Abstract
This study was designed to evaluate which of several T-cell-specific, immune response assays are the most relevant in measuring the key characteristics of an effective immune response to HIV-1. Using 5 HIV-1 antigens as stimulants, we assessed lymphocyte proliferation, supernatant gamma interferon (IFN-γ) cytokine production (CP), single-cell IFN-γ production by enzyme-linked immunospot (ELISPOT) assay, with and without Epstein-Barr virus-transformed B-lymphoblastoid cell lines (B-LCLs), and intracellular cytokine production (ICC) for IFN-γ and interleukin 2 (IL-2) by flow cytometry. We used these to compare specimens from HIV-1-infected subjects who were virally suppressed with a stable antiretroviral therapy (ART) regimen (group A) with specimens from subjects not on ART but with HIV-1 viremia of <3,000 copies/ml (group B). The lymphocyte proliferation assay (LPA) did not significantly differentiate between the two groups. Using fresh peripheral blood mononuclear cells (PBMCs), the CP and ELISPOT assays for IFN-γ detected the greatest differences between the two groups, specific for three of the five HIV-1 antigens, whereas significant differences were seen only in response to one antigen when cryopreserved cells were used. The strongest correlations were seen between the CP and ELISPOT assays. The ELISPOT B-LCL assay showed a cell concentration-dependent increase in IFN-γ production compared to that shown by the standard ELISPOT assay but did not differentiate between the groups. In the ICC assay, greater numbers of IFN-γ-producing T cells were seen in group B, and little or no detectable IL-2 production was seen in both groups. These studies highlight complexities of immunologic monitoring of T-cell responses in multisite clinical trials in HIV infection and outline considerations for optimizing these efforts.
During acute HIV-1 infection, a potent T-cell immune response is generated soon after the peak of viremia and continues until such time as a steady state is reached among immune control, viral replication, and availability of targets (12). The mechanism(s) by which HIV-infected individuals come to a steady-state viremia within several months of initial infection is a central issue in HIV disease pathogenesis. There is a wide variation in the steady-state level among individuals, with rare patients able to control viral replication at levels associated with slow disease progression. It is hypothesized that the T-cell-mediated immune response helps determine the level of viremia in patients who are not on antiretroviral drugs and is therefore a fundamental measure of viral control (6-8). Since persistent antigen stimulation is thought to be necessary to maintain a continuous level of effector function, one might predict that removal of antigen, such as in the setting of potent antiretroviral therapy (ART), would result in a coordinated decrease in T-cell effector activity. In these cases, exogenous viral antigens could be used as immunogens to increase the magnitude of the antiviral immune response (11, 13, 14, 23).
There are many obstacles to the implementation of such a therapeutic strategy. One of these is devising a panel of immunologic readouts predictive of an effective antiviral immune response that could control HIV replication. Another challenge is to utilize antigens for in vitro testing that are sufficiently reflective of the sequence diversity of the circulating virus for each subject. Finally, the analytical characteristics of our current assays have not been directly compared across multiple laboratories. Given the urgency of developing an effective vaccine and methods to evaluate vaccine activity, the AIDS Clinical Trials Group (ACTG) implemented this study to evaluate current assays that may inform the optimal monitoring of a therapeutic immunization trial. Results of this study may then be used by investigators in evaluating which vaccine candidates are effective and should continue to be studied further in clinical trials.
We designed these studies to ask which of the commonly used T-cell-specific immune response assays are the most relevant in measuring the key characteristics of an effective immune response by evaluating their ability to distinguish between two HIV-1-infected populations. As a surrogate cohort for subjects with “vaccine”-induced immunologic control of HIV replication, subjects who maintained low plasma HIV levels (<3,000 copies/ml over at least 6 months in the absence of ART) were chosen for examination. We enrolled chronically HIV-1-infected subjects maintained on a stable ART regimen with undetectable plasma virus levels for at least 9 months to be the comparison group, in which we expected to see less robust immune responses. We also characterized the relations between results using fresh and cryopreserved specimens and the correlation between the assays studied.
Our results showed evidence that the cytokine production (CP) and enzyme-linked immunospot (ELISPOT) assays could reliably distinguish between effective and less effective immune responses. However, the variations between the assay runs and between fresh and frozen cell analyses are larger than has been commonly realized.
MATERIALS AND METHODS
Study design and population.
This was a stratified observational study conducted by the Immunology Specialty Laboratories (ISLs) of the AIDS Clinical Trials Group (ACTG), protocol A5181, in which blood samples were obtained from HIV-1-infected study participants at entry, week 12, and week 24 for plasma HIV-1 RNA and CD4+/CD8+ T-cell count and for multiple immunologic assays designed to measure HIV-1 antigen-specific T-cell function. Local review boards in each study site approved the study, and written informed consent was obtained prior to enrollment. Study participants were stratified into two groups upon entry. Group A consisted of adult HIV-1-positive subjects who had effective control of HIV-1 replication (i.e., <75 copies/ml) with the same antiretroviral regimen for at least 9 months prior to study entry. Subjects in this group continued taking their current antiretroviral regimen for the entire 6-month follow-up. Group B consisted of adult HIV-1-infected patients who had not been on any antiretroviral drugs for 6 months prior to study entry but had a plasma HIV-1 RNA viral level of <3,000 copies/ml within the 6 months prior to entry. Participants in both groups also had a CD4+ T-cell count greater than 300 cells/mm3 within 6 months prior to study entry. Exclusion criteria included pregnancy or breast-feeding and any history of an AIDS-defining opportunistic infection. Medical history was obtained during screening to determine if the participants had any diseases that may reflect altered immunologic status. Each of the 6 ACTG sites enrolled 9 subjects, 5 for group A and 4 for group B. Subjects participated in the study for a maximum of 6 months, with no additional follow-up required.
