Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) infection induces both humoral and cellular immune responses. In this study, we investigated the changes in cytokine levels in peripheral blood between the highly pathogenic PRRSV HuN4 strain and its derivative strain HuN4-F112 obtained by serial propagation in MARC145 cells to 112 passages. The results demonstrated that pigs infected with HuN4 showed a loss of appetite, decrease in body weight, raised body temperature, and respiratory symptoms, along with interstitial pneumonia lesions. The PRRSV amounts in the pigs infected with HuN4 were 105 to 109 copies/ml in the blood and 1010 to 1011 copies/g in the lung tissues, whereas the virus amounts with HuN4-F112 were 102.15 to 103.13 copies/ml in the blood and 103.0 to 103.6 copies/g in the lungs. Moreover, the levels of interleukin 1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), and alpha interferon (IFN-α) in peripheral blood were upregulated 7 days postinoculation with HuN4, which was earlier than in the HuN4-F112 group. Furthermore, cytokine levels in the pigs infected with HuN4 returned to normal on the 21st day postinoculation, while the levels in those infected with HuN4-F112 continued to increase. These results demonstrated that the pigs infected with the highly pathogenic PRRSV HuN4 strain generated earlier and higher levels of inflammatory cytokines, and the results also indicated that HuN4 may aggravate inflammation and damage tissues and organs. The low-pathogenic PRRSV HuN4-F112 strain induced lower levels of inflammatory cytokines, which may enhance the immune responses against the infection.
Porcine reproductive and respiratory syndrome virus (PRRSV) is a single-stranded RNA virus that belongs to the Arteriviridae and can cause porcine reproductive and respiratory syndrome (PRRS) (15). PRRSV is one of the main causes of reproductive disorders in female pigs and also causes secondary infection of the respiratory system in young pigs. In early 2006, a highly pathogenic infectious disease appeared in pigs in the central region of China, with morbidity rates from 50% to 100% and mortality rates from 20% to 100% in some outbreaks (10, 19, 28). A highly pathogenic PRRSV strain was isolated from swine farms experiencing the disease (20). The genomic analysis revealed that the virus was significantly different from any previously identified isolates (10, 19). The highly pathogenic PRRSV has caused great economic losses to the Chinese swine industry (7, 19, 20).
It is thought that the main immune response of the host against PRRSV infection is cell-mediated immunity, the core of which consists of CD4+ T lymphocytes and the related cytokines (2). Cytokines play an important role in the induction and regulation of the immune responses. When hosts are infected by various kinds of pathogens, including viruses, the expression of various cytokines is up- or downregulated (25). Under most conditions, the degree of pathological damage is not positively correlated with the number of virus particles in the infected tissues, but it may be the result of immunologic injury mediated by the immune system (9).
When a host is infected by a virus, the early cytokines, such as interleukin 1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), and alpha interferon (IFN-α), are produced first (24). These are important inflammatory factors that cause pyrexia, inflammation, histological lesions, respiratory system disorders, and even shock or the death of the host. IL-1, IL-6, and TNF are expressed early in pulmonary infection, promoting infiltration and activated leukocytes, and thus increased vascular permeability and dysfunction, in the lungs (1, 12, 22).
Several previous studies had shown that PRRSV infection, particularly at the active stage, resulted in systemic and local production of a potent immunosuppressive cytokine, IL-10 (16, 17). The expression of inflammatory cytokines induced by the synergism of PRRSV and lipopolysaccharide (LPS) is 10 to 100 times higher than that induced by either of the two factors alone (23). Suradhat and Thanawongnuwech found that pigs infected by American and European types of PRRSV showed transcriptional levels of IL-10 and IFN-γ mRNAs in bronchoalveolar lavage cells (BALC) higher than those in control pigs (17). In addition, the pigs infected with PRRSV and vaccinated against classical swine fever virus showed up- and downregulation in the expression of IL-10 and IFN-γ, respectively (16).
To determine the relationship between the changes in the expression of cytokines in the peripheral blood and the immunity of the host after different PRRSV infections, we reported the changes in the levels of IL-1, IL-6, TNF-α, IFN-α, and IFN-γ in pigs infected with the PRRSV HuN4 and HuN4-F112 strains. In addition, the results of this study provide an idea of the effects of inflammatory cytokines on pulmonary disease after PRRSV infection in an in vivo model.
MATERIALS AND METHODS
Viruses.
The highly pathogenic PRRSV HuN4 strain and its derivative HuN4-F112 were stored in our laboratory after propagation in MARC145 cells to 112 passages. The virus titers were determined based on a procedure described previously (18).
Animals and experimental design.
