Abstract
Highly pathogenic avian H5N1 influenza viruses are endemic in poultry in Asia and pose a pandemic threat to humans. Since the deployment of vaccines against a pandemic strain may take several months, adequate antiviral alternatives are needed to minimize the effects and the spread of the disease. Passive immunotherapy is regarded as a viable alternative. Here, we show the development of an IgA monoclonal antibody (DPJY01 MAb) specific to H5 hemagglutinin. The DPJY01 MAb showed a broad hemagglutination inhibition (HI) profile against Asian H5N1 viruses of clades 0, 1.0, 2.1, 2.2, and 2.3 and also against H5 wild bird influenza viruses of the North American and Eurasian lineages. DPJY01 MAb displayed also high neutralization activity in vitro and in vivo. In mice, DPJY01 MAb provided protection via a single dose administered intranasally before or after inoculation with a sublethal dose of H5N1 viruses of clades 1.0 and 2.2. Pretreatment with 50 mg of DPJY01 MAb kg of body weight at either 24, 48, or 72 h before highly pathogenic H5N1 virus (A/Vietnam/1203/2004 [H5N1]) inoculation resulted in complete protection. Treatment with 50 mg/kg at either at 24, 48, or 72 h after H5N1 inoculation provided 100%, 80%, and 60% protection, respectively. These studies highlight the potential use of DPJY01 MAb as an intranasal antiviral treatment for H5N1 influenza virus infections.
Influenza type A viruses are negative-sense segmented RNA viruses that belong to the family Orthomyxoviridae (11). They are further subdivided into subtypes based on the antigenic properties of the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Among these subtypes, the highly pathogenic avian H5N1 influenza viruses have been intensively studied since the first report of lethal human infections in 1997 (36). H5N1 viruses continue to circulate in poultry in Asia and occasionally are transmitted from birds to humans, posing a potential pandemic threat (1). As of 6 April 2010, the World Health Organization (WHO) had reported 493 human infections with 292 deaths, a fatality rate exceeding 60%. These strains have shown significant evolutionary changes and are currently divided into 10 HA clades (36). Among these clades, clade 2 is further classified into five subclades (2.1 to 2.5), and within each subclade there are several lineages (35). Clade 2.1 is predominant in Indonesia, the country in which H5N1 has become endemic and in which the highest number of human infections and associated fatalities have been reported. In Indonesia, of the 163 cases confirmed to date by the WHO, 135 have been fatal. The latest human infections with H5N1 viruses have been reported in Egypt, where viruses from clade 2.2.1 are endemic. In Egypt since 2006, H5N1 viruses have been identified as the causative agent in 109 human infections with 34 deaths according to the WHO. More importantly, some of these strains have developed resistance to available antiviral drugs (17, 21). For example, most clade 1 H5N1 viruses are resistant to adamantanes (10), and oseltamivir-resistant H5N1 viruses with neuraminidase mutations (H274Y and N294S) have been also identified in infected patients during or after treatment (7, 12). These limitations and others, such as the poor immunogenicity of H5N1 vaccines (3, 16, 26, 31), call for the development of alternative intervention strategies.
Several groups have reported the development of monoclonal antibodies (MAbs) against the HA of influenza viruses, particularly against the H1, H3, and H5 subtypes (9, 14, 38). Some of these MAbs have broad subtype cross-reactions (38). Human and mouse monoclonal antibodies against H5 HA have been shown to provide protection against lethal infection in a mouse model (4, 20, 24). These anti-H5 MAbs are usually of the IgG1 or IgG2a subtypes and are administered by parenteral routes. Retrospective studies have suggested that those patients with influenza pneumonia during the 1918 Spanish influenza pandemic who received influenza convalescent-phase human blood products may have experienced a reduction in the risk of death (15), and H5N1-infected patients treated with convalescent H5N1 plasma recovered from the infection (39). Therefore, passive antibody immunotherapy is an attractive and potentially efficient alternative for the treatment of H5N1 infections. To our knowledge, intranasal administration of antibodies against H5N1 has not been reported. Although intranasal administration of drugs depends largely on the health status of the patient, it does represent an alternative intervention strategy. Intranasal administration of antibodies would allow the antibodies to directly reach their target in the respiratory track, which is the major site for influenza virus replication in humans and other mammals (29, 33). IgA-mediated neutralization monoclonal antibody therapy against H5N1 has not been reported, and only a few IgA MAbs against A/Puerto Rico/8/34 (H1N1) have been reported to show antiviral activity when given intravenously (2). In this study, we generated an IgA monoclonal antibody (DPJY01) with a broad HI profile and high neutralization activity against the H5N1 virus in vitro and in vivo. Remarkably, DPJY01 provided protection against sublethal H5N1 infection after a single dose through intranasal administration.
MATERIALS AND METHODS
Cells and virus.
s/p20 myeloma cells (ATCC) were cultured in modified Eagle's medium (MEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO). Madin-Darby canine kidney (MDCK) cells (ATCC) were maintained in MEM containing 5% FBS. 293-T human embryonic kidney cells (ATCC) were cultured in Opti-MEM (Gibco, Grand Island, NY) containing 5% FBS. The A/egret/Egypt/1162-NAMRU3/2006 (H5N1) (H5/egret) and A/Vietnam/1203/2004 (H5N1) (H5/Vietnam) strains were kindly provided by Ruben Donis, Centers for Disease Control and Prevention, Atlanta, GA. The attenuated avian influenza virus ΔH5N1-WF10tsHA has been previously described in detail (25). Other viruses are listed in Table 1, below. These viruses were propagated in 10-day-old embryonated specific-pathogen-free (SPF) chicken eggs at 35°C and stored at −70°C. The viruses were titrated by the Reed and Muench method to determine the 50% tissue culture infective dose (TCID50) and minimal 50% mouse lethal dose (MLD50) (22, 32).
