 (CCR5) in DCs and macrophages by the secreted T. gondii cyclophilin 18 (TgCyp18) (3). TgCyp18 recruits immature mouse DCs in vitro (3). Moreover, it appears to act as a structural mimic of CCR5-binding ligands, albeit one with no sequence similarity to the known host ligands, macrophage inflammatory protein 1α (MIP-1α)/chemokine (C-C motif) ligand 3 (CCL3), MIP-1β/CCL4, regulated on activation normal T-cell expressed and secreted (RANTES)/CCL5, or monocyte chemotactic protein 2 (MCP-2)/CCL8, for this receptor (3, 6, 42). High et al. in 1994 isolated genes encoding two Toxoplasma gondii cyclophilins, TgCyp18 and TgCyp20, as cyclosporine-binding proteins on affinity columns (24). Both were highly similar to human cyclophilin (hCyp18) in the central core region, but TgCyp20 differed in a 7-amino-acid insertion in the same region as in the Plasmodium falciparum cyclophilins (24).
TgCyp18, but neither hCyp18 nor PfCyp19A, appears to induce IL-12 production by interacting directly with CCR5, an effect that is blocked by the addition of cyclosporine A (CsA) (3, 6, 42). These observations implied that structural determinants of TgCyp18, related to CsA binding, are responsible for the induction of IL-12 synthesis (6, 42). This idea was confirmed by modeling the TgCyp18 structure on that of P. falciparum Cyp19A (PfCyp19A) and site-directed mutagenesis of putative surface-exposed residues that were absent in PfCyp19A (42). Two of the TgCyp18 mutants, namely, 17GEH19 to 17AAA19 and 149RP150 to 149YV150, located in the N and C termini of the protein, respectively, had reduced interactions with CCR5 and reduced IL-12 induction (42). Moreover, TgCyp18 peptidyl-prolyl cis-trans isomerase (PPIase) activity was not required for its interaction with CCR5, but IL-12 induction by TgCyp18 required both CCR5 binding and PPIase enzymatic activities (42). There is also evidence that the closely related protozoan Neospora cyclophilin plays a role in stimulating IFN-γ production by bovine peripheral blood mononuclear cells and Neospora caninum-specific CD4+ T cells (40). This effect is also blocked by CsA (40). IFN-γ production induced by N. caninum tachyzoites is thought to be critical in controlling the acute phase of neosporosis (40).
As an intracellular pathogen, T. gondii has mechanisms to interfere with host signaling pathways to subvert innate immunity (18). T. gondii profilin induces IL-12 via toll-like receptor 11 (TLR11)/myeloid differentiation factor 88 (MyD88), promotes parasite actin assembly, and contributes to gliding motility and invasion and egress (21, 32). Glycosylphosphatidylinositol-anchored proteins on the surface of T. gondii trigger tumor necrosis factor alpha (TNF-α) production from macrophages in a TLR2/TLR4/MyD88-dependent manner (14). T. gondii has three genotypic lineages, types I, II, and III, which differ widely in terms of virulence and effects on host cell signaling. Recent data indicate that these differences are controlled by specific T. gondii molecules (35). Types I and III, but not type II, isoforms of the rhoptry protein 16 (ROP16) kinase induce activation of signal transducer and activator of transcription 3/6 (STAT3/6) and suppress IL-12 production from macrophages (35). Moreover, it was reported that T. gondii heat shock protein 70 (HSP70)-induced nitric oxide (NO) release is dependent on TLR2, MyD88, and interleukin-1 receptor-associated kinase 4 (IRAK4) (31), which means that T. gondii has several proteins that work through different pathways to interfere with the host immune response. The identification of such molecules and determination of how they impact host cell behavior are very important when attempting to control the parasite. Because of the strong cooperation between macrophages and spleen cells (mainly lymphocytes) during infection, we focused our study on the role of TgCyp18 on the proliferation and migration of these cells in order to determine the effect of TgCyp18 on the parasite-host interaction.
MATERIALS AND METHODS
Animals.
C57BL/6J female mice (B6 mice), 6 to 8 weeks of age, were obtained from CLEA Japan (Tokyo, Japan). CCR5 knockout mice (CCR5−/−; B6.129P2-Ccr5tm1Kuz/J; stock no. 005427) were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed under specific-pathogen-free conditions in the animal facility of the National Research Center for Protozoan Diseases at the Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan. Animals used in this study were treated and used according to the Guiding Principles for the Care and Use of Research Animals published by the Obihiro University of Agriculture and Veterinary Medicine.
Reagents.
Lipopolysaccharide (LPS) from Escherichia coli (10-μg/ml stock solution in RPMI 1640 medium), concanavalin A (ConA) from Canavalia ensiformis (5-mg/ml stock solution in RPMI 1640 medium) were obtained from Sigma (Sigma, St. Louis, MO). Recombinant mouse MIP-3α (25-μg/ml stock solution in RPMI 1640 medium) was obtained from R&D Systems, Inc. (Minneapolis, MN).
Construction and expression of recombinant TgCyp18 and MTgCyp18.
Construction and expression of recombinant TgCyp18 and recombinant mutant TgCyp18 (MTgCyp18), with changed amino acids of 17GEH19 to 17AAA19 and 149RP150 to 149YV150, were performed as described previously (25). Wild-type and mutant TgCyp18s were expressed as glutathione S-transferase (GST) fusion proteins in the E. coli DH5α strain (Takara, Bio Inc., Japan). The GST tags of the recombinant proteins were removed with thrombin protease (GE Healthcare, Buckinghamshire, England) according to the manufacturer's instructions. Proteins were dialyzed in phosphate-buffered saline (PBS) and purified with Detoxi-Gel endotoxin-removing gel (Pierce Biotechnology Inc., Rockford, IL). For cell culture use, the proteins were filtered using a 0.45-μm low-protein-binding Supor membrane (Pall Life Sciences, Ann Arbor, MI). The purity and quantity of the proteins were detected as a single band by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie brilliant blue R250 (CBBR; MP Biomedicals Inc., France) staining. The concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Inc., Rockford, IL).