Forty-eight 4- to 5-week-old PRRSV-seronegative pigs with PRRSV-free and non-PRRSV-vaccinated status were divided into 3 groups with 16 pigs per group. All of the pigs were bled prior to inoculation. The pigs in group 1 and group 2 were intranasally inoculated with the HuN4 and HuN4-F112 strains of PRRSV, respectively, at 3 × 105.12 50% tissue culture infective doses (TCID50) of virus per pig. The pigs in group 3 were intranasally inoculated with phosphate-buffered saline (PBS). The clinical presentation of all pigs was observed. Prior to inoculation and euthanasia, peripheral blood was collected from all pigs, with sample numbers as follows: 16 samples at 0 days postinfection (p.i.) and 16 at 1 day p.i., 14 at 3 days p.i., 12 at 5 days p.i., 10 at 7 days p.i., 8 at 10 days p.i., 6 at 14 days p.i., 4 at 21 days p.i., and 2 at 28 days p.i. from each group. Two pigs from each group were euthanized on those days p.i., and samples of the lungs and blood were collected for virus detection, while samples of the brain and lungs were used for histopathological tests.
Clinical and pathological examinations.
The clinical conditions of the pigs were observed and recorded for at least 10 min per day, including appetite, coughing, respiratory rate, anhelation, and changes in behavior. The clinical scores ranged from 0 to 4 (0, normal; 1, tachypnea when stressed; 2, tachypnea at rest; 3, tachypnea and dyspnea at rest; 4, severe tachypnea and dyspnea with labored, jerky breathing). The rectal temperature was measured every day until the end of the experiment.
Macroscopic lung and brain lesions were evaluated by visual inspection. For histological examination, the lung and brain tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and processed for routine histology.
RNA extraction and reverse transcription.
Tissue suspensions were prepared by grinding tissue samples (0.1 g) under liquid nitrogen with RNase-free PBS (1 ml). From each sample, a 300-μl tissue suspension was used to extract total RNA according to the instructions for the RNeasy Plus Mini kit (Qiagen). A reverse transcription step was performed with 10.5 μl total RNA, 4 μl 5× reverse transcription buffer, 2 μl deoxynucleoside triphosphate (dNTP) mixture (10 mmol/liter), 1 μl 9-mer random primer (50 pmol/liter), 2 μl avian myeloblastosis virus (AMV) (5 U/μl), and 0.5 μl RNase inhibitor (40 U/μl). The reactants were mixed gently, placed in a water bath at 42°C for 1 h, and incubated on ice for 2 min, and the mixture was examined by fluorescence quantitative PCR.
TaqMan fluorescent quantitative PCR.
All primers and conditions for TaqMan fluorescence quantitative PCR were based on the previously described procedure (27). The primer sequences, reference sequences, and anticipated lengths of the PCR products are shown in Table 1. The first set of primers, designated PNF and PNR, was designed for the amplification of a conserved region of PRRSV open reading frame (ORF) 7 sequence (250 bp), and the second set, designated PNPF and PNPR, was for another region of ORF 7 (60 bp). The NP probe (FAM-TCTGTCGTCGATCCAGA-MGB) was labeled at the 5′ end with 6-carboxyfluorescein (FAM) dye as a reporter and had a nonfluorescent quencher and a minor groove binder (MGB) at the 3′ end.
TABLE 1.
Oligonucleotide sequences designed for PCR
| Primer | Oligonucleotide sequence (5′-3′) | Product size (bp) |
|---|---|---|
| PNF (forward) | 5′-AAAACCAGTCCAGAGGCAAG-3′ | 250a |
| PNR (reverse) | 5′-CGGATCAGACGCACAGTATG-3′ | |
| PNPF (forward) | 5′-CCCTAGTGAGCGGCAATTGT-3′ | 60b |
| PNPR (reverse) | 5′-TCCAGCGCCCTGATTGAA-3′ |
PNF and PNR were designed for the amplification of a conserved region of PRRSV ORF7 sequence (250 bp).
Designed for another region of the amplification of a conserved region of PRRSV ORF7 sequence (250 bp).
The PCR system was 25 μl, including 2 μl cDNA template, 1 μl (20 pmol/liter) special primers, 1 μl (10 mM) dNTPs, 2.5 U Taq DNA polymerase, and 5 μl 5× PCR buffer (all from Takara). The final volume was obtained by the addition of RNase-free water.
The PCR conditions were as follows: predenaturing at 95°C for 15 min and 45 cycles of denaturing at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 45 s.
Cytokine bioassays.
The levels of IL-1, IL-6, TNF-α, IFN-α, and IFN-γ in serum were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (Market Inc.) according to the manufacturer's instructions. The standard curve was obtained using a known standard. All detections were performed in parallel.
Statistical analysis.
Statistical analyses were performed using GraphPad PRISM software (version 5.02 for Windows; GraphPad Software Inc.) for analysis of variance (ANOVA).
Nucleotide sequence accession number.
The oligonucleotide sequences designed for PCR have been deposited in GenBank with accession number EF635006.
RESULTS
Clinical signs and pathology.