Cryopreserved and fresh peripheral blood mononuclear cells (PBMCs) were used in the immunologic assays. PBMC isolation and cryopreservation were done using standard operating procedures utilized in Division of AIDS network protocols as described in the HIV/AIDS Network Coordination website at http://www.hanc.info/labs/Pages/PBMCSOP.aspx. All sites assessed the percent and absolute count of CD4+ and CD8+ cells by flow cytometry at entry and at weeks 12 and 24. Each of the six study sites performed three conventional assays, i.e., the lymphocyte proliferation assay (LPA), ELISPOT assay for gamma interferon (IFN-γ), and supernatant cytokine production of IFN-γ detected by enzyme immunoassay (EIA). Week 12 responses from these assays were used to analyze differences using fresh and cryopreserved specimens. In addition, additional assays were performed in selected laboratories. These were intracellular cytokine staining by flow cytometry (ICC-flow, also termed the intracellular cytokine staining [ICS] assay) and the ELISPOT assay with autologous Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell lines (B-LCLs) as antigen-presenting cells (APCs) (ELISPOT B-LCL).
Immunologic assays.
A combination of any of five HIV-1 antigens together with corresponding negative and positive controls was used for each of the assays tested. These HIV-1 antigens were HIV-1 MN Aldrithiol-inactivated virions (final concentration, 5.8 μg/ml; J. Lifson, Frederick, MD), Gag peptide pool (75 15-mer Gag peptides overlapping by 11; p17 [aa 11 to 48, 71 to 107], p17-to-p24 transition, p24 [aa 133 to 363], p15 [aa 364 to 493]; SynPep, Dublin, CA; 1 μg/well), PEN peptide pool (45 15-mer Pol peptides [aa 311 to 345, 356 to 370, 398 to 434, 448 to 474, 496 to 538, 547 to 561, 571 to 612, 640 to 695], 24 15-mer Env peptides [gp120 aa 1 to 15, 31 to 61, 103 to 129, 192 to 217, 237 to 261, 298 to 321, 369 to 391, 416 to 430], and 20 15-mer Nef peptides [68 to 146, 175 to 198], all overlapping by 11 peptides; SynPep, Dublin, CA; 1 μg per well), recombinant HIV-1 p24 protein (aa 241 to 255, 245 to 259, 249 to 263, 329 to 343, 333 to 347, 337 to 351; Protein Sciences, Meriden, CT; 1 μg/well), and a vaccinia virus construct expressing env, gag, and pol (vHIV; multiplicity of infection [MOI], 0.2) (where aa stands for amino acid). Positive controls were staphylococcus enterotoxin B (SEB) superantigen (10 μg/ml), pokeweed mitogen (PWM; 0.1 μg/ml), tetanus toxoid (TT; 1 limit of flocculation unit [LFU]/ml), and Candida albicans (10 μg/ml); negative controls were mock recombinant antigen (for the HIV-1 p24; 1 μg/well), vLacZ (vaccinia virus vector control; MOI, 0.2), and CEMx174 cell extract (5.8 μg/ml; HIV-1 MN control).
For the LPA, the ACTG consensus method was utilized. One hundred thousand PBMCs (fresh for weeks 0 and 24 and thawed and fresh for week 12) were placed in each well of a 96-well plate and were cocultured with SEB, PWM, TT, Candida albicans, and the following HIV-1 antigens: the Gag peptide pool, the PEN peptide pool, HIV-1 MN and a control, and HIV-1 p24 antigen and a control. The cells were allowed to proliferate for 6 days at 37°C in a 5% CO2 humidified incubator. On day 6, the plates were pulsed with 25 μl/well of [3H]thymidine (1 μCi/well; NEN Life Science Products, Boston, MA). After 6 h, cells were harvested on glass fiber filters using a cell harvester. The amount of radioactivity incorporated into DNA was measured in a scintillation counter. The results are reported as stimulation indices (SI = counts per minute experimental/counts per minute background unstimulated).
The CP assay for production of IFN-γ was done using the same antigens used for the LPA plus vHIV. Cryopreserved PBMCs were used at weeks 0 and 24, while both fresh and cryopreserved cells were used at week 12. One million PBMCs were cocultured with Gag and PEN peptide pools, p24 antigen, HIV-1 MN, or vHIV in a 96-well plate at 37°C in a 5% CO2 humidified incubator for 48 h. From each well, 150 μl of supernatant was obtained and placed in a separate, labeled 96-well plate and stored at −20°C. All supernatants were shipped to the University of Alabama laboratory, and the enzyme immunoassay (EIA) was performed in a single batch.
The ACTG consensus method was also used for the ELISPOT assay. This was performed on both fresh and frozen PBMCs at week 12 and on frozen cells at weeks 0 and 24. Using sterile techniques in a biological hood, 96-well ELISPOT plates were coated with mouse anti-human IFN-γ (1-D1K; 100 μl/well of 5-μg/ml solution; Mabtech, Sweden) and incubated overnight at 4°C. The plates were then washed four times in sterile phosphate-buffered saline (PBS) and blocked with 200 μl of sterile RPMI 1640-10% fetal calf serum (FCS) (Gemini Bio-Products, West Sacramento, CA). Three cell concentrations were prepared in RPMI 1640-10% FCS, i.e., 3 × 106, 1 × 106, and 3 × 105 cells/ml, and were added to the wells (at 100 μl/well to deliver final concentrations of 3 × 105, 1 × 105, and 0.3 × 105 cells/well in duplicate) after decanting the blocking medium. Stimulants (SEB, p24, Gag and PEN peptide pools, HIV-1 MN, and vHIV and controls) were added to the appropriate wells at a final culture volume of 125 μl/well. The plates were then incubated in a 5% CO2 incubator for 18 h at 37°C. After overnight incubation, the plates were washed three times with PBS and three times with PBS-0.05% Tween 20 (number BP337-50; Fisher Scientific, Pittsburgh, PA). They were then washed twice with distilled H2O to lyse residual cells. Biotinylated detecting antibody was added to each well (1 μl/ml of mouse anti-human IFN-γ biotinylated monoclonal antibody [MAb]; 100 μl/well), incubated at room temperature (RT) for 3 h, and washed four times with PBS-0.05% Tween 20, with a 5-min soak in PBS-0.05% Tween 20 during the third wash. Enzyme-avidin conjugate diluted in 1% FCS-PBS (ABC-horseradish peroxidase [HRP]; 100 μl/well) was added, and the plates were incubated for 1 h at RT and then washed four times in the same manner as was described earlier. Diaminobenzidine solution (D-0426 at 100 μl/well; Sigma, St. Louis, MO) was added to each well and incubated for 5 min at RT. Plates were washed with tap water three times and were then air dried overnight. Spots were counted the following day with an automated ELISPOT reader system. Background was subtracted from results in the stimulated wells. For the ELISPOT B-LCL assay (performed only in the Pittsburgh ISL on both fresh and frozen cells at weeks 12 and 24), antigens (10 μl/well at 100 μg/ml solution) were loaded into autologous B-LCLs and cocultured with the PBMCs for 2 h at 37°C, as in the antigen-stimulation systems (17). Controls for this assay included medium alone and SEB.