Monolayer cultures of peritoneal macrophages.
Mouse peritoneal macrophages were collected from B6 and CCR5−/− mice 4 days after intraperitoneal injection of 1 ml of 4.05% brewer modified BBL thioglycolate medium (Becton Dickinson, Sparks, MD) by peritoneal washing with 5 ml of cold PBS. After harvesting, the cells were centrifuged at 800 × g for 10 min and suspended in RPMI 1640 medium (Sigma) containing 10% fetal bovine serum (FBS). The macrophage suspension was then added to 24-well tissue culture microplates at 1 × 106 cells/well. The suspensions were incubated at 37°C for 3 h, washed thoroughly to remove nonadherent cells, and further incubated at 37°C. The purity of the peritoneal macrophage cultures was quantified by flow cytometry using anti-CD11b and anti-CD3 monoclonal antibodies (MAb). The percentage of CD11b+ cells was 99.4 ± 0.17% at 0 h and 98.6 ± 1.01% at 24 h (n = 3). Moreover, the percentage of CD3+ cells was 0.02% ± 0% at 0 h and 0.04 ± 0.06% at 24 h. These results indicate the high purity of the peritoneal macrophages used in this study.
Spleen cell isolation.
B6 and CCR5−/− mice were sacrificed and their spleens were aseptically removed. The splenocytes were suspended in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin. The mononuclear cells (the majority of which are lymphocytes) were isolated by using Histopaque 1077 (Sigma) according to the manufacturer's instructions.
IL-12 p40 ELISA.
Macrophage culture supernatants were collected for measurement of IL-12 p40 levels by enzyme-linked immunosorbent assay (ELISA; Pierce Biotechnology Inc.) according to the manufacturer's recommendations.
Measurement of nitric oxide.
Supernatants from peritoneal macrophages cultured in Dulbecco's modified Eagle's medium (DMEM) contain 10% FBS were collected for analysis of NO. Nitrate and nitrite production in the culture medium was measured using a nitrite/nitrate assay kit (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer's recommendations.
Sandwich ELISA for detection of secreted TgCyp18.
TgCyp18 secretion from extracellular parasites was determined as described previously (11). Purified T. gondii tachyzoites (3 × 107) were incubated in 1.5 ml of GIT medium (Nihon Pharmaceutical Co., Ltd., Tokyo, Japan) at 37°C. Before transfer of the parasite suspensions from ice to 37°C for the secretion assay, 250 μl was removed and processed as the time zero reading. The rest of the parasite suspension was incubated at 37°C in a water bath. After 15, 30, 60, and 120 min of incubation, 250-μl aliquots of the parasite suspensions were removed. The culture supernatants were centrifuged sequentially (760 × g for 10 min at 4°C and then 7,000 ×g for 10 min at 4°C), together with the ascitic fluid from the in vivo experiment, and subjected to a sandwich ELISA.
Rabbit anti-rTgCyp18 polyclonal immunoglobulin G (IgG) was purified using protein A chromatography columns (Bio-Rad Laboratories). Protein concentrations were measured using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). One microgram of the IgG diluted in 0.05 M carbonate buffer (pH 9.6) was used as the capture antibody to coat microtiter plates at 4°C overnight. Blocking was performed with a blocking solution (3% PBS-SM [PBS containing 3% skim milk], pH 7.2) at 37°C for 2 h. The plates were incubated at 37°C for 30 min with each supernatant in triplicate. After washing six times with a washing solution (0.05% Tween 20 in PBS), anti-rTgCyp18 mouse serum diluted 1:100 in blocking solution was added to each well as a detection antibody. After a further six washes, the plates were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Amersham Pharmacia Biotech) diluted 1:2,500 in blocking solution. Binding was visualized with substrate solution [0.3 mg/ml 2,2′-azino-bis-(3-ethlbenz-thiazoline-6-sulfonic acid), 0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2]. The absorbance at 415 nm was measured by using a MTP-500 microplate reader (Corona Electric, Tokyo, Japan). The TgCyp18 concentration of each sample was calculated by standardization with recombinant TgCyp18.
Parasite infection of mice.
Parasites purified from an in vitro culture were washed in sterile PBS, and 103 tachyzoites were inoculated intraperitoneally into B6 mice. After the indicated time periods, peritoneal cells were collected from the peritoneal cavities of naïve or parasite-infected mice by peritoneal washing with 5 ml of cold PBS. After harvesting, the cells were centrifuged at 800 × g for 10 min and suspended in cold PBS. The supernatants were used to measure the TgCyp18 secretion.
Macrophage or spleen cell proliferation assay.
Macrophages (5 × 105) from B6 and CCR5−/− mice were cultured for 24 h with TgCyp18, MTgCyp18, GST, or LPS. Spleen cells (5 × 105) from B6 and CCR5−/− mice were cultured for 48 h with TgCyp18, MTgCyp18, GST, or ConA. Then, 10 μl of cell counting kit 8 reagent (Dojindo Laboratories, Japan) was added. After 3 h of incubation at 37°C in 5% CO2, the optical density at 450 nm was determined (Corona Electric, Tokyo, Japan).
Flow cytometry.
Phycoerythrin (PE)-labeled anti-mouse CD11b MAb, CCR5, CD3e (CD3ɛ chain; 145-2C11), and Fc block were purchased from BD Biosciences (San Jose, CA).
After washing with cold PBS, 1 × 106 cells (macrophages or spleen cells) were suspended in cold PBS containing 0.5% bovine serum albumin (BSA) and treated with Fc block to avoid nonspecific adherence of MAb to Fc receptors. Cells were subsequently incubated with PE-labeled anti-mouse MAbs for 30 min at 4°C followed by a final washing step with cold PBS. Labeled macrophages (5 × 103) or spleen cells (1 × 104) were examined using an EPICS XL flow cytometer (Beckman Coulter, Hialeah, FL).
Migration assays.
