Abstract
T cells are likely to play an important role in the host defense against Salmonella enterica serovar Typhi, the causative agent of typhoid fever. We have shown that HLA-E can function as a restriction element for S. Typhi-specific CD8+ T cells. Because of the potential importance of HLA-E-restricted CD8+ responses in resistance to Salmonella infection, we characterized these responses and investigated their kinetics of appearance and persistence in volunteers immunized orally with the licensed attenuated Ty21a strain typhoid vaccine. Cells were obtained from volunteers before and at days 2, 4, 7, 10, 14, 28, 42, 56, 120, 180, 360, and 720 after immunization. An ex vivo multicolor staining panel including antibodies to CD107a and -b, interleukin-2, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) was used to functionally assess memory T-cell subsets by flow cytometry. Increases in cytokine-secreting CD8+ cells were observed in the T effector/memory (TEM) and CD45RA+ TEM (TEMRA) subsets as early as 4 days after immunization and persisted, particularly in the TEMRA subset, up to 2 years after immunization. The majority of HLA-E-restricted CD8+ cells 28 to 56 days after immunization coexpressed CD107, IFN-γ, and TNF-α, showing characteristic features of multifunctional T cells. In summary, the multifunctionality and longevity of the HLA-E-restricted CD8 responses observed in this study highlight their significance in adaptive immunity to S. Typhi. Finally, this is the first demonstration, in either animals or humans, of the presence of long-term multifunctional HLA-E-restricted CD8+ cells after immunization.
Salmonella enterica serovar Typhi, the causative agent of typhoid fever, is a facultative intracellular bacterial pathogen (5, 7, 37). In industrialized countries, Salmonella infection is rare, with most infections occurring in military personnel and in individuals traveling to areas where typhoid fever is endemic. However, it remains an important public health priority in undeveloped parts of the world. Overall, it is estimated that 16 million new cases and 600,000 deaths occur annually (5, 12, 18). Our laboratory has demonstrated that oral immunization of volunteers with attenuated typhoid vaccine strain Ty21a triggers the generation of CD8+ T cells that kill S. Typhi-infected cells displaying human leukocyte antigen E (HLA-E) on the cell surface (27). T cells are likely to play an important role in the host defense against S. Typhi. They might contribute to Salmonella control by secreting cytokines such as gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-2 (IL-2) and by directly killing bacterium-infected cells (27, 28, 30-32, 38-40).
HLA-E is a protein that is part of a family of molecules known as class Ib or nonclassical HLA molecules (26). HLA-E is expressed ubiquitously on the peripheral blood mononuclear cell (PBMC; e.g., B cells, T cells, natural killer [NK] cells, and macrophages) surface at various levels, depending on the cell type (15, 34). Because HLA-E molecules are essentially monomorphic, they are likely to present a more conserved set of bacterial peptides that could be universally recognized by CD8+ T cells from most, if not all, individuals.
Given the potential significance of HLA-E-restricted CD8+ responses in resistance to Salmonella infection, it is of great importance to study these responses in depth, including their kinetics of appearance and persistence, which currently remains largely unknown. These responses might provide key insights into the open question concerning the dual role of the HLA-E molecules in short-lived innate and long-lived adaptive immunity (35). In fact, HLA-E molecules were primarily described as ligands for CD94/NKG2A, -B, and -C receptors present on the innate NK cell surface (3). However, HLA-E-restricted T cells have recently been implicated in adaptive immunity not only to Salmonella but also to cytomegalovirus and Mycobacterium (10, 22).
Oral immunization with the licensed Ty21a typhoid vaccine is a particularly relevant model of Salmonella infection, which is transmitted by the ingestion of virulent S. Typhi. In the present studies, volunteers were immunized with the standard regimen of four spaced doses of Ty21a given every other day (8, 28). This vaccination regimen results in levels of protection ranging from 60 to 80% (9). Using cells from Ty21a vaccinees, we investigated the kinetics of appearance and longevity of HLA-E-restricted CD8+ T-cell responses for up to 2 years after immunization with the Ty21a typhoid vaccine and defined in detail the various memory T-cell subsets and effector functions mediating these responses.
MATERIALS AND METHODS
Volunteers.
Seven healthy volunteers 24 to 41 years old recruited from the Baltimore-Washington area and the University of Maryland at Baltimore campus participated in this study. Volunteers were screened for good health by medical history, physical examination, laboratory tests (including blood counts), and the absence of antibiotic treatment. The purpose of this study was explained to the volunteers, and they gave informed, signed consent. Five volunteers were immunized with S. Typhi strain Ty21a typhoid vaccine, and their blood was collected before and at days 2, 4, 7, 10, 14, 28, 42, 56, 120, 180, 360, and 720 after immunization. Two unvaccinated volunteers were used as negative controls, and their blood was collected at the same times as that of the vaccinees. PBMC were isolated by density gradient centrifugation and cryopreserved in liquid N2. These PBMC were used ex vivo as effectors cells.
The human experimentation guidelines of the U.S. Department of Health and Human Services and those of the University of Maryland, Baltimore, were followed in the conduct of the present clinical research. All blood specimens were collected from volunteers who participated in University of Maryland Institutional Review Board-approved protocols HP-00040025 and HP-00040022, which authorized the collection of blood specimens for the studies described in this report.
Target cells.
