Abstract
Scabies, a parasitic skin infestation by the burrowing “itch” mite Sarcoptes scabiei, causes significant health problems for children and adults worldwide. Crusted scabies is a particularly severe form of scabies in which mites multiply into the millions, causing extensive skin crusting. The symptoms and signs of scabies suggest host immunity to the scabies mite, but the specific resistant response in humans remains largely uncharacterized. We used 4 scabies mite recombinant proteins with sequence homology to extensively studied house dust mite allergens to investigate a differential immune response between ordinary scabies and the debilitating crusted form of the disease. Subjects with either disease form showed serum IgE against recombinant S. scabiei cysteine and serine proteases and apolipoprotein, whereas naive subjects showed minimal IgE reactivity. Significantly (P < 0.05) greater serum IgE and IgG4 binding to mite apolipoprotein occurred in subjects with crusted scabies than in those with ordinary scabies. Both subject groups showed strong proliferative responses (peripheral blood mononuclear cells) to the scabies antigens, but the crusted scabies group showed increased secretion of the Th2 cytokines interleukin 5 (IL-5) and IL-13 and decreased Th1 cytokine gamma interferon (IFN-γ) in response to the active cysteine protease. These data confirm that a nonprotective allergic response occurs in the crusted disease form and demonstrate that clinical severity is associated with differences in the type and magnitude of the antibody and cellular responses to scabies proteins. A quantitative IgE inhibition assay identified IgE immunoreactivity of scabies mite antigens distinct from that of house dust mite antigens, which is potentially important for specific scabies diagnosis and therapy.
Scabies is a disease of the skin caused by the burrowing “itch” mite Sarcoptes scabiei. The predominant disease manifestations are mediated through inflammatory and allergic-like reactions to mite products, leading to intensely pruritic skin lesions. A spectrum of disease is recognized, from the more common ordinary scabies (OS), with an average infestation of 10 to 15 mites per person, to a rare and severely debilitating form of the disease termed crusted (Norwegian) scabies (CS). In this form, mite infestations can number in the millions, and hyperkeratotic skin crusts develop (41, 50). Due to the extreme burden of mites, CS is considerably more infectious, and infectivity persists far longer, as eradicating mites from heavily crusted skin is extremely difficult.
Crusted scabies is an important public health problem, not only for the individuals concerned, but also for their families and communities, in which sufferers may act as “core transmitters” who continue to reinfect others. In many remote Aboriginal communities in northern Australia, scabies prevalence is high: up to 65% in children, with first presentation peaking at 2 months of age (13).
Adult female mites reside in burrows within the stratum granulosum of the epidermis. The clinical rash and itch present as papules or vesicles that may contain individual mites, eggs, egg cases, mite fecal pellets, and debris present in the burrow. A secondary, more generalized papular immune response also occurs. The associated underlying inflammatory response varies in intensity, with combinations of lymphocytes, histiocytes, and polymorphonuclear leukocytes (10, 47). Due to difficulties in isolating sarcoptic mites on the host in OS and to the clinical symptoms imitating those of other skin diseases, scabies is a challenging disease to diagnose (48). To date, limited investigations of humoral immunity to the scabies mite (SM) in patients have yielded contradictory results and have used scabietic extracts from other hosts, such as dogs (37). Interpretation of these studies is complex, as scabies mites are highly host specific and generally produce only a transient, self-limiting reaction in the nonpermissive host.
In Darwin, Northern Territory, Australia, patients with CS were noted to have extremely high levels of total IgE and IgG in serum (41). Western blotting with plasma from these patients demonstrated that 6 of 7 individuals had strong IgE binding to S. scabiei var. canis protein extracts (2). Immunochemical studies have previously demonstrated that sera from rabbits infested with S. scabiei var. canis bind to house dust mite (HDM) extracts and, conversely, that sera from rabbits immunized with HDM extract bind with S. scabiei var. canis whole-mite protein extract (4-6, 18, 20, 36). Allergens from HDMs are recognized as major causes of allergic respiratory disease in humans (17, 43). As SMs and HDMs are phylogenetically related arthropods with similar nutritional requirements, it is not surprising that these mites or their excreta have homologous allergens. However, it is likely that only a few of these allergens are cross-reactive. For example, Der p 5 from the HDM Dermatophagoides pteronyssinus and Blo t 5 from the storage mite Blomia tropicalis have been studied extensively, and although they have 43% amino acid identity, they are not IgE cross-reactive (31). The identities of the specific cross-reactive molecules between S. scabiei and D. pteronyssinus remain undefined but may be glycan related (33).
The recent development of S. scabiei cDNA libraries and expressed sequence tag (EST) databases (21, 29) allows more precise characterization of the specific antigens responsible for the immune reactions to the SM. The cDNAs encoding S. scabiei var. hominis cysteine proteases (27), serine proteases (26), glutathione S-transferase (GST) (16), and apolipoprotein (25) show homology to the D. pteronyssinus HDM group 1, 3, 8, and 14 allergens, respectively (43). As with HDMs, the availability of recombinant proteins and identification of key immunoreactive allergens for the SM would facilitate development of refined diagnosis and potential immunotherapy. Thus, more effective control of mite infestations at both an individual and a community level may be possible. We report here the characterization of specific antibody binding profiles and cellular immune responses of subjects with clinical scabies by using purified S. scabiei var. hominis recombinant proteins. Quantitative IgE inhibition analysis of cross-reactivity with HDM allergens identified IgE epitopes of scabies mite proteins distinct from HDM epitopes, a prerequisite for using purified allergens in scabies diagnosis and therapy.
MATERIALS AND METHODS
Study groups.
Blood samples were collected from people living in both remote and urban regions of Australia. The donors included a total of 82 indigenous and nonindigenous subjects (33 male and 49 female; mean age, 45 years) from northern Australia and 13 subjects attending the Allergy Clinic at the Alfred Hospital, Melbourne (Victoria, Australia). Collection of blood was approved by the Human Research Ethics Committees of the Northern Territory Department of Health and Families and the Menzies School of Health Research (approval number 97/21) and by the Alfred Hospital. Written informed consent was obtained from each donor. Together, the subjects comprised 32 subjects with CS; 24 subjects with OS (endemic infested); 20 naïve, never-exposed subjects without HDM allergy (nonendemic negative controls; N); and 19 naive, never-exposed subjects with HDM allergy (nonendemic allergic controls; N-HDM).
Crusted scabies was diagnosed clinically (37) and confirmed microscopically on the basis of skin scrapings containing more than 5 mites. Demographic information, risk factors, and immunological parameters are routinely collected for these patients on admission to the hospital, and only those with no overt immunosuppression (e.g., malignancies, chemotherapy, HIV, or leprosy) were included in the study. Due to considerable difficulties in isolating mites from patients with OS and the lack of a diagnostic blood test for scabies, OS was diagnosed clinically based on typical lesions and rash. Control (N and N-HDM) subjects were sourced from the Darwin and Melbourne urban regions, where scabies is rare. These subjects had no known current scabies or history of scabies.
Collection of blood samples.
Heparinized venous blood (50 ml) was centrifuged at 645 × g for 10 min. Approximately 5 ml of plasma was removed for serological studies and subsequently replaced with heparinized RPMI 1640 (Gibco Life Technologies, Invitrogen Pty Ltd., Victoria, Australia). Peripheral blood mononuclear cells (PBMC) were separated by Ficoll-Hypaque (Amersham Pharmacia, GE Healthcare Pty Ltd., NSW, Australia) density gradient centrifugation using standard methods. The samples were either used immediately or cryopreserved in liquid nitrogen for later testing.