Cell migration was assessed using 24-well chemotaxis chambers and inserts with 8-μm-pore polycarbonate filters (Falcon, Beckton Dickinson, Franklin Lakes, NJ). Macrophages or spleen cells (1 × 106) from B6 and CCR5−/− mice suspended in 0.4 ml of RPMI 1640 supplemented with 10% FBS were applied to the upper compartment of the chamber. In the lower chamber, TgCyp18, MTgCyp18, or MIP-3α (R&D Systems) in 1.2 ml RPMI 1640 supplemented with 10% FBS was added as a chemoattractant. Chambers were incubated at 37°C for 24 h. The cells that migrated into the lower compartment were counted by microscopy. Each treatment was performed in triplicate. Results are expressed as the percentage of macrophages or spleen cells in the lower compartment in relation to the number of total macrophages or spleen cells used in the experiment as follows: percentage of migrating cells = [(number of cells in the lower chambers)/(total cell number)] × 100.
Statistical analysis.
Data are expressed as the means ± standard deviations. Various assay conditions were evaluated by using the independent Student t test and/or an analysis of variance (ANOVA) test followed by a post hoc analysis of group differences that was performed with the least significant differences (LSD) test. P values of <0.05 were considered statistically significant, and P values of <0.01 were considered highly statistically significant.
RESULTS
IL-12 p40 and NO production in response to TgCyp18 and physiologically relevant doses of TgCyp18.
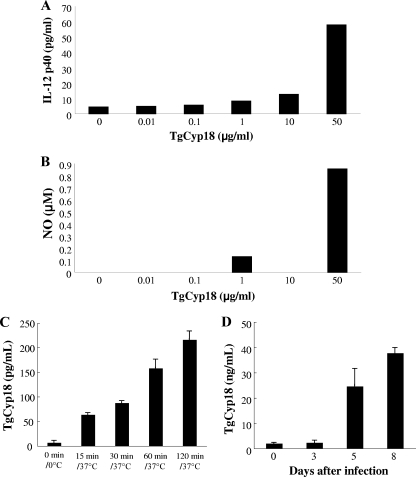
TgCyp18 has been shown to stimulate macrophages to produce IL-12 and NO in a CCR5-dependent manner (25). However, the dose-dependent effect of TgCyp18 has not yet been elucidated. We investigated the production of IL-12 p40 and NO by peritoneal macrophages treated with different concentration of TgCyp18. As shown in Fig. 1 A and B, the higher dose (50 μg/ml) of TgCyp18 showed potent effects on the production of IL-12 p40 and NO. Next, we examined the effects of physiologically relevant doses of TgCyp18 using in vitro and in vivo models (Fig. 1C and D). TgCyp18 was secreted from extracellular tachyzoites in a time-dependent manner (Fig. 1C), with detectable endogenous levels of 216.3 ± 17.5 pg/ml from 5 × 106 parasites after 120 min. Moreover, higher levels of secreted endogenous TgCyp18 (24.57 ± 7.1 and 37.74 ± 2.1 ng/ml) were detected in the ascitic fluid from mice infected with T. gondii tachyzoites (1,000 tachyzoites) at 5 and 8 days postinfection, respectively. These results suggested that huge numbers of tachyzoites were required to secrete a threshold concentration of TgCyp18 capable of IL-12 and NO production. Thus, TgCyp18 might have paracrine effects (locally or systemically) at the site of infection.
FIG. 1.
IL-12 p40 and NO production induced by TgCyp18. Peritoneal macrophages (1 × 106) from B6 mice were incubated for 24 h with TgCyp18 (0.01, 0.1, 1, 10, or 50 μg/ml), and the supernatants were collected to measure the production of IL-12 p40 (A) and NO (B). Each value represents the mean of duplicate samples. (C) Secretion of TgCyp18 from extracellular parasites. Purified T. gondii tachyzoites were incubated in GIT medium at 37°C. After 15, 30, 60, and 120 min of incubation, the parasite suspension was removed and the supernatants were prepared by centrifugation and subjected to a sandwich ELISA. Each value represents the mean ± the standard deviation of triplicate samples. The results are representative of two repeated experiments with similar results. (D) TgCyp18 secretion in response to infection with parasites. A total of 103 tachyzoites were inoculated intraperitoneally into B6 mice. After the indicated time periods, ascitic fluid was collected from the peritoneal cavities of mice. The ascitic fluids were used to measure TgCyp18 secretion. At day zero, the level of TgCyp18 was detected at 7.64 ± 5.29 pg/ml as a background reaction. Each value represents the mean ± the standard deviation of quadruplicate samples (four mice). The results are representative of two repeated experiments with similar results.
Effects of TgCyp18 on macrophage and spleen cell proliferation.
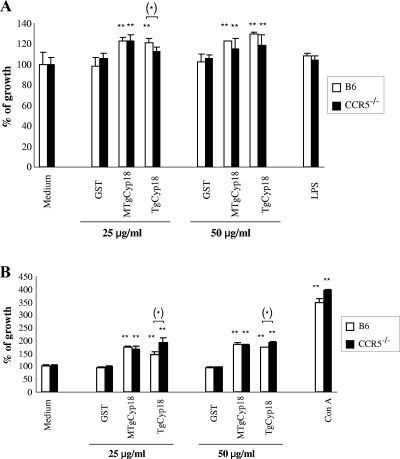
The mitogenic effects of TgCyp18 and MTgCyp18 were evaluated in peritoneal macrophages and spleen cells. Since serial doses of TgCyp18 (0.01, 0.1, 1, and 10 μg/ml) did not have significant effects on the proliferation of these cells (data not shown), we examined the effects of higher concentration of TgCyp18 (25 and 50 μg/ml) (Fig. 2). Significant proliferative responses were observed after stimulation with 25 μg/ml of TgCyp18 in wild-type macrophages (P < 0.05) compared to CCR5−/− macrophages (Fig. 2A). The proliferative responses were not significant after stimulation with 50 μg/ml of TgCyp18 or with 25 and 50 μg/ml of MTgCyp18 in wild-type macrophages compared to CCR5−/− macrophages. Although the result from the 25 μg/ml TgCyp18 stimulation in wild-type macrophages may give some evidence about the dependence on CCR5, the results of the 50 μg/ml of TgCyp18 and both doses of MTgCyp18 give clear-cut evidence that the responses are independent of CCR5. The MTgCyp18 results also give evidence about the role of other receptors and TgCyp18. This observation is important toward determining the active binding site on TgCyp18 that is responsible for macrophage proliferation. It may also suggest that cytokine induction (e.g., IL-12), as shown by Yarovinsky et al. 2004 (42), is dependent on this site (17GEH19 and 149RP150) but not the macrophage proliferation site. A previous report demonstrated that treating RAW 264.7 macrophages with LPS inhibited their proliferation (10). Our results demonstrated that no significant differences were detected upon treatment of B6 or CCR5−/− macrophages with LPS compared to controls (complete medium or GST). The difference between our results and the previously mentioned report may be related to the use different types of cells and different methods of detection. On the level of macrophages, although significant proliferative responses were observed after stimulation with both TgCyp18 and MTgCyp18 in wild-type and CCR5−/− macrophages compared to controls (complete medium or GST), these effects were marginal, suggesting that the effects of TgCyp18 on macrophage growth might be questionable under physiological conditions.
