Abstract
The usefulness of a specific immunoglobulin G (IgG) avidity enzyme-linked immunosorbent assay (ELISA) based on recombinant GRA6 antigen for distinguishing between acute and chronic Toxoplasma infection was investigated. Two sets of serum samples obtained from pregnant women with acute, chronic, or no Toxoplasma infection collected in France and Iran were used. Among the French subjects, 19 of 20 (95%) women who experienced seroconversion during the past 4 months before sampling displayed low-avidity IgG antibodies against GRA6, while all 17 (100%) women with chronic infection had high-avidity antibodies. When the Euroimmun IgG avidity ELISA was used, 15 of 19 (78.9%) recently infected women had low-avidity antibodies, and 20 of 22 (90.9%) women with chronic infection displayed high-avidity antibodies. The results suggested better performance of the GRA6 avidity ELISA than the Euroimmun avidity ELISA for exclusion of a recent infection occurring less than 4 months previously. Similarly, all 35 Iranian women with acute Toxoplasma infection had low-avidity antibodies against GRA6, whereas all 34 women with chronic infection displayed IgG antibodies of high avidity, indicating the value of GRA6 avidity testing for ruling out a recent infection. Avidity tests based on lysed whole-cell Toxoplasma gondii antigen are currently used to exclude recently acquired infections; however, the use of recombinant antigen(s) might improve the diagnostic performance of avidity tests and facilitate the development of more standardized assays.
Congenital toxoplasmosis may occur when maternal infection is acquired during pregnancy and results in severe fetopathy or miscarriage (28, 38). While the rate of fetal infection with Toxoplasma gondii is extremely low in preconception infections, the transmission rate increases and the severity of fetal infection decreases as gestational age progresses (7, 18). Fortunately, prenatal treatment of the infection is effective for reducing both the incidence of clinical manifestations in infected newborns (8) and the maternal-fetal transmission rate (6, 29, 37). It is, therefore, essential to estimate the gestational age of the primary Toxoplasma infection as precisely as possible for the proper clinical management of pregnant women.
Diagnosis of acute toxoplasmosis depends mainly on serological testing, as the infection is asymptomatic in 93% to 97% of pregnant women (10, 27). In countries where a prenatal screening of T. gondii infection is performed, detection of seroconversion or a significant rise in the specific immunoglobulin G (IgG) titer establishes a recently acquired infection. In most countries, however, a single serum sample from a pregnant woman is available to determine whether the infection occurred during gestation. IgM antibodies have been traditionally known as the markers of acute infection; however, persistent specific IgM has been found for up to several years after primary infection (15, 24, 39). Measurement of specific IgG avidity was shown to be an effective confirmatory test to help discriminate between recently acquired and distant infections (4, 16, 20, 22, 30, 31). In fact, it was shown that the combination of a sensitive test for T. gondii IgM and a specific IgG avidity assay is the best tool for determining the time of infection (34). The functional affinity (avidity) of specific IgG antibodies is low in the beginning of infection and usually increases with time by antigen-driven B-cell selection. However, low-avidity antibodies may persist for several months after acute infection, indicating that the avidity test is best used to rule out a recent infection in individuals with high-avidity results (5, 20, 30). Some studies reported the presence of very rare borderline- or high-avidity antibodies in acute infection with T. gondii (11, 20-22). The discordant results of avidity assays are attributed to the presence of antiparasitic treatment and immunodeficiency status; however, the complex nature of lysed whole-cell T. gondii antigen might cause such results (4, 35). Recombinant antigens might improve the performance of avidity assays for distinguishing between acute and chronic infection (1, 32, 33). Furthermore, preparation of recombinant antigens with more constant quality, compared to T. gondii antigen prepared from parasites grown either in cell culture or in mice peritoneal cavities, would facilitate development of better standardized assays (2).
In this study, we developed an IgG avidity test based on recombinant GRA6 antigen and evaluated its value for differentiation between recently acquired and distant Toxoplasma infections in pregnant women. The potential usefulness of the GRA6 avidity test was compared with that of the Euroimmun IgG avidity enzyme-linked immunosorbent assay (ELISA; Euroimmun, Lübeck, Germany) for exclusion of a recent Toxoplasma infection that occurred less than 4 months before.
MATERIALS AND METHODS
Serum samples.