Antigens: protein sequence and expression.
The cDNA was amplified from S. scabiei var. hominis cDNA libraries (21) by PCR using specific primers. The primers were designed to amplify the mature forms of an active cysteine protease molecule, Yv5032CO8 (CO8) (27); an inactive cysteine protease paralogue, Yv5009FO4 (FO4); an active serine protease, Sar s 3 (GO3) (26); and Ssag1.2, a 1.2-kb fragment of an S. scabiei apolipoprotein (AF462196) (21) (Table 1). The PCR products were cloned into the pQE-9 expression vector (Qiagen Ltd., Victoria, Australia) in frame with the 6-His tag, and then expressed in the Escherichia coli strain BL21(pREP4) and purified using Ni-nitrilotriacetic acid (NTA) agarose (Qiagen Ltd., Victoria, Australia), according to the manufacturer's instructions. The concentration of each protein was determined by Bradford assay using Bradford reagent (Bio-Rad Laboratories, Regents Park, NSW, Australia).
TABLE 1.
Primer sequences for proteases and apolipoprotein
| Protein | Name | Primer sequences (5′-3′)a | Primer restriction sites | Size (aa) | Molecular mass (kDa) | GenBank accession no. | Reference(s) |
|---|---|---|---|---|---|---|---|
| Active cysteine protease | Yv5032C08 | aactgcagAAATTGGGCGAGCTCGATCTG, cccaagcttTCAAGCTACAGTTTCTTTGCCCAG | PstI, HindIII | 227 | 25 | AY525149 | 27 |
| Inactive cysteine protease | Yv5009F04 | cgggatccAAACTACCAAAATGGTTCGATC, ttctgcagTCATTCAAAATCTTCAGGCTC | BamHI, PstI | 240 | 28 | AY525155 | 27 |
| Active serine protease | Yv7016G03 | accggtcgacATTGTCGGCGGTCGTTTAGCTAAGCC, accgctgcagTTAATTATTTCTAAGGATATTTTTGATCCATTGG | SalI, PstI | 231 | 26 | AY333071 | 7 |
| Apolipoprotein | Ssag1.2 | ggggatccTCGAATGTGAAACGAAACAATG, ggctgcagnnCTGCAAATATTGTCTGATAGC | BamHI, PstI | 393 | 46 | AF462196 | 25, 21 |
S. scabiei-specific sequence shown in uppercase.
Endotoxin levels in the recombinant proteins were undetectable (<3 endotoxin units [EU]; QCL-1000 Endotoxin Detection Kit; BioWhittaker, Oslo, Norway). Mitogenicity of the SM recombinant proteins was excluded by culturing increasing concentrations of the proteins with negative-control PBMC and comparing the proliferative responses with that for CS subjects by [3H]thymidine incorporation. Toxicity was excluded by culturing PBMC with phytohemagglutinin with and without added recombinant protein and comparing the proliferative responses (data not shown).
Electrophoresis and Western immunoblotting.
Purified proteins (0.5 to 1 μg/lane), including S. scabiei GST as a control protein (16), were resolved on 12% SDS-PAGE reducing gels for 60 min at 125 V DC, with Benchmark prestained protein ladders (Invitrogen Pty Ltd., Victoria, Australia) as molecular mass standards. Gels were stained with Coomassie blue R250 (Sigma-Aldrich, Sydney, NSW, Australia) or, for Western blotting, transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) for 60 min using standard methods (9, 44). For Western blots, primary human plasma was diluted 1:50, and antibody binding was detected using 1:1,000 anti-human IgE alkaline phosphatase conjugate (Sigma-Aldrich) developed with BCIP (5-bromo-4-chloro-3-indolylphosphate)/nitroblue tetrazolium (NBT) substrate (Promega Australia, Annandale, NSW, Australia).
Immunohistochemistry.
Ethics approval for the use of rabbits and mice for generation of antibodies against SM recombinant antigens was granted by the Animal Ethics Committee of the Northern Territory University, Darwin, Northern Territory, Australia (approval number A99019). Antisera were prepared at the Veterinary Services Division of the Institute of Medical and Veterinary Sciences, Gilles Plains, South Australia, using their standard immunization schedule.
Shed skin tissue highly infested with SMs was obtained from subjects with CS, and 5-mm3 blocks were fixed in 10% formalin solution. Samples were dehydrated in ethanol and embedded in paraffin at 60°C, and 6- to 10-μm sections were dried overnight at 34°C. The sections were dewaxed in histolene, rehydrated, and blocked with 10% goat serum (Sigma-Aldrich)-1% bovine serum albumin (BSA) (Roche Diagnostic GmbH, Mannheim, Germany)-phosphate-buffered saline (PBS) for 30 min. Endogenous peroxidase activity was then blocked with 3% (vol/vol) H2O2 (Fluka Analytical, Sigma-Aldrich, Steinheim, Switzerland)-1% BSA-PBS for 10 min. After the sections were washed, mouse anti-CO8 or rabbit anti-Ssag1.2 (1/500 and 1/5,000 in 1% BSA-PBS, respectively) was added for 1 h at room temperature. After further washing, either Dako EnVision anti-mouse immunoglobulin (Ig) horseradish peroxidase (HRP)-labeled polymer (Dako Cytomation, Carpinteria, CA), for detection of CO8, or Dako EnVision+ Dual Link anti-mouse/anti-rabbit HRP-labeled polymer, for detection of Ssag1.2, was added for 30 min at room temperature. After another washing, the chromogenic reaction was conducted using Nova Red (Vector, Burlingame, CA) and previously described methodology (51). Prebleeds from mice or rabbits used for raising antibodies were used as negative controls.
For anti-human IgE staining, slides were stained as described above, apart from an extra incubation step (45 min at 37°C) at the beginning for antigen retrieval with trypsin (Zymed Laboratories, Invitrogen Corporation, CA). Mouse anti-human IgE (Zymed Laboratories) was applied (1:500) at room temperature for 1 h, followed by the secondary antibody, Dako EnVision+ HRP-labeled polymer anti-mouse Ig (Dako Cytomation). Chromogenic reactions were then conducted as described above. Slides were counterstained with Mayer's hematoxylin and bluing solution (0.45% ammonium hydroxide in 70% ethanol).
ELISA.
Plasma collected from CS, OS, and N subject groups was tested for antibody reactivity to CO8, FO4, GO3, and Ssag1.2 by enzyme-linked immunosorbent assay (ELISA) using IgA-, IgG-, IgG1-, IgG4-, and IgE-specific secondary antibodies. Optimum dilutions of plasma, secondary antibodies, and tertiary antibodies were first determined by checkerboard titration, using plasma from subjects with and without scabies and HDM allergy. Antigen-specific assays were conducted in triplicate and no-antigen background controls in duplicate. For specific IgE assays, IgG was not adsorbed out prior to plating the plasma.