TABLE 1.
HI profiles of MAb DPJY01 against H5 viruses of different lineages and other HA subtypes
| Clade or lineage group | Virusa | HI titer/50 μl |
|---|---|---|
| 0 | RG-A/Hong Kong/156/97 (H5N1)* | 12,800 |
| 1.0 | RG-A/Vietnam/1194/2004 (H5N1)* | 12,800 |
| 1.0 | RG-A/Vietnam/1203/2004(H5N1)* | 16,384 |
| 2.1 | RG-A/Indonesia/5/2005 (H5N1)* | 3,200 |
| 2.2 | A/egret/Egypt/1162-NAMRU3/2006 (H5N1) | 4,096 |
| 2.2 | RG-A/Turkey/1/2005 (H5N1)* | 3,200 |
| 2.3 | RG-A/Anhui/1/2005 (H5N1)* | 3,200 |
| North American lineage | A/Mallard Duck/Pensylvania/10218/84 (H5N2) | 4,096 |
| Eurasian lineage | RG-A/Mallard/NL/3/99 (H5N1) | 12,800 |
| A/WSN/1933 (H1N1) | <2 | |
| A/Mallard/New York/6750/78 (H2N2) | <2 | |
| A/Duck/Hongkong/3/75 (H3N2) | <2 | |
| A/Mallard/Alberta/206/96 (H6N8) | <2 | |
| A/Chicken/Delaware/VIVA/2004 (H7N2) | <2 | |
| A/Mallard/Alberta/194/92 (H8N4) | <2 | |
| A/Guinea fowl/Hong Kong/WF10/99 (H9N2) | <2 | |
| A/Pintail/Alberta/202/2000 (H10N7) | <2 | |
| A/Duck/MD/2T70/2004 (H11N9) | <2 | |
| A/Mallard/Alberta/238/96 (H12N5) | <2 | |
| A/Mallard/Alberta/146/2001 (H13N6) | <2 |
Viruses marked with an * are attenuated recombinant viruses generated by reverse genetics (RG). The HA genes of RG viruses were derived from the indicated virus. RG viruses contain the NA gene derived from A/egret/Egypt/1162-NAMRU3/2006 (H5N1) and a truncated NS1-73 gene derived from A/guinea fowl/Hong Kong/WF10/99 (H9N2). The other five genes of the RG viruses were derived from the WF10att backbone (25), with the exception of RG-A/Mallard/NL/3/99 (H5N1), for which the genes were derived from the WF10 wild-type backbone virus.
Immunization.
Eight-week-old female BALB/c mice (n = 8; National Cancer Institute, Frederick, MD) were immunized by intraperitoneal injections with attenuated avian influenza virus ΔH5N1-WF10tsHA (25). Doses consisted of 200 μl of the allantoic fluid containing 2,048 HA units of virus. Boost immunization was given at 10, 20, and 30 days postvaccination. Animal studies using attenuated ΔH5N1-WF10tsHA recombinant viruses were conducted under animal biosafety level 2 (ABSL-2) conditions and performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland, College Park.
Production of MAbs.
Mouse spleen cells collected at day 4 post-third boost with ΔH5N1-WF10tsHA virus were fused to the s/p20 myeloma cells as previously described (37). After selection of the hybridomas in hypoxanthine-aminopterin-thymidine medium (HAT; Invitrogen, Carlsbad, CA), antibody-producing cells were screened by the hemagglutination inhibition (HI) method (32) and subcloned by limiting dilution. Positive clones were checked for isotype by using the Iso-Gold Rapid mouse monoclonal isotyping test kit (BioAssay Works, Ijamsville, MD) as described in the manufacturer's protocol. The MAbs from ascites fluids were generated as previously described (37).
Western blot assays.
The culture supernatants from MAb-expressing cells were collected, and the proteins (10 μg total protein/well) were analyzed by polyacrylamide gel electrophoresis (PAGE) under nonreducing conditions. After PAGE, the proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) and analyzed by Western blotting. Western blots were revealed using horseradish peroxidase (HRP)-conjugated goat anti-mouse Ig or IgA (Santa Cruz Biotechnology, Santa Cruz, CA) and enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) followed by X-ray film exposure (Thermo Scientific, Rockford, IL).
Neutralization assays.
For microneutralization assays (MN) (see Table 2), serially diluted MAbs were first incubated with 100 TCID50 of virus for 1 h at 37°C. The virus-MAb mixture was then adsorbed to MDCK cells for 1 h. Infected cells were washed twice with phosphate-buffered saline (PBS) and replenished with Opti-MEM (Gibco, Grand Island, NY). The infected cell supernatants were harvested at 4 days postinoculation (dpi) and were analyzed by HA assay (32). In vivo neutralization followed by in vitro evaluation were performed by incubating the MAbs (1.5 mg/ml) with 1,000 PFU of H5/Vietnam virus for 1 h at 37°C. Then the virus-MAb mixture was inoculated intranasally into 5-week-old mice (n = 5). Body weight, morbidity, and survival of mice were monitored for 14 dpi.
TABLE 2.
MN profile of MAb DPJY01 against different HA subtypes
| Virus | MN titer/50 μl |
|
|---|---|---|
| DPJY01 | 2B9 | |
| A/guinea fowl/Hong Kong/WF10/99 (H9N2) | <10 | <10 |
| A/Chicken/Delaware/VIVA/2004 (H7N2) | <10 | <10 |
| A/Vietnam/1203/2004 (H5N1) | >10,240 | <10 |
| A/WSN/1933 (H1N1) | <10 | <10 |
Escape mutants.