As shown in Table 2, no clinical signs were observed in pigs (group 2) inoculated with the HuN4-F112 strain or the control pigs (group 3). The pigs in group 1 inoculated with HuN4 showed loss of appetite and a decrease in weight from 3 to 21 days p.i. Phenomena such as rest, coughing, wheezing, and anhelation were observed from 4 to 15 days p.i. After 16 days p.i., these signs began to ease gradually. From 7 to 21 days p.i., purulent secretion was found in the nose. The conch scope was dry, and some pigs had paralyzed hind legs and limp red skin. At 10 days p.i., the skin at the edges of the ears turned blue, and some pigs had muscle tremor and diarrhea.
TABLE 2.
Respiratory scores and weight measurements of the three groups of pigsa
| Day p.i.b | Mean respiratory scorec for group: |
Wt ratio (postinfection/pre-infection) for group: |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 1 | 0 | 0 | 0 | 1.04 | 1.05 | 1.47 |
| 3 | 1.07 ± 0.61 | 0 | 0 | 1.04 | 1.15 | 1.26 |
| 5 | 1.75 ± 0.62 | 0 | 0 | 0.951 | 1.16 | 1.36 |
| 7 | 2.50 ± 0.71 | 0 | 0 | 0.899 | 1.16 | 1.18 |
| 10 | 3.00 ± 0.76 | 0 | 0 | 0.899 | 1.40 | 1.27 |
| 14 | 3.38 ± 0.74 | 0 | 0 | 0.891 | 1.40 | 1.54 |
| 21 | 2.00 ± 0.82 | 0 | 0 | 1.256 | 1.56 | 1.33 |
| 28 | 0.5 ± 0.71 | 0 | 0 | 1.35 | 1.40 | 1.64 |
Group 1 and group 2 pigs were infected with PRRSV HuN4 and HuN4-F112, respectively. Group 3 was the control group with pigs injected with PBS.
The numbers of pigs measured at each day p.i. were as follows: 16 at 0 days p.i., 16 at 1 day p.i., 14 at 3 days p.i., 12 at 5 days p.i., 10 at 7 days p.i., 8 at 10 days p.i., 6 at 14 days p.i., 4 at 21 days p.i., and 2 at 28 days p.i.
Respiratory scores ranged from 0 to 4 (see the text for the definition of each score).
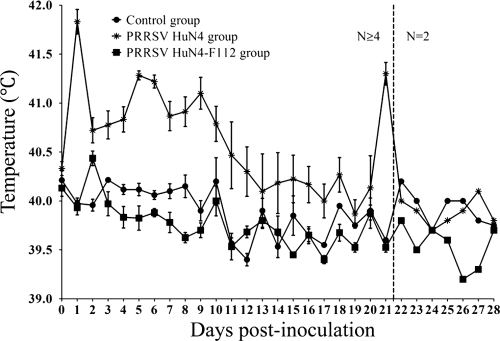
The rectal temperatures of group 1 pigs were raised greatly at 2 days p.i. The body temperatures of 13 of the 16 pigs reached over 41°C at 5, 6, and 9 days p.i. and remained above 40.5°C for most of the experiment; temperatures started to drop to 40.2°C from 10 to 13 days p.i. The temperature did not significantly increase from 14 to 20 days p.i., reached 41.3°C at 21 days p.i., and then dropped to about 40°C from 22 to 28 days p.i. The body temperatures of group 2 pigs were 40.7°C at 3 days p.i. and then returned to normal and remained stable. No significant increase in the body temperature was observed in pigs in the control group (Fig. 1).
FIG. 1.
Body temperature changes in pigs before and after infection. Shown are daily average temperatures of animals in group 1 infected with PRRSV strain HuN4, group 2 pigs infected with PRRSV HuN4-F112, and group 3 control pigs. Each point represents the mean (±standard deviation [SD]) generated from all pigs at 0 to 21 days p.i. or the mean generated from 2 pigs at 22 to 28 days p.i.
Visual and histological observations.