Briefly, Nunc-Immuno 96-well plates with MaxiSorp (Nalge Nunc International, New York, NY) were coated overnight at 4°C with 50 μl per well of recombinant antigen adjusted to 2 μg/ml of protein in 3 M urea buffer (3 M urea, 100 mM NaH2PO4, 10 mM Tris base, pH 8.2). Blank control wells were coated with 0.01% BSA in 3 M urea buffer. The plates were washed in PBS with 0.5% Tween 20 (PBST) and then blocked with 1% BSA in PBST for 2 h at 37°C. After another washing, plasma diluted in 0.1% BSA in PBST (Table 2) was added (50 μl per well), and the plates were incubated for 2 h at 37°C. The plates were washed again, and then either streptavidin-HRP-conjugated polyclonal rabbit anti-human antibody (IgA and IgM; Dako, Camberfield, Australia) or unlabeled polyclonal rabbit anti-human antibody (IgG and IgE; Dako, Australia) in 0.1% BSA in PBST was added (50 μl per well). The plates were incubated for 2 h at 37°C. For IgG and IgE detection, the plates were washed again and incubated with HRP-conjugated goat anti-rabbit IgG (50 μl per well; Promega, Sydney, Australia) for a further 2 h at 37°C. All plates were given a final wash with PBST and PBS before the addition of a solution of 5 mg O-phenylenediamine dihydrochloride (Sigma, Australia) in phosphate citrate buffer with sodium perborate (50 μl per well). The plates were incubated for 10 min at 37°C, according to the manufacturer's instructions. Color development was stopped with 4 M HCl (50 μl per well), and absorbance was measured at 490 nm. Background (no-antigen)-corrected absorbance of replicate wells was determined, and the data are presented as the mean optical density at 490 nm (OD490) ± standard deviation (SD). The IgE positive cutoff value was determined as 3 SD above the mean absorbance for a panel of 7 N plasmas.
TABLE 2.
Antibody dilutions for detection of antibodies to recombinant scabies antigens in human plasma samples by ELISA
| Antibody type | Dilution |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO8 |
FO4 |
GO3 |
Ssag1.2 |
|||||||||
| First | Second | Third | First | Second | Third | First | Second | Third | First | Second | Third | |
| IgE | 100 | 500 | 500 | 100 | 500 | 500 | 50 | 1,000 | 1,000 | 100 | 4,000 | 4,000 |
| IgM | 100 | 2,000 | NAa | 100 | 4,000 | NA | NA | NA | NA | 50 | 1,000 | NA |
| IgA | 100 | 2,000 | NA | 90 | 1,000 | NA | NA | NA | NA | 50 | 500 | NA |
| IgG | 2,000 | 8,000 | 8,000 | NA | NA | NA | NA | NA | NA | 200 | 1,000 | NA |
| IgG4 | 2,000 | 1,000 | 1,000 | NA | NA | NA | 50 | 500 | 1,000 | 10 | 1,000 | 2,000 |
| IgG1 | 160 | 500 | 1,000 | NA | NA | NA | NA | NA | NA | 100 | 1,000 | 2,000 |
NA, not applicable.
Levels of Der p 1-specific IgE were determined by dissociation enhanced lanthanide fluoroimmunoassays (DELFIA) (Wallac, Turku, Finland). Total IgE was determined by ImmunoCap assay (Pharmacia Diagnostics AB, Uppsala, Sweden).
IgE cross-reactivity studies.
To investigate the cross-reactivity of IgE with SM and HDM allergens, the ability of whole HDM extract to inhibit IgE immunoreactivity with S. scabiei Ssag1.2 and CO8 was tested by inhibition ELISA (42). Briefly, ELISA plates were coated with Ssag1.2 (2 μg/ml in 3 M urea buffer; 50 μl per well) or CO8 (1 μg/ml in 1 M urea buffer; 50 μl per well) and incubated overnight at 4°C. The plates were washed and blocked as described above. Plasma samples were diluted 1:160 (Ssag 1.2) or 1:80 (CO8) in 0.1% BSA in PBST. The diluted plasmas were preincubated with increasing concentrations of HDM extract or, as positive and negative controls, Ssag 1.2 or CO8 or no inhibitor, for 90 min at room temperature. The plasma and HDM extract mixtures or diluted plasma samples were added to the coated plates, and the IgE ELISA protocol was followed as described above. The percentage of inhibition of IgE binding was calculated as follows: percent inhibition = 100 − (OD490 of plasma with HDM extract/OD490 of plasma without HDM extract × 100).
PBMC proliferation studies.
PBMC were cultured in triplicate (1 × 105 cells/200 μl/well) in 96-well plates (Nunc Maxisorp) in complete medium (RPMI 1640 supplemented with 100 IU/ml penicillin or streptomycin, 2 mol/liter l-glutamine [Gibco Life Technologies, Auckland, NZ], and 5% screened heat-inactivated human AB+ serum [Sigma, St. Louis, MO]). The PBMC were stimulated with recombinant SM antigen at concentrations predetermined to induce maximum proliferation (1 μg for CO8 and FO4, 10 μg for GO8, and 3 μg for Ssag) for 7 days at 37°C in 5% CO2. Control wells contained no antigen or phytohemagglutinin (final concentration, 2.5 μg/ml). After 6 days, the cells were pulsed with [3H]thymidine (1 μCi/well; Amersham, Pharmacia, GE Healthcare Pty Ltd., NSW, Australia) overnight and then harvested. CPM were determined with a Wallac Winspectral Liquid Scintillation Counter (Perkin Elmer, Victoria, Australia). The results are presented as the mean stimulation index (SI) of triplicates, where the SI is equal to CPM challenged cells/non-CPM-challenged cells. An SI of ≥5 was considered a positive response.
Cytokine analysis.
Supernatants (20 μl per well) were collected from day 3 and day 6 cultures of fresh PBMC stimulated with S. scabiei cysteine protease CO8 and apolipoprotein Ssag1.2. Triplicate samples were pooled and stored at −80°C until analysis was done. The supernatants were analyzed for levels of interleukin 2 (IL-2), IL-4, IL-5, IL-10, tumor necrosis factor (TNF), and gamma interferon (IFN-γ) with the Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit (Becton Dickinson, NSW, Australia) and a four-color FACSCalibur flow cytometer (Becton Dickinson, CA), according to the manufacturer's instructions. Levels of IL-13 were tested with the Human IL-13 CBA Flex Set (Becton Dickinson, Australia). After fluorescence data were obtained, the results were generated in data format with the BD CBA software.
Statistical analysis.
Differences between the distributions of 2 unmatched groups were analyzed with nonparametric Mann-Whitney U tests, using GraphPad PRISM version 5.0 (GraphPad Software, Inc.). The nonparametric Kruskal-Wallis test was used to assess differences between 3 or more groups. Differences were considered statistically significant when the P value was <0.05.
RESULTS
Expression, purification, and localization of recombinant S. scabiei proteins.
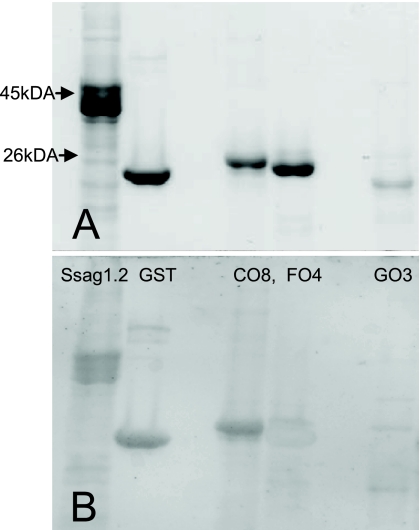
The expressed, purified SM recombinant proteins migrated with molecular masses between 25 and 46 kDa (Fig. 1A). The Ssag1.2 protein migrated as 2 or 3 multiple bands due to the formation of aggregates on refolding under denaturing conditions, as observed previously for this protein (25). Western blotting demonstrated that the sample from the subject with CS showed strong IgE binding to CO8 and Ssag1.2 and weaker binding to FO4 and GO3 (Fig. 1B).