A total of 138 serum samples collected from pregnant women, each sample corresponding to a woman, at 3 medical diagnostic labs in Tehran, Iran, or at Parasitology and Mycology Laboratory, Grenoble Teaching Hospital A. Michallon, Grenoble, France, were divided into 5 groups. Serum samples in group 1 (French acute-phase sera) were collected from 23 women who experienced seroconversion during the past 4 months before sampling. These sera were tested in Grenoble by Vidas Toxo IgG II, Vidas Toxo IgM (bioMérieux, Marcy l'Etoile, France), indirect immunofluorescence (IIF) IgG and IgM (in-house tests), and the IgM immunosorbent agglutination assay (IgM ISAGA; bioMérieux) (9). The occurrence of seroconversion in these women was confirmed by analyzing 2 consecutive samples collected within an interval of 3 weeks, as follows: (i) the first sample tested negative, whereas the second one was positive, or (ii) the first sample was IgM positive and either IgG negative or weakly positive, and the second sample showed an increase in the IgG titer by at least 2-fold. These samples were further tested in Iran by Euroimmun IgG, IgM, and avidity ELISA. Group 2 (Iranian acute-phase sera) consisted of 35 serum samples obtained in Tehran from pregnant women with clinical signs of suspected acute toxoplasmosis. The samples were collected from the women as soon as they showed clinical signs of toxoplasmosis. They were IgG and IgM positive and have low avidity indices (AIs), as tested by Euroimmun ELISA kits (Euroimmun, Lübeck, Germany). Group 3 (French chronic sera) consisted of 22 serum samples collected in Grenoble, France, from women whose serodiagnostic test results were suggestive of the infection acquired in the distant past. They were positive by Vidas Toxo IgG II but scored negative by Vidas Toxo IgM and IgM IIF. These samples were further tested in Iran by Euroimmun IgG, IgM, and avidity ELISA. Group 4 (Iranian chronic sera) consisted of 40 serum samples collected in Tehran, Iran, from women with chronic infection. These serum samples were IgG positive, IgM negative, and had high avidity indices, as tested by Euroimmun ELISA kits. Group 5 (T. gondii-negative sera) consisted of 10 serum samples collected in Iran and 8 serum samples collected in France from pregnant women who tested negative for both T. gondii IgG and IgM by the standard kits.
Preparation of GRA6 recombinant antigen.
GRA6 antigen (amino acids 40 to 230) from the T. gondii RH strain was used in this study. Recombinant GRA6 was expressed in bacterial cells and purified by affinity chromatography as previously described (13). Recombinant Escherichia coli was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), centrifuged, and resuspended in lysis buffer A (10 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl at pH 8.3, 0.1% Triton X-100, and proteases inhibitor cocktail without EDTA [Roche, Mannheim, Germany]). The mixture was sonicated at 4°C and centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was recovered, incubated with Ni-nitrilotriacetic acid resin (Qiagen, Courtaboeuf, France), and transferred to an empty column. Following sequential washes of the column with buffers B, C, and D (buffers having the same composition as buffer A but containing 20, 40 and 80 mM imidazole, respectively), the recombinant proteins were eluted with buffer E (buffer having the same composition as buffer A but containing 400 mM imidazole and 0.01% Triton X-100). Purified protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), dialyzed against phosphate-buffered saline (PBS), and stored in aliquots at −70°C.
E. coli lysate.
E. coli BL21(DE3) pLysS bacteria were grown at 32°C until the optical density ay 600 nm (OD600) reached 0.5. The culture was then incubated for 4 h at 32°C. Bacteria were collected by centrifugation, resuspended in PBS, and lysed by sonication. The volume of the lysate was adjusted to 1:20 of the original culture volume and was added to the serum diluent of ELISA experiments. This lysate contained 4 mg of protein per milliliter, as determined by the DC protein assay kit (Bio-Rad Laboratories, Hercules, CA).
GRA6 IgG ELISA.
Maxisorp multiwell plates (Nunc, Roskilde, Denmark) were coated with GRA6 at a concentration of 10 μg/ml in 50 mM carbonate buffer (pH 9.6). After overnight incubation at 4°C, the plates were washed with PBS-0.05% Tween 20 (PBS-T) and blocked at 37°C for 1 h with 200 μl of blocking buffer (PBS-T containing 1% bovine serum albumin [BSA]). Subsequently, the plates were incubated at room temperature with 100 μl of human sera diluted 1:100 in blocking buffer containing 2% E. coli lysate. Plates were incubated for 1 h at 37°C and washed, and 100 μl of horseradish peroxidase-conjugated goat anti-human IgG (Sigma, St. Louis, MO) diluted 1:4,000 in blocking buffer was added to each well. After 30 min of incubation at room temperature, the plates were washed and incubated with tetramethylene benzidine dihydrochloride (TMB; KemEnTech, Copenhagen, Denmark) at room temperature. The reaction was stopped 15 min later by adding 100 μl of 2 M H2SO4, and the optical density at 450 nm (OD450) was measured by an ELISA reader (BioTek Instruments, Highland Park, VT), with a reference wavelength of 630 nm. Each serum sample was tested in duplicate wells. The mean plus two standard deviations (SD) of OD values obtained for negative sera were considered the cutoff OD values.
Recombinant IgG avidity ELISA.
Serum samples with OD values greater than the cutoff in IgG ELISA with GRA6 were applied to an avidity ELISA. Maxisorp plates were coated with GRA6 as described above. Serum samples were tested in duplicate in two different rows at 1:100 dilutions. After 1 h of incubation at room temperature, plates were washed once with PBS-T washing buffer. Row A was then incubated with 200 μl of dissociation buffer (PBS-T containing 8 M urea) per well, except in the preliminary experiment where different concentrations of urea were tested, and row B was incubated with 200 μl of PBS-T per well. After 30 min of incubation at 37°C with gentle shaking, plates were washed twice with PBS-T and processed as described above. The results were expressed as avidity indices (AIs) and calculated for each serum sample as the percent ratio of OD values obtained with and without the urea washing step.
Comparison of avidity calculation methods.
The IgG avidity ELISA was performed with 5 acute, 5 chronic, and 4 negative serum samples obtained from Iranian women, and the AI was calculated either as a percent ratio of OD values with and without the urea washing step at a 1:100 dilution (single-dilution method) or as a percent ratio of IgG titers with and without the urea washing step (end titer method). For the end titer method, sera were used in increasing serial dilutions from 1:100 to 1:3,200. The highest dilution yielding an OD value greater than the cutoff was considered the end titer. A cutoff value was calculated in each dilution as the mean plus 2 SD of OD values of negative sera in the presence or absence of urea washing buffer.