The 721.221.AEH cell line, generously provided by D. Geraghty (10, 15), were used as target cells. The 721.221.AEH cell line is an HLA class I-defective Epstein-Barr virus-transformed lymphoblastoid B-cell line which has been transfected with HLA-E fused to the HLA-A2 leader peptide and therefore expresses HLA-E*0101, but not HLA-A, -B, -C, or -G, molecules on the cell surface. The 721.221.AEH cells were cultured in RPMI 1640 (Gibco, Grand Island, NY) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 2 mM l-glutamine, 2.5 mM sodium pyruvate, 10 mM HEPES buffer, and 10% heat-inactivated fetal bovine serum (R10) supplemented with 200 mU/ml hygromycin B (Sigma). Cells were infected as previously described (27). Briefly, cells were incubated for 3 h at 37°C in RPMI (without antibiotics) in the presence of S. Typhi strain ISP1820 (obtained from J. Nataro, Center for Vaccine Development) at a multiplicity of infection of 10:1 (27, 28, 31, 32). After incubation, cells were washed and incubated overnight at 37°C in medium containing gentamicin (100 μg/ml) to kill and remove extracellular bacteria. The targets were then irradiated and surface stained with antibody to CD45, a marker abundantly expressed on the hematopoietic cell surface (4). After staining, the target cells were cocultured with PBMC (effector cells rested overnight) collected before and at days 2, 4, 7, 10, 14, 28, 42, 56, 120, 180, 360, and 720 after immunization at an effector-to-target cell ratio of 5:1.
MAbs for surface staining.
Cells were surface stained with monoclonal antibodies (MAbs) to CD3 (clone UCHT1) (Beckman-Coulter, Miami, FL), CD4 (clone SK3), CD14 (clone M5E2) (BD Pharmingen, San Diego, CA), CD57 (clone TB01), CD107a (clone eBioH4A3), CD107b (clone eBioH4B4) (eBioscience, San Diego, CA), CD8 (clone 3B5), CD19 (clone SJ25-C1), CD45 (clone H130), CD27 (clone CLB-27/1), CD45RA (clone MEM-56), and CD62L (clone Dreg-56) (Invitrogen, Carlsbad, CA). Antibodies conjugated to the following fluorochromes were used in these studies: fluorescein isothiocyanate, phycoerythrin (PE), peridinin chlorophyll protein-Cy5.5, PE-Cy7, energy-coupled dye or PE-Texas Red conjugate, Pacific Blue, Pacific Orange, Quantum Dot (QD) 605, QD 655, QD 705, Alexa 647, allophycocyanin (APC)-Alexa 700, and APC-Cy7.
Surface and intracellular staining.
PBMC from vaccinees or unvaccinated controls were stimulated with S. Typhi-infected 721.221.AEH target cells as described in the target cell section above, in the presence of MAbs to CD107a and CD107b (15 μl of each/1 × 106 cells in 500 μl of R10 medium). The CD107a and -b antibodies were used to measure degranulation, a mechanism essential for the killing of Salmonella-infected targets by cytotoxic CD8+ cells (1). Uninfected 721.221.AEH target cells and Staphylococcus enterotoxin B (SEB; 10 μg/ml, Sigma, St. Louis, MO) were used as negative and positive controls, respectively. After 1 to 2 h of stimulation with S. Typhi antigens, the protein transport blockers monensin (1 μg/ml, Sigma) and brefeldin A (2 μg/ml; Sigma) were added to the PBMC. After overnight incubation (16 to 18 h), PBMC were harvested; stained with a dead-cell discriminator, a violet fluorescent viability dye (ViViD; Invitrogen) (14); surface stained with MAbs against surface antigens (CD3, CD4, CD8, CD14, CD19, CD27, CD45RA, CD57, and CD62L); and fixed and permeabilized with Fix & Perm cell buffers (Invitrogen, Carlsbad, CA) (Table 1). Cells were then stained intracellularly for IFN-γ (clone B27), TNF-α (clone MAb11), IL-2 (clone 5344.111) (B-D Pharmingen), and CD69 (clone TPI-55-3; Beckman-Coulter) to complete the 13-color staining panel. Cells were then resuspended in fixation buffer (1% formaldehyde) and analyzed as soon as possible by flow cytometry on an LSR-II instrument (BD Biosciences). Data were analyzed with WinList v6.0 (Verity Software House, Topsham, ME). Lymphocytes were gated based on their scatter characteristics. Single lymphocytes were gated based on forward scatter height versus forward scatter area. A “dump” channel was used to eliminate dead cells (ViViD+), as well as macrophages/monocytes (CD14+), B lymphocytes (CD19+), and targets (CD45+), from the analysis. This was followed by additional gating on CD3, CD4, and CD8 to identify cytokine-producing CD8+ T cells. Each cytokine was gated individually. During sample acquisition, 300,000 to 500,000 events were routinely collected in the forward and side scatter lymphocyte gate. This large number of gated lymphocyte events was essential to ensure that sufficient numbers of positive cells for defined subsets would be collected for each tube analyzed.
TABLE 1.
Cell phenotypes and corresponding markers
| Phenotype | Marker | Reference(s) |
|---|---|---|
| T-cell subsets | ||
| Naïve | CD45RA+ CD62L+ | 25, 29, 33 |
| TCM | CD45RA− CD62L+ | 25, 29, 33 |
| TEM | CD45RA− CD62L− | 25, 29, 33 |
| TEMRA | CD45RA+ CD62L− | 25, 29, 33 |
| Degranulation | ||
| LAMP-1 | CD107a | 1 |
| LAMP-2 | CD107b | 1 |
| Dump channel | ||
| Dead cells | ViViD | 14 |
| Monocytes | CD14 | 14 |
| B cells | CD19 | 14 |
| Hematopoietic cells | CD45 | 4 |
Statistical analysis.