FIG. 1.
Purified S. scabiei var. hominis recombinant antigens Ssag1.2, GST, CO8, FO4, and GO3. (A) SDS-12% PAGE stained with Coomassie brilliant blue. (B) Western blot with plasma from a subject with crusted scabies.
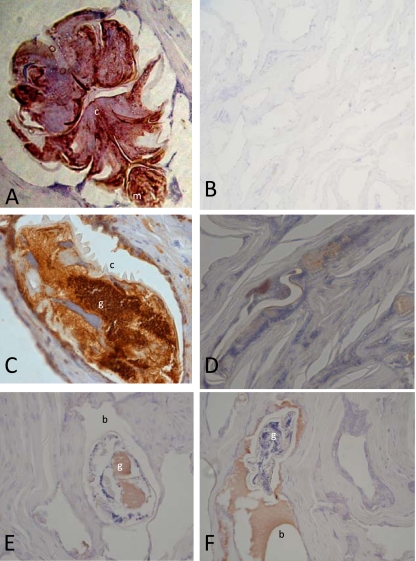
Immunolocalization studies using mouse anti-CO8 and rabbit anti-Ssag1.2 antisera and sections of human skin infested with mites clearly demonstrated the localization of Ssag1.2 as a component of the external cuticle and CO8 within the gastrointestinal tracts of the mites (Fig. 2). The CO8 was also localized to the scybala (fecal pellets; data not shown). Sections probed with polyclonal anti-human IgE antibodies showed IgE inundating the mite burrow (Fig. 2E) or localized inside the gastrointestinal tract of the mite (Fig. 2F).
FIG. 2.
Sections of human skin infested with S. scabiei mites probed with rabbit anti-Ssag1.2, showing staining of mite external cuticle (A); probed with rabbit prebleed (B); probed with mouse anti-CO8, showing staining of the mite gastrointestinal tract (C); probed with mouse prebleed (D); and probed with anti-human IgE showing in vivo localization of IgE in the mite gastrointestinal tract and burrow (E and F). (m, mouthparts; c, cuticle; g, gut; b, burrow).
Characterization of human humoral immune response to S. scabiei cysteine proteases and apolipoprotein and evaluation of cross-reactivity with HDM allergens.
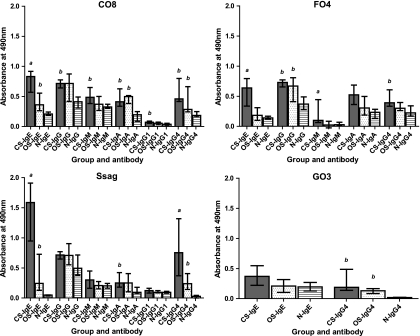
Total IgE levels (kU/liter) for subjects with CS were significantly higher than the levels observed for subjects with OS (P < 0.0364; data not shown). Furthermore, IgE binding to the purified recombinant mite proteins C08, F04, and Ssag1.2 was stronger for subjects with CS than for subjects with OS (P < 0.0037, P < 0.0019, and P < 0.0062, respectively) and for control (N) subjects (P < 0.0004, P < 0.0004, and P < 0.0005, respectively) (Fig. 3). No significant IgE binding of plasma from subjects with CS to GO3, the serine protease construct, was observed (Fig. 3). Compared to naive subjects, significant IgE binding of plasma from subjects with OS to CO8 (P < 0.0047) and Ssag1.2 (P < 0.0003) was also observed. However, significant binding to the inactive form of the cysteine protease molecule (F04) or the serine protease (GO3) molecule was not observed. Compared with the cysteine (CO8; P < 0.0279) and serine (GO3; P < 0.0039) protease constructs, the apolipoprotein fragment Ssag1.2 was more highly recognized by IgE from subjects with CS than by that from subjects with OS. An IgE reactivity threshold was determined for CO8 and Ssag1.2 by calculating the mean of the naive group plus 3 standard deviations for each protein. Based on these results, the sensitivity of the IgE ELISA for determining scabies infestation was 88% and the specificity was 100%, using the Ssag1.2 fragment. Similarly, the sensitivity was 76% and the specificity was 100% using CO8.
FIG. 3.
Specific anti-CO8, -FO4, -GO3, and -Ssag1.2 plasma antibody levels measured by ELISA in subjects with CS and OS and naïve (N) subjects. The bars represent the median with interquartile range. a, significantly different (P < 0.05) from OS and N groups; b, significantly different (P < 0.05) from the N group only. Specific P values are given in the text.
Subjects with CS showed significantly increased IgG4 binding to C08 (P < 0.0104), F04 (P < 0.0044), GO3 (P < 0.0003), and Ssag1.2 (P < 0.0001) than did naïve subjects but only significantly increased IgG4 binding to Ssag1.2 (P < 0.0343) compared to subjects with OS (Fig. 3). Plasma from subjects with OS also showed increased binding levels of IgG4 to CO8 (P < 0.0104), GO3 (P < 0.0003), and Ssag1.2 (P < 0.0012) compared with naïve subjects.
Interestingly, increased binding of IgA to CO8 was observed for both the CS (P < 0.0003) and OS (P < 0.0002) groups, and to Ssag1.2 for the CS group (P < 0.0012), compared with the control (N) group (Fig. 3). Comparison of binding levels for IgG, IgG1, and IgM ELISA data between groups or proteins was not informative.
To investigate the cross-reactivity of SM proteins with HDM proteins, plasma from 7 N-HDM subjects was tested for IgE reactivity against the S. scabiei Ssag1.2 construct. No IgE binding was observed (data not shown). In addition, IgE binding of plasma from subjects with CS or OS to the recombinant HDM group 1 allergen Der p 1 was also tested, but no binding was observed (data not shown).
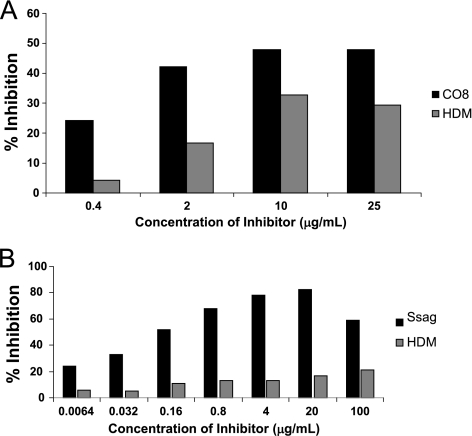
The ability of whole HDM extract to inhibit IgE immunoreactivity with S. scabiei CO8 and Ssag1.2 was tested in inhibition ELISA (Fig. 4A and B). The maximum percent inhibition by HDM extract of scabies subject plasma IgE binding to CO8 was 30% and to Ssag1.2 was 17% (25 μg/ml and 20 μg/ml of HDM extract as the inhibitor, respectively).
FIG. 4.
(A) Inhibition ELISA by HDM extract for plasma IgE from a subject with CS. CO8 was immobilized on an ELISA plate, and the inhibition of CS IgE binding by increasing concentrations of HDM extract or CO8 as a positive control was assessed. (B) Same as for panel A, with apolipoprotein Ssag1.2 immobilized on the plate and as the positive control.
Characterization of the human cellular response to S. scabiei proteases and apolipoprotein.
One subject allocated to the OS group had excessively elevated SI values for all 4 SM recombinant proteins (CO8, 155.52; FO4, 137.65; GO3, 97.68; Ssag1.2, 71.11), in combination with a proliferative response to phytohemagglutinin within the normal range (data not shown). As the SI values far exceeded even those observed for subjects with CS, these data were excluded from the analysis.