To find escape mutant H5N1 viruses, studies were designed as previously described (27). In brief, 100 TCID50 of 2ΔH5N1-WF10 were propagated in MDCK cells in the presence of MAb DPJY01 (100 μg/ml) in a six-well plate format. After 72 h, 1 ml of the supernatant was further propagated in MDCK cells in the presence of MAb DPJY01. The supernatant from the second passage was harvested for the third virus propagation. We also performed similar studies in eggs. Briefly, MAb (100 μg/ml) was incubated with 100 TCID50 of virus at room temperature for 30 min and then the mixture was inoculated into 10-day-old embryonated chicken eggs (n = 5). After 48 h, the allantoic fluid was harvested and used for two additional cycles of selection. A monoclonal antibody against NP (2B9) was used as control. Escape mutants were also evaluated using viruses found in lung, brain, and tracheal homogenates of treated mice at 48 and 72 h postinoculation (hpi) (see results shown in Fig. 5, below). The volume of tissue homogenate used corresponded to 100 TCID50 of virus and it was preincubated with MAb (100 μg/ml) for 30 min at room temperature prior to infection of MDCK cells.
Protection studies in mice.
The ascites fluids were filtered using a 0.22-μm filter and then heat inactivated at 56°C for 30 min. Six-week-old mice (n = 5) were treated intranasally with a single dose of 50 μl PBS/mouse containing either 50 mg/kg of body weight or 10 mg/kg of MAb (the concentration corresponded to the total protein concentration, which was quantified by two independent methods, bicinchoninic acid protein assay kit [Pierce, Rockford, IL] and protein absorbance at 280 nm using the NanoDrop ND-1000 photometer [Thermo Scientific, Wilmington, DE]). The treatment was performed at different time points before or after virus inoculation as indicated in Results. Two different H5N1 strains were used for inoculation, H5/Vietnam from clade 1.0 and H5/egret from clade 2.2.1 at doses of 3 PFU of H5/Vietnam and 300 50% egg infective doses (EID50) of H5/egret, respectively. The inoculation doses were lethal to between 80 and 100% (∼2 MLD50) of untreated or mock-treated mice. Body weight change was monitored daily until day 21 postinoculation. Additionally, infected mice were monitored for other morbidity signs, including rough coat, inactivity, and neurological signs, following an approved scoring method. Mice showing body weight losses of ≥25% of preinoculation values were euthanized for humane reasons and counted as dead.
Virus titers in brain, lungs, and trachea were determined in 6-week-old mice that were administered intranasally a single dose of 50 μl PBS/mouse containing 50 mg/kg of DPJY01 MAb at different time points before or after inoculation with 3 PFU of H5/Vietnam. An IgA MAb 1D9 directed against the HA of 2009 H1N1 pandemic influenza viruses (H. Shao and D. R. Perez, unpublished data) was used as a control. Brain, lungs, and tracheas were collected at 6 dpi and titrated in MDCK cells by a TCID50 assay with a limit of virus detection of ≤0.6999 log10 TCID50/g of tissue. H5N1 inoculation studies were conducted under ABSL-3 conditions approved by the U.S. Department of Agriculture and performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland, College Park.
RESULTS
Generation of IgA MAb specific to H5 virus.
After fusion between spleen cells from H5N1 virus-immunized mice and s/p20 myeloma cells, screening by HI assay yielded six monoclonal antibodies specific to the HA protein of the H5 virus. Subtyping showed that one of these MAbs was an IgA subtype, herein referred to as DPJY01. Because previous passive immunotherapy studies against H5 influenza virus have not made use of IgA antibodies, DPJY01 was tested as a suitable antiviral alternative. The subtype and polymeric structure of DPJY01 were confirmed by Western blotting under nonreducing conditions. The DPJY01 MAb was produced in monomeric (I) and polymeric (II) forms as revealed with an anti-IgA specific antibody or anti-mouse antibody (Fig. 1). An IgG MAb was used as a control, which as expected did not react with the anti-IgA antibody (Fig. 1). In contrast, both the IgG control and DPJY01 showed positive reactions against an anti-mouse antibody (Fig. 1).
FIG. 1.
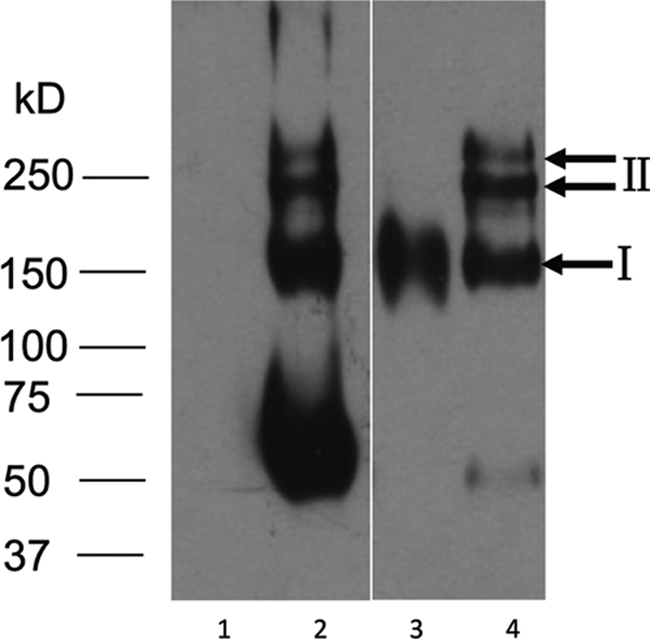
Polymeric structure analysis of DPJY01 based on Western blotting under nonreducing conditions. Culture supernatants of 2B9 IgG MAb (lanes 1 and 3) and DPJY01 IgA MAb (lanes 2 and 4) were analyzed by PAGE and Western blotting and revealed with HRP-labeled goat anti-mouse IgA antibody (lanes 1 and 2) or HRP-labeled goat anti-mouse Ig antibody (lanes 3 and 4). Numbers on the left correspond to a standard protein molecular mass marker, and bands I and II correspond to IgA monomeric and polymeric forms, respectively.