Statistical analysis.
Data were analyzed using SPSS version 12 software (SPSS, San Francisco, CA). All equivocal or intermediate values were considered false negatives in subjects with acute toxoplasmosis and false positives in subjects with chronic toxoplasmosis. Spearman's test was used to analyze the correlation between avidity indices and time after infection. The means of avidity indices were compared by Student's t test.
RESULTS
Reactivity of GRA6 antigen with French sera.
Twenty-three serum samples obtained from pregnant women seroconverted between 2 to 15 weeks before blood collection (group 1) and 22 serum samples obtained from women with chronic T. gondii infection (group 3) were tested in IgG ELISA with GRA6. The cutoff value was determined as the mean plus 2 standard deviations of optical density (OD) values of 8 serum samples obtained from French women with no Toxoplasma infection (group 5). A total of 20 of 23 (87.0%) acute-phase serum samples and 17 of 22 (72.3%) chronic serum samples had OD values above the cutoff of 0.22 (Table 1). The three acute-phase serum samples that scored negative in the GRA6 ELISA also tested negative by Vidas Toxo IgG II and Euroimmun IgG ELISA. These sera were collected 3 or 4 weeks after seroconversion. On the other hand, serum samples 5 and 23, both collected 2 weeks after seroconversion, showed positive results in the GRA6 ELISA, while sample 23 was negative in both the Euroimmun IgG ELISA and Vidas Toxo IgG II, and sample 5 was equivocal in Vidas Toxo IgG II. None of the negative sera scored positive in the GRA6 ELISA. Taken together, the IgG ELISA using GRA6 displayed slightly better sensitivity (87%), and thus a better precocity, than Vidas Toxo IgG II (78.2%) and Euroimmun IgG ELISA (82.6%) for detection of IgG in sera obtained from women with recent Toxoplasma infection.
TABLE 1.
Results from the GRA6 avidity ELISA and standard serologic tests on acute-phase sera obtained from pregnant French women with known times of Toxoplasma infection
| Sample no. | Time after infection (weeks) | Treatmentg | ELISA resultsh |
|||||
|---|---|---|---|---|---|---|---|---|
| IgG |
Avidity (% AI) |
|||||||
| Euroimmuna | VIDASb | GRA6 (PBS)c | GRA6 (urea)d | GRA6e | Euroimmunf | |||
| 1 | 2 | NT | 16.8 | 12 | 0.816 | 0.612 | 75.0 | 21.2 |
| 2 | 2 | NT | 18.5 | 9 | 2.264 | 0.133 | 5.90 | 26.7 |
| 3 | 3 | NT | 32.2 | 25 | 2.483 | 0.188 | 7.60 | 16.6 |
| 4 | 12 | Rovamycin | 37.2 | >300 | 2.587 | 0.710 | 27.4 | 43.5 |
| 5 | 2 | Rovamycin | 41.7 | 6 | 0.453 | 0.145 | 32.0 | 33.2 |
| 6 | 3 | Rovamycin | 49.7 | 16 | 1.433 | 0.161 | 11.2 | 15.8 |
| 7 | 4 | Rovamycin | 18.9 | 24 | 0.357 | 0.070 | 19.6 | 24.7 |
| 8 | 3 | NT | 20.5 | 10 | 0.748 | 0.098 | 13.1 | 18.1 |
| 9 | 4 | Rovamycin | 42.9 | 16 | 0.364 | 0.076 | 20.9 | 14.3 |
| 10 | 4 | NT | 37.1 | 34 | 1.596 | 0.100 | 6.30 | 14.7 |
| 11 | 4 | Rovamycin | 43.8 | 34 | 1.000 | 0.083 | 8.30 | 12.7 |
| 12 | 4 | NT | 19.6 | 28 | 1.881 | 0.177 | 9.40 | 10.0 |
| 13 | 8 | Rovamycin | 53.4 | 181 | 2.368 | 0.272 | 11.5 | 58.7 |
| 14 | 10 | Rovamycin | 70.0 | >300 | 2.667 | 0.474 | 17.7 | 52.4 |
| 15 | 10 | NT | 90.6 | >300 | 0.642 | 0.193 | 30.1 | 77.0 |
| 16 | 10 | NT | 45.3 | 64 | 0.599 | 0.078 | 13.0 | 18.2 |
| 17 | 15 | Rovamycin | 65.2 | >300 | 2.153 | 0.207 | 9.60 | 35.1 |
| 18 | 15 | Fansidar | 80.7 | 231 | 2.462 | 0.469 | 19.0 | 38.7 |
| 19 | 6 | Rovamycin | 69.0 | 128 | 1.863 | 0.177 | 9.50 | 21.7 |
| 20 | 4 | NT | <8 | 0 | 0.203 | 0.063 | ND | ND |
| 21 | 4 | Rovamycin | <8 | 4 | 0.156 | 0.059 | ND | ND |
| 22 | 3 | NT | <8 | 2 | 0.186 | 0.079 | ND | ND |
| 23 | 2 | NT | <8 | 1 | 0.296 | 0.082 | 27.7 | ND |
Positive results, values ≥ 11 IU/ml; equivocal results, values ≥ 8 to <11 IU/ml; negative results, values < 8 IU/ml.