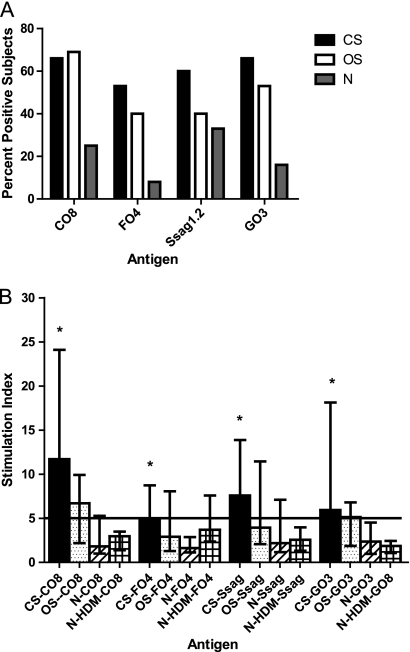
The overall percentage of subjects showing positive proliferative responses to scabies recombinant antigens CO8, FO4, GO3, and Ssag1.2 was significantly higher in S. scabiei-infected individuals than in non-S. scabiei-infected individuals (P < 0.03). No significant differences in percentages of positive subjects were observed for the CS group compared with the OS group (Fig. 5A), although there was a trend toward higher reactivity to the FO4, GO3, and Ssag1.2 antigens in the CS group.
FIG. 5.
(A) Percentage of subjects showing positive responses (stimulation index ≥ 5) after culture of PBMCs stimulated with S. scabiei recombinant proteins CO8, FO4, Ssag1.2, and GO3. All CS and OS group results were significantly different (P < 0.05) from those of the N group. (B) Mean SI (with 95% confidence interval) after culture of PBMC stimulated with S. scabiei recombinant proteins CO8, FO4, Ssag1.2, and GO3. *, significantly different (P < 0.05) from the N group for each protein. Specific P values are given in the text. CS, n = 15; OS, n = 12; N, n = 12; N-HDM, n = 5.
The PBMC proliferative responses to the 4 SM recombinant antigens varied in the 4 subject groups (Fig. 5B). The responses to all antigens were significantly higher in the CS group than in the N group (CO8, P < 0.0037; FO4, P < 0.0073; GO3, P < 0.0128; Ssag1.2, P < 0.0264). However, no significant differences in responses to any antigens were observed when the CS and OS groups, the OS and N groups, or the OS and N-HDM groups were compared. Additionally, there were no significant differences between stimulatory responses to the 4 recombinant antigens within the CS group. However, significant differences were observed between the 4 subject groups in reactivity with CO8 (P < 0.0084) and GO3 (P < 0.0195), but not with FO4 (P < 0.0514) or Ssag1.2 (P < 0.0811).
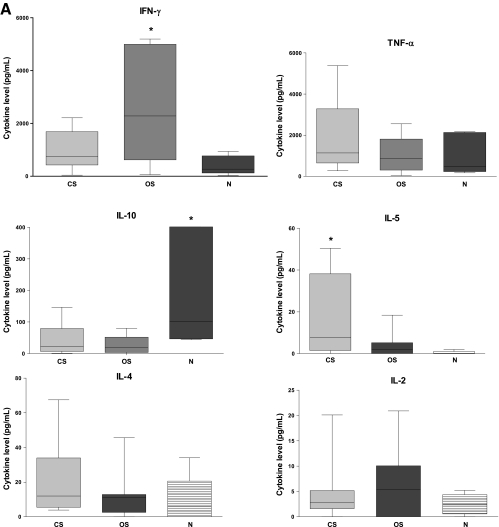
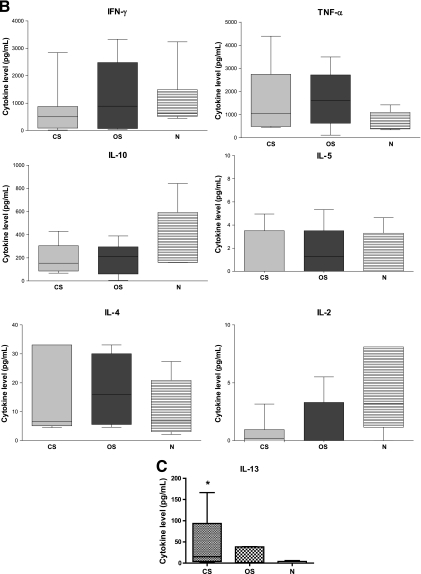
The cellular immune response to SM antigens was also characterized by evaluating IFN-γ, IL-4, IL-5, IL-10, TNF, IL-2, and IL-13 cytokine profiles in PBMC culture supernatants collected after 72 h of CO8 or Ssag1.2 stimulation. A clear trend of decreased IFN-γ production was observed in CO8- and Ssag1.2-stimulated PBMC from subjects with CS compared to subjects with OS (Fig. 6A and B). However, significant differences between IFN-γ levels were observed only in the OS and N groups for the CO8-stimulated PBMC (P < 0.0480). No differences in IL-4 levels were observed between groups for either stimulation protein, but the IFN-γ/IL-4 ratio for CO8 stimulation trended lower in the CS group than in the OS group (P < 0.0734) (data not shown). A trend of increased IL-5 expression was also observed in subjects with CS, with 3 of 7 subjects secreting high levels of IL-5 cytokine in response to CO8 stimulation. However, significant differences between IL-5 levels were observed only between the CS and N groups (P < 0.0286). The levels of IL-10 cytokine were significantly higher in CO8-stimulated PBMC from the N group than in those from the CS group (P < 0.0480), and the OS group showed a trend toward a decreased level when similarly compared (P < 0.0732). No differences in TNF and IL-2 levels were observed between any groups for either SM protein. The IL-13 levels in PBMC supernatants at 48 h after stimulation with CO8 were significantly increased in the CS group compared to the N group (P < 0.0317) (Fig. 6C). However, there were no significant differences between IL-13 levels in the CS and OS groups or the OS and N groups.
FIG. 6.
(A) Secreted IFN-γ, TNF, IL-10, IL-5, IL-4, and IL-2 cytokine levels analyzed at 72 h from CO8-stimulated PBMCs. CS, n = 7; OS, n = 7; N, n = 5. Note that one N value (IL-10) is outside the graph limits (797 pg/ml). (B) Secreted IFN-γ, TNF, IL-10, IL-5, IL-4, and IL-2 cytokine levels analyzed at 72 h from Ssag1.2-stimulated PBMCs. CS, n = 6; OS, n = 7; N, n = 5. (C) Secreted IL-13 cytokine levels analyzed at 48 h from CO8-stimulated PBMCs. CS, n = 5; OS, n = 5; N, n = 5. The bars represent box and whiskers displaying minima and maxima. *, significantly different (P < 0.05) from the N group. Specific P values are given in the text. Horizontal lines within the bars indicate medians.