In order to determine the specificity and the HI profile of DPJY01 for the H5 HA viruses, HI assays were performed using viruses of the H5, H1, H2, H3, H6, H7, H8, H9, H10, H11, H12, and H13 subtypes (Table 1). HI assays revealed that DPJY01 did not cross-react with H1, H2, H3, H6, H7, H8, H9, H10, H11, H12, and H13 HA subtype viruses and reacted exclusively with H5 HA viruses (Table 1). Moreover, DPJY01 showed a broad HI profile against Asian H5N1 viruses of different clades, including clades 0, 1.0, 2.1, 2.2, and 2.3. DPJY01 also showed strong HI profiles against a low-pathogenic H5 virus of the North American lineage and a low-pathogenic H5 virus from the Eurasian lineage (Table 1), suggesting that this IgA binds to a highly conserved epitope in the H5 HA protein.
To further test if the conserved epitope would be easily mutated and recognition by DPJY01 would be lost, we attempted to generate escape mutant viruses. Despite several attempts, we could not isolate a mutant virus that would not be neutralized by the DPJY01 MAb. Selection in the presence of 100 μg/ml of DPJY01 IgA in tissue culture MDCK cells or in eggs produced no escape mutant viruses. Although the use of various virus/antibody ratios could be attempted in order to select for escape mutants, this was beyond the scope of the present report.
Neutralization activity of DPJY01.
To evaluate the neutralization activity of DPJY01 MAb, a microneutralization assay was performed in MDCK cells. As shown in Table 2, the neutralization titer of DPJY01 for H5N1 virus reached more than 10,240/50 μl, whereas there was no neutralization for H9, H7, and H1 viruses. More importantly, prior incubation of 1,000 PFU of H5/Vietnam virus with DPJY01 resulted in complete block of virus replication in mice (Fig. 2). Control mice that receive an inoculum containing the H5/Vietnam virus and MAb 2B9 (obtained against the influenza virus nucleoprotein and which cannot neutralize the virus in vitro) showed a rapid decline in body weight and did not survive (Fig. 2A and B). It is important to note that 3 PFU of H5/Vietnam represents a sublethal dose in mice, sufficient to produce between 80 and 100% mortality. Moreover, DPJY01 was ineffective against the mouse-adapted lethal strain A/WSN/33 (H1N1) and the 2009 pandemic mouse-adapted lethal strain ma-Ca/04 (data not shown). These results indicate high specificity and inhibitory activity of DPJY01 for H5 viruses in vitro and in vivo.
FIG. 2.
Neutralization activity in mice. DPJY01 MAb (1.5 mg/ml) or 2B9 MAb (1.5 mg/ml) was first incubated with 1,000 PFU of H5/Vietnam virus for 1 h at 37°C. The mixture was then inoculated intranasally into 5-week-old mice (n = 5). Body weight changes (A) and survival (B) were monitored for 14 dpi.
Intranasal delivery of DPJY01: effects on prophylaxis against H5N1.
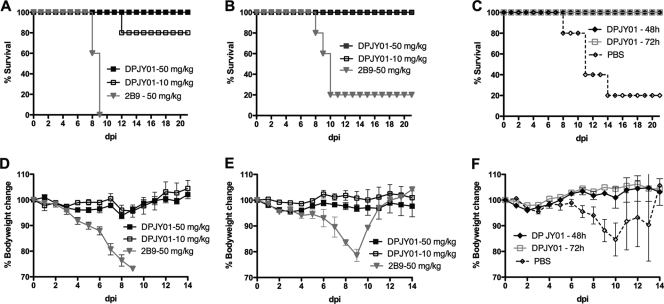
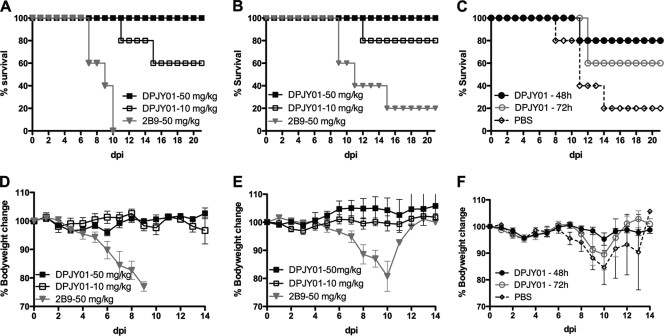
An acute respiratory disease characterizes H5N1 influenza virus infection in humans (1, 5, 13). The respiratory tract is the site of virus replication, and a combination of host-virus interactions modulates the severity and outcome of the disease. It is known that mucosal, virus-specific IgA antibodies play a vital role in the antiviral immune response (29, 33). To evaluate the efficacy of DPJY01 against H5N1 in vivo, the antibody was delivered to mice via intranasal droplets before H5N1 inoculation. In a prophylactic study, pretreatment with 50 mg/kg or 10 mg/kg of DPJY01 at 24 h before inoculation with H5/Vietnam provided 100% and 80% protection, respectively (Fig. 3A). Additionally, mice were fully protected against inoculation with the H5/egret virus (Fig. 3B), regardless of the dose of MAb used. Thus, DPJY101 shows efficient antiviral activity. As expected, MAb 2B9 showed no prophylactic protection against the H5N1 viruses (Fig. 3A and B). These results further support our in vitro neutralization studies with respect to the effective inhibitory activity of DPJY01 against H5 viruses of different clades. We also explored whether prophylactic protection could be achieved by providing the MAb at 48 h or at 72 h before inoculation (Fig. 3C). Here too, 100% protection against H5/Vietnam was observed with a single dose of 50 mg/kg administered at either 48 h or 72 h before inoculation (Fig. 3C), suggesting that the IgA antibody could be maintained in an active form for at least 72 h before virus inoculation and was sufficient to neutralize the virus. DPJY01 administration prevented signs of disease and body weight loss in mice that received the 50-mg/kg dose regardless of the time of administration before virus inoculation. Administration of 10 mg/kg of DPJY01 also resulted in no signs of disease and no significant body weight loss except for one mouse that died in the 24-h pretreatment group at 12 dpi (Fig. 3A). In contrast, mock-treated mice showed exacerbated signs of disease and significant body weight losses, including the few mice that ultimately recovered from the infection. These results show that DPJY01 not only increases the chances of survival but also prevents the disease associated with H5N1 infection.