All statistical tests were performed using Prism software (version 5.02; GraphPad Software, La Jolla, CA). Comparisons between groups were performed using Kruskal-Wallis one-way analysis of variance on ranks or Pearson product-moment correlation tests. P values of <0.05 were considered significant. Cubic spline curves were used for a better representation of the complex responses observed. Cubic spline curves are smooth curves through every point with the capability to be used as a standard curve or to calculate the area under the curve (AUC) (21).
RESULTS
Kinetics of S. Typhi-specific HLA-E-restricted CD8+ cells over a 2-year postvaccination follow-up.
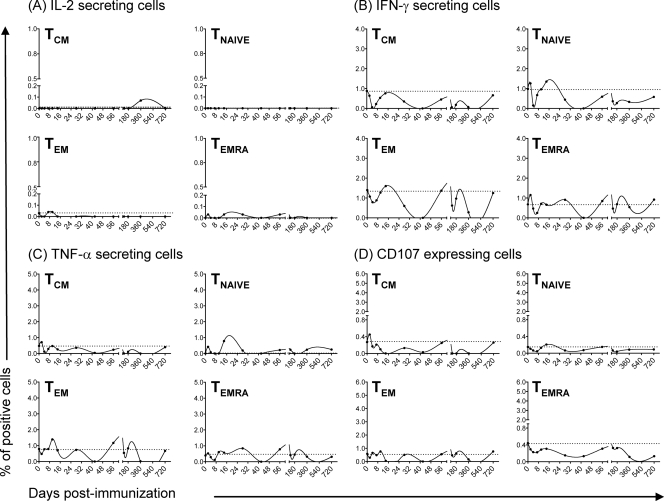
Because of the potential importance of HLA-E-restricted CD8+ cells in resistance to Salmonella infection (27), we investigated their kinetics of appearance and persistence in volunteers immunized with the S. Typhi Ty21a strain typhoid vaccine. Based on previous data showing that CD8+ responses can be detected as early as 14 days after immunization (32), we hypothesized that these responses might appear at even earlier time points. We also evaluated the longevity of these responses up to 2 years after immunization. To this end, PBMC collected before and at days 2, 4, 7, 10, 14, 28, 42, 56, 120, 180, 360, and 720 after immunization were stimulated with S. Typhi-infected 721.221.AEH target cells in the presence of MAbs to CD107a and -b. The 721.221.AEH targets are HLA class Ia-defective lymphoblastoid B cells which constitutively express HLA-E on their surface. The CD107a and -b antibodies were used to measure degranulation (1). Previously, we have shown that HLA-E-restricted S. Typhi-specific CD8+ T cells effectively kill S. Typhi-infected targets by a granule-dependent mechanism (27). In the present studies, uninfected 721.221.AEH target cells and SEB were used as negative and positive controls, respectively. After overnight incubation, PBMC were surface stained with MAbs to CD3, CD4, CD8, CD14, CD19, CD27, CD45RA, CD57, and CD62L. We selected overnight incubation to minimize bystander stimulation while maintaining a close parallel with the in vivo cytokine response (6). Cells were then stained intracellularly with MAbs to CD69 and IL-2, IFN-γ, and TNF-α cytokines analyzed by multichromatic flow cytometry. We have previously demonstrated that these cytokines have the ability to serve as surrogate markers of S. Typhi-specific CD8+ cell responses (28, 30-32, 40). For data analysis, CD8+ cells (CD3+ CD4−) were divided into three main subsets: naïve T cells (CD45RA+ CD62L+), central memory T cells (TCM [CD45RA− CD62L+]), and effector memory T cells (TEM [CD45RA− CD62L−] or TEMRA [CD45RA+ CD62L−]) (25, 29, 33). In agreement with previous results, cytokine-secreting (e.g., IFN-γ and TNF-α), as well as CD107-expressing, CD8+ cells were predominantly classical TEM cells (41). Unexpectedly, these responses were multiphasic, with a first peak response observed as early as 4 days (in two out of five volunteers) after immunization (Fig. 1 and 2), followed by a second, more pronounced and persistent, peak between days 42 and 56 (in four out of five volunteers) (Fig. 1). This second peak decreased sharply over the next few months. In three out of five volunteers, a much smaller third peak could be observed at around 180 days postimmunization. Interestingly, CD8+ TEMRA and TCM subsets secreting IFN-γ and TNF-α and expressing CD107 were also present, albeit at a much lower magnitude than the TEM subset (Fig. 1 and 2). Like those of the TEM subset, the TEMRA subset responses were multiphasic, with a first peak between days 4 and 14 (day 7 in three out of five volunteers) after immunization, followed by a second peak between days 14 and 28. A third peak, if present, was observed between days 42 and 720 after immunization (day 42 in two out of three volunteers). Surprisingly, responses in the TEMRA subset were more persistent than those observed in the TEM and TCM subsets, found to be present 720 days after immunization in two out of four volunteers. These results are in agreement with published data suggesting that TEMRA subset cells are not necessarily terminally differentiated and may maintain their ability to proliferate and secrete cytokines after antigenic exposure (20). We also observed, for the first time, the presence of IL-2-secreting CD8+ cells. As expected, these cells were predominantly of the TCM subset. However, unexpectedly, we observed IL-2 production by TEMRA cells, followed, albeit at a lower levels, by TEM cells. These TCM subset cells appeared as early as 2 to 4 days (in four out of five volunteers) after immunization, with the fifth volunteer showing peak IL-2 production at day 7. A second peak was present in all five volunteers at days 28 to 56 and a third peak, if present (in two of five volunteers), could be observed between days 180 and 720 after immunization (Fig. 1 and 2). It is important to note that the level and timing of cytokine production varied among of the various volunteers. This is not surprising, since differences in the degree of response, as well as differences in the background level before immunization, among individuals are a common finding in human studies (32, 40, 41). Finally, as expected, only minor changes without defined patterns in cytokine secretion by S. Typhi-infected cells were observed in TNAÏVE cells or in the four subsets (TEM, TEMRA, TCM, and TNAIVE) from two healthy volunteers used as controls in these studies (Fig. 1, 2, and 3). Regardless of the subpopulation(s) showing the most pronounced responses, all five volunteers showed an increase at least one of the times examined compared to those observed before immunization. Based on these results, we conclude that HLA-E-restricted CD8+ cells might play a dual role, i.e., (i) at early time points bridging innate and acquired immunity and (ii) at later times protecting from Salmonella infection by an adaptive mechanism.