Positive results, values ≥ 8 IU/ml; equivocal results, values ≥ 6 to <8 IU/ml; negative results, values < 6 IU/ml.
OD values in the GRA6 IgG ELISA with PBS in washing buffer. Positive results, OD values > 0.22.
OD values in the GRA6 IgG ELISA with 8 M urea in washing buffer (avidity ELISA).
Low avidity, AI ≤ 32.0%; high avidity, AI ≥37.0%.
Low avidity, AI < 40%; equivocal avidity, AI of ≥40 to ≤60%; high avidity, AI > 60%.
Fansidar, pyrimethamine-sulfadoxine; Rovamycin, spiramycin; NT, No treatment.
ND, not determined; % AI, avidity index as a percent ratio of the well washed with urea to the well washed with PBS. Negative or equivocal results in IgG ELISAs are printed in boldface, and equivocal or high-avidity results in GRA6 and Euroimmun avidity ELISAs are printed in boldface.
Setting up the conditions of recombinant avidity ELISA.
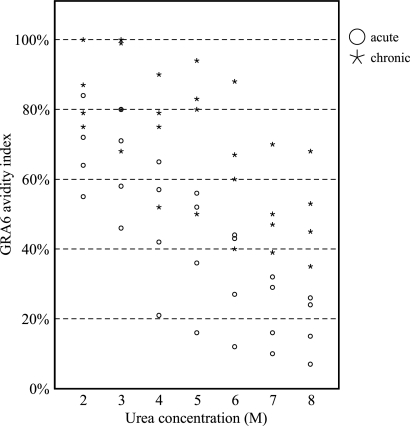
Determination of IgG avidity is usually based on the separation of low- and high-avidity antibodies by elution of antigen-bound IgG using urea-containing washing buffer; low-avidity IgG dissociates from antigen in the presence of urea, while high-avidity IgG remains bound to antigen. The avidity result, generally stated as the avidity index (AI), is expressed mainly as the percent ratio of antibody titers or OD values with and without the urea washing step. The urea concentration in washing buffer and urea washing time are among parameters affecting the level of dissociation of antigen-antibody complex. These parameters were empirically determined to reach the maximum performance of the GRA6 avidity ELISA for differentiating between acute and chronic Toxoplasma infection. Urea concentrations from 2 to 8 M were applied in parallel avidity ELISA experiments, and the AIs of 4 acute and 4 chronic serum samples obtained from Iranian women were determined in a single serum dilution of 1:100. Figure 1 shows that an increasing urea concentration from 2 to 8 M improved differentiation between AIs calculated for acute and chronic sera, but complete separation between the two groups of sera was achieved in urea concentrations of 7 and 8 M.
FIG. 1.
Evaluation of different concentrations of urea, as the elution agent, in a GRA6 IgG avidity ELISA to discriminate between acute and chronic T. gondii infection.
To evaluate the effect of urea washing time on differentiation between acute and chronic infection, different washing times with 8 M urea, from 5 to 30 min, were tested in parallel experiments, and AIs of a pool of 5 acute and 5 chronic serum samples obtained from Iranian women were determined in a single serum dilution of 1:100. The results showed that increasing the urea washing time from 5 to 30 min resulted in improved discrimination between AIs of the pooled acute and chronic sera, and the maximum differentiation was observed with a urea washing time of 30 min (data not shown).
Comparison of avidity calculation methods.
Calculation of AIs in avidity assays has been performed by various methods in the literature. In the most straightforward way, the AI is expressed as the percent ratio of OD values obtained with and without the urea washing step in single dilutions of serum (single-dilution method). In the end titer method, several dilutions of the serum sample are simultaneously tested, and the AI is calculated as the percent ratio of end titers, the highest titer giving an OD value greater than the cutoff OD, in the presence and absence of urea. Avidity determination based on end titers of IgG is considered the gold standard method for determination of avidity results, as it is not influenced by IgG concentration.
We compared the discriminative performance of the two avidity calculation methods in our assay. A panel of 5 acute, 5 chronic, and 4 T. gondii-negative serum samples from Iranian women were serially diluted from 1:100 to 1:3,200 and tested in an avidity ELISA using GRA6. The AIs of acute and chronic sera were determined by both single dilution and end titer methods, as explained in Material and Methods. The results showed that both methods were capable of discriminating acute infection from chronic infection (Table 2).
TABLE 2.
Comparison of avidity indices calculated by the single-dilution or end titer method in the GRA6 avidity ELISA
| Toxoplasma infection status | AI (%) |
|
|---|---|---|
| Single-dilution method | End titer method | |
| Acute | 11.7 | 6.25 |
| 21.0 | 25.0 | |
| 16.7 | 12.5 | |
| 20.0 | 12.5 | |
| 12.0 | 6.25 | |
| Chronic | 59.7 | 50.0 |
| 65.0 | 100 | |
| 64.0 | 50 | |
| 85.0 | 100 | |
| 81.0 | 50 | |
Recombinant avidity ELISA using French sera.