FIG. 3.
Prophylactic effect of DPJY01 against H5N1 inoculation in mice. (A) Results for 6-week-old mice (n = 5) previously treated with a single dose of DPJY01 (50 or 10 mg/kg) or control 2B9 antibody (50 mg/kg) via the intranasal route at 24 h before inoculation with 3 PFU of H5/Vietnam virus. The graph shows the percent survival over time in dpi. (B) The same experiments as show in panel A, but mice were inoculated with 300 EID50 of H5/egret virus. (C) The same experiment as shown in panel A, except that mice received a single dose of 50 mg/kg of DPJY01 at either 48 h or 72 h before inoculation with 3 PFU of H5/Vietnam virus. Mock-treated mice received PBS. (D, E, and F) Percent body weight changes over a 14-day period for the experiments shown in panels A, B and C, respectively.
Intranasal delivery of DPJY01: therapeutic effect against H5N1 inoculation.
In order to determine the therapeutic potential of DPJY01, the MAb was administered to mice at either 50 mg/kg or 10 mg/kg at 24 h after virus inoculation. Our results showed that the 50-mg/kg dose offered 100% protection against H5/Vietnam, whereas the 10-mg/kg dose attained 60% protection, although mortality was in general delayed 3 to 4 days compared to the mock-treated control group (Fig. 4A). As expected, the mock-treated group (treatment with 50 mg/kg of the NP MAb) showed 100% mortality within 10 dpi (Fig. 4A). Additionally, the 50-mg/kg and 10-mg/kg doses of DPJY01 also provided 100% and 80% protection against H5/egret infection (Fig. 4B). Again, mortality was delayed compared to the mock-treated group. DPJY01, at the 50-mg/kg single dose, was effective in preventing signs of disease and significant body weight loss after inoculation with either H5/Vietnam or H5/egret for the duration of the study (Fig. 4D and E). At the 10-mg/kg single dose, DPJY01 was also effective at preventing significant body weight losses for the first 14 dpi, despite the fact that some mice were euthanized due to ethical reasons (Fig. 4D). To monitor therapeutic efficacy at different times postinoculation, 50 mg/kg DPJY01 was given at 48 or at 72 after virus inoculation. As shown in Fig. 4C, DPJY01 provided 80% and 60% protection when administered at 48 and 72 hpi, respectively. Once again, mortality was delayed compared to the mock-treated group, suggesting that DPJY01 has the potential to significantly limit virus replication once the infection is well under way. Although body weight losses and signs of disease were more evident in mice that received the DPJY01 treatment at 48 and 72 h after virus inoculation (Fig. 4F), they were less severe and less significant than in the mock-treated group. We would like to emphasize that these results represent effects after a single intranasal dose administration of DPJY01 and thus suggest a strong antiviral activity by this IgA MAb.
FIG. 4.
Therapeutic effect of DPJY01 against H5N1 inoculation in mice. (A) Six-week-old mice (n = 5) were infected intranasally with 3 PFU of H5/Vietnam virus and treated 24 hpi with a single dose of DPJY01 (50 or 10 mg/kg) or control 2B9 antibody (50 mg/kg). The graph shows the percent survival over time in dpi. (B) The same experiment as shown in panel A, except mice were infected with 300 EID50 of H5/egret virus. (C) The same experiment as in panel A, except that mice received a single dose of 50 mg/kg of DPJY01 at either 48 or 72 hpi with 3 PFU of H5/Vietnam virus. Mock-treated mice received PBS. (D, E, and F) The corresponding percent body weight changes over a 14-day period for the experiments shown in panels A, B, and C, respectively.
DPJY01 significantly decreases virus replication in the lungs, trachea, and brain of H5N1-inoculated mice.