FIG. 1.
Kinetics of S. Typhi-specific HLA-E-restricted CD8+ cells over a 2-year postvaccination follow-up. PBMC were stimulated with S. Typhi-infected 721.221.AEH target cells in the presence of CD107a and -b MAbs. After overnight incubation, PBMC were stained with ViViD, followed by surface staining with MAbs to CD3, CD4, CD8, CD14, CD19, CD45RA, and CD62L. After fixation and permeabilization, cells were intracellularly stained with MAbs to CD69, as well as to the cytokines IL-2, IFN-γ, and TNF-α, and analyzed by multichromatic flow cytometry. For the analysis, first a dump channel was used to eliminate dead cells (ViViD+), as well as macrophages (CD14+), B cells (CD19+), and stimulators (CD45+), from the results. This was followed by additional gating on CD3, CD4, and CD8, as well as CDR45RA versus CD62L, to analyze the three main CD8+ cell subsets: naïve T cells (CD45RA+ CD62L+), central memory T cells (TCM [CD45RA− CD62L+]), and effector memory T cells (TEM [CD45RA− CD62L−] or TEMRA [CD45RA+ CD62L−]). Represented are cubic spline curves based on all data points to allow a better representation of the complex responses observed. The spline curves were generated by grouping the data of all five volunteers. The curves represent the mean of the results from the five different volunteers who participated in these studies. The filled area denotes the standard error bands. The dashed lines represent the baseline values (day 0).
FIG. 2.
Kinetics of S. Typhi-specific HLA-E-restricted CD8+ cells over a 2-year postvaccination follow-up in a Ty21a vaccinee. PBMC from one Ty21a vaccinee were stimulated with S. Typhi-infected 721.221.AEH target cells in the presence of CD107a and -b MAbs. After overnight incubation, PBMC were stained with ViViD, followed by surface staining with MAbs to CD3, CD4, CD8, CD14, CD19, CD45RA, and CD62L. After fixation and permeabilization, cells were stained intracellularly for CD69, as well as for the cytokines IL-2, IFN-γ, and TNF-α, and analyzed by multichromatic flow cytometry as described in the legend to Fig. 1. Represented are cubic spline curves based on all data points to allow a better representation of the complex responses observed. The curves represent the results from one vaccinee (CVD5000#04S). The dashed lines represent the baseline values (day 0).
FIG. 3.
Kinetics of S. Typhi-specific HLA-E-restricted CD8+ cells over a 2-year postvaccination follow-up in an unvaccinated volunteer. PBMC from one healthy unvaccinated volunteer were stimulated with S. Typhi-infected 721.221.AEH target cells in the presence of CD107a and -b MAbs. After overnight incubation, PBMC were stained with ViViD, followed by surface staining with MAbs to CD3, CD4, CD8, CD14, CD19, CD45RA, and CD62L. After fixation and permeabilization, cells were stained intracellularly for CD69, as well as for the cytokines IL-2, IFN-γ, and TNF-α, and analyzed by multichromatic flow cytometry as described in the legend to Fig. 1. Represented are cubic spline curves based on all data points to allow a better representation of the complex responses observed. The curves represent the results of one healthy unvaccinated volunteer (CVD4000#33C). The dashed lines represent the baseline values (day 0).
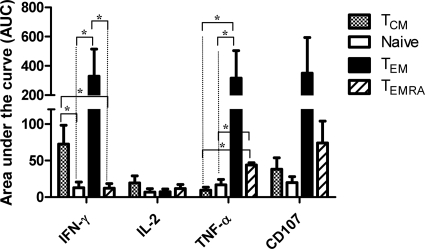
Cumulative HLA-E-restricted CD8+ cell responses over the 2-year follow-up.
One of the hallmarks of successful vaccination is the induction of strong and persistent memory cell responses (19, 25, 29). We studied this phenomenon by combining the level and duration of HLA-E-restricted CD8+ cell responses into a single parameter to measure differences among responses in defined cell subsets. In this case, we used AUC analysis as a cumulative measurement of the rise and persistence of HLA-E-restricted CD8+ cell responses above the baseline (day 0) over the 2-year period. The AUC was defined as the number of positive responses divided by the number of postvaccination visits. Comparisons of the various CD8+ cell subsets were performed using Kruskal-Wallis one-way analysis of variance on ranks. Nominal variables were analyzed by the Student-Newman-Keuls test. The cumulative analyses of IFN-γ production demonstrated that the CD8+ TEM and TCM cell subsets exhibited a significantly greater AUC than the naïve and TEMRA subsets. Interestingly, the AUC analyses of TNF-α production demonstrated that the CD8+ TEM and TEMRA subsets had a significantly greater AUC than the naïve and TCM subsets (Fig. 4). Although we observed greater AUCs in IL-2-producing TCM and CD107-expressing TEM and TEMRA cells than in the other populations, these differences did not reach statistically significance. This phenomenon might be due to the limited number of volunteers studied (Fig. 4). Taken together, these data confirm and extend the notion that CD8+ TEM cells are the most effective producers of the IFN-γ and TNF-α cytokines. These data also demonstrate the relative importance of IFN-γ-secreting CD8+ TCM, as well as TNF-α-secreting CD8+ TEMRA, cells.