For the ICC assay, fresh and cryopreserved cells were stimulated with three HIV-1 antigens: the Gag and PEN peptide pools and the HIV-1 p24 antigen. Cell suspension (160 μl; final concentration, 1 × 106 cells) was dispensed to designated wells of a 96-well round-bottom plate. The HIV-1 antigens were added to the corresponding wells (40 μl/well), and the plates were incubated at 37°C in a 5% CO2 humidified incubator for an hour. Brefeldin A solution (20 μl at 500 μg/ml; BD, Franklin Lakes, NJ; 10 μl/106 cells) was added to each culture and was incubated for an additional 5 h. After the cells were harvested and washed, they were incubated with FACS permeabilizing solution (BD, Franklin Lakes, NJ; 10× solution diluted 1:10 with deionized water) for 10 min at RT and subsequently washed prior to staining. For each stimulant, 3 sets of 0.3 × 106 PBMCs were stained with three different cocktails of labeled MAb (20 μl; BD, Franklin Lakes, NJ): isotype control MAbs, anti-interleukin 2 (IL-2)-fluorescein isothiocyanate (FITC) plus anti-CD69-phycoerythrin (PE) plus anti-CD4-PerCP (peridinin chlorophyll protein)-Cy5.5 plus anti-CD3-APC MAbs, and anti-IFN-γ-FITC plus anti-CD69-PE plus anti-CD4-PerCP-Cy5.5 plus anti-CD3-APC MAbs. Samples were incubated at RT for 30 min in the dark and then washed twice before adding 200 μl of 1% paraformaldehyde in PBS. The samples were then stored at 4°C in the dark prior to flow cytometric analysis. The samples were analyzed using four-color flow cytometry. At least 50,000 lymphocyte events were acquired and recorded per tube. A template report form that runs under BD CellQuest software was used to analyze the list mode data and calculate the indices for analysis.
Statistical analysis.
Differences in each assay between the two groups were tested using the Wilcoxon rank sum test. Correlations between two different types of assays or between fresh and cryopreserved PBMCs used in the same assay were assessed using rank-based Spearman correlation coefficients. All available data at each time point were included in the analyses. With a sample size of 30 subjects in group A and 24 in group B, the study was expected to have a 94% power to detect a 0.75 log10 shift (e.g., 2 SI to 11.05 SI for LPA responses, 4 spot-forming cells [SFC]/105 cells to 22.10 SFC/105 cells for ELISPOT responses, or 20 ng/ml to 1,105 ng/ml for CP responses) in the distribution of a given assay result on a given type of specimen between the two groups with a type I error of 0.05. Reported P values are two-sided at the 5% level and unadjusted for multiple testing. Comparisons and correlations with a P value of ≤0.05 are considered statistically significant.
RESULTS
Group demographics.
Fifty-four subjects were enrolled, 30 in group A and 24 in group B. Differences between the baseline demographic information of the two groups were not statistically significant (Table 1). The median age was 43 years. Median CD4 counts were 682 and 776 cells/mm3 for groups A and B, respectively. Two subjects from group A had plasma viral loads >50 copies/ml (97 and 1,170 copies/ml) at entry. For group B, the median plasma viral load was 165 copies/ml, with 25th and 75th percentiles being 50 and 1,110 copies/ml, respectively. None of the subjects were lost to follow-up.
TABLE 1.
Baseline demographics of the A5181 study groups (P = not significant)
| Demographics | Valuea |
||
|---|---|---|---|
| Total (n = 54) | Group A (n = 30) | Group B (n = 24) | |
| Age | |||
| Medianb | 43 | 42 | 43 |
| 18-29 | 3 (6) | 2 (7) | 1 (4) |
| 30-39 | 15 (28) | 9 (30) | 6 (25) |
| 40-49 | 22 (41) | 13 (43) | 9 (38) |
| 50-59 | 12 (22) | 5 (17) | 7 (29) |
| Over 60 | 2 (4) | 1 (3) | 1 (4) |
| Gender | |||
| Male | 41 (76) | 25 (93) | 16 (67) |
| Female | 13 (24) | 5 (17) | 8 (33) |
| Race/ethnicity | |||
| White | 30 (56) | 17 (57) | 13 (54) |
| Black | 20 (37) | 10 (33) | 10 (42) |
| Hispanic | 4 (7) | 3 (10) | 1 (4) |
| CD4 countc | |||
| Median | 736 | 682 | 776 |
| Q1, Q3 | 557, 887 | 549, 851 | 598, 918 |
| RNA entry | |||
| <50 copies/ml | 35 (65) | 28 (93) | 7 (29) |
| >50 copies/ml | 19 (35) | 2 (7) | 17 (71) |
| Median (Q1, Q3)d | 165 (50, 1,110) | ||
Values are the numbers of patients (percentages) except where otherwise noted.
Values are the median ages in years.
Values are the numbers of CD4 cells/mm3.
Q values are median HIV-1 RNA expressed in copies per milliliter.
T-cell reactivity in the two groups of subjects assessed by the LP, CP, and ELISPOT assays.