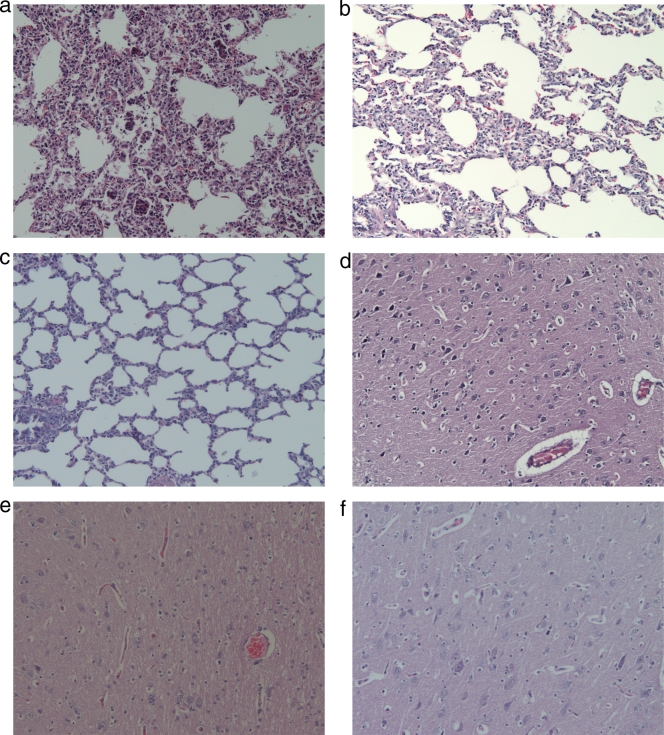
The lung tissues from group 1 pigs (infected with PRRSV HuN4) showed red and beige variegated appearance, and pulmonary cirrhosis could be observed under the microscope. The cerebral cortex was atrophic, the convolutions were flat, and the cerebral groove had widened (Fig. 2a). Histologically, interstitial pneumonia lesions were observed in group 1 pigs. The lung tissues from group 2 pigs showed slight atelectasis with unchanged cerebral convolutions. The alveolar septum was slightly widened, and a few microthrombi had formed in the lungs (Fig. 2b).
FIG. 2.
Pathological examination of lung and brain tissues. (a and d) Lung (a) and brain (d) tissues from group 1 pig no. 5; (b and e) lung (b) and brain (e) tissues from group 2 pig no. 20; (c and f) lung (c) and brain (f) tissues from a control pig. The tissues were stained with hematoxylin and eosin (magnification, ×200).
Brain edema, liquefaction necrosis, and many microthrombi from group 1 pigs could be observed under the microscope (Fig. 2d), while some microthrombi were seen in group 2 pigs (Fig. 2e). There were no macro- or micropathological changes in lung and brain tissues from the control pigs (group 3) (Fig. 2f).
Amounts of virus in blood and lung tissues.
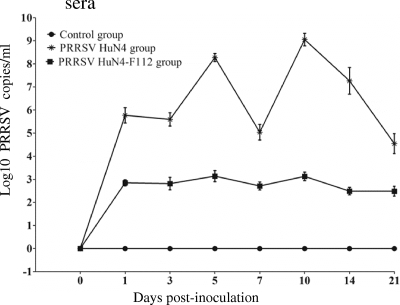
The amounts of PRRSV in the blood and lung tissues are shown in Fig. 3 and Table 3. As shown in Fig. 3, PRRSV was detected in the blood of group 1 pigs, with 105.36 to 109.56 copies/ml at 5 to 14 days p.i. and 104 copies/ml and 103.13 copies/ml at 21 days p.i., and 28 days p.i., respectively. PRRSV amounts in the blood from group 2 pigs were 102.15 to 103.13 copies/ml from 1 to 28 days p.i., which was significantly lower than the amounts in group 1 pigs (P < 0.05). The viral load in lung tissues is given in Table 3. In group 1, the PRRSV level was up to 106.09 copies/ml at 1 day p.i. and 1010.65 copies/ml at 3 days p.i., reached a peak at 5 days p.i. with 1011.75 copies/ml, remained over 109 copies/ml from 7 to 21 days p.i., and dropped to 107 copies/ml at 28 days p.i. In group 2, PRRSV was not detected from 1 to 3 days p.i. in the lung tissues and was detected from 5 to 28 days p.i. at 102.50 to 104.39 copies/ml, which was significantly lower than the data for group 1 pigs (P < 0.05) (Table 3). No virus was detected in the blood and lung tissues from the control group (Fig. 3 and Table 3).
FIG. 3.
Virus amounts in the peripheral blood. Shown are the virus amounts in the blood collected from group 1, group 2, and group 3 pigs. The data represent the means (±SD) of the log10 PRRSV copies/ml determined by TaqMan fluorescence quantitative PCR.
TABLE 3.
Virus amounts in lung tissues from two pigs in each groupa
| Day p.i.b | Amt.c of virus |
|||||
|---|---|---|---|---|---|---|
| Group 1 |
Group 2 |
Group 3 |
||||
| Pig 1 | Pig 2 | Pig 1 | Pig 2 | Pig 1 | Pig 2 | |
| 1 | 6.09 | 3.09 | 0 | 0 | 0 | 0 |
| 3 | 9.87 | 10.65 | 0 | 0 | 0 | 0 |
| 5 | 11.75 | 11.00 | 4.39 | 2.50 | 0 | 0 |
| 7 | 10.39 | 10.44 | 3.39 | 3.71 | 0 | 0 |
| 10 | 9.84 | 10.40 | 3.22 | 3.98 | 0 | 0 |
| 14 | 9.74 | 10.48 | 3.33 | 3.24 | 0 | 0 |
| 21 | 10.11 | 9.29 | 2.72 | 3.28 | 0 | 0 |
| 28 | 7.02 | 7.93 | 2.52 | 3.87 | 0 | 0 |
See Table 2, footnote a.