FIG. 4.
Comparison of the AUCs of different CD8+ subsets and their production of IL-2, TNF-α, and IFN-γ and expression of CD107 molecules. Bar graphs represent means plus standard errors of the pooled data from the five different volunteers. AUCs are expressed in arbitrary units. *, positive response (statistically significant at P < 0.05).
Induction of long-lasting multifunctional HLA-E-restricted CD8+ memory T-cell subsets by Ty21a immunization.
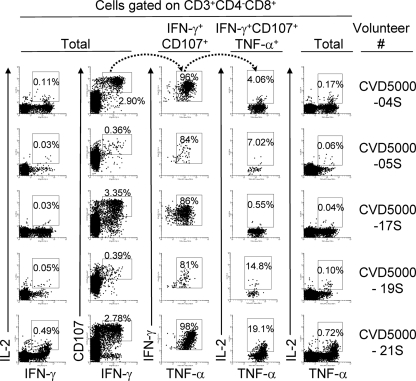
Because previous human studies have demonstrated a relationship between the presence of multifunctional T cells and the quality of the immune responses (2, 23), here we simultaneously measured four HLA-E-restricted CD8+ T-cell functions (CD107 mobilization and IFN-γ, TNF-α, and IL-2 secretion) by multichromatic flow cytometry. Analyses of multiple cytokine patterns revealed that the majority of HLA-E-restricted CD8+ TEM and TEMRA cells, particularly between days 28 and 56 after immunization, showed characteristics typical of multifunctional cells, with the specific cells exhibiting predominantly a response pattern characterized by three functions (Fig. 5). The major populations of multifunctional cells were those that concurrently secrete IFN-γ and TNF-α and express CD107. IL-2 production was also detected in combination with these three functions, but at a much lower frequency (Fig. 5). Virtually no correlations were observed between IL-2 secretion and the other three functions (Fig. 6). The only exception of a statistically significant correlation was observed between IL-2- and IFN-γ-secreting CD8+ TCM cells at days 28 to 56 after immunization (Fig. 6). In contrast, highly significant correlations were observed between the kinetics of IFN-γ production and the expression of CD107 in all three major CD8+ T-cell subsets, i.e., TCM, TEM, and TEMRA (Fig. 6). IFN-γ production was also correlated with TNF-α production in the TEM (at all time points) and TEMRA (at days 0 to 4 and 28 to 56) subsets. Of note, the vast majority of these multifunctional T cells did not express either CD27 or CD57 on their cell membrane (∼90%) at any time point. Less than 10% of multifunctional T cells expressed either CD27 or CD57, but none expressed both CD27 and CD57. In conclusion, these data support the idea that multifunctional HLA-E-restricted CD8+ cells are long-lived and might contribute to effective Salmonella immunity.
FIG. 5.
Induction of multifunctional CD8+ TEM cells by Ty21a immunization. PBMC were stimulated and analyzed as described in the legend to Fig. 1. As shown by the arrows, sequential gating was used to identify multifunctional CD8+ TEM cells from five volunteers 42 days after vaccination. Four T-cell functions were evaluated: expression of CD107 and secretion of IL-2, IFN-γ, and TNF-α.
FIG. 6.
Correlations among different CD8+ subsets that produce IFN-γ and those that produce IL-2 or TNF-α or express CD107 molecules. PBMC were stimulated and analyzed as described in the legend to Fig. 1. Results were divided into four groups: 1, responses at days 2 to 4; 2, responses at days 7 to 14; 3, responses at days 28 to 56; 4, responses at days 120 to 720. Coefficients of determination (r values) and P values are shown. Significant P values are in bold. P values of <0.05 were considered statistically significant.
Correlation between early and late cytokine production patterns by the different CD8+ memory T-cell subsets.
Another important aim in vaccine development is to determine whether early responses can predict the long-term persistence of immunity to the organism used for immunization. This information could be important in the selection of the best early T-cell markers of systemic immune responses to evaluate future S. Typhi vaccine candidates. To this end, we compared the responses of the three main CD8+ memory T-cell subsets (i.e., TEM, TCM, and TEMRA) to predict long-term immunity. Each of these three subsets was arranged in two groups based on the timing postvaccination. One group included early positive responses (days 2, 4, 7, 10, 14, and 28), and the other group included long-term positive responses after immunization (days 42, 56, 120, 180, 360, and 720). Comparisons among the different CD8+ cell subsets were performed using Pearson product-moment correlation. Interestingly, we found a striking correlation between volunteers exhibiting early IL-2- and IFN-γ-secreting CD8+ TCM subsets and those CD8+ TCM, TEM, and TEMRA subsets that later produced IFN-γ (Table 2). We also found significant correlations between the early IL-2- and IFN-γ-secreting CD8+ TCM subsets and the CD8+ TEM and TEMRA subsets that later produced TNF-α, as well as the CD8+ TCM and TEM subsets that later expressed CD107 (Table 2). Unexpectedly, we also found a good correlation between the early IFN-γ-secreting CD8+ TEMRA responses and the CD8+ TCM and TEM subsets that later secreted IFN-γ, as well as the TCM and TEM subsets that expressed CD107 (Table 2). No other early predictors of positive late responses were observed. In conclusion, these data suggest that the presence at early postvaccination of responses by CD8+ TCM and TEMRA subsets that produce sizable amounts of IL-2 and/or IFN-γ might be good indicators of long-lasting immune responses.