We performed 3 conventional assays for anti-HIV-1 T-cell-specific immunologic function, i.e., the LPA (at entry and weeks 12 and 24 on fresh PBMCs and at week 12 on cryopreserved PBMCs), the CP assay (at week 12 on fresh PBMCs and at entry and weeks 12 and 24 on cryopreserved PBMCs), and the ELISPOT assay (same as for the CP assay). Our initial analysis suggested no discernible variability within each of these assays at the three different time points using the same type of PBMCs, and in order to effectively summarize and present data, results from the three time points using the same type of PBMCs were averaged using the geometric mean for further analysis of each assay. Although there were no subjects lost to follow-up, we were unable to obtain some specimens, and certain samples were classified as technical failures due to inadequate positive-control responses and were excluded from the analyses. A technical failure was defined for each of the assays as follows: for the LPA, an SEB response of SI < 10; for the CP assay, an SEB response of <10,000 ng/ml IFN-γ; for the ELISPOT assay, an SEB response of <100 spot-forming units (SFU)/105 cells; for the ICC-flow assay, fewer than 10 CD8+ CD69+ IFN-γ cells per 50,000 cells examined in the sample stimulated with SEB.
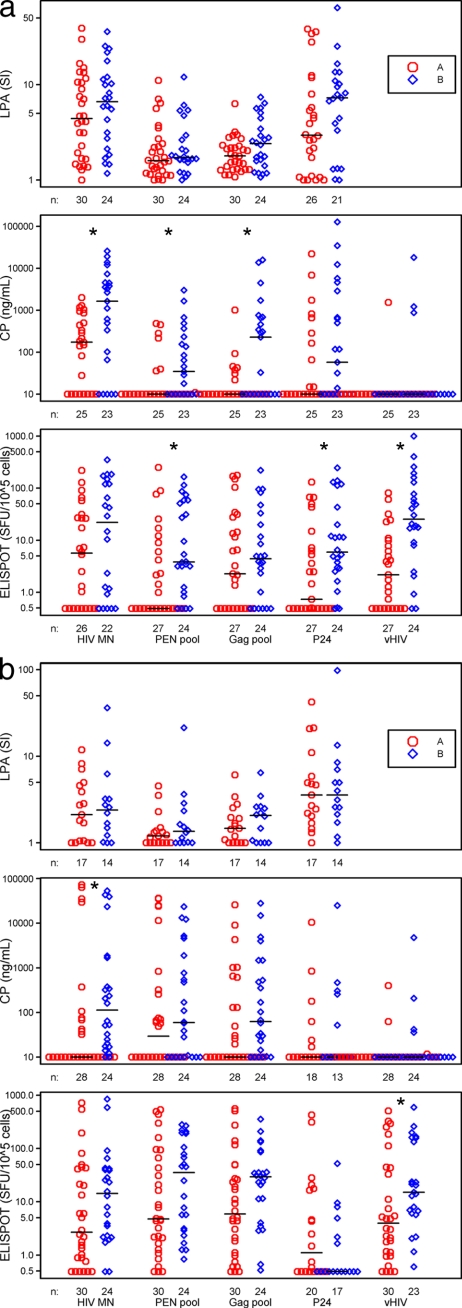
We examined LPA responses to inactivated HIV-1, to both HIV-1 peptide pools, and to recombinant HIV-1 p24 antigen in fresh PBMC samples at all time points and on cryopreserved PBMCs at week 12. The HIV-1 peptide pools should stimulate both CD8+ and CD4+ T cells, whereas the whole protein should predominately activate CD4+ T cells. The results show that the PBMCs from both groups of subjects had proliferative responses to the HIV-1 antigens, with the greatest responses to whole inactivated HIV-1 MN and to p24 Gag (Fig. 1 a). Although there were higher levels of T-cell proliferative responses for group B subjects than for group A to all four types of HIV-1 antigens using fresh PBMCs, these differences were not significant. Similarly, there were no significant differences observed between the groups when using cryopreserved specimens (Fig. 1b).
FIG. 1.
T-cell responses assessed by the LP, CP, and ELISPOT assays in group A versus group B using fresh PBMC specimens (a) and using cryopreserved specimens (b). The CP and ELISPOT assays were used to detect IFN-γ. For each assay, data were averaged over 1 to 3 separate time points for each individual and each antigen, and differences between the two arms were tested by the Wilcoxon rank sum tests. Comparisons marked with asterisks reached clinical significance.
We assessed PBMC supernatants by EIA (CP) for production of IFN-γ in response to the same 4 HIV-1 antigens as in the LPA, as well as to vHIV. Fresh specimens were used from week 12 only, while cryopreserved cells were used for all time points. Fresh PBMCs from group B produced more IFN-γ than group A PBMCs in response to HIV-1 MN and the two HIV-1 peptide pools (Fig. 1a). Indeed, fresh PBMCs from group A produced detectable median IFN-γ in response to HIV-1 MN only. When cryopreserved PBMC samples were used, greater IFN-γ levels were produced in group B than in group A in response to HIV-1 MN only (group A, <10 ng/ml; group B, 113 ng/ml; P = 0.004), with loss of response to the peptide pools (Fig. 1b).