FIG. 2.
TgCyp18-induced macrophage and spleen cell proliferation. (A) Macrophages (5 × 105) from B6 and CCR5−/− mice were cultured for 24 h with 25 or 50 μg/ml of TgCyp18, MTgCyp18, or GST or 10 ng/ml LPS. (B) Spleen cells (5 × 105) from B6 and CCR5−/− mice were cultured for 48 h with 25 or 50 μg/ml of TgCyp18, MTgCyp18, or GST or 5 μg/ml ConA. Then, 10 μl of Cell Counting kit 8 reagent was added. After 3 h of incubation at 37°C, the optical density was determined at 450 nm. The percentage of growth was calculated by dividing each value (control or tested) by the average mean of the control samples (nontreated cells, complete medium) multiplied by 100. Each value represents the mean ± the standard deviation of triplicate samples. The results are representative of three repeated experiments with similar results. Statistical significance was calculated compared to the control groups (complete medium or GST) with an ANOVA and follow-up test (LSD). **, high significance (P < 0.01). In addition, the statistical difference between the wild-type B6 and CCR5−/− cells upon stimulation with the same dose of TgCyp18 was calculated using an independent Student t test. *, significance at P < 0.05.
On the level of spleen cells, significant proliferative responses were elicited after incubation of wild-type or CCR5−/− spleen cells with both doses (25 and 50 μg/ml) of TgCyp18 or MTgCyp18 (P < 0.01) compared to controls (complete medium or GST) (Fig. 2B). Moreover, high proliferative responses were observed with a known inducer of proliferation, ConA. Furthermore, a significant proliferative response was observed after stimulation with 25 or 50 μg/ml of TgCyp18 in CCR5−/− spleen cells (P < 0.05) compared to stimulation with the same doses in wild-type spleen cells. On the other hand, no significant proliferative response was demonstrated after stimulation with 25 or 50 μg/ml of MTgCyp18 in knockout spleen cells compared to wild-type spleen cells. Taken together, treatment of macrophages or spleen cells with TgCyp18 resulted in the enhancement of macrophage and spleen cell proliferation in a CCR5-independent manner.
Effects of TgCyp18 on CCR5 expression by macrophages and spleen cells.
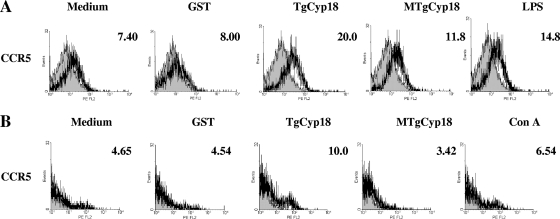
To determine the quality of the recombinant proteins in altering CCR5 expression on macrophages and spleen cells, the expression of CCR5 on macrophages and spleen cells after contact with TgCyp18 and MTgCyp18 was examined by flow cytometry (Fig. 3). After 24 h of incubation with TgCyp18, expression of CCR5 on macrophages and spleen cells was enhanced in comparison to the control groups (complete medium or GST). Although incubation of macrophages with MTgCyp18 showed a slight enhancement in the expression of CCR5 compared to the control groups, treatment of spleen cells with MTgCyp18 failed to enhance the expression of CCR5 compared with the control groups. Interestingly, clear enhancements of the expression of CCR5 were observed in LPS-treated macrophages and ConA-treated spleen cells. A recent report suggested that CCR5-mediated neuron-glia signaling functions to protect neurons by suppressing microglia toxicity through downregulation of the expression of mRNAs for inflammatory cytokines (IL-1β and TNF-α) and inducible NO synthetase induced by LPS (22). Our results together with the previously reported data might clarify the direct or the indirect interaction between LPS and CCR5 in regulating the immune response. Our data demonstrated that TgCyp18 could control CCR5 expression on macrophages and spleen cells.
FIG. 3.
CCR5 expression on macrophages and spleen cells after incubation with TgCyp18. (A) Peritoneal macrophages (1 × 106) from B6 mice were incubated with 50 μg/ml TgCyp18, MTgCyp18, or GST or 10 ng/ml LPS for 24 h. (B) Spleen cells (1 × 106) from B6 mice incubated with 50 μg/ml TgCyp18, MTgCyp18, or GST or 5 μg/ml ConA for 24 h; the cells were subjected to flow cytometry. Shaded profiles correspond to cells labeled with isotype controls. Numbers correspond to the percentage of labeled cells. Data are representative of two separate experiments with similar results.
TgCyp18 induces macrophage and spleen cell migration in a CCR5-dependent manner.