DISCUSSION
Scabies mite proteins have considerable similarity to the extensively studied HDM proteins that are a major cause of allergic disease. The cloning of SM proteins corresponding to those of the predominant HDM allergens has now facilitated studies of their sequence homology (25, 26, 34, 45, 46, 49, 50), localization (47, 51), enzymatic activity (7, 16, 22, 35, 40), and interactions with the immune system (8, 11, 30). Here, we have studied differences in the types and magnitudes of the systemic antibody and cellular responses to characterize the specific immune response in OS and compare it to the more severe and debilitating crusted form of the disease (CS). Our studies also looked for any cross-reactivity between SM and HDM proteins to assess the potential future design of specific immunodiagnostics for scabies infestation. Our results demonstrate that patients with OS and CS have a specific allergic IgE response to S. scabiei cysteine proteases and apolipoprotein. Clinical severity was reflected in differences in the types and magnitudes of the antibody and cellular responses to these proteins. Furthermore, our results show that a nonprotective Th2 response occurs in patients with CS, which may, in part, account for the pathology of this disease form.
In Darwin, patients with CS have extremely high levels of total IgE in serum (41). In this study, subjects with CS demonstrated significantly higher levels of total IgE (data not shown) and scabies-specific IgE and IgG4 than subjects with OS and naïve control subjects. A correlation was observed between levels of CO8- and Ssag1.2-specific IgE and IgG4 antibody responses and the classification of subjects into disease types in conjunction with observed inflammatory processes. These results demonstrate that Ssag1.2 and CO8 are present in scabies infestations at sufficient levels to elicit the production of scabies-specific IgE and IgG4 and are consistent with the conclusion that they are abundant allergens. Previous studies revealed that sequences corresponding to an apolipoprotein are the third most frequent cDNA clones in an S. scabiei EST library (21), with the abundance of the group 1 scabies allergen cDNA 10 times lower (27). These frequencies may account for the greater immunological response to Ssag1.2 compared with the other proteins in our study.
In this study, levels of specific IgG, IgG1, and IgM were not informative for disease status. Increased levels of total IgG and IgM have previously been observed in subjects with OS. However, it is unclear whether these increased antibody levels represent specific or nonspecific immunological reactions against scabies antigens or a response to the secondary cutaneous bacterial infections commonly associated with scabies (14). Synthesis of IgG4 is related to the Th2 immune response, in combination with chronic or repeated antigenic exposure, and may be of particular benefit to the host in protection against anaphylactic responses by blocking IgE receptor sites on antigens. Protective mechanisms may also be modulated by the IgE/IgG4 ratio, which tends to be high in heavy infections of schistosomiasis and lymphatic filariasis (1, 32). A correlation between IgE responses and protective immunity has been established in human schistosomiasis (23, 24, 39). The reasons for the extreme nonprotective IgE and IgG4 scabies-specific antibody responses and the eosinophilia seen in subjects with CS remain unknown and appear to be related to an inappropriate Th2-polarized immune response. Future studies need to determine the roles of mast cells, basophils, and eosinophils in CS and how levels of specific IgE and IgG4 are regulated in CS. Additionally, investigating the clinical effect of selectively boosting IgG4 over IgE is important. Finally, understanding the immunoregulatory mechanism involved in the protective immune response in OS is also necessary to develop rational strategies for intervention. This understanding is especially important when a Th2 response is associated with disease susceptibility.
Secretory IgA is important in local (mucosal) immunity and is the predominant antibody in external secretions, such as sweat, saliva, and tears, as well as in intestinal and respiratory secretions, after stimulation. Total serum IgA levels have been reported to be significantly lower during OS infestation than following successful treatment (19, 38). However, in this study, specific IgA binding levels to the SM recombinant construct CO8 were significantly increased in both the CS and OS groups compared to the control (N) group. The observation that mouse anti-CO8 polyclonal antibody predominantly bound to the gastrointestinal tracts of the mites suggests that S. scabiei cysteine proteases play a role in skin digestion and mite burrowing and that specific anti-CO8 IgA is also involved in the skin immune response.
Our studies demonstrated minimal cross-reactivity between IgE from HDM-allergic individuals (N-HDM) and the S. scabiei allergens CO8 and Ssag1.2. Similarly, plasma from subjects with scabies demonstrated little or no IgE cross-reactivity with purified Der p 1 allergens. S. scabiei proteins that fail to show cross-reactivity with HDM proteins, but which are recognized by serum antibody from scabies-infested subjects, provide potential target antigens for subsequent development into a high-performance serodiagnostic test for scabies infestation.
Regulation of the immune response directed against S. scabiei is critical to establish effective control of the disease. To gain a better understanding of the possible roles that T cells and their associated cytokines have in the disease progression and the protective immune responses to infection, a detailed study of the antigen-specific, cytokine-producing T cells was performed. The S. scabiei recombinant proteins CO8, FO4, and Ssag1.2 induced strong proliferative responses of PBMC from scabies subjects compared to control subjects. Subjects with OS tended to show less stimulation than subjects with CS. These results demonstrate the importance of the cell-mediated immune response to S. scabiei, with all antigens inducing a stimulatory response in PBMC from scabies-exposed subjects.
Of note, the subject classified as OS who was excluded from the cell proliferation analysis due to extremely high responses to scabies antigens was a 48-year-old man who had been admitted 3 times to the hospital for suspected crusted scabies (in 2002, 2005, and 2006). However, mites were found in skin scrapings only on the second admission (only 4 mites were observed). The blood used in the assay was taken on the 2006 admission, in which crusting on the subject's feet was documented, but no mites were observed in multiple skin scrapings. To maintain consistency in the study and based on our criteria of subjects with CS having more than 5 mites in skin scrapings, he was placed in the OS group. This situation highlights the difficulties faced by clinicians in diagnosing suspected CS and the need for more robust diagnostic tests.
The role of T cells secreting Th2-type cytokines in asthma and allergic diseases has been well documented (28). The cysteine and serine proteases of the dust mite; the serine protease of Aspergillus fumigatus; the aspartic protease of the cockroach, Bla g 2; and helminth proteases have all been documented to induce Th2-driven inflammatory responses dominated by elevated IgE, eosinophilia, and Th2 cells (15). Therefore, the present study analyzed for cytokines that might be involved in the activation of an effective anti-Sarcoptes immune response. Our sample sizes were small, and thus, the nonparametric tests used for analysis had less power or were less likely to detect true differences between groups. Nevertheless, the in vitro responses of cytokines to S. scabiei antigens observed in cultures confirmed the in vivo immune modulation to scabies infestation and the antibody class switches detected in plasma samples. In subjects with CS versus those with OS, trends toward increased levels of IL-5 and IL-13, in combination with lower levels of IFN-γ, were observed in the supernatants of CO8-stimulated PBMC. These findings are similar to those seen with Der p 1 and HDM allergy. Importantly, the proteolytic activity of HDM cysteine proteases is now believed to play a significant role in its ability to elicit Th2 responses (12). In this study, the recombinant SM cysteine and serine proteases were not expressed as active protease enzymes. However, CO8 is clearly recognized as a major allergen. The Th2 cytokines IL-4 and IL-13 play important roles in the class switching of B cells to an IgE-secreting phenotype and in the generation of Th2-type immune responses. The cytokine IL-5 promotes the maturation of eosinophils, and eosinophils are known to be extremely high in CS (42). Interestingly, in this analysis, no significant differences in IL-4 levels could be demonstrated between subjects with CS and those with OS. However, the IFN-γ/IL-4 ratio indicated a trend toward a Th2 response in CS and a Th1 response in OS (P < 0.07). Statistically significantly elevated levels of IL-4 have been reported previously in pilot studies on cytokine production in fresh PBMC collected from subjects with CS and from noninfested control subjects in northern Australia (50). In this study, the subjects with CS were at different stages of disease progression. Therefore, it is possible that greater differences in PBMC-secreted levels would be revealed by a detailed time course analysis of IL-4 production in CS. The in vivo findings of significantly increased levels of scabies-specific IgE and IgG4 in plasma from subjects with CS and documented clinical eosinophilia in CS are collectively indicative of the trend toward increased production of the cytokines IL-4, IL-5, and IL-13 in CS subjects.