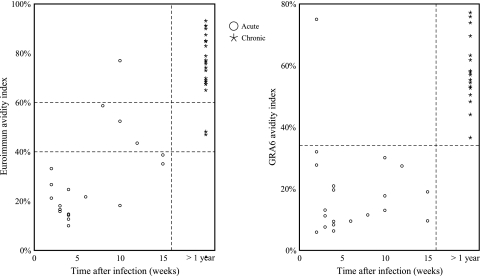
Serum samples that scored positive in the GRA6 ELISA were applied to an avidity ELISA. A total of 19 of 20 (95.0%) women who seroconverted during the past 4 months before blood collection were found to have AIs of less than or equal to 32.0%, while all 17 (100.0%) serum samples obtained from women with chronic infection displayed AIs that were equal to or greater than 37.0% (Fig. 2). The difference between mean indices of sera from recent infection (i.e., 18.7%) and those of sera from chronic infection (i.e., 55.4%) was highly significant (P < 0.0001; Student's t test), indicating that avidity of antibodies to GRA6 increases with time; however, no correlation between avidity and estimated time after the acquisition of infection in acutely infected women was observed.
FIG. 2.
Comparison of the performance of Euroimmun and GRA6 avidity ELISAs to distinguish between recently acquired and chronic T. gondii infection in pregnant French women.
Serum sample 1, which had an AI of 0.75 in the GRA6 avidity ELISA, was obtained from a pregnant woman who seroconverted 2 weeks before blood sampling and received no treatment for Toxoplasma infection. The OD value of the sample was 0.816, and further analysis of the sample at a 1:200 dilution yielded a similar avidity index, excluding the probability of a high-avidity result due to a large amount of specific IgG. This sample had positive IgM titers in both the Euroimmun IgM ELISA and Vidas Toxo IgM test (data not shown) and showed a low-avidity result in Euroimmun avidity testing (Table 1).
Analysis of French sera by Euroimmun avidity ELISA.
The value of the Euroimmun avidity assay for distinguishing between recent and chronic Toxoplasma infection in French patients was evaluated. Among 19 acutely infected women with positive results in the Euroimmun IgG ELISA, 15 had AIs of less than 40% (78.9%), the cutoff below which recent toxoplasmosis is suggested, 3 had AIs between 40% and 60% (borderline values), and 1 had an AI of 77%. On the other hand, 20 of 22 (90.9%) women with chronic infection displayed an AI of greater than 60%, the cutoff above which toxoplasmosis is considered chronic, and 2 had borderline values. The mean avidity indices for acute and chronic sera were 29.1 and 75.6%, respectively, and the difference between them was highly significant (P < 0.0001; Student's t test). In contrast to the GRA6 avidity ELISA, a weak correlation (r = 0.480; P = 0.038; Spearman's test) between avidity and estimated time after the acquisition of infection in sera obtained from women with recent infection was observed.
The results presented here showed the superior performance of GRA6 avidity, compared to Euroimmun avidity, for exclusion of recent Toxoplasma infection occurred in the past 4 months.
Recombinant avidity ELISA using Iranian sera.
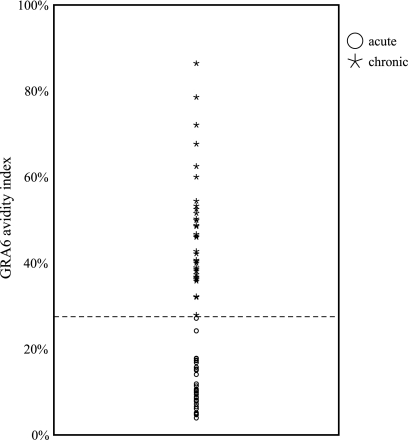
Thirty-five serum samples obtained from pregnant women with clinical signs of acute toxoplasmosis (group 2) and with serologic profiles suggesting acute infection (IgG positive, IgM positive, and low avidity index) and forty serum samples obtained from women with chronic Toxoplasma infection (group 4; IgG positive, IgM negative, and high avidity index) were tested in a GRA6 ELISA. The cutoff value was determined as the mean plus 2 SD of OD values of 10 serum samples obtained from women with no Toxoplasma infection (group 5). Serum samples from all 35 (100%) women with acute infection had OD values greater than the cutoff value (i.e., 0.24), while 34 of 40 (85%) serum samples obtained from women with chronic infection scored positive. None of the negative sera had OD values higher than the cutoff. Sera that scored positive in the GRA6 ELISA were tested in an avidity ELISA to further evaluate performance of the GRA6 avidity ELISA for diagnosis of acute Toxoplasma infection. Figure 3 shows complete separation between acute and chronic infection by the GRA6 avidity ELISA. All women with acute infection had AIs equal to or less than 27.0%, while all women with chronic infection displayed AIs equal to or greater than 28%. The mean avidity index of acute-phase sera was 10.8%, and that of chronic sera was 47.9%.
FIG. 3.
Diagnostic performance of the GRA6 avidity ELISA for distinguishing between acute and chronic T. gondii infection in pregnant Iranian women.
DISCUSSION
Accurate diagnosis of recent Toxoplasma infection and estimation of time of infection is of utmost importance in pregnant women, since timely treatment can reduce the risk of congenital infection and subsequent clinical manifestations in the infected infants (29). Although detection of T. gondii IgM antibodies, as markers of acute infection, suggests a recent infection, IgM assays frequently detect low-level specific antibodies months or years after infection, making them unreliable for dating Toxoplasma infection (15, 24, 39). Measurement of T. gondii IgG avidity has been well established as a confirmatory test to exclude recently acquired infections and avoid unnecessary antibiotic treatment in pregnant women (4, 16, 20, 22, 30, 31, 35). Current avidity tests make use of lysed whole-cell T. gondii antigen and frequently detect persistent low- or borderline-avidity results for months or years after infection (5, 20, 30). Several studies report that development of avidity assays based on recombinant T. gondii antigens is useful for improvement of avidity assays and differentiation of recent versus late infection (1, 32, 33).