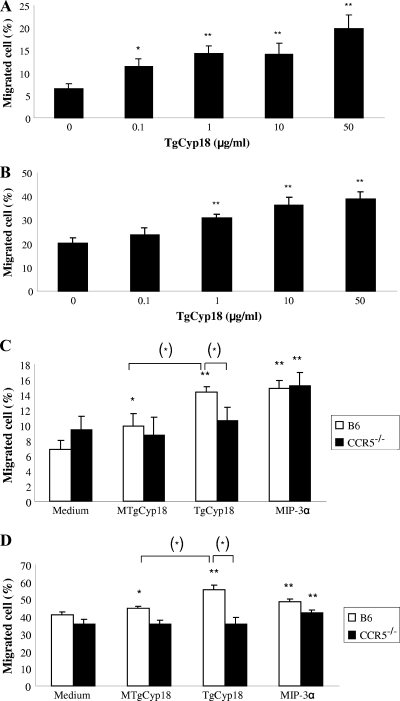
To investigate the ability of TgCyp18 to chemoattract macrophages and spleen cells, wild-type cells were placed in the upper chamber and permitted to migrate to different concentrations of TgCyp18 (Fig. 4 A and B). TgCyp18 elicited a typical chemotactic response in both cell types compared to the negative control (no TgCyp18). All tested concentration of TgCyp18 elicited macrophage migration compared to the negative control (P < 0.05 and P < 0.01) (Fig. 4A). All TgCyp18 concentrations (1, 10, and 50 μg/ml) enhanced spleen cell migration compared to the negative control (P < 0.01) (Fig. 4B). Altogether, TgCyp18 elicited macrophage and spleen cell migration in a dose-dependent fashion.
FIG. 4.
The effects of TgCyp18 on macrophage and spleen cell migration. (A and B) Macrophages (A) or spleen cells (B) (1 × 106) from wild-type mice were applied to the upper compartment of the chamber. In the lower chamber, serial concentration of TgCyp18 (0.1, 1, 10, and 50 μg/ml) were added as chemoattractant. Chambers were incubated at 37°C for 24 h. The cells that migrated into the lower compartment were counted by microscopy. The percentage of migrating cells shown represents the mean ± the standard deviation of triplicate samples. Statistical significance was calculated compared to the complete medium control (0 μg/ml) based on an ANOVA and an LSD follow-up test. *, P < 0.05; **, P < 0.01. (C and D) TgCyp18-induced cell migration in a CCR5-dependent manner. Macrophages (C) or spleen cells (D) (1 × 106) from B6 and CCR5−/− mice were applied to the upper compartment of the chamber. In the lower chamber, 0.25 μg/ml MIP-3α, 50 μg/ml TgCyp18, or 50 μg/ml MTgCyp18 was added as chemoattractant. Chambers were incubated at 37°C for 24 h. The cells that migrated into the lower compartment were counted by microscopy. The percentage of migrating cells represents the mean ± the standard deviation of triplicate samples. The results are representative of two repeated experiments with similar results. Statistical significance was calculated compared to the complete medium control group using an ANOVA and LSD follow-up test. *, P < 0.05; **, P < 0.01. The statistical difference between the wild-type B6 and CCR5−/− cells upon stimulation with the same dose of TgCyp18 was calculated using an independent Student t test (*, P < 0.05). In addition, the difference between TgCyp18 and MTgCyp18 wild-type B6-stimulated cells was significant (*, P < 0.05).
To determine the importance of the CCR5 receptor for the chemotactic effect of TgCyp18 on macrophages and spleen cells, we evaluated the ex vivo migration of wild-type cells and CCR5−/− cells using different chemoattractants (TgCyp18, MTgCyp18, and MIP-3α, a known ligand of CCR6). Significant migratory responses were observed after incubation of wild-type macrophages and spleen cells with TgCyp18 (P < 0.01) compared to the complete medium control (Fig. 4C and D). In contrast, CCR5−/− macrophages and CCR5−/− spleen cells showed insignificant migratory responses upon incubation with TgCyp18. Although the incubation of wild-type macrophages and wild-type spleen cells with MTgCyp18 showed slight migratory responses (P < 0.05), MTgCyp18 failed to attract CCR5−/− cells. As expected, wild-type and CCR5−/− cells were significantly attracted to MIP-3α. Moreover, incubation of wild-type macrophages or spleen cells with TgCyp18 showed significant migratory responses (P < 0.05) compared to incubation of these cells with MTgCyp18. No significant difference was detected upon incubation of CCR5−/− cells with TgCyp18 compared to MTgCyp18-treated cells. This observation suggests that these two amino acid sequences (17GEH19 and 149RP150) are critical for cell migration. Although incubation of CCR5−/− cells with TgCyp18 showed a significant reduction in cell migration compared with wild-type cells (P < 0.05), incubation with MTgCyp18 displayed no significant reduction in knockout cell migration. This observation supports the notion that cell migration in response to TgCyp18 is carried out in a CCR5-dependent manner.
DISCUSSION
Cellular immunity is an important immune defense mechanism against intracellular pathogens. During T. gondii infection, production of high levels of IL-12 and IFN-γ by the innate immune system elicits a very strong adaptive Th1-biased CD4+ and CD8+ T-cell-mediated immune response (30). Secreted products from T. gondii can extensively modify its host cell environment (33). Toxoplasma possesses two mechanisms to trigger IL-12 production from DCs (3, 18, 37). One is the interaction of T. gondii profilin with TLR11, triggering signaling pathways via the MyD88 pathway (18, 37, 43). The other is through an 18-kDa cyclophilin, TgCyp18, which is released by extracellular tachyzoites, triggering IL-12 production through binding to CCR5, a transmembrane receptor expressed by multiple cell types that transmit signals through a Gi-like protein (3, 4, 18, 33). Both CCR5−/− and MyD88−/− mice display defects in IL-12 production after T. gondii infection (2, 28, 37). The present study aimed to clarify the immunomodulatory role of TgCyp18 in relation to CCR5 on the proliferation and migration of macrophages and spleen cells (mainly lymphocytes) in order to understand the immunobiology of the immune response toward T. gondii as one step on the way to controlling the parasite.