On each day p.i., two pigs from each group were euthanized to measure the virus amounts in the lung tissues.
Log10 PRRSV copies/g.
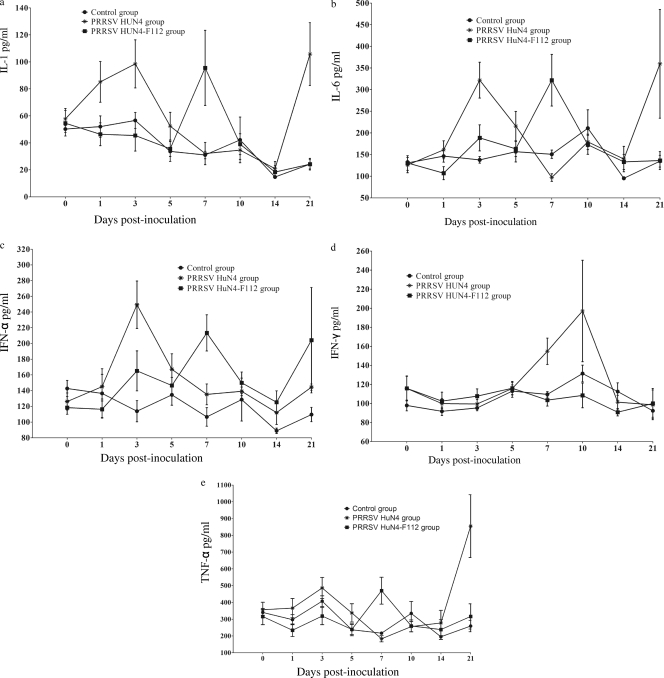
Cytokine level changes.
The levels of IL-1, TNF-α, IL-6, IFN-α, and IFN-γ in blood collected from pigs from 0 to 21 days p.i. are shown in Fig. 4. The levels of IL-1 for group 1 pigs were 57.73 pg/ml at 0 days p.i., gradually increased to 99.90 pg/ml at 3 days p.i., and then declined to the preinoculation level but bounced back to 105.77 pg/ml at 21 days p.i. (Fig. 4a). In group 2 pigs, the levels of IL-1 showed two peaks: one was at 7 days p.i. (95.57 pg/ml), and the other was at 28 days p.i. (32.91 pg/ml). There was no change of cytokine levels at any time point for pigs in the control group (Fig. 4a).
FIG. 4.
Levels of cytokines in peripheral blood. The levels of IL-1 (a), IL-6 (b), IFN-α (c), IFN-γ (d), and TNF-α (e) were measured with a commercial ELISA kit. Each point represents the mean value (±SD) generated from all pigs on each day p.i.
For group 1 pigs, the level of IL-6 started to rise at 1 day p.i. (125.76 pg/ml) and reached the first peak at 3 days p.i. (321.86 pg/ml), then declined to the preinoculation level (139.52 pg/ml) and increased, with the second peak at the highest level of 359.56 pg/ml at 21 days p.i. (Fig. 4b). For group 2 pigs, the level of IL-6 was 130.47 pg/ml at 0 days p.i., decreased to 106.93 pg/ml at 1 day p.i., and then reached the first peak at 7 days p.i. (321.68 pg/ml). The second peak was detected at 21 days p.i. (287.73 pg/ml).
The levels of IFN-α for group 1 pigs quickly reached 248.36 pg/ml at 3 days p.i. and soon returned to nearly base levels (Fig. 4c). In group 2 pigs, the levels started to rise at 3 days p.i. (165.09 pg/ml) and reached the first peak at 7 days p.i. (213.64 pg/ml). After a 14-day gradual decrease, the level started to rise again at 21 days p.i. and reached the second peak at 28 days p.i. (238.68 pg/ml) (Fig. 4c).
The levels of IFN-γ from group 1 pigs began to rise at 7 days p.i., continued until 14 days p.i., and then decreased to normal levels (Fig. 4d). The TNF-α levels began to rise at 3 days p.i. (489.91 pg/ml), decreased to normal, and rose again at 21 days p.i. (854.67 pg/ml) (Fig. 4e). The levels of IFN-γ showed no significant changes from 0 to 28 days p.i. in group 2 pigs (Fig. 4d). The expression of TNF-α reached maximum levels at 7 days p.i. (469.83 pg/ml) and then decreased (Fig. 4e).
Since only two pigs were left at 28 days p.i., the results for the levels of peripheral blood cytokines from each pig are presented individually in Table 4. In general, the levels of cytokines were highest in group 2 pigs, followed by group 1, and lowest in group 3 pigs. In the control group pigs, there were no significant changes in the levels of IL-1, IL-6, TNF-α, IFN-α, and IFN-γ before or during the experiment.