We previously reported that GRA6 is a marker of acute infection with T. gondii and induces immune response very early in the infection (12). In this study, we confirmed the previous results and showed that the IgG ELISA with GRA6 displayed better sensitivity than the Vidas and Euroimmun IgG assays for sera taken early in the infection. We then developed a GRA6 avidity ELISA by optimizing assay conditions affecting dissociation of antigen and antibody. Optimal differentiation between high- and low-avidity antibodies in GRA6 avidity was achieved using 8 M urea and a urea washing time of 30 min. It seems that the optimal conditions vary in different assays, since different concentrations of urea and different urea washing times have been successfully used in Toxoplasma avidity assays (1, 19).
Numerous studies reported that determination of avidity in a single dilution is both easy to perform and quite sensitive and specific for distinguishing between primary (recent) and chronic infection (3, 14, 17, 25, 36). The major drawback to this method is possible variation of AIs, depending on the total amount of T. gondii-specific IgG in the serum sample (17). Calculation of avidity based on end titration of antibodies is independent to the level of specific IgG; however, some limitations are associated with this method, including the necessity of testing several dilutions of the serum sample, the frequent requirement for repeating the test because of insufficient serial dilution of the sample (i.e., the highest dilution shows an OD value greater than the cutoff OD value), and relatively complex calculations (19). The advantage of the method used in this study was that only a single dilution of the serum was assayed with and without urea treatment, and consequently, the amount of reagents and technical time required to perform the test was reduced. Calculation of avidity for a series of acute and chronic sera in a GRA6 avidity ELISA showed both end titer and single-dilution methods discriminated well between low-avidity sera and high-avidity sera.
In a panel of French acute-phase sera obtained from women with known times of infection and from women with chronic infection, the avidity ELISA with GRA6 was capable of distinguishing between recent and chronic infection. GRA6 avidity showed better performance than Euroimmun avidity for exclusion of a recent infection that happened less than 4 months before and resulted in less discordant results. Only 1 acute sample had a high avidity index in the GRA6 avidity ELISA, while 4 samples had borderline- or high-avidity results in the Euroimmun avidity ELISA.
The presence of borderline- or high-avidity antibodies in pregnant women with recent infections has been documented. A total of 2 of 73 serum samples collected from 23 women within 20 weeks after infection had IgG avidity indices above 20%, while most patients developed avidity indices of greater than 15% after 180 days (20, 21). Interestingly, one of these serum samples was taken from a woman 2 weeks after the estimated time of infection. Lappalainen et al. (22) reported that a seroconverting mother had a borderline-avidity result 15 weeks after the IgG-negative sample was taken. In a GRA6 avidity assay, one mother who seroconverted 2 weeks before sampling had an avidity index of 75%, higher than that of most chronically infected women. The woman had no underlying immune disorder and displayed a low-avidity result in a Euroimmun avidity assay. The result might be explained by a different pattern of maturation of GRA6 antibodies in this patient.
Avidity assays based on whole-cell T. gondii antigen detect low- or borderline-avidity antibodies in many individuals with chronic infection, whereas recombinant antigens reportedly produce more mature avidity indices in chronically infected individuals and better distinguish between acute and chronic infection (1, 32, 33).
Several studies investigated the usefulness of avidity assays using single antigens of T. gondii for differentiation between acute and chronic infection. Marcolino et al. (26) developed an avidity immunoblotting method and showed that, using 8 M urea in washing buffer, the frequency of the antigenic p32 (former name of GRA6) band recognized by acute-phase sera was reduced from 90 to 20%, while the frequency of the p32 band recognized by chronic sera was reduced from 95 to 80%, suggesting that an avidity assay based on p32 might be useful in differentiating acute and chronic infection.
Beghetto et al. (1) showed that low-avidity antibodies to MIC3 were exclusively present in sera collected within 2 months after onset of infection and high-avidity antibodies were merely detected in sera collected after 2 months after acquisition of infection. In our study, the presence of low-avidity antibodies to GRA6 until 4 months after the onset of infection and the absence of significant correlation between avidity and time after infection, while could be produced by a small number of acute-phase serum samples, might indicate that antibodies to GRA6 mature more than 4 months after infection. In other words, a low avidity index in GRA6 avidity might not necessarily indicate a recent infection, as for avidity assays using whole-cell T. gondii antigen (20, 30). Further research using a sufficient number of serum samples with known times of infection expanding the first year of infection would allow us to determine the time frame when antibodies to GRA6 matured to high avidity.
Some studies reported delayed maturation of IgG affinity upon antiparasitic treatment in pregnant women (23, 35). In our study, 11 of 20 IgG-positive French women with precise estimated times of infection were receiving antiparasitic treatment at the time of blood collection (Table 1). We did not find any significant difference in mean avidity values between sera obtained from treated and nontreated women using GRA6 or Euroimmun avidity assays.