The results of the present study demonstrated that treatment with TgCyp18 enhanced expression of CCR5 on macrophages and spleen cells compared with the control groups (complete medium or GST). In contrast, treatment with MTgCyp18 failed to enhance the expression of CCR5 compared with the TgCyp18-treated group. Previous studies confirmed the binding abilities of TgCyp18 with CCR5 (3, 42). The induction of mutations in two amino acid sequences, namely, 17GEH19 to 17AAA19 and 149RP150 to 149YV150, located in the N and C termini of TgCyp18, respectively, reduced interactions with CCR5 (42). Altogether, TgCyp18 could control CCR5 expression on macrophages and spleen cells.
In the present study, the purities of the spleen cell cultures were quantified by flow cytometry using anti-CD3 and anti-CD11b monoclonal antibodies. The percentage of CD3+ cells was 90.6 ± 0.85% (n = 3). Moreover, the percentage of CD11b+ cells was 0.17 ± 0.05%. These results indicate the high purity of the lymphocytes (the majority of cells are T lymphocytes) used in this study. T. gondii possesses superantigen-like properties that contribute to T-cell proliferation and activation (15, 16). Incubation of ex vivo spleen cells cultured with tachyzoite lysates or irradiated parasites resulted in vigorous T-cell proliferation (15). Furthermore, previous studies on human peripheral blood T lymphocytes from unexposed donors also suggested that T. gondii acts as a strong T-lymphocyte mitogen, inducing high proliferative responses (39). TgCyp18 and MTgCyp18 stimulate the proliferation of spleen cells in wild-type and CCR5−/− spleen cells (Fig. 2B). Our data demonstrated that treatment of spleen cells with TgCyp18 enhanced their proliferation in a CCR5-independent manner. It has been reported that TgHSP70 induces splenic B-cell proliferation in a TLR4-dependent manner (5), indicating that T. gondii has several proteins that act as mitogens to induce lymphocyte proliferation. Furthermore, a significant proliferative response was observed after stimulation with 25 or 50 μg/ml of TgCyp18 in CCR5−/− spleen cells compared to stimulation with the same doses in wild-type spleen cells (P < 0.05). In contrast, no significant proliferative response was demonstrated after stimulation with 25 or 50 μg/ml of MTgCyp18 in CCR5−/− spleen cells compared to wild-type spleen cells. Interestingly, this effect was supported by the result with MTgCyp18, where the induced mutations (17GEH19 to 17AAA19 and 149RP150 to 149YV150) of the TgCyp18 region abrogated this effect. This observation provides further evidence that TgCyp18 binding to CCR5 is antagonistic for spleen cell proliferation, but further study will be required to confirm this. Altogether, TgCyp18 enhanced the proliferation of macrophages and spleen cells in a CCR5-independent manner.
Moreover, TgCyp18 enhanced the proliferation of macrophages in a CCR5-independent way. Treatment with TgCyp18 and MTgCyp18 enhanced the proliferation of wild-type and CCR5−/− macrophages (Fig. 2A). A previous report revealed that cyclophilin A (CypA), which shows functional similarity to TgCyp18, contributes to macrophage proliferation through activation of the Raf-1/MEK/extracellular signal-regulated kinase pathway and regulation of cyclin-dependent kinase activities, which are required for cell cycle progression (36). Cytokines and/or soluble factors might contribute to the cell proliferation via a CCR5-independent manner, but determination of the potential roles of the factors is needed.
With respect to macrophages, while the marginal effect on production of IL-12 p40, NO, and cellular proliferation induced by high doses of TgCyp18 could be questionable, there are other possible interpretations to our results. First, we interpreted that the activity of our recombinant TgCyp18 relative to native TgCyp18 might be reduced. Therefore, higher doses of recombinant TgCyp18 expressed in E. coli would be equivalent to lower doses of native protein with high activity. Aliberti et al. in 2003 reported that low IL-12-inducing activity of recombinant TgCyp18 was observed compared to native TgCyp18, that was included in the soluble tachyzoite extract (3). Those authors explained that the reduced activity of recombinant TgCyp18 expressed in E. coli relative to native TgCyp18 resulted from a missing cofactor rather than improper expression (3). It is also possible that a threshold concentration of TgCyp18 is needed to enhance the production of NO and trigger cellular proliferation. We found low levels of secreted endogenous TgCyp18 from extracellular parasites that appeared to be time dependent (Fig. 1C). Aliberti et al. proved that TgCyp18 was actively secreted in a time- and temperature-dependent fashion (3). Moreover, we demonstrated higher levels of secreted endogenous TgCyp18 upon challenge with the tachyzoites (Fig. 1D). Therefore, these lower levels compared to the doses used in our experiments suggest that huge numbers of tachyzoites (proliferated asexually through many cycles) are required to secrete a threshold dose of TgCyp18 capable of having effects on NO and IL-12 production and cell proliferation. Thus, this possibility indicates that TgCyp18 might have paracrine effects (locally or systemically) at the site of infection.
