Abstract
A simple method suitable for self-administration of vaccine would improve mass immunization, particularly during a pandemic outbreak. Influenza virus-like particles (VLPs) have been suggested as promising vaccine candidates against potentially pandemic influenza viruses, as they confer long-lasting immunity but are not infectious. We investigated the immunogenicity and protective efficacy of influenza H5 VLPs containing the hemagglutinin (HA) of A/Vietnam/1203/04 (H5N1) virus delivered into the skin of mice using metal microneedle patches and also studied the response of Langerhans cells in a human skin model. Prime-boost microneedle vaccinations with H5 VLPs elicited higher levels of virus-specific IgG1 and IgG2a antibodies, virus-specific antibody-secreting cells, and cytokine-producing cells up to 8 months after vaccination compared to the same antigen delivered intramuscularly. Both prime-boost microneedle and intramuscular vaccinations with H5 VLPs induced similar hemagglutination inhibition titers and conferred 100% protection against lethal challenge with the wild-type A/Vietnam/1203/04 virus 16 weeks after vaccination. Microneedle delivery of influenza VLPs to viable human skin using microneedles induced the movement of CD207+ Langerhans cells toward the basement membrane. Microneedle vaccination in the skin with H5 VLPs represents a promising approach for a self-administered vaccine against viruses with pandemic potential.
Influenza viruses typically cause seasonal epidemics resulting in over 200,000 hospitalizations and approximately 36,000 annual deaths in the United States (45). In addition to seasonal outbreaks, a new pandemic influenza virus strain may emerge at any time. For example, the novel 2009 H1N1 virus has spread rapidly throughout the world, resulting in the first influenza pandemic in the 21st century (6). Indeed, the recent experience with the 2009 H1N1 virus demonstrates the need to develop improved methods of immunization, as conventional vaccination programs showed a significant delay in controlling the new pandemic spread despite a half-century of experience with influenza vaccines.
In 1997, the first human cases of infection by highly pathogenic H5N1 avian influenza viruses were reported, with 6 fatalities out of 18 confirmed cases (7, 41). Since 2003, more than 400 human infections with H5N1 viruses have occurred from recurring H5N1 outbreaks. Accumulating data indicated that the fatality rates among H5N1-infected individuals are about 60% (http://www.who.int/csr/disease/avian_influenza). Although H5N1 viruses isolated from humans retain characteristic features of avian influenza viruses, direct transmission of this virus among family members has been observed in Vietnam, Thailand, and Indonesia (31, 46, 50). If these H5N1 viruses were to acquire the properties for efficient transmission among humans, like the 2009 H1N1 pandemic virus, and if the fatality rate remains high, this virus would pose a significant health threat.
It is highly desirable to develop pandemic influenza vaccines that can be rapidly produced on a large scale and at low cost, as well as vaccine delivery methods that can achieve mass vaccination within weeks rather than months. Virus-like particles (VLPs) have been suggested as a promising candidate vaccine against influenza viruses. Such influenza VLPs have been demonstrated to provide protective immunity in experimental animal models (12, 15, 19, 37, 44), and VLP vaccines against other diseases are in widespread clinical use (18).
The skin is considered an attractive site for vaccination due to the abundance of Langerhans and dermal dendritic cells (11, 17, 27, 30). Intradermal (i.d.) immunization, i.e., delivering antigens to the dermal layer in the skin, has been investigated in many clinical trials (1, 3, 20). In particular, i.d. delivery of influenza vaccines was reported to induce greater protective immunity in the high-risk elderly population (16), possibly by stimulating effective cellular immune responses (51). While the use of syringes and needles to deliver a liquid formulation of vaccines is the most common method for delivering i.d. vaccines, the injection is painful and difficult to perform in a reproducible manner and requires highly trained medical personnel.