Notably, CO8-stimulated PBMC from control (N) subjects showed significantly increased levels of IL-10, similar to previous studies using whole SM extract (3). The cytokine IL-10 is capable of inhibiting synthesis of the proinflammatory cytokines IFN-γ, IL-2, and TNF and is also stimulatory toward certain T cells and mast cells and stimulates B-cell maturation and antibody production. These results suggest IL-10 may play a role in the human delayed immune response, depressing the inflammatory and allergic responses so that clinical symptoms are not seen until 4 to 6 weeks after a person becomes infested with scabies mites.
In summary, patients with OS and CS have a specific allergic IgE response to S. scabiei cysteine proteases and apolipoprotein. Clinical severity is reflected in differences in the types and magnitudes of the antibody and cellular responses to these proteins. Our results demonstrate a nonprotective Th-2 response in patients with CS, as previously hypothesized (50), and suggest that dysfunctional effector cells may, in part, account for the pathology of CS. Difficulties associated with the collection and culture of S. scabiei var. hominis have previously confounded studies on the characterization of the immune responses in scabies. These studies using recombinant proteins are the first to describe the specific immune response in scabies. This information will assist in developing tools for the early diagnosis of scabies carriers and in better control of the infestation in communities where the disease is endemic. This research considerably advances our understanding of the immunopathogenesis of scabies and helps direct future development of specific immunotherapy and vaccines.
Acknowledgments
This study was supported by National Health and Medical Research Council of Australia grant 283300 and the Channel 7 Children's Research Foundation of South Australia. D.J.K. was supported by program grant 496600 and an NHMRC Fellowship.
We acknowledge Leigh Findlay for editorial assistance with the manuscript and Katja Fischer for technical support.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Aalberse, R. C., P. H. Dieges, V. Knul-Bretlova, P. Vooren, M. Aalbers, and J. van Leeuwen. 1983. IgG4 as a blocking antibody. Clin. Rev. Allergy 1:289-302. [DOI] [PubMed] [Google Scholar]
- 2.Arlian, L. G., M. S. Morgan, S. A. Estes, S. F. Walton, D. J. Kemp, and B. J. Currie. 2004. Circulating IgE in patients with ordinary and crusted scabies. J. Med. Entomol. 41:74-77. [DOI] [PubMed] [Google Scholar]
- 3.Arlian, L. G., M. S. Morgan, and C. C. Paul. 2006. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J. Med. Entomol. 43:283-287. [DOI] [PubMed] [Google Scholar]
- 4.Arlian, L. G., C. M. Rapp, and M. S. Morgan. 1995. Resistance and immune response in scabies-infested hosts immunised with Dermatophagoides mites. Am. J. Trop. Med. Hyg. 52:539-545. [DOI] [PubMed] [Google Scholar]
- 5.Arlian, L. G., D. L. Vyszenski-Moher, S. G. Ahmed, and S. A. Estes. 1991. Cross-antigenicity between the scabies mite, Sarcoptes scabiei, and the house dust mite, Dermatophagoides pteronyssinus. J. Invest. Dermatol. 96:349-354. [DOI] [PubMed] [Google Scholar]
- 6.Arlian, L. G., D. L. Vyszenski-Moher, and A. M. Gilmore. 1988. Cross-antigenicity between Sarcoptes scabiei and the house dust mite, Dermatophagoides farinae (Acari: Sarcoptidae and Pyroglyphidae). J. Med. Entomol. 25:240-247. [DOI] [PubMed] [Google Scholar]
- 7.Beckham, S. A., S. E. Boyd, S. Reynolds, C. Willis, M. Johnstone, A. Mika, P. Simerska, L. C. Wijeyewickrema, A. I. Smith, D. J. Kemp, R. N. Pike, and K. Fischer. 2009. Characterisation of a serine protease homologous to house dust mite group 3 allergens from the scabies mite sarcoptes scabiei. J. Biol. Chem. 284:34413-34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergström, F. C., S. Reynolds, M. Johnstone, R. N. Pike, A. M. Buckle, D. J. Kemp, K. Fischer, and A. M. Blom. 2009. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J. Immunol. 182:7809-7817. [DOI] [PubMed] [Google Scholar]
- 9.Burnette, W. 1981. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacylamide gel to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera, R., and M. V. Dahl. 1993. The immunology of scabies. Semin. Dermatol. 12:15-21. [PubMed] [Google Scholar]
- 11.Casais, R., M. Prieto, A. Balseiro, P. Solano, F. Parra, and J. M. Martin Alonso. 2007. Identification and heterologous expression of a Sarcoptes scabiei cDNA encoding a structural antigen with immunodiagnostic potential. Vet. Res. 38:435-450. [DOI] [PubMed] [Google Scholar]
- 12.Chapman, M. D., S. Wunschmann, and A. Pomes. 2007. Proteases as Th2 adjuvants. Curr. Allergy Asthma Rep. 7:363-367. [DOI] [PubMed] [Google Scholar]
- 13.Clucas, D., K. Carville, C. Connors, B. Currie, J. Carapetis, and R. Andrews. 2008. Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bull. World Health Organ. 86:241-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl, M. V. 1985. The immune system in scabies, p. 75-83. In M. Orkin and H. I. Maibach (ed.), Cutaneous infestations and insect bites. Marcel Dekker, New York, NY.
- 15.Donnelly, S., J. P. Dalton, and A. Loukas. 2006. Proteases in helminth- and allergen-induced inflammatory responses. Chem. Immunol. Allergy 90:45-64. [DOI] [PubMed] [Google Scholar]
- 16.Dougall, A., D. C. Holt, K. Fischer, B. J. Currie, D. J. Kemp, and S. F. Walton. 2005. Identification and characterization of Sarcoptes scabiei and Dermatophagoides pteronyssinus glutathione S-transferases: implication as a major potential allergen in crusted scabies. Am. J. Trop. Med. Hyg. 73:977-984. [PubMed] [Google Scholar]
- 17.Douglass, J., and R. O'Hehir. 2000. Asthma. A multisystem disease. In R. S. Walls and C. R. Jenkins (ed.), Understanding asthma. McLennan and Petty, Sydney, Australia.