Diana et al. in 2005 demonstrated that T. gondii-excreted/secreted antigens induced the recruitment and migration of human DCs in a CCR5-dependent fashion (19). Other studies in mice have reported that T. gondii can activate DCs and trigger their migration to the spleen to activate T-cell proliferation or to potentiate parasite dissemination (27, 38). Moreover, TgCyp18, which is similar in action to MIP-1β, which binds CCR5, can attract mouse DCs in vitro (3). Furthermore, it has been reported that the secretory form of CypA possesses chemotactic activity, regulating the migration of DCs, monocytes, neutrophils, eosinophils, B lymphocytes, and T lymphocytes through its interaction with CD147, a type I transmembrane glycoprotein (13, 26, 41, 44). Our study showed that TgCyp18 elicited macrophage and spleen cell migration in a dose- and CCR5-dependent fashion. In contrast, CCR5−/− cells showed insignificant migratory responses upon incubation with the same dose of TgCyp18. Although treatment of macrophages and spleen cells with MTgCyp18 failed to attract CCR5−/− cells, the incubation of wild-type cells with MTgCyp18 showed significant migratory responses (P < 0.05). Two explanations may help to clarify the MTgCyp18 migratory response. First, the induced mutation in TgCyp18 to produce MTgCyp18 might enhance partial binding of MTgCyp18 with other receptors rather than CCR5 on macrophages and spleen cells. This partial binding might be responsible for these migratory properties related to MTgCyp18. It is also possible that the binding between the CCR5 and MTgCyp18 was not blocked completely. Yarovinsky et al. in 2004 reported that two amino acid sequences (17GEH19 and 149RP150) are critical for the binding of TgCyp18 with CCR5 (42). In the previous report they demonstrated that two of the TgCyp18 mutants, namely, 17GEH19 to 17AAA19 and 149RP150 to 149YV150, detected one by one, respectively, had reduced interactions with CCR5 without complete blocking of binding (42). This minimal binding with CCR5 might be responsible for the MTgCyp18 migratory properties. A previous study demonstrated that CCR5 plays an important role in the migration of intraepithelial CD8+ T cells and the regulation of the inflammatory response following T. gondii infection (29). Altogether, TgCyp18 appears to control cellular migration in a dose- and CCR5-dependent manner.
Because the effects of TgCyp18 are very similar in macrophages and spleen cells, the question comes up whether TgCyp18 will exert the same effects in every cell type. Thus, the discussed role of TgCyp18 may not be specific for cells of the immune system but a more general cell biological effect. Further studies will be required to estimate the underlying mechanisms responsible for the induction of proliferation and migration to demonstrate this hypothesis.
In conclusion, our data suggested that T. gondii, through producing TgCyp18, has the ability to recruit macrophages to the site of infection, to invade them, and to use their migratory properties. T. gondii enhances the proliferation of macrophages to enable the egressed parasites to find more host cells. In the same way, the parasite may control the migration and proliferation of lymphocytes in order to control the parasite infection and protect the host. T lymphocytes have been demonstrated to be important in controlling T. gondii infection (1, 16, 17). The ability of T. gondii to survive within the host and establish a successful infection while under attack from potent immune responses implicates a balance between parasite immune evasion strategies and the host immune response. The data presented herein, concerning the ability of TgCyp18 to enhance the proliferation and migration of macrophages and lymphocytes, provide new evidence for the complex interaction between the parasite and the host. Further investigation is required to understand how T. gondii controls its own fate in the face of this potent immune response.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (19041008) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. H.M.I. was supported by the Egyptian Ministry of High Education and Scientific Research.
We deeply thank Alaa M. Terkawi for beneficial help.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Alexander, J., and C. A. Hunter. 1998. Immunoregulation during toxoplasmosis. Chem. Immunol. 70:81-102. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 α+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 3.Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485-490. [DOI] [PubMed] [Google Scholar]
- 4.Aliberti, J. 2005. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat. Rev. Immunol. 5:162-170. [DOI] [PubMed] [Google Scholar]
- 5.Aosai, F., M. Chen, H. K. Kang, H. S. Mun, K. Norose, L. X. Piao, M. Kobayashi, O. Takeuchi, S. Akira, and A. Yano. 2002. Toxoplasma gondii-derived heat shock protein HSP70 functions as a B cell mitogen. Cell Stress Chaperones 7:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell, A., P. Monaghan, and A. P. Page. 2006. Peptidyl-prolyl cis-trans isomerases (immunophilins) and their roles in parasite biochemistry, host-parasite interaction and antiparasitic drug action. Int. J. Parasitol. 36:261-276. [DOI] [PubMed] [Google Scholar]
- 7.Bliss, S. K., A. J. Marshall, Y. Zhang, and E. Y. Denkers. 1999. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J. Immunol. 162:7369-7375. [PubMed] [Google Scholar]
- 8.Bliss, S. K., Y. Zhang, and E. Y. Denkers. 1999. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J. Immunol. 163:2081-2088. [PubMed] [Google Scholar]
- 9.Buzoni-Gatel, D., J. Schulthess, L. C. Menard, and L. H. Kasper. 2006. Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell. Microbiol. 8:535-544. [DOI] [PubMed] [Google Scholar]
- 10.Chang, C. Y., M. Tucci, and R. C. Baker. 2000. Lipopolysaccharide-stimulated nitric oxide production and inhibition of cell proliferation is antagonized by ethanol in a clonal macrophage cell line. Alcohol 20:37-43. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi, S., H. Qi, D. Coleman, A. Rodriguez, P. I. Hanson, B. Striepen, D. S. Roos, and K. A. Joiner. 1999. Constitutive calcium-independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. J. Biol. Chem. 274:2424-2431. [DOI] [PubMed] [Google Scholar]
- 12.Courret, N., S. Darche, P. Sonigo, G. Milon, D. Buzoni-Gâtel, and I. Tardieux. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damsker, J. M., I. Okwumabua, T. Pushkarsky, K. Arora, M. I. Bukrinsky, and S. L. Constant. 2009. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology 126:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debierre-Grockiego, F., M. A. Campos, N. Azzouz, J. Schmidt, U. Bieker, M. G. Resende, D. S. Mansur, R. Weingart, R. R. Schmidt, D. T. Golenbock, R. T. Gazzinelli, and R. T. Schwarz. 2007. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 179:1129-1137. [DOI] [PubMed] [Google Scholar]
- 15.Denkers, E. Y., P. Caspar, and A. Sher. 1994. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor V beta 5-bearing CD8+ lymphocytes. J. Exp. Med. 180:985-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denkers, E. Y. 1999. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect. 1:699-708. [DOI] [PubMed] [Google Scholar]
- 18.Denkers, E. Y. 2003. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS. Immunol. Med. Microbiol. 39:193-203. [DOI] [PubMed] [Google Scholar]
- 19.Diana, J., C. Vincent, F. Peyron, S. Picot, D. Schmitt, and F. Persat. 2005. Toxoplasma gondii regulates recruitment and migration of human dendritic cells via different soluble secreted factors. Clin. Exp. Immunol. 141:475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey, J. P. 2007. The history and life cycle of Toxoplasma gondii, p. 1-17. In L. M. Weiss and K. Kim (ed.), Toxoplasma gondii, the model apicomplexan: perspectives and methods. Academic Press, London, United Kingdom.