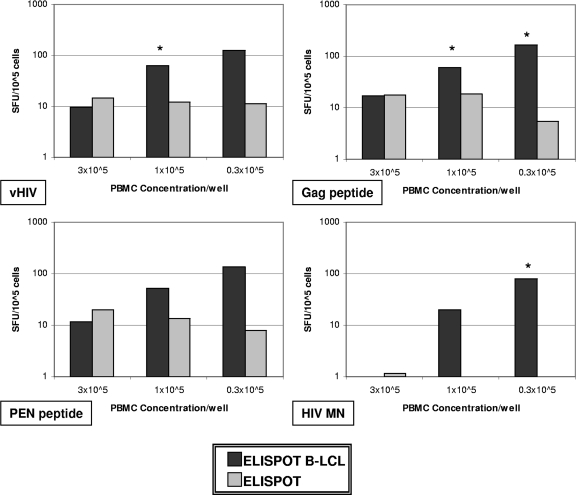
Our third approach to defining T-cell reactivity specific for HIV-1 was enumerating the number of IFN-γ-producing cells as detected by the ELISPOT assay. For this, we tested all 5 HIV-1 antigens. Figure 1a shows results from 4 antigens. Using fresh PBMC specimens, group B subjects had higher IFN-γ responses by ELISPOT assay than group A subjects for the PEN peptide pool, the p24 Gag antigen, and vHIV (P < 0.02). Although IFN-γ production was noted in response to all of the HIV-1 antigens with the cryopreserved specimens for both groups, the assay could differentiate between them only in response to vHIV (group A, 3.97 SFC/105 cells; group B, 15.2 SFC/105 cells; P = 0.037) (Fig. 1b). In a subset of seven subjects, we compared stimulation of IFN-γ production in the ELISPOT assay by coculturing both the fresh and cryopreserved PBMCs with autologous EBV-transformed B-LCLs acting as antigen-presenting cells to the response in the standard ELISPOT assay. The cumulative results show that there was a cell concentration-dependent increase in IFN-γ production in response to B-LCLs loaded with any of the 4 different HIV-1 antigens (vHIV, Gag pool, PEN, or HIV-1 MN) compared to the responses to antigens without B-LCLs. Figure 2 shows a comparison of the two assays using cryopreserved specimens obtained at week 12 and assayed using three different PBMC concentrations. Proportional T-cell reactivity decreased as the PBMC concentration increased, with the greatest T-cell response at the lowest concentration of PBMCs. Although there were significant differences between the results of the ELISPOT B-LCL and the standard ELISPOT assays, the ELISPOT B-LCL assay did not reveal significant differences in T-cell responses between the two study groups (data not shown).
FIG. 2.
Median ELISPOT B-LCL and standard ELISPOT responses in week 12 cryopreserved PBMC samples (n = 7; n = 6 in all 3 × 105-cell concentrations). ELISPOT B-LCL points with asterisks indicate statistically significant differences compared to results from the standard ELISPOT assay.
T-cell reactivity detected by the ICC assay.
Intracellular production of IFN-γ and IL-2 was measured by the ICC assay using flow cytometry in both CD4+ and CD8+ T cells and in both fresh and cryopreserved specimens (Table 2). The two HIV-1 peptide pools and the p24 antigen were used as stimulants. Using fresh specimens, group A IFN-γ production in CD8+ T cells was only found for the PEN antigen, whereas T-cell responses were induced by all of the antigens in cells from group B. The ICC assay significantly differentiated the two groups when using the peptide pools. Significant differences were also observed with the peptide pools when using cryopreserved specimens, but there were greater numbers of reactive T cells in the fresh PBMC responses. There was no detectable IL-2 production by fresh CD8+ T cells in response to either peptide pool. Although IL-2 production was observed in cryopreserved CD8+ T cells, the assay was not able to significantly differentiate between the two groups. Finally, there was little or no detectable IL-2 and IFN-γ production by fresh or cryopreserved CD4+ T cells in response to any of the antigens in group A or B. Where some positive responses were seen, the assay was not able to significantly differentiate between the two groups (Table 2).
TABLE 2.
Expression of IFN-γ and IL-2 in groups A and B as measured by intracellular cytokine staining by flow cytometrya
| Assay | PBMC prep | Group (no. of samples) | Median antigenic stimulus for: |
||
|---|---|---|---|---|---|
| Gag pool | PEN pool | p24 | |||
| ICC-flow IFN-γ CD8+ | Fresh | A (17) | <5 | 6.3 | <5 |
| Fresh | B (14) | 23.2 | 33.9 | 8.7 | |
| P value | 0.048 | 0.019 | 0.14 | ||
| Cryopreserved | A (11) | 5.5 | 21.9 | <5 | |
| Cryopreserved | B (10) | 196.7 | 112.5 | <5 | |
| P value | 0.036 | 0.042 | 0.31 | ||
| ICC-flow IL-2 CD8+ | Fresh | A (17) | <5 | <5 | <5 |
| Fresh | B (14) | <5 | <5 | 20.7 | |
| P value | 0.61 | 0.80 | 0.36 | ||
| Cryopreserved | A (11) | 42.7 | 30.8 | <5 | |
| Cryopreserved | B (10) | 58.6 | 33.6 | 33.0 | |
| P value | 0.47 | 0.76 | 0.07 | ||
| ICC-flow IFN-γ CD4+ | Fresh | A (17) | <5 | <5 | <5 |
| Fresh | B (14) | <5 | <5 | 11.4 | |
| P value | 0.31 | 1.0 | 0.25 | ||
| Cryopreserved | A (11) | 23.7 | <5 | <5 | |
| Cryopreserved | B (10) | 23.9 | 11.5 | 9.2 | |
| P value | 0.82 | 0.52 | 0.67 | ||
| ICC-flow IL-2 CD4+ | Fresh | A (17) | <5 | <5 | <5 |
| Fresh | B (14) | <5 | 10.4 | 6.2 | |
| P value | 0.66 | 0.70 | 0.60 | ||
| Cryopreserved | A (11) | 49.9 | 18.3 | <5 | |
| Cryopreserved | B (10) | 27.1 | 11.9 | 79.7 | |
| P value | 0.63 | 0.97 | 0.29 | ||
For each assay, data were averaged over 1 to 3 separate time points for each individual and each antigen, and medians are for the two arms tested for differences by the Wilcoxon rank sum tests. Comparisons that reached statistical significance are in bold.
Correlations between assays.