TABLE 4.
Concentrations of cytokines in peripheral blood collected at 28 days p.i. from two pigs in each groupa
| Cytokine | Concn of cytokines (pg/ml) |
|||||
|---|---|---|---|---|---|---|
| Group 1 |
Group 2 |
Group 3 |
||||
| Pig 1 | Pig 2 | Pig 1 | Pig 2 | Pig 1 | Pig 2 | |
| IL-1 | 34.91 | 30.91 | 93.20 | 39.42 | 15.73 | 20.73 |
| IL-6 | 190.32 | 205.33 | 355.72 | 219.74 | 118.00 | 100.00 |
| IFN-α | 154.11 | 170.11 | 301.60 | 175.77 | 110.28 | 116.28 |
| IFN-γ | 89.33 | 98.33 | 65.77 | 95.84 | 73.29 | 81.81 |
| TNF-α | 272.89 | 202.89 | 999.79 | 381.05 | 225.10 | 265.10 |
See Table 2, note a.
DISCUSSION
The virulence of the PRRSV HuN4 strain used in this study was greater than that of any other PRRSV strain (20), which made it very important to study the host cell-mediated immunity against the highly pathogenic PRRSV. Cytokines, such as IL-1, IL-6, IFN, and TNF-α, secreted by alveolar macrophages have various effects on the organism that protect the hosts from infection. Despite their effects against pathogens, cytokines may also induce inflammation and tissue damage when they are overexpressed.
Fever is a typical clinical sign in the acute immune response. PRRSV HuN4 could cause long-lasting high fever compared with other PRRSV strains. Inflammatory cytokines, including IL-1, IL-6, TNF-α, IFN-α, and IFN-γ, affect the prechiasma area to activate the synthesis and release of prostaglandin E2 (PGE2), which is a neurotransmitter that can cause fever by affecting the hypothalamus and other related areas (11, 13). Furthermore, PGE2 can induce local inflammation with local angiotelectasis developing and permeability increasing. A lower concentration of PEG2 could promote platelet aggregation and thrombogenesis (4, 5, 6). High levels of IL-1, IL-6, TNF-α, IFN-α, and IFN-γ were detected in group 1 pigs, which may increase the synthesis and secretion of PGE2. The skin of the ears turned blue, and thrombi could be found in hematoxylin and eosin (HE)-stained sections of the brain. No skin cyanosis was found in group 2 pigs, and thrombi were not obvious in HE-stained brain sections in group 1 pigs, which may partly be due to the lower and later secretion of the cytokines.
As an endogenous pyrogen, IL-1 can induce the generation of leptin, fever, and loss of appetite and can stimulate effector cells in the immune and inflammatory response (3). High body temperature of group 1 pigs started at 1 day p.i. and lasted 6 days (Fig. 1). Meanwhile, loss of appetite and a decrease in body weight were also found, which were significantly different from those in the other two groups. Due to its high virulence, PRRSV HuN4 stimulated the expression of IL-1 to a higher level and thus aggravated the inflammatory reaction and caused an adverse reaction. Generation of IL-1 in group 2 was later than that in group 1, and there were no changes in body temperature or weight, which may be related to the lower virulence of the PRRSV HuN4-F112 strain (Fig. 4a).
It has been reported that the concentrations of IFN-α and IL-6 in the nasal cavity and IL-6 in the blood are related to symptoms of upper respiratory tract disease (24). We have demonstrated that the pigs infected with PRRSV HuN4 did show obvious clinical signs and pathological changes with a lower level of IL-6 (179.35 pg/ml) at 10 days p.i. (Fig. 4b). A hysteresis effect also existed in the expression of IL-6 in group 2 pigs, which may be related to the joint interaction of host and virus, between which a balance had been reached. This was helpful in immune protection, as well as in avoiding the cell and tissue damage caused by the severe upregulation of the inflammatory cytokines (Fig. 4b).
IFN-α can be produced by virus infection, and viruses have a greater ability to induce the cytokine than bacteria and their products (14). The level of IFN-α in group 1 pigs was highest at 3 days p.i. and then returned to the same level as before PRRSV inoculation (Fig. 4c). In group 2 pigs, expression of IFN-α began to be upregulated at 3 days p.i. and reached a higher level at 28 days p.i. There was a significant difference in the times of IFN-α expression between these two groups. The early appearance of the high level of IFN-α may have aggravated the inflammatory reaction, which may also be one of the reasons for the differences in clinical signs. The expression time of IFN-α in group 2 allowed a stronger antiviral effect than that in group 1. We speculate that if PRRSV HuN4 were given to group 2 pigs after 21 days p.i., immunoprotection could be obtained. IFN-γ is produced mainly by activated T cells and NK cells, which participate in regulating the immune and inflammatory responses. From 7 to 10 days p.i., group 1 pigs had high levels of IFN-γ (titers, 154.81 to 197.12 pg/ml), obvious clinical signs (mean respiratory score ± SD, 2.50 ± 0.71 to 3.00 ± 0.76), high fever (40.87 to 41.1°C), and up to 107 to 1010 copies/ml of viruses in the blood. Elevation of the IFN-γ level may aggravate the inflammatory response, which was demonstrated by the obvious clinical signs. In accordance with the results of Suradhat et al. (16, 17), no obvious changes were found in the expression of IFN-γ in the PRRSV HuN4-F112 group (Fig. 4d).