TABLE 2.
Correlations among early and long-term responses in CD8+ T-cell subsets
| Subset and cytokine (early positive responses [<28 days]) | Long-term positive responses (>42 days) (P values)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCM |
TEM |
TEMRA |
||||||||||
| IL-2 | IFN-γ | TNF-α | CD107 | IL-2 | IFN-γ | TNF-α | CD107 | IL-2 | IFN-γ | TNF-α | CD107 | |
| TCM | ||||||||||||
| IL-2 | 0.4 | <0.0001 | 0.472 | 0.0003 | 0.317 | 0.0009 | 0.041 | 0.0115 | 0.704 | 0.0006 | 0.031 | 0.784 |
| IFN-γ | 0.568 | 0.002 | 0.419 | 0.012 | 0.732 | 0.036 | 0.272 | 0.0496 | 0.918 | 0.0001 | 0.049 | 0.824 |
| TEMRA, IL-2 | 0.638 | 0.001 | 0.488 | 0.002 | 0.704 | 0.047 | 0.245 | 0.042 | 0.969 | 0.878 | 0.743 | 0.316 |
Significant P values are in bold. P values of <0.05 were considered statistically significant.
DISCUSSION
In spite of the putative importance of HLA-E-restricted responses in protection from infectious agents, very little information is available about either mice or humans (11, 35, 36). In this report, we present the first detailed ex vivo kinetic analysis and characterization of the induction and persistence of HLA-E-restricted CD8+ memory T (TM) cells up to 2 years after immunization of volunteers with the licensed Ty21a typhoid vaccine. Interestingly, we observed two or three waves of elevated levels of HLA-E-restricted CD8+ TM cells over time in the absence of additional exposure to the organism beyond day 7 (the last day of vaccination). This phenomenon is remarkably different than the typical one-wave (expansion-contraction) responses observed in classical class Ia HLA-A-, B-, and C-restricted TM responses (24). The first wave occurred as early as 2 to 4 days after immunization with S. Typhi, with an increase in the levels of all three major HLA-E-restricted CD8+ TM subsets (TEM, TEMRA, and TCM). This phenomenon further supports the contention advanced by us and others (27, 35) that these HLA-E-restricted responses mediated by CD8+ cells might bridge the innate and adaptive immune responses. The first wave was followed by a decline in the levels of HLA-E-restricted CD8+ TM cells in circulation. We speculate that this might be the result of T-cell recirculation, with some T-cell effectors homing to the gut. This assumption is based on our previous work showing the presence of S. Typhi-specific CD8+ TM cells coexpressing gut-homing molecules (high levels of integrin α4/β7, intermediate levels of CCR9, and low levels of CD103) in the circulation (31, 41). The second wave, the one with the most pronounced levels of HLA-E-restricted CD8+ TM cells, occurred between days 28 and 56. Interestingly, we observed that these HLA-E-restricted CD8+ memory cells consisted of heterogeneous populations that exhibited a distinct kinetic behavior depending on the memory T-cell subset. For example, while high levels of TEM responses were observed 56 days after immunization, they declined sharply afterward. In contrast, low levels of TEMRA responses persisted for up to 2 years after immunization. These results are in agreement with our previous studies showing the presence of specific CD8+ T-cell responses even 3 years after immunization, although these responses could only be evidenced following in vitro expansion in the presence of specific antigens (28, 31). It is widely accepted that strong immune responses are determined not only by their magnitude but also by their quality and persistence. Consequently, it is reasonable to speculate that TEM responses of high magnitude but short duration and TEMRA responses of lower magnitude but long duration might have synergistic and/or complementary roles in the control of Salmonella infection. In this scenario, some antigen-specific T-cell clones may remain for long periods of time as memory T cells that, upon subsequent exposure to antigen, provide a stronger, rapid, and sometimes qualitatively different specific immune response (13, 24). The third wave was present in three of the five volunteers between days 42 and 720, depending on the TM subset evaluated. These long-term cell-mediated immune responses might help explain the observations in field trials showing that three doses of the enteric-coated formulations of Ty21a given within 1 week provided 67% efficacy during the first 3 years of follow-up (17) and 62% protection over 7 years of follow-up (16) and that four doses of Ty21a in enteric-coated capsules given within 8 days are significantly more protective than two or three doses (8).