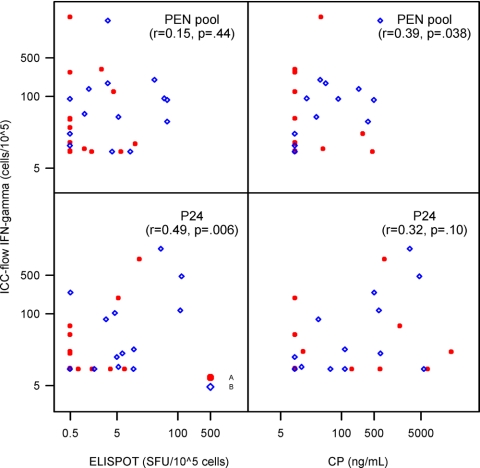
Correlations were assessed between the LP, CP, and ELISPOT assays (Table 3). A modest correlation was seen between the ELISPOT assay and the LPA for both fresh and cryopreserved PBMC samples in response to HIV-1 MN (r = 0.37, P = 0.01 and r = 0.43, P = 0.02, respectively). Similar correlations were noted between the ELISPOT and the CP assays in response to HIV-1 MN (r = 0.39, P = 0.01 and r = 0.53, P < 0.001 for fresh and cryopreserved PBMCs, respectively). Greater correlations were also observed between the ELISPOT and CP responses for cryopreserved PBMCs when stimulated with the Gag and PEN peptide pools (r = 0.60, P < 0.001 and r = 0.68, P < 0.001, respectively). Modest correlations were also noted between the ICC and ELISPOT assays when using the p24 antigen (r = 0.49, P < 0.006) (Fig. 3) and between the ICC and CP assays when using the PEN peptide pool (r = 0.39, P = 0.038) (Fig. 3).
TABLE 3.
Correlation between the ELISPOT and the LP and CP assaysa
| Antigen | Correlation between ELISPOT and: |
|||||||
|---|---|---|---|---|---|---|---|---|
| LPA |
CP assay |
|||||||
| Fresh samples |
Cryopreserved samples |
Fresh samples |
Cryopreserved samples |
|||||
| n | r (P value) | n | r (P value) | n | r (P value) | n | r (P value) | |
| HIV-1 MN | 48 | 0.37 (0.010) | 31 | 0.43 (0.016) | 43 | 0.39 (0.010) | 52 | 0.53 (<0.001) |
| Gag pool | 51 | −0.18 (0.21) | 31 | −0.07 (0.70) | 45 | 0.003 (0.98) | 52 | 0.60 (<0.001) |
| PEN pool | 51 | −0.15 (0.29) | 31 | 0.37 (0.38) | 45 | 0.11 (0.49) | 52 | 0.68 (<0.001) |
| p24 | 44 | 0.03 (0.050) | 24 | 0.28 (0.18) | 45 | −0.03 (0.85) | 30 | 0.04 (0.98) |
| vHIV | Not done | Not done | 45 | 0.05 (0.74) | 51 | 0.11 (0.45) | ||
| vLacZ | Not done | Not done | 42 | −0.08 (0.61) | 44 | 0.11 (0.49) | ||
Correlations in bold indicate statistical significance using rank-based Spearman correlations.
FIG. 3.
Correlation between the ICC-flow assay for IFN-γ (sum of IFN-γ-producing CD4+ and CD8+ T cells) versus the ELISPOT and CP assays using fresh PBMCs (rank-based Spearman correlation).
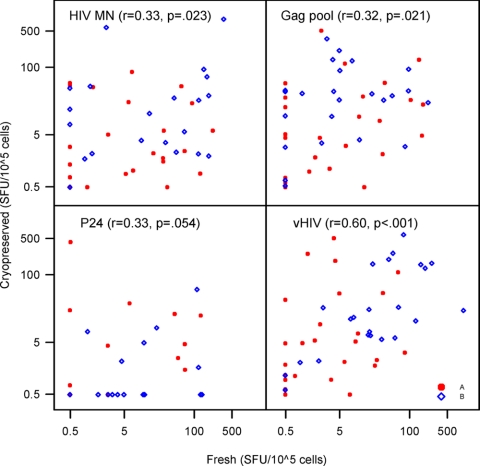
The HIV-1 antigen-specific T-cell responses of fresh and cryopreserved PBMC samples were examined for correlations at week 12 using the three assays. In the LPA, significant correlations were observed between responses of fresh and cryopreserved samples when using HIV-1 MN and the Gag peptide pool. Of the HIV-1 antigens used in the CP assay, only the response to vHIV was found to have a significant correlation in fresh and cryopreserved samples. In the ELISPOT assay, significant albeit marginal correlations between fresh and cryopreserved PBMC samples were found for responses to HIV-1 MN and the Gag peptide pool. A stronger correlation was seen when the stimulant used was vHIV (r = 0.6, P < 0.001) (Fig. 4).
FIG. 4.
Correlations between fresh and cryopreserved PBMCs in the ELISPOT assay (rank-based Spearman correlation).
DISCUSSION
As immunotherapeutic strategies for HIV-1 infection are continuously being developed, so are the techniques for assessing their immunologic activities. In HIV-1 immune-based therapy trials, the standard assays have included the ELISPOT, LP, CP, and ICC assays. Of these, the ELISPOT assay has been considered the gold standard in a number of vaccine trials because of its sensitivity and extensive validation (18, 20). However, although the ELISPOT assay is more sensitive than the ICC assay, the latter has the advantage of simultaneously identifying production of many different cytokines, chemokines, and cytotoxic factors (26).
The rationale for this study was to compare T-cell reactivity using different, highly controlled immunologic assays in order to determine which of these commonly used assays is the most relevant in measuring an effective immune response to HIV. Our data suggested that no single assay showed clear-cut differences between the study groups for all of the HIV-1 antigens. Among the four assays tested, the LPA did not differentiate between the two groups with any of the HIV antigens tested. Use of fresh or cryopreserved specimens did not make a difference. Despite not being able to differentiate between the two groups, lymphocyte proliferation was observed with all the antigens tested. The inability to distinguish between the two groups may be because the LPA has been shown to detect T-cell immunity whether the immune response is low or robust, whereas IFN-γ secretion detected by the ELISPOT assay was accurate in assessing a robust T-cell response (15).