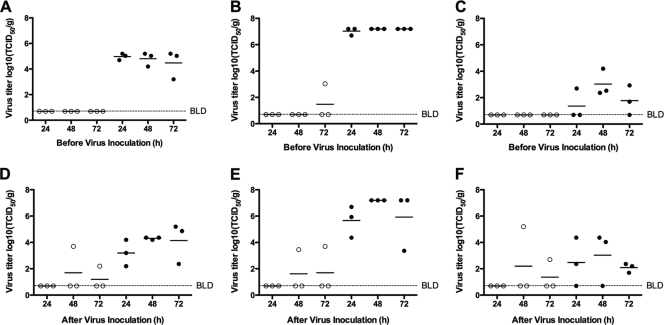
In order to test whether DPJY01 could provide protection by decreasing virus replication, 6-week-old mice were administered 50 mg/kg of DPJY01 at 24, 48, or 72 h pre- or postinoculation with 3 PFU of H5/Vietnam (Fig. 5). Virus titers in the trachea, lung, and brain of infected mice were determined by 6 dpi in MDCK cells as described in Materials and Methods. Pretreatment with DPJY01 prior to inoculation resulted in complete inhibition of virus replication in trachea, lung, and brain of infected mice regardless of the day of DPJY01 inoculation. The exception was one mouse in the 72-h group (Fig. 5A, B, and C); however, lung virus titers in this mouse were 10,000-fold lower than in the control group mice (Fig. 5B). Treatment after inoculation resulted in complete block in virus replication in the trachea, lungs, or brain if DPJY01 was administered 24 hpi. When administrated at 48 or 72 hpi, DPJY01 could also completely block virus replication in two out of three mice in each group. The mice in which DPJY01 was ineffective showed virus titers not higher than in the control groups, with the exception of virus titers in the brain of one mouse in the 48-hpi treatment group (Fig. 5E). In order to determine whether failure of protection in these mice was the result of the emergence of H5N1 escape mutants in vivo, tissue homogenates containing the equivalent of 100 TCID50 of virus were incubated in the presence of DPJY01 or 1D9 control antibody, and virus growth was analyzed in MDCK cells. No evidence of escape mutants was found in the tissue homogenates (data not shown). These results suggest that the mechanism of protective action of DPJY01 in vivo is by binding and neutralizing the virus. Failure of the antibody to control infection at 6 dpi may be due to a combination of ongoing replication and inefficient access of DPJY01 to the site of replication; however, the data presented in Fig. 5 suggest that DPJY01 can extend survival beyond the window of 6 to 10 days in which most control mice succumb to infection.
FIG. 5.
DPJY01 inhibits virus replication in infected mice. Three mice/group were treated with a single dose of 50 mg/kg of DPJY01 (open cirlces) or control 1D9 IgA antibody (black circles) at the indicated time points (24, 48, or 72 h) before or after inoculation with 3 PFU of H5/Vietnam virus. At 6 dpi, the trachea, lungs, and brain from each mouse were collected and homogenized. Viruses in homogenates were titrated in MDCK cells; virus titers (log10 TCID50/g) of tissue homogenate are shown on the y axis, and the limit of detection was 0.669 log10 TCID50/g. Virus titers are shown in trachea (A and D), lung (B and E), and brain (C and F) in mice that were treated at the indicated time points (h) before or after inoculation.
DISCUSSION
In this study, we developed a novel IgA monoclonal antibody (DPJY01) specific to H5 HA subtype influenza virus. PAGE under nonreducing conditions followed by Western blot analysis revealed that DPJY01 has polymeric forms (Fig. 1), characteristic of IgA. DPJY01 showed broad HI activity against H5 HA proteins from Eurasian and North American influenza viruses. Furthermore, DPJY01 showed no reactivity against other HA subtypes, such as H1, H2, H3, H6, H7, H8, H9, H10, H11, H12, and H13. Although, we have not tested the recognition pattern of DPJY01 against a full panel of HA subtypes, our studies suggest a high degree of conservation of an epitope among H5 HA proteins. It is important to note that several attempts were made to obtain H5 escape mutants viruses by using the DPJY01 IgA MAb; however, none of the attempts was successful. Although we cannot completely rule out the possibility of limitations in our approach to select for escape mutants, it is tempting to speculate that DPJY01 binds to an epitope with restricted flexibility for mutations. If this is indeed the case, then the DPJY01 binding site makes it an ideal antiviral target. Future studies are needed to reveal the site of recognition of DPJY01 and whether viable escape mutants can readily emerge.
Since the respiratory tract is the target for influenza virus infection in mammals, it is generally accepted that mucosal IgA antibody responses play an essential role in the antiviral immune response (29, 33). In this regard, DPJY01 shows remarkable activity against H5N1 infection under prophylactic and therapeutic conditions. Previous studies have shown the potential of IgG MAbs for passive immunotherapy against H5N1 influenza virus when administered parentally (4, 9, 18, 19, 24, 27, 28, 30, 38). Instead, we developed an IgA MAb that can be administered intranasally. DPJY01 showed great stability and activity when administered prophylactically as a single dose at 72 h prior to virus inoculation. This effect might be related to the polymeric form of DPJY01, since IgA is more resistant to proteases, and one IgA molecule has the potential to bind to two HA molecules instead of one, as is the case with IgG antibodies. More importantly, DPJY01 greatly improved survival of H5N1-infected mice even when provided as a single dose at 72 h postinoculation. It is important to note that adamantanes and NA inhibitors are very effective prophylactically but their effectiveness beyond the 48-h postinoculation window is controversial (6, 8). More importantly, neuraminidase inhibitors provide minimal benefit in shortening the duration of illness in children with seasonal influenza (23). H5N1 patients showing exacerbated signs of disease require hospitalization and respiratory assistance (34), in which case it is plausible that an IgA antibody like DPJY01 could be administered through the respiratory route. Management of critically ill H5N1 patients is complex, and a multicomponent antiviral strategy is critical for a positive outcome (34). We speculate that an antibody like DPJY01 could be a valuable tool in combinatorial therapy with other anti-influenza virus drugs.