We also observed that Ty21a immunization elicited multifunctional HLA-E-restricted CD8+ cells that can degranulate (CD107+), a mechanism essential for cytotoxic responses against S. Typhi-infected targets, and produce IFN-γ, TNF-α, and IL-2, which are critical components of a robust inflammatory response. However, the observations regarding IL-2 appear to be in contrast to our previous results obtained with volunteers immunized with the attenuated S. Typhi strain CVD 909, which resulted in significant increases in IFN-γ, TNF-α, and IL-10, but not IL-2, production (40). It is likely that this discrepancy is the result of the use of different methodologies to analyze CD8+ T-cell responses. Here, CD8+ T cells from Ty21a vaccinees were stimulated with S. Typhi-infected targets whereas CD8+ T cells from CVD 909 vaccinees were stimulated with S. Typhi flagella or particulate S. Typhi antigen. Interestingly, we also observed that S. Typhi-specific multifunctional HLA-E-restricted TM cells do not express either CD27 or CD57. These results are in agreement with those obtained with CD8+ T cells of volunteers immunized with vaccinia virus (23) but in contrast to the observations of CD8+ cells in volunteers immunized with cytomegalovirus (42). These observations strongly suggest that different pathogens elicit different TM and effector populations. Multifunctional T cells are widely believed to be of great importance in determining the quality of immune responses to vaccination or exposure to wild-type organisms (2, 23). The fact that the multifunctional HLA-E-restricted TM cells described herein appear to dominate early responses and persist for long periods of time adds further support to the contention that this might be an important mechanism of the host immune response to S. Typhi.
Finally, we observed a striking correlation among volunteers who showed strong CD8+ TCM subsets which secreted IL-2 and IFN-γ at early times and the presence in these volunteers of long-term immune responses. We speculate that this phenomenon might be due to the fact that IL-2- and/or IFN-γ-secreting CD8+ TCM subsets at early times after vaccination results in the development of a larger pool of long-lived specific CD8+ TM cell subsets (CD8+ TCM, TEM, and TEMRA), which might lead to an improved ability of the host to control reinfection. In this scenario, following the contraction phase, only low levels of CD8+ TEMRA cells remain detectable in circulation. These CD8+ TEMRA cells might play an important role in the expansion of the other CD8+ TM subsets upon reexposure to the pathogen.
In sum, this is, to our knowledge, the first demonstration in humans of the presence of persistent multifunctional HLA-E-restricted CD8+ cells up to 2 year after immunization with attenuated S. Typhi. These findings argue that HLA-E-specific CD8+ cells play an integral role in the innate/adaptive immune response against Salmonella infection. These observations are particularly important because they could guide the selection of appropriate systemic immune responses to evaluate in the development of future vaccine candidates, not only for S. Typhi, but also for other infectious enteric pathogens.
Acknowledgments
We are indebted to the volunteers who allowed us to perform this study. We also thank Robin Barnes and the staff from the recruiting section of the Center for Vaccine Development for their help in collecting blood specimens and Guillermo Sahaniuk, Regina Harley, and Catherine Storrer for excellent technical assistance.
This work was supported, in part, by NIAID, NIH, DHHS federal research grants R01 AI036525 and U19 AI082655 (CCHI) to M.B.S.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Disease or the National Institutes of Health.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 2.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braud, V. M., D. S. Allan, C. A. O'Callaghan, K. Soderstrom, A. D'Andrea, G. S. Ogg, S. Lazetic, N. T. Young, J. I. Bell, J. H. Phillips, L. L. Lanier, and A. J. McMichael. 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391:795-799. [DOI] [PubMed] [Google Scholar]
- 4.Coren, L. V., T. Shatzer, and D. E. Ott. 2008. CD45 immunoaffinity depletion of vesicles from Jurkat T cells demonstrates that exosomes contain CD45: no evidence for a distinct exosome/HIV-1 budding pathway. Retrovirology 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump, J. A., S. P. Luby, and E. D. Mintz. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346-353. [PMC free article] [PubMed] [Google Scholar]
- 6.Di Genova, G., J. Roddick, F. McNicholl, and F. K. Stevenson. 2006. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood 107:2806-2813. [DOI] [PubMed] [Google Scholar]
- 7.Eisenstein, T. K. 1999. Mucosal Immune defense: the Salmonella typhimurium model, p. 51-108. In Y. Paterson (ed.), Intracellular bacterial vaccine vectors. Wiley-Liss, New York, NY.
- 8.Ferreccio, C., M. M. Levine, H. Rodriguez, and R. Contreras. 1989. Comparative efficacy of two, three, or four doses of Ty21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J. Infect. Dis. 159:766-769. [DOI] [PubMed] [Google Scholar]
- 9.Guzman, C. A., S. Borsutzky, M. Griot-Wenk, I. C. Metcalfe, J. Pearman, A. Collioud, D. Favre, and G. Dietrich. 2006. Vaccines against typhoid fever. Vaccine 24:3804-3811. [DOI] [PubMed] [Google Scholar]
- 10.Heinzel, A. S., J. E. Grotzke, R. A. Lines, D. A. Lewinsohn, A. L. McNabb, D. N. Streblow, V. M. Braud, H. J. Grieser, J. T. Belisle, and D. M. Lewinsohn. 2002. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J. Exp. Med. 196:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoare, H. L., L. C. Sullivan, G. Pietra, C. S. Clements, E. J. Lee, L. K. Ely, T. Beddoe, M. Falco, L. Kjer-Nielsen, H. H. Reid, J. McCluskey, L. Moretta, J. Rossjohn, and A. G. Brooks. 2006. Structural basis for a major histocompatibility complex class Ib-restricted T cell response. Nat. Immunol. 7:256-264. [DOI] [PubMed] [Google Scholar]