The GRA6 antigen was shown to be a good marker for diagnosis of acute Toxoplasma infection, and high-avidity results can be used to determine the time of infection. A high avidity index in GRA6 avidity excludes a recent infection occurred less than 4 months ago, since only 1 of 20 serum samples from women infected during the past 4 months displayed high-avidity antibodies to GRA6. The conclusion was further supported as none of the 35 Iranian women with acute toxoplasmosis displayed high-avidity antibodies to GRA6.
Similarly, avidity tests based on whole-cell T. gondii antigen are currently used to rule out recently acquired infections (4, 11, 31). We showed better clinical usefulness of GRA6 avidity, compared to Euroimmun avidity, for exclusion of recent infection in pregnant women occurring less than 4 months before; however, more sera obtained from women with known times of infection are required to further evaluate the conclusion.
Recombinant antigens could enhance the clinical usefulness of avidity tests for dating Toxoplasma infections in pregnant women. In addition, standardization of avidity tests would be more feasible by using constant-quality recombinant antigens. Further research is required to identify more useful antigens in avidity assays. Combined use of such antigens with GRA6 might improve the value of avidity assays for accurate diagnosis of T. gondii infection during pregnancy.
Acknowledgments
We appreciate Corinne Mercier for reviewing the manuscript.
This work was supported by research grants 410 and 428 from the Pasteur Institute of Iran.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Beghetto, E., W. Buffolano, A. Spadoni, M. Del Pezzo, M. Di Cristina, O. Minenkova, E. Petersen, F. Felici, and N. Gargano. 2003. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 41:5414-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beghetto, E., A. Spadoni, L. Bruno, W. Buffolano, and N. Gargano. 2006. Chimeric antigens of Toxoplasma gondii: toward standardization of toxoplasmosis serodiagnosis using recombinant products. J. Clin. Microbiol. 44:2133-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodeus, M., S. Feyder, and P. Goubau. 1998. Avidity of IgG antibodies distinguishes primary from non-primary cytomegalovirus infection in pregnant women. Clin. Diagn. Virol. 9:9-16. [DOI] [PubMed] [Google Scholar]
- 4.Candolfi, E., R. Pastor, R. Huber, D. Filisetti, and O. Villard. 2007. IgG avidity assay firms up the diagnosis of acute toxoplasmosis on the first serum sample in immunocompetent pregnant women. Diagn. Microbiol. Infect. Dis. 58:83-88. [DOI] [PubMed] [Google Scholar]
- 5.Cozon, G. J., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 17:32-36. [DOI] [PubMed] [Google Scholar]
- 6.Desmonts, G., and J. Couvreur. 1974. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N. Engl. J. Med. 290:1110-1116. [DOI] [PubMed] [Google Scholar]
- 7.Dunn, D., M. Wallon, F. Peyron, E. Petersen, C. Peckham, and R. Gilbert. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353:1829-1833. [DOI] [PubMed] [Google Scholar]
- 8.Foulon, W., I. Villena, B. Stray-Pedersen, A. Decoster, M. Lappalainen, J. M. Pinon, P. A. Jenum, K. Hedman, and A. Naessens. 1999. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at age 1 year. Am. J. Obstet. Gynecol. 180:410-415. [DOI] [PubMed] [Google Scholar]
- 9.Fricker-Hidalgo, H., C. Saddoux, A. S. Suchel-Jambon, S. Romand, A. Foussadier, H. Pelloux, and P. Thulliez. 2006. New Vidas assay for Toxoplasma-specific IgG avidity: evaluation on 603 sera. Diagn. Microbiol. Infect. Dis. 56:167-172. [DOI] [PubMed] [Google Scholar]
- 10.Gard, S., and J. H. Magnusson. 1951. A glandular form of toxoplasmosis in connection with pregnancy. Acta Med. Scand. 141:59-64. [DOI] [PubMed] [Google Scholar]
- 11.Gay-Andrieu, F., H. Fricker-Hidalgo, E. Sickinger, A. Espern, M. P. Brenier-Pinchart, H. B. Braun, and H. Pelloux. 2009. Comparative evaluation of the ARCHITECT Toxo IgG, IgM, and IgG avidity assays for anti-Toxoplasma antibodies detection in pregnant women sera. Diagn. Microbiol. Infect. Dis. 65:279-287. [DOI] [PubMed] [Google Scholar]
- 12.Golkar, M., K. Azadmanesh, G. Khalili, B. Khoshkholgh-Sima, J. Babaie, C. Mercier, M. P. Brenier-Pinchart, H. Fricker-Hidalgo, H. Pelloux, and M. F. Cesbron-Delauw. 2008. Serodiagnosis of recently acquired Toxoplasma gondii infection in pregnant women using enzyme-linked immunosorbent assays with a recombinant dense granule GRA6 protein. Diagn. Microbiol. Infect. Dis. 61:31-39. [DOI] [PubMed] [Google Scholar]
- 13.Golkar, M., M. A. Shokrgozar, S. Rafati, K. Musset, M. Assmar, R. Sadaie, M. F. Cesbron-Delauw, and C. Mercier. 2007. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine 25:4301-4311. [DOI] [PubMed] [Google Scholar]
- 14.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944-946. [DOI] [PubMed] [Google Scholar]