The beneficial effects of TNF-α in defense against infection are due to its quantity in the infected area. However, when TNF-α is generated in large quantities, adverse physiological effects or even death may occur because of its distribution throughout the body or in organs, such as the brain, that are hypersensitive to toxins. Antibodies against TNF-α can help infected animals fight against the septic shock caused by the bacterium (21).
At 3 days p.i. in group 1, the expression level of TNF-α was higher than that before PRRSV inoculation, which may be the result of the immune responses against the highly pathogenic PRRSV infection (Fig. 4e), and similar results were found by Krajcsi and Wold and Van Reeth et al. (8, 26). At 21 days p.i., the amount of PRRSV in group 1 pigs was 100,000 times lower than that at 10 days p.i., and the expression level of TNF-α was slightly upregulated, which may be associated with clearance of the virus. The TNF-α levels in group 2 pigs were higher at 7 and 28 days p.i. than before the inoculation. However, the level in group 2 pigs was lower and later than that in group 1 pigs, which may be due to the lower pathogenicity of the PRRSV HuN4-F112 strain. Both our results and the data previously reported by others suggest that the PRRSV HuN4-F112 strain induced an immune response at a later point, depressing the inflammatory reaction.
When hosts are infected by the highly pathogenic PRRSV, the specific mechanism by which the immune system reacts on the cellular and subcellular levels is still unknown. Our in vivo study showed that the level of inflammatory cytokines induced by PRRSV HuN4 was higher and the changes appeared earlier, which intensified the inflammatory reaction and damaged the tissues and organs. Cytokines induced by low-pathogenic PRRSV (HuN4-F112) developed later and at lower levels, which made it possible for the animal to relieve the inflammatory reaction and enhance the immune response effectively. In summary, detection of the inflammatory cytokines may be useful to investigate whether the PRRSV infection mechanism is affected by other aspects of the host immune system.
Acknowledgments
We thank Z. F. Sun for his valuable suggestions and comments.
This work was supported by grant 2008041 from Chinese Academy of Agricultural Sciences fund for basic scientific research and the National Natural Science Foundation of China (no. 30972075).
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Bielefeldt-Ohmann, H. 1995. Role of cytokines in the pathogenesis and treatment of respiratory disease, p. 291-332. In M. J. Myers and M. P. Murtaugh (ed.), Cytokines in animal health and disease. Marcel Dekker, Inc., New York, NY.
- 2.Buista, E. M., and T. W. Molitor. 1997. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. J. Viral Immunol. 2:83-94. [DOI] [PubMed] [Google Scholar]
- 3.Duff, G. W., and S. K. Durum. 1983. The pyrogenic and mitogenic actions of interleukin-1 are related. Nature 304:449-451. [DOI] [PubMed] [Google Scholar]
- 4.Fabre, J. E., M. Nguyen, K. Athirakul, K. Coggins, J. D. McNeish, S. Austin, L. K. Parise, G. A. FitzGerald, T. M. Coffman, and B. H. Koller. 2001. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J. Clin. Invest. 107:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray, S. J., and S. Heptinstall. 1991. Interactions between prostaglandin E2 and inhibitors of platelet aggregation which act through cyclic AMP. J. Eur. Pharmacol. 194:63-70. [DOI] [PubMed] [Google Scholar]
- 6.Gross, S., P. Tilly, D. Hentsch, J. L. Vonesch, and J. E. Fabre. 2007. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J. Exp. Med. 204:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halbur, P. G. 1998. Porcine viral respiratory diseases, p. 1-10. In Proceedings of the International Pig Veterinary Society. International Pig Veterinary Society, Birmingham, United Kingdom.
- 8.Krajcsi, P., and W. S. Wold. 1998. Inhibition of tumor necrosis factor and interferon triggered responses by DNA viruses. Semin. Cell Dev. Biol. 9:351-358. [DOI] [PubMed] [Google Scholar]
- 9.Lager, K. M., and W. L. Mengeling. 2000. PRRS: nature of the RNA virus and how it causes disease, p. 538-543. In Proceedings of the International Pig Veterinary Society. International Pig Veterinary Society, Melbourne, Australia.