- 18.Falk, E., and R. Bolle. 1980. IgE antibodies to house dust mite in patients with scabies. Br. J. Dermatol. 102:283-288. [DOI] [PubMed] [Google Scholar]
- 19.Falk, E. S. 1980. Serum immunoglobulin values in patients with scabies. Br. J. Dermatol. 102:57-61. [DOI] [PubMed] [Google Scholar]
- 20.Falk, E. S., S. Dale, R. Bolle, and B. Haneberg. 1981. Antigens common to scabies and house dust mites. Allergy 36:233-238. [DOI] [PubMed] [Google Scholar]
- 21.Fischer, K., D. C. Holt, P. Harumal, B. J. Currie, S. F. Walton, and D. J. Kemp. 2003. Generation and characterisation of cDNA clones from Sarcoptes scabiei var. hominis for an expressed sequence tag library: identification of homologues of house dust mite allergens. Am. J. Trop. Med. Hyg. 68:61-64. [PubMed] [Google Scholar]
- 22.Fischer, K., C. G. Langendorf, J. A. Irving, S. Reynolds, C. Willis, S. Beckham, R. H. Law, S. Yang, T. A. Bashtannyk-Puhalovich, S. McGowan, J. C. Whisstock, R. N. Pike, D. J. Kemp, and A. M. Buckle. 2009. Structural mechanisms of inactivation in scabies mite serine protease paralogues. J. Mol. Biol. 390:635-645. [DOI] [PubMed] [Google Scholar]
- 23.Hagan, P. 1993. IgE and protective immunity to helminth infections. Parasite Immunol. 15:1-4. [DOI] [PubMed] [Google Scholar]
- 24.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 25.Harumal, P., M. S. Morgan, S. F. Walton, D. C. Holt, J. Rode, L. G. Arlian, B. J. Currie, and D. J. Kemp. 2003. Identification of a homologue of a house dust mite allergen in a cDNA library from Sarcoptes scabiei var. hominis and evaluation of its vaccine potential in a rabbit/S. scabiei var. canis model. Am. J. Trop. Med. Hyg. 68:54-60. [PubMed] [Google Scholar]
- 26.Holt, D. C., K. Fischer, G. E. Allen, D. Wilson, P. Wilson, R. Slade, B. J. Currie, S. F. Walton, and D. J. Kemp. 2003. Mechanisms for a novel immune evasion strategy in the scabies mite Sarcoptes scabiei: a multigene family of inactivated serine proteases. J. Invest. Dermatol. 121:1419-1424. [DOI] [PubMed] [Google Scholar]
- 27.Holt, D. C., K. Fischer, S. J. Pizzutto, B. J. Currie, S. F. Walton, and D. J. Kemp. 2004. A multigene family of inactivated cysteine proteases in Sarcoptes scabiei. J. Invest. Dermatol. 123:240-241. [DOI] [PubMed] [Google Scholar]
- 28.Kay, A. B. 2001. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 344:30-37. [DOI] [PubMed] [Google Scholar]
- 29.Kemp, D., S. Walton, P. Harumal, and B. Currie. 2002. The scourge of scabies. Biologist 49:19-24. [PubMed] [Google Scholar]
- 30.Kuhn, C., R. Lucius, H. F. Matthes, G. Meusel, B. Reich, and B. H. Kalinna. 2008. Characterisation of recombinant immunoreactive antigens of the scab mite Sarcoptes scabiei. Vet. Parasitol. 153:329-337. [DOI] [PubMed] [Google Scholar]
- 31.Kuo, I. C., N. Cheong, M. Trakultivakorn, B. W. Lee, and K. Y. Chua. 2003. An extensive study of human IgE cross-reactivity of Blo t 5 and Der p 5. J. Allergy Clin. Immunol. 111:603-609. [DOI] [PubMed] [Google Scholar]
- 32.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 33.Malandain, H. 2005. IgE-reactive carbohydrate epitopes-classification, cross-reactivity, and clinical impact. Allerg. Immunol. 37:122-128. [PubMed] [Google Scholar]
- 34.Mattsson, J. G., E. L. Ljunggren, and K. Bergstrom. 2001. Paramyosin from the parasitic mite Sarcoptes scabiei: cDNA cloning and heterologous expression. Parasitology 122:555-562. [DOI] [PubMed] [Google Scholar]
- 35.Molin, E. U., and J. G. Mattsson. 2008. Effect of acaricides on the activity of glutathione transferases from the parasitic mite Sarcoptes scabiei. Parasitology 135:115-123. [DOI] [PubMed] [Google Scholar]
- 36.Morgan, M. S., L. G. Arlian, K. C. Barnes, and E. Fernandez-Caldes. 1997. Characterisation of the allergens of the house dust mite Euroglyphus maynei. J. Allergy Clin. Immunol. 100:222-228. [DOI] [PubMed] [Google Scholar]
- 37.Morgan, M. S., L. G. Arlian, and S. A. Estes. 1997. Skin test and radioallergosorbent test characteristics of scabietic patients. Am. J. Trop. Med. Hyg. 57:190-196. [DOI] [PubMed] [Google Scholar]
- 38.Morsy, T. A., M. Z. Kenawi, H. A. Zohdy, K. F. Abdalla, and A. F. El Fakahany. 1993. Serum immunoglobulin and complement values in scabietic patients. J. Egypt. Soc. Parasitol. 23:221-229. [PubMed] [Google Scholar]
- 39.Nyindo, M., T. M. Kariuki, P. W. Mola, I. O. Farah, L. Elson, R. E. Blanton, and C. L. King. 1999. Role of adult worm antigen-specific immunoglobulin E in acquired immunity to Schistosoma mansoni infection in baboons. Infect. Immun. 67:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson, E. U., E. L. Ljunggren, D. A. Morrison, and J. G. Mattsson. 2005. Functional analysis and localisation of a delta-class glutathione S-transferase from Sarcoptes scabiei. Int. J. Parasitol. 35:39-48. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, L. J., S. E. Huffam, S. F. Walton, and B. J. Currie. 2005. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J. Infect. 50:375-381. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland, M. F., R. E. O'Hehir, D. Czarny, and C. Suphioglu. 1999. Macadamia nut anaphylaxis: demonstration of specific IgE reactivity and partial cross-reactivity with hazelnut. J. Allergy Clin. Immunol. 104:889-890. [DOI] [PubMed] [Google Scholar]
- 43.Thomas, W. R., W. A. Smith, B. J. Hales, K. L. Mills, and R. M. O'Brien. 2002. Characterization and immunobiology of house dust mite allergens. Int. Arch. Allergy Immunol. 129:1-18. [DOI] [PubMed] [Google Scholar]
- 44.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walton, S., J. Low Choy, A. Bonson, A. Valle, J. McBroom, D. Taplin, L. Arlian, J. Mathews, B. Currie, and D. Kemp. 1999. Genetically distinct dog-derived and human-derived Sarcoptes scabiei in scabies-endemic communities in northern Australia. Am. J. Trop. Med. Hyg. 61:542-547. [DOI] [PubMed] [Google Scholar]
- 46.Walton, S., J. McBroom, J. Mathews, D. Kemp, and B. Currie. 1999. Crusted scabies: a molecular analysis of Sarcoptes scabiei var. hominis populations in patients with repeated infestations. Clin. Infect. Dis. 29:1226-1230. [DOI] [PubMed] [Google Scholar]
- 47.Walton, S. F., D. Beroukas, P. Roberts-Thomson, and B. J. Currie. 2008. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br. J. Dermatol. 158:1247-1255. [DOI] [PubMed] [Google Scholar]
- 48.Walton, S. F., and B. J. Currie. 2007. Problems in diagnosing scabies, a global disease in human and animal populations. Clin. Microbiol. Rev. 20:268-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walton, S. F., B. J. Currie, and D. J. Kemp. 1997. A DNA fingerprinting system for the ectoparasite Sarcoptes scabiei. Mol. Biochem. Parasitol. 85:187-196. [DOI] [PubMed] [Google Scholar]
- 50.Walton, S. F., D. C. Holt, B. J. Currie, and D. J. Kemp. 2004. Scabies: new future for a neglected disease. Adv. Parasitol. 57:309-376. [DOI] [PubMed] [Google Scholar]
- 51.Willis, C., K. Fischer, S. F. Walton, B. J. Currie, and D. J. Kemp. 2006. Scabies mite inactivated serine protease paralogues are present both internally in the mite gut and externally in feces. Am. J. Trop. Med. Hyg. 75:683-687. [PubMed] [Google Scholar]