Recent studies by Simmons et al. have shown the potential of human monoclonal antibodies against H5N1 virus infections in a mouse model (24). Human monoclonal antibodies were generated via Epstein-Barr virus-mediated immortalization of B cells obtained from patients that had recovered from H5N1 infection (24). Simmons et al. showed that at least four such antibodies were effective for survival during prophylactic and therapeutic intraperitoneal administration against clade 1 and clade 2 viruses, although their effects in reducing morbidity were not discussed in detail (24). It is important to note that the dose of virus used by Simmons et al. was higher than in our studies, although we used a dose that was lethal for 80 to 100% of the mice (5 MLD50 versus ∼2 MLD50, respectively). More recently, Throsby et al. described a highly cross-reactive human monoclonal antibody effective in vivo against at least H5 and H1 viruses (30). Again, Throsby et al. used an amount of H5 virus significantly higher than in our report (10 MLD50), although the antibody was administered intravenously multiple times at 24-h intervals starting at 3 or 4 days postinoculation. Immunotherapy is an effective method to control infectious diseases, and it is commonly used in the prevention and transmission of hepatitis A and B, rabies, and varicella. It is also used for the prevention and treatment of respiratory syncytial virus (RSV) infections in high-risk babies under 1 year. In the case of RSV prevention, a large dose of a “humanized” IgG MAb is injected intramuscularly every 28 to 30 days to prevent the virus from causing exacerbated lung infections. In our studies we showed an IgA MAb that is stable in vivo and highly effective against a highly lethal respiratory virus, the H5N1 influenza virus. Future studies are needed to determine whether DPJY01 can be “humanized” without losing its capacity to form polymers and, more importantly, maintain powerful antiviral activity.
Acknowledgments
We are indebted to Yonas Araya and Ivan Gomez-Osorio for their excellent laboratory techniques and animal handling assistance. We thank Andrea Ferrero and Theresa Wolter-Marth for their excellent laboratory managerial skills and Annabelle Morano Pascua for proofreading the manuscript.
This work was supported by NIAID NIH contract HHSN266200700010C and USDA grant numbers 2005-35605 and 2007-04981.
Footnotes
Published ahead of print on 28 July 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Abdel-Ghafar, A. N., T. Chotpitayasunondh, Z. Gao, F. G. Hayden, D. H. Nguyen, M. D. de Jong, A. Naghdaliyev, J. S. Peiris, N. Shindo, S. Soeroso, and T. M. Uyeki. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261-273. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, S. J., and N. J. Dimmock. 1992. Neutralization of influenza virus by low concentrations of hemagglutinin-specific polymeric immunoglobulin A inhibits viral fusion activity, but activation of the ribonucleoprotein is also inhibited. J. Virol. 66:3823-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel, J. H., J. Voell, C. Y. Huang, P. D. Burbelo, and H. C. Lane. 2009. Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J. Infect. Dis. 200:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Y., K. Qin, W. L. Wu, G. Li, J. Zhang, H. Du, M. H. Ng, J. W. Shih, J. S. Peiris, Y. Guan, H. Chen, and N. Xia. 2009. Broad cross-protection against H5N1 avian influenza virus infection by means of monoclonal antibodies that map to conserved viral epitopes. J. Infect. Dis. 199:49-58. [DOI] [PubMed] [Google Scholar]
- 5.Chokephaibulkit, K., M. Uiprasertkul, P. Puthavathana, P. Chearskul, P. Auewarakul, S. F. Dowell, and N. Vanprapar. 2005. A child with avian influenza A (H5N1) infection. Pediatr. Infect. Dis. J. 24:162-166. [DOI] [PubMed] [Google Scholar]
- 6.Cram, P., S. G. Blitz, A. Monto, and A. M. Fendrick. 2001. Influenza. Cost of illness and considerations in the economic evaluation of new and emerging therapies. Pharmacoeconomics 19:223-230. [DOI] [PubMed] [Google Scholar]
- 7.de Jong, M. D., T. T. Tran, H. K. Truong, M. H. Vo, G. J. Smith, V. C. Nguyen, V. C. Bach, T. Q. Phan, Q. H. Do, Y. Guan, J. S. Peiris, T. H. Tran, and J. Farrar. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353:2667-2672. [DOI] [PubMed] [Google Scholar]
- 8.Gillissen, A., and G. Hoffken. 2002. Early therapy with the neuraminidase inhibitor oseltamivir maximizes its efficacy in influenza treatment. Med. Microbiol. Immunol. 191:165-168. [DOI] [PubMed] [Google Scholar]
- 9.Hanson, B. J., A. C. Boon, A. P. Lim, A. Webb, E. E. Ooi, and R. J. Webby. 2006. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden, F. G. 2006. Antiviral resistance in influenza viruses-implications for management and pandemic response. N. Engl. J. Med. 354:785-788. [DOI] [PubMed] [Google Scholar]
- 11.Lamb, R. A. 1989. Genes and proteins of the influenza viruses. In R. M. Krug (ed.), The influenza viruses, 1st ed. Plenum Press, New York, NY.