- 21.Egan, C. E., W. Sukhumavasi, B. A. Butcher, and E. Y. Denkers. 2009. Functional aspects of Toll-like receptor/MyD88 signalling during protozoan infection: focus on Toxoplasma gondii. Clin. Exp. Immunol. 156:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamo, K., S. Kiryu-Seo, H. Konishi, S. Aoki, K. Matsushima, K. Wada, and H. Kiyama. 2008. G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity. J. Neurosci. 28:11980-11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. U. S. A. 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.High, K. P., K. A. Joiner, and R. E. Handschumacher. 1994. Isolation, cDNA sequences, and biochemical characterization of the major cyclosporin-binding proteins of Toxoplasma gondii. J. Biol. Chem. 269:9105-9112. [PubMed] [Google Scholar]
- 25.Ibrahim, H. M., H. Bannai, X. Xuan, and Y. Nishikawa. 2009. Toxoplasma gondii cyclophilin 18-mediated production of nitric oxide induces bradyzoite conversion in a CCR5-dependent manner. Infect. Immun. 77:3686-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khromykh, L. M., N. L. Kulikova, T. V. Anfalova, T. A. Muranova, V. M. Abramov, A. M. Vasiliev, V. S. Khlebnikov, and D. B. Kazansky. 2007. Cyclophilin A produced by thymocytes regulates the migration of murine bone marrow cells. Cell. Immunol. 249:46-53. [DOI] [PubMed] [Google Scholar]
- 27.Lambert, H., N. Hitziger, I. Dellacasa, M. Svensson, and A. Barragan. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell. Microbiol. 8:1611-1623. [DOI] [PubMed] [Google Scholar]
- 28.Liu, C. H., Y. T. Fan, A. Dias, L. Esper, R. A. Corn, A. Bafica, F. S. Machado, and J. Aliberti. 2006. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J. Immunol. 177:31-35. [DOI] [PubMed] [Google Scholar]
- 29.Luangsay, S., L. H. Kasper, N. Rachinel, L. A. Minns, F. J. Mennechet, A. Vandewalle, and D. Buzoni-Gatel. 2003. CCR5 mediates specific migration of Toxoplasma gondii-primed CD8 lymphocytes to inflammatory intestinal epithelial cells. Gastroenterology 125:491-500. [DOI] [PubMed] [Google Scholar]
- 30.Miller, C. M., N. R. Boulter, R. J. Ikin, and N. C. Smith. 2009. The immunobiology of the innate response to Toxoplasma gondii. Int. J. Parasitol. 39:23-39. [DOI] [PubMed] [Google Scholar]
- 31.Mun, H. S., F. Aosai, K. Norose, L. X. Piao, H. Fang, S. Akira, and A. Yano. 2005. Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70. Infect. Immun. 73:4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plattner, F., F. Yarovinsky, S. Romero, D. Didry, M. F. Carlier, A. Sher, and D. Soldati-Favre. 2008. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3:77-87. [DOI] [PubMed] [Google Scholar]
- 33.Pollard, A. M., L. J. Knoll, and D. G. Mordue. 2009. The role of specific Toxoplasma gondii molecules in manipulation of innate immunity. Trends Parasitol. 25:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeij, J. P., S. Coller, J. P. Boyle, M. E. Jerome, M. W. White, and J. C. Boothroyd. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sànchez-Tilló, E., M. Wojciechowska, M. Comalada, C. Farrera, J. Lloberas, and A. Celada. 2006. Cyclophilin A is required for M-CSF-dependent macrophage proliferation. Eur. J. Immunol. 36:2515-2524. [DOI] [PubMed] [Google Scholar]
- 37.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 38.Sher, A., S. Hieny, H. Charest, T. Scharton-Kersten, C. Collazo, R. N. Germain, and C. Reis e Sousa. 1998. The role of dendritic cells in the initiation of host resistance to Toxoplasma gondii. Adv. Exp. Med. Biol. 452:103-110. [DOI] [PubMed] [Google Scholar]
- 39.Subauste, C. S., F. Fuh, R. de Waal Malefyt, and J. S. Remington. 1998. Alpha beta T cell response to Toxoplasma gondii in previously unexposed individuals. J. Immunol. 160:3403-3411. [PubMed] [Google Scholar]
- 40.Tuo, W., R. Fetterer, M. Jenkins, and J. P. Dubey. 2005. Identification and characterization of Neospora caninum cyclophilin that elicits gamma interferon production. Infect. Immun. 73:5093-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, Q., M. C. Leiva, S. A. Fischkoff, R. E. Handschumacher, and C. R. Lyttle. 1992. Leukocyte chemotactic activity of cyclophilin. J. Biol. Chem. 267:11968-11971. [PubMed] [Google Scholar]
- 42.Yarovinsky, F., J. F. Andersen, L. R. King, P. Caspar, J. Aliberti, H. Golding, and A. Sher. 2004. Structural determinants of the anti-HIV activity of a CCR5 antagonist derived from Toxoplasma gondii. J. Biol. Chem. 279:53635-53642. [DOI] [PubMed] [Google Scholar]
- 43.Yarovinsky, F., D. Zhang, J. F. Andersen, G. L. Bannenberg, C. N. Serhan, M. S. Hayden, S. Hieny, F. S. Sutterwala, R. A. Flavell, S. Ghosh, and A. Sher. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626-1629. [DOI] [PubMed] [Google Scholar]
- 44.Yurchenko, V., G. Zybarth, M. O'Connor, W. W. Dai, G. Franchin, T. Hao, H. Guo, H. C. Hung, B. Toole, P. Gallay, B. Sherry, and M. Bukrinsky. 2002. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 277:22959-22965. [DOI] [PubMed] [Google Scholar]