- 12.Ivanoff, B., M. M. Levine, and P. H. Lambert. 1994. Vaccination against typhoid fever: present status. Bull. World Health Organ. 72:957-971. [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom, B., and W. W. Agace. 2007. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 215:226-242. [DOI] [PubMed] [Google Scholar]
- 14.Lamoreaux, L., M. Roederer, and R. Koup. 2006. Intracellular cytokine optimization and standard operating procedure. Nat. Protoc. 1:1507-1516. [DOI] [PubMed] [Google Scholar]
- 15.Lee, N., D. R. Goodlett, A. Ishitani, H. Marquardt, and D. E. Geraghty. 1998. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J. Immunol. 160:4951-4960. [PubMed] [Google Scholar]
- 16.Levine, M. M., C. Ferreccio, P. Abrego, O. S. Martin, E. Ortiz, and S. Cryz. 1999. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 17:S22-S27. [DOI] [PubMed] [Google Scholar]
- 17.Levine, M. M., C. Ferreccio, R. E. Black, and R. Germanier. 1987. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet 1:1049-1052. [DOI] [PubMed] [Google Scholar]
- 18.Levine, M. M., and O. S. Levine. 1997. Influence of disease burden, public perception, and other factors on new vaccine development, implementation, and continued use. Lancet 350:1386-1392. [DOI] [PubMed] [Google Scholar]
- 19.Levine, M. M., and M. B. Sztein. 2004. Vaccine development strategies for improving immunization: the role of modern immunology. Nat. Immunol. 5:460-464. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. D., R. G. van der Most, R. S. Akondy, J. T. Glidewell, S. Albott, D. Masopust, K. Murali-Krishna, P. L. Mahar, S. Edupuganti, S. Lalor, S. Germon, C. Del Rio, M. J. Mulligan, S. I. Staprans, J. D. Altman, M. B. Feinberg, and R. Ahmed. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28:710-722. [DOI] [PubMed] [Google Scholar]
- 21.Motulsky, H., and A. Christopoulos. 2004. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford University Press, New York, NY.
- 22.Pietra, G., C. Romagnani, P. Mazzarino, M. Falco, E. Millo, A. Moretta, L. Moretta, and M. C. Mingari. 2003. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 100:10896-10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Precopio, M. L., M. R. Betts, J. Parrino, D. A. Price, E. Gostick, D. R. Ambrozak, T. E. Asher, D. C. Douek, A. Harari, G. Pantaleo, R. Bailer, B. S. Graham, M. Roederer, and R. A. Koup. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 204:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849-863. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, H. L., and R. R. Amara. 2005. T cell vaccines for microbial infections. Nat. Med. 11:S25-S32. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers, J. R., and R. G. Cook. 2005. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 5:459-471. [DOI] [PubMed] [Google Scholar]
- 27.Salerno-Gonçalves, R., M. Fernandez-Vina, D. M. Lewinsohn, and M. B. Sztein. 2004. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 173:5852-5862. [DOI] [PubMed] [Google Scholar]
- 28.Salerno-Goncalves, R., M. F. Pasetti, and M. B. Sztein. 2002. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 169:2196-2203. [DOI] [PubMed] [Google Scholar]
- 29.Salerno-Gonçalves, R., and M. B. Sztein. 2006. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 14:536-542. [DOI] [PubMed] [Google Scholar]
- 30.Salerno-Goncalves, R., and M. B. Sztein. 2009. Priming of Salmonella enterica serovar Typhi-specific CD8(+) T cells by suicide dendritic cell cross-presentation in humans. PLoS One 4:e5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salerno-Gonçalves, R., R. Wahid, and M. B. Sztein. 2005. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect. Immun. 73:3521-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salerno-Gonçalves, R., T. L. Wyant, M. F. Pasetti, M. Fernandez-Vina, C. O. Tacket, M. M. Levine, and M. B. Sztein. 2003. Concomitant induction of CD4(+) and CD8(+) T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain CVD 908-htrA. J. Immunol. 170:2734-2741. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 34.Shawar, S. M., J. M. Vyas, J. R. Rodgers, and R. R. Rich. 1994. Antigen presentation by major histocompatibility complex class I-B molecules. Annu. Rev. Immunol. 12:839-880. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, L. C., C. S. Clements, J. Rossjohn, and A. G. Brooks. 2008. The major histocompatibility complex class Ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens 72:415-424. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan, L. C., H. L. Hoare, J. McCluskey, J. Rossjohn, and A. G. Brooks. 2006. A structural perspective on MHC class Ib molecules in adaptive immunity. Trends Immunol. 27:413-420. [DOI] [PubMed] [Google Scholar]
- 37.Sztein, M. B. 2007. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica serovar Typhi strains used as live oral vaccines in humans. Clin. Infect. Dis. 45(Suppl. 1):S15-S19. [DOI] [PubMed] [Google Scholar]
- 38.Sztein, M. B., M. K. Tanner, Y. Polotsky, J. M. Orenstein, and M. M. Levine. 1995. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J. Immunol. 155:3987-3993. [PubMed] [Google Scholar]
- 39.Sztein, M. B., S. S. Wasserman, C. O. Tacket, R. Edelman, D. Hone, A. A. Lindberg, and M. M. Levine. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J. Infect. Dis. 170:1508-1517. [DOI] [PubMed] [Google Scholar]
- 40.Wahid, R., R. Salerno-Goncalves, C. O. Tacket, M. M. Levine, and M. B. Sztein. 2007. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine 25:1416-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahid, R., R. Salerno-Goncalves, C. O. Tacket, M. M. Levine, and M. B. Sztein. 2008. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue-homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol. 1:389-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wills, M. R., A. J. Carmichael, M. P. Weekes, K. Mynard, G. Okecha, R. Hicks, and J. G. Sissons. 1999. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J. Immunol. 162:7080-7087. [PubMed] [Google Scholar]