The CP assay detected the most number of differences between the two groups of study subjects, indicating that this simple EIA for IFN-γ on cell culture supernatants may be adequate in distinguishing between endogenous immune control of HIV replication and its absence. The ELISPOT assay detected as many differences as the CP assay but in response to different HIV-1 antigens. As the ELISPOT assay has been standardized and is sensitive to low-frequency responses, as low as 25 IFN-γ-producing cells per million (25), results of this assay still prove to be useful readouts of immunologic control. However, it is important to consider that results of previous studies have shown that the ELISPOT assay for IFN-γ production does not necessarily correlate with viral control (1, 27), hence the need for other assays that would better predict immune control of HIV.
The use of B-LCLs as antigen-presenting cells for the ELISPOT assay also yielded interesting results. Although this assay did not show significant differences between the groups, there was an increase in the numbers of IFN-γ-producing cells in this system compared to results using the standard ELISPOT assay in response to most of the HIV-1 antigens. This has been found in previous studies wherein the use of B-LCLs yielded higher numbers of HIV-1-specific cytotoxic T lymphocyte (CTL) and IFN-γ-producing cells (10, 17). Due to the presence of EBV and the relatively high background responses to the B-LCL assay, current approaches to enhance the magnitude of T-cell reactivity in vitro include using autologous dendritic cells (16, 22) and nontransformed B cells (28) as antigen-presenting cells or an extension of the incubation period in the ELISPOT assay (9). It is important to note that the ELISPOT B-LCL assay was conducted in only a subset of patients (n = 7), which could possibly explain why, statistically, it was not able to distinguish between the two groups.
Although the ICC assay was able to differentiate between the two groups only when using the peptide pools, it was able to do so in both fresh and cryopreserved specimens, unlike the CP and ELISPOT assays, where the ability to distinguish between the groups was decreased when cryopreserved specimens were used. Our results confirm previously published findings which found that in flow cytometry, peptides were more effective in stimulating responses in both fresh and cryopreserved than whole-protein antigens (21). Moreover, previous studies have suggested that using 15-mer peptides overlapping by 11 amino acids can effectively stimulate responses in both CD4+ and CD8+ T cells (19, 21). Our results showed that even if we were able to show T-cell responses in both CD4+ and CD8+ T cells using these types of peptides, it was only in the CD8+ T cells that the ICS was able to distinguish between the two groups. Secretion of IL-2 did not distinguish between the two groups. Moreover, CD8+ IL-2 secretion was lower than CD8+ IFN-γ secretion. This could be due to CD8+ T-cell dysfunction or depletion as a consequence of sustained HIV replication. IL-2 production is lost early in the transition into viral persistence (5). Indeed, in chronic viral infections, there is a hierarchical loss of effector cytokines, with IL-2 secretion as the first to go and IFN-γ the last (29). The advantage of using flow cytometry is its ability to combine multiple parameters in evaluating T-cell function. As recent studies have shown that an effective HIV-1 response is characterized by the presence of a polyfunctional (i.e., secreting two or more immune mediators) CD8+ T-cell response to the virus (2, 4, 24), multiparameter flow cytometry could have a better role in evaluating HIV immunotherapeutic strategies.
Since multiple sites are necessary for large studies, such as those needed to explore the utility of prophylactic vaccines, the use of cryopreserved PBMCs permits centralized analyses of samples in these multisite trials. In the ELISPOT and CP assays, the ability to detect a difference between the two groups was decreased when cryopreserved specimens were used. Indeed, in the study by Best et al. (3), IFN-γ release as detected by enzyme-linked immunosorbent assay (ELISA) was lower in samples that were cryopreserved than that in fresh samples. Specimen processing may be a factor contributing to the disparity. As such, strict quality control in cryopreservation and thawing is necessary. Although participating sites in the ACTG follow the same procedures in processing specimens for all network protocols, laboratory performance must be regularly evaluated to ensure strict adherence and uniformity among laboratory processing sites. As with frozen specimens, the use of fresh samples also requires strict processing guidelines. In protocols with multiple study sites, proper and efficient shipping procedures must be in place if real-time assays are to be done. In ICC-flow assays, the same number of statistically significant comparisons was observed with the use of fresh and frozen samples.
In conclusion, we found that both the CP and ELISPOT assays were able to discriminate in vitro immune responses associated with endogenous control of HIV replication from those that were not. However, the wide variation between the assays studied provides a cautionary note to the use of multiple laboratories to monitor results of HIV immunotherapeutic vaccine trials, specifically those which use in vitro assays of T-cell function as primary endpoints. The basics of specimen handling, cryopreservation, and thawing should also be emphasized, as they will necessarily affect the performance and reliability of these assays. Quality control of immunologic techniques should be performed regularly in order to validate these tests, allowing them to be used in large-scale immunotherapeutic studies.
Acknowledgments
The project described was supported by the following grants from the National Institute of Allergy and Infectious Diseases: U01AI068636, SDMC grant AI68634, ACTG grants AI69477, AI27675, AI73961, AI69494, AI69501, AI68638, AI069471, AI038858, AI069452, AI69450, AI54907, and RR25780.