- 15.Gras, L., R. E. Gilbert, M. Wallon, F. Peyron, and M. Cortina-Borja. 2004. Duration of the IgM response in women acquiring Toxoplasma gondii during pregnancy: implications for clinical practice and cross-sectional incidence studies. Epidemiol. Infect. 132:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedman, K., M. Lappalainen, I. Seppaia, and O. Makela. 1989. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:736-740. [DOI] [PubMed] [Google Scholar]
- 17.Hedman, K., and I. Seppala. 1988. Recent rubella virus infection indicated by a low avidity of specific IgG. J. Clin. Immunol. 8:214-221. [DOI] [PubMed] [Google Scholar]
- 18.Hohlfeld, P., F. Daffos, J. M. Costa, P. Thulliez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 19.Holliman, R. E., R. Raymond, N. Renton, and J. D. Johnson. 1994. The diagnosis of toxoplasmosis using IgG avidity. Epidemiol. Infect. 112:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenum, P. A., B. Stray-Pedersen, and A. G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenum, P. A., B. Stray-Pedersen, K. K. Melby, G. Kapperud, A. Whitelaw, A. Eskild, and J. Eng. 1998. Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. J. Clin. Microbiol. 36:2900-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lappalainen, M., P. Koskela, M. Koskiniemi, P. Ammala, V. Hiilesmaa, K. Teramo, K. O. Raivio, J. S. Remington, and K. Hedman. 1993. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J. Infect. Dis. 167:691-697. [DOI] [PubMed] [Google Scholar]
- 23.Lefevre-Pettazzoni, M., C. S. Le, M. Wallon, and F. Peyron. 2006. Delayed maturation of immunoglobulin G avidity: implication for the diagnosis of toxoplasmosis in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 25:687-693. [DOI] [PubMed] [Google Scholar]
- 24.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) Toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz, E., K. N. Ward, R. Szydlo, and J. M. Goldman. 1996. Cytomegalovirus antibody avidity in allogeneic bone marrow recipients: evidence for primary or secondary humoral responses depending on donor immune status. J. Med. Virol. 49:61-65. [DOI] [PubMed] [Google Scholar]
- 26.Marcolino, P. T., D. A. Silva, P. G. Leser, M. E. Camargo, and J. R. Mineo. 2000. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin. Diagn. Lab. Immunol. 7:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe, R. E., R. G. Brooks, R. F. Dorfman, and J. S. Remington. 1987. Clinical spectrum in 107 cases of toxoplasmic lymphadenopathy. Rev. Infect. Dis. 9:754-774. [DOI] [PubMed] [Google Scholar]
- 28.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 29.Montoya, J. G., and J. S. Remington. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47:554-566. [DOI] [PubMed] [Google Scholar]
- 30.Pelloux, H., E. Brun, G. Vernet, S. Marcillat, M. Jolivet, D. Guergour, H. Fricker-Hidalgo, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1998. Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the Vidas system (bioMerieux). Diagn. Microbiol. Infect. Dis. 32:69-73. [DOI] [PubMed] [Google Scholar]
- 31.Petersen, E., M. V. Borobio, E. Guy, O. Liesenfeld, V. Meroni, A. Naessens, E. Spranzi, and P. Thulliez. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 43:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfrepper, K. I., G. Enders, M. Gohl, D. Krczal, H. Hlobil, D. Wassenberg, and E. Soutschek. 2005. Seroreactivity to and avidity for recombinant antigens in toxoplasmosis. Clin. Diagn. Lab. Immunol. 12:977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietkiewicz, H., E. Hiszczynska-Sawicka, J. Kur, E. Petersen, H. V. Nielsen, M. Paul, M. Stankiewicz, and P. Myjak. 2007. Usefulness of Toxoplasma gondii recombinant antigens (GRA1, GRA7 and SAG1) in an immunoglobulin G avidity test for the serodiagnosis of toxoplasmosis. Parasitol. Res. 100:333-337. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, A., K. Hedman, V. Luyasu, J. Zufferey, M. H. Bessieres, R. M. Blatz, E. Candolfi, A. Decoster, G. Enders, U. Gross, E. Guy, M. Hayde, D. Ho-Yen, J. Johnson, B. Lecolier, A. Naessens, H. Pelloux, P. Thulliez, and E. Petersen. 2001. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 20:467-474. [DOI] [PubMed] [Google Scholar]
- 35.Sensini, A., S. Pascoli, D. Marchetti, R. Castronari, M. Marangi, G. Sbaraglia, C. Cimmino, A. Favero, M. Castelletto, and A. Mottola. 1996. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: a multicenter study. Clin. Microbiol. Infect. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 36.Soderlund, M., C. S. Brown, B. J. Cohen, and K. Hedman. 1995. Accurate serodiagnosis of B19 parvovirus infections by measurement of IgG avidity. J. Infect. Dis. 171:710-713. [DOI] [PubMed] [Google Scholar]
- 37.Stray-Pedersen, B. 1992. Treatment of toxoplasmosis in the pregnant mother and newborn child. Scand. J. Infect. Dis. Suppl. 84:23-31. [PubMed] [Google Scholar]
- 38.Wallon, M., L. Kodjikian, C. Binquet, J. Garweg, J. Fleury, C. Quantin, and F. Peyron. 2004. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 113:1567-1572. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, M., J. S. Remington, C. Clavet, G. Varney, C. Press, and D. Ware. 1997. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J. Clin. Microbiol. 35:3112-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]