- 10.Li, Y., X. Wang, K. Bo, X. Wang, B. Tang, B. Yang, W. Jiang, and P. Jiang. 2007. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet. J. 174:577-584. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura, K., Y. Watanabe, K. Imai-Matsumura, M. Connolly, Y. Koyama, H. Onoe, and Y. Watanabe. 1992. Mapping of prostaglandin E2 binding sites in rat brain using quantitative autoradiography. Brain Res. 581:292-298. [DOI] [PubMed] [Google Scholar]
- 12.Murtaugh, M. P., M. J. Baarsch, Y. Zhou, R. W. Scamurra, and G. Lin. 1996. Inflammatory cytokines in animal health and disease. Vet. Immunol. Immunopathol. 54:45-55. [DOI] [PubMed] [Google Scholar]
- 13.Saper, C. B., and C. D. Breder. 1994. The neurologic basis of fever. N. Engl. J. Med. 330:1880-1886. [DOI] [PubMed] [Google Scholar]
- 14.Skidmore, S., and M. J. Jarlow. 1987. Interferon as a viral diagnostic test. J. Virol. Methods 16:155-158. [DOI] [PubMed] [Google Scholar]
- 15.Snijder, E. J., and J. J. M. Meulenberg. 1998. The molecular biology of arteriviruses. J. Gen. Virol. 79:961-979. [DOI] [PubMed] [Google Scholar]
- 16.Suradhat, S., R. Thanawongnuwech, and Y. Poovorawan. 2003. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 84:453-459. [DOI] [PubMed] [Google Scholar]
- 17.Suradhat, S., and R. Thanawongnuwech. 2003. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 84:2755-2760. [DOI] [PubMed] [Google Scholar]
- 18.Thanawongnuwech, R., E. L. Thacker, and P. G. Halbur. 1998. Influence of pig age on virus titer and bactericidal activity of porcine reproductive and respiratory syndrome virus (PRRSV)-infected pulmonary intravascular macrophages (PIMs). Vet. Microbiol. 63:177-187. [DOI] [PubMed] [Google Scholar]
- 19.Tian, K., X. Yu, T. Zhao, Y. Feng, Z. Cao, C. Wang, Y. Hu, X. Chen, D. Hu, X. Tian, D. Liu, S. Zhang, X. Deng, Y. Ding, L. Yang, Y. Zhang, H. Xiao, M. Qiao, B. Wang, L. Hou, X. Wang, X. Yang, L. Kang, M. Sun, P. Jin, S. Wang, Y. Kitamura, J. Yan, and G. F. Gao. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS. One 2:e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong, G. Z., Y. J. Zhou, X. F. Hao, Z. J. Tian, H. J. Qiu, J. M. Peng, and T. Q. An. 2007. Identification and molecular epidemiology of the very virulent porcine reproductive and respiratory syndrome viruses emerged in China. Chin. J. Prev. Vet. Med. 31:323-327. [Google Scholar]
- 21.Tracey, K. J., Y. Fong, D. G. Hesse, K. R. Manogue, A. T. Lee, G. C. Kuo, S. F. Lowry, and A. Cerami. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662-664. [DOI] [PubMed] [Google Scholar]
- 22.Ulich, T. R., L. R. Watson, S. M. Yin, K. Z. Guo, P. Wang, H. Thang, and J. Del Castillo. 1991. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am. J. Pathol. 138:1485-1496. [PMC free article] [PubMed] [Google Scholar]
- 23.van Gucht, S., K. van Reeth, and M. Pensaert. 2003. Interaction between porcine reproductive-respiratory syndrome virus and bacterial endotoxin in the lungs of pigs: potentiation of cytokine production and respiratory disease. J. Clin. Microbiol. 41:960-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Reeth, K., and H. Nauwynck. 2000. Proinflammatory cytokines and viral respiratory disease in pigs. Vet. Res. 31:187-213. [DOI] [PubMed] [Google Scholar]
- 25.Van Reeth, K., S. Van Gucht, and M. Pensaert. 2002. In vivo studies on cytokine involvement during acute viral respiratory disease of swine: troublesome but rewarding. Vet. Immunol. Immunopathol. 87:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Reeth, K., G. H. Labarque, H. Nauwynck, and M. Pensaert. 1999. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 67:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei, T. C., Z. J. Tian, T. Q. An, Y. J. Zhou, Y. Xiao, Y. F. Jiang, X. F. Hao, S. R. Zhang, J. M. Peng, H. J. Qiu, and G. Z. Tong. 2008. Development and application of assay for detection of porcine reproductive and respiratory syndrome virus. Chin. J. Prev. Vet. Med. 12:944-948, 991. [Google Scholar]
- 28.Zhou, Y.-J., X.-F. Hao, Z.-J. Tian, G.-Z. Tong, D. Yoo, T.-Q. An, T. Zhou, G.-X. Li, H.-J. Qiu, T.-C. Wei, and X.-F. Yuan. 2008. Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound. Emerg. Dis. 55:152-164. [DOI] [PubMed] [Google Scholar]