We gratefully acknowledge the study volunteers and the A5181 study sites and site staff, particularly Margaret Fischl and Leslie Thompson (University of Miami School of Medicine), Sharon Riddler, Carol Oriss, Xiao-Li Huang, and Nancy Connolly (University of Pittsburgh), Kathleen Medvik and Dominic Dorazio (Case Western Reserve University), Allan Tenorio and Janet Rindells (Rush University Medical Center), Xiao-Dong Li (UC Davis), Karen Savage and Dana Green (University of Alabama Therapeutics), and Graham Ray and Monica Carten (University of Colorado Hospital).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Elridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, B. M., B. L. Block, A. C. Rothchild, and B. D. Walker. 2009. Elite control of HIV infection: implications for vaccine design. Expert Opin. Biol. Ther. 9:55-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best, A., G. Hidalgo, K. Mitchell, and J. R. Yannelli. 2007. Issues concerning the large scale cryopreservation of peripheral blood mononuclear cells (PBMC) for immunotherapy trials. Cryobiology 54:294-297. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, D. G., L. Teyoton, M. B. Oldstone, and D. B. McGavern. 2005. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79:10514-10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucy, R. P. 1999. Immune clearance of HIV type 1 replication-active cells: a model of two patterns of steady state HIV infection. AIDS Res. Hum. Retroviruses 15:223-227. [DOI] [PubMed] [Google Scholar]
- 7.Bucy, R. P. 2001. Viral and cellular dynamics in HIV disease. Curr. Infect. Dis. Rep. 3:295-301. [DOI] [PubMed] [Google Scholar]
- 8.Bucy, R. P., and J. M. Kilby. 2001. Perspectives on inducing efficient immune control of HIV-1 replication—a new goal for HIV therapeutics? AIDS 15(Suppl. 2):S36-S42. [DOI] [PubMed] [Google Scholar]
- 9.Calarota, S. A., A. Foli, R. Maserati, F. Baldanti, S. Paolucci, M. A. Young, C. M. Tsoukas, J. Lisziewicz, and F. Lori. 2008. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J. Immunol. 180:5907-5915. [DOI] [PubMed] [Google Scholar]
- 10.Connick, E., R. L. Schlichtemeier, M. B. Purner, K. M. Schneider, D. M. Anderson, S. MaWhinney, T. B. Campbell, D. R. Kuritzkes, J. M. Douglas, Jr., F. N. Judson, and R. T. Schooley. 2001. Relationship between human immunodeficiency virus type 1 (HIV-1)-specific memory cytotoxic T lymphocytes and virus load after recent HIV-1 seroconversion. J. Infect. Dis. 184:1465-1469. [DOI] [PubMed] [Google Scholar]
- 11.Connolly, N. C., T. L. Whiteside, C. Wilson, V. Kondragunta, C. R. Rinaldo, and S. A. Riddler. 2008. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin. Vaccine Immunol. 15:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406-416. [DOI] [PubMed] [Google Scholar]
- 13.Endsley, A. N., N. N. Salama, and R. J. Ho. 2008. Combining drug and immune therapy: a potential solution to drug resistance and challenges of HIV vaccines? Curr. HIV Res. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 14.Fuse, S., M. J. Molloy, and E. J. Usherwood. 2008. Immune responses against persistent viral infections: possible avenues for immunotherapeutic interventions. Crit. Rev. Immunol. 28:159-183. [DOI] [PubMed] [Google Scholar]
- 15.Goodell, V., C. dela Rosa, M. Slota, B. McLeod, and M. L. Disis. 2007. Sensitivity and specificity of tritiated thymidine incorporation and ELISPOT assays in identifying antigen specific T cell immune responses. BMC Immunol. 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, X. L., Z. Fan, L. Borowski, and C. R. Rinaldo. 2008. Maturation of dendritic cells for enhanced activation of anti-HIV-1 CD8(+) T cell immunity. J. Leukoc. Biol. 83:1530-1540. [DOI] [PubMed] [Google Scholar]
- 17.Huang, X. L., Z. Fan, J. Liebmann, and C. Rinaldo. 1995. Detection of human immunodeficiency virus type 1-specific memory cytotoxic T lymphocytes in freshly donated and frozen-thawed peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 2:678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janetzki, S., S. Schaed, N. E. Blachere, L. Ben-Porat, A. N. Houghton, and K. S. Panageas. 2004. Evaluation of ELISPOT assays: influence of method and operator on variability of results. J. Immunol. Methods 291:175-183. [DOI] [PubMed] [Google Scholar]
- 19.Kiecker, F., M. Streitz, G. Cherepnev, H. D. Volk, R. Volkmer-Engert, and F. Kern. 2004. Analysis of antigen-specific T-cell responses with synthetic peptides—what kind of peptide for which purpose? Hum. Immunol. 65:523-536. [DOI] [PubMed] [Google Scholar]
- 20.Kutscher, S., C. J. Dembek, S. Allgayer, S. Heltai, B. Stadibauer, P. Biswas, S. Nozza, G. Tambussi, J. R. Bogner, H. J. Stellbrink, F. D. Goebel, P. Lusso, M. Tinelli, G. Poli, F. Erfle, H. Pohla, M. Mainati, and A. Cosma. 2008. The intracellular detection of MIP-1beta enhances the capacity to detect IFN-gamma mediated HIV-1-specific CD8 T-cell responses in a flow cytometric setting providing a sensitive alternative to the ELISPOT. AIDS Res. Ther. 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 22.Rinaldo, C. R. 2009. Dendritic cell-based human immunodeficiency virus vaccine. J. Intern. Med. 265:138-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Routy, J. P., M. R. Boulassel, B. Yassine-Diab, C. Nicolette, D. Healey, R. Jain, O. Yegorov, I. Tcherepanova, T. Monesmith, L. Finke, and R. P. Sekaly. 2010. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin. Immunol. 134:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247-258. [DOI] [PubMed] [Google Scholar]
- 25.Streeck, H., N. Frahm, and B. D. Walker. 2009. The role of IFN-gamma ELISPOT assay in HIV vaccine research. Nat. Protoc. 4:461-469. [DOI] [PubMed] [Google Scholar]
- 26.Tobery, T. W., S. A. Dubey, K. Anderson, D. C. Freed, K. S. Cox, J. Lin, M. T. Prokop, K. J. Sykes, R. Mogg, D. V. Mehrotra, T. M. Fu, D. R. Casimiro, and J. W. Shiver. 2006. A comparison of standard immunogenicity assays for monitoring HIV type 1 gag-specific T cell responses in Ad5 HIV type 1 gag vaccinated human subjects. AIDS Res. Hum. Retroviruses 22:1081-1090. [DOI] [PubMed] [Google Scholar]
- 27.Valentine, L. E., S. M. Piaskowski, E. G. Rakasz, N. L. Henry, N. A. Wilson, and D. I. Watkins. 2008. Recognition of escape variants in ELISPOT does not always predict CD8+ T-cell recognition of simian immunodeficiency virus-infected cells expressing the same variant sequences. J. Virol. 82:575-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Bergwelt-Baildon, M., A. Shimabukuro-Vornhagen, A. Popov, et al. 2006. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood 107:2786-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]