- 12.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. Nguyen, N. D. Pham, H. H. Ngyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437:1108. [DOI] [PubMed] [Google Scholar]
- 13.Liem, N. T., C. V. Tung, N. D. Hien, T. T. Hien, N. Q. Chau, H. T. Long, N. T. Hien, Q. Mai le, W. R. Taylor, H. Wertheim, J. Farrar, D. D. Khang, and P. Horby. 2009. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004-2006. Clin. Infect. Dis. 48:1639-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipatov, A. S., A. K. Gitelman, and Y. A. Smirnov. 1997. Prevention and treatment of lethal influenza A virus bronchopneumonia in mice by monoclonal antibody against haemagglutinin stem region. Acta Virol. 41:337-340. [PubMed] [Google Scholar]
- 15.Luke, T. C., E. M. Kilbane, J. L. Jackson, and S. L. Hoffman. 2006. Meta-analysis. Convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann. Intern. Med. 145:599-609. [DOI] [PubMed] [Google Scholar]
- 16.Nolan, T. M., P. C. Richmond, M. V. Skeljo, G. Pearce, G. Hartel, N. T. Formica, K. Hoschler, J. Bennet, D. Ryan, K. Papanaoum, R. L. Basser, and M. C. Zambon. 2008. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine 26:4160-4167. [DOI] [PubMed] [Google Scholar]
- 17.Park, J. W., and W. H. Jo. 2009. Infiltration of water molecules into the oseltamivir-binding site of H274Y neuraminidase mutant causes resistance to oseltamivir. J. Chem. Inf. Model. 49:2735-2741. [DOI] [PubMed] [Google Scholar]
- 18.Prabakaran, M., N. Prabhu, F. He, Q. Hongliang, H. T. Ho, J. Qiang, T. Meng, M. Goutama, and J. Kwang. 2009. Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants. PLoS One 4:e5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhu, N., M. Prabakaran, H. T. Ho, S. Velumani, J. Qiang, M. Goutama, and J. Kwang. 2009. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J. Virol. 83:2553-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhu, N., M. Prabakaran, Q. Hongliang, F. He, H. T. Ho, J. Qiang, M. Goutama, A. P. Lim, B. J. Hanson, and J. Kwang. 2009. Prophylactic and therapeutic efficacy of a chimeric monoclonal antibody specific for H5 haemagglutinin against lethal H5N1 influenza. Antivir. Ther. 14:911-921. [DOI] [PubMed] [Google Scholar]
- 21.Reece, P. A. 2007. Neuraminidase inhibitor resistance in influenza viruses. J. Med. Virol. 79:1577-1586. [DOI] [PubMed] [Google Scholar]
- 22.Reed, L. J., and H. Muench. 1938. A simple method for estimating 50 percent endpoints. Am. J. Hyg. (Lond.) 27:493-497. [Google Scholar]
- 23.Shun-Shin, M., M. Thompson, C. Heneghan, R. Perera, A. Harnden, and D. Mant. 2009. Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials. BMJ 339:b3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons, C. P., N. L. Bernasconi, A. L. Suguitan, K. Mills, J. M. Ward, N. V. Chau, T. T. Hien, F. Sallusto, D. Q. Ha, J. Farrar, M. D. de Jong, A. Lanzavecchia, and K. Subbarao. 2007. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 4:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song, H., G. R. Nieto, and D. R. Perez. 2007. A new generation of modified live-attenuated avian influenza viruses using a two-strategy combination as potential vaccine candidates. J. Virol. 81:9238-9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suguitan, A. L., Jr., M. P. Marino, P. D. Desai, L. M. Chen, Y. Matsuoka, R. O. Donis, H. Jin, D. E. Swayne, G. Kemble, and K. Subbarao. 2009. The influence of the multi-basic cleavage site of the H5 hemagglutinin on the attenuation, immunogenicity and efficacy of a live attenuated influenza A H5N1 cold-adapted vaccine virus. Virology 395:280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sui, J., W. C. Hwang, S. Perez, G. Wei, D. Aird, L. M. Chen, E. Santelli, B. Stec, G. Cadwell, M. Ali, H. Wan, A. Murakami, A. Yammanuru, T. Han, N. J. Cox, L. A. Bankston, R. O. Donis, R. C. Liddington, and W. A. Marasco. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun, L., X. Lu, C. Li, M. Wang, Q. Liu, Z. Li, X. Hu, J. Li, F. Liu, Q. Li, J. A. Belser, K. Hancock, Y. Shu, J. M. Katz, M. Liang, and D. Li. 2009. Generation, characterization and epitope mapping of two neutralizing and protective human recombinant antibodies against influenza A H5N1 viruses. PLoS One 4:e5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura, S., and T. Kurata. 2004. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect. Dis. 57:236-247. [PubMed] [Google Scholar]
- 30.Throsby, M., E. van den Brink, M. Jongeneelen, L. L. Poon, P. Alard, L. Cornelissen, A. Bakker, F. Cox, E. van Deventer, Y. Guan, J. Cinatl, J. ter Meulen, I. Lasters, R. Carsetti, M. Peiris, J. de Kruif, and J. Goudsmit. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1343-1351. [DOI] [PubMed] [Google Scholar]
- 32.Webster, R. G., N. J. Cox, and K. Sthor. 2002. WHO manual on animal influenza diagnosis and surveillance. World Health Organization, Geneva, Switzerland.
- 33.Weltzin, R., and T. P. Monath. 1999. Intranasal antibody prophylaxis for protection against viral disease. Clin. Microbiol. Rev. 12:383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White, N. J., R. G. Webster, E. A. Govorkova, and T. M. Uyeki. 2009. What is the optimal therapy for patients with H5N1 influenza? PLoS Med. 6:e1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2009. Continuing progress towards a unified nomenclature for the highly pathogenic H5N1 avian influenza viruses: divergence of clade 2.2 viruses. Influenza Other Respir Vir. 3:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. 2008. Update: WHO-confirmed human cases of avian influenza A (H5N1) infection, November 2003-May 2008. Wkly. Epidemiol. Rec. 83:415-420. [PubMed] [Google Scholar]
- 37.Yang, M., Y. Berhane, T. Salo, M. Li, K. Hole, and A. Clavijo. 2008. Development and application of monoclonal antibodies against avian influenza virus nucleoprotein. J. Virol. Methods 147:265-274. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida, R., M. Igarashi, H. Ozaki, N. Kishida, D. Tomabechi, H. Kida, K. Ito, and A. Takada. 2009. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog. 5:e1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, B., N. Zhong, and Y. Guan. 2007. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 357:1450-1451. [DOI] [PubMed] [Google Scholar]