Microneedles have been developed to facilitate simple and effective vaccination without using hypodermic needles (13, 34). Because they can be prepared in a patch format, microneedles are envisioned to be administered easily and quickly by minimally trained personnel, or possibly by patients themselves. Metal microneedles coated with whole inactivated influenza viruses were demonstrated to deliver the antigen cargo through the restrictive stratum corneum skin barrier, eliciting protective immunity (23, 36, 53). In this study, we tested the feasibility of microneedle vaccination using influenza H5 (A/Vietnam/1203/04) VLPs. Vaccination by microneedles coated with H5 VLPs in the skin induced protective immunity in mice equivalent to or higher than that from conventional intramuscular immunization. Importantly, we provide evidence that these findings could be relevant for human vaccines because human Langerhans cells (LCs) responded to influenza VLP vaccines delivered by coated microneedles.
MATERIALS AND METHODS
Cells, virus, and reagents.
SF9 insect cells for the production of recombinant baculoviruses (rBVs) and VLPs were cultured in SF900-II SFM medium at 27°C. The wild-type influenza A/Vietnam/1203/04 virus (A/VN/1203/04) isolated from a fatal human infection was used in animal challenge studies, and a reassortant virus, termed rgΔH5N1, which contains hemagglutinin (HA) and neuraminidase derived from H5/VN and internal proteins from A/PR/8/34 (H1N1) virus, was inactivated and used as an enzyme-linked-immunosorbent assay (ELISA) antigen as described previously (19).
Preparation and characterization of influenza H5 VLPs.
Influenza H5 VLPs containing H5 HA and M1 proteins derived from influenza A/VN/1203/04 virus were produced using the rBV expression system, as previously described (19). The protein concentration of H5 VLPs was quantified with a protein assay kit (Bio-Rad), and the content of HA was determined by Western blotting using the purified H5 HA protein as a standard. The biological activity of the HA protein of VLPs was determined by a hemagglutination assay as previously described (19).
Fabrication and coating of microneedle arrays.
Stainless steel microneedle arrays were fabricated using laser cutting and electropolishing (21). For vaccine coating, a microneedle array was dipped six times into a coating solution using a dip-coating device (21) and then air dried at room temperature. The coating solution consisted of 1% (wt/vol) carboxymethylcellulose sodium salt (Carbo-Mer), 0.5% Lutrol F-68 NF (BASF) in the absence or presence of 15% (wt/vol) d-(+)-trehalose dihydrate (Sigma Aldrich) and 1 mg/ml H5 VLPs in phosphate-buffered saline (PBS). Images of uncoated and coated microneedle arrays were taken by bright-field microscopy (Olympus) with a charge-coupled device (CCD) camera as described previously (21).
Immunization and viral-challenge infection.
Female 7- to 8-week-old BALB/c mice (n = 15; Harlan) were vaccinated in the skin using microneedles as described previously (21). Briefly, an array with five microneedles coated with a total of 0.4 μg of H5 VLP vaccine protein was manually inserted into the back skin of the mice and left for 5 to 10 min. For intramuscular (i.m.) immunization groups, 0.4 μg unprocessed H5 VLPs in PBS or redissolved H5 VLPs from coated microneedles in 50 μl PBS (i.m.-R) were injected intramuscularly in the upper quadriceps muscles of mice. Both microneedle and i.m. immunization sites were close to the draining inguinal lymph nodes, where antigen presentation occurs (22). The control mice received microneedles without VLPs (mock). Treatment with the microneedle patches apparently did not induce irritation or distress to the animals. To determine the amount of H5 VLP vaccine with which the microneedles were coated, vaccine-coated microneedles were incubated in PBS for 12 h at 4°C, and the amount of protein was measured. Mice (n = 9 out of 15) that were immunized using microneedles or intramuscularly at weeks 0 and 8 were transferred to enhanced animal biosafety level (BSL) 3 facilities (CDC, Atlanta, GA) for challenge studies at 16 weeks after boost. The challenge dose of 500 PFU A/VN/1203/04 virus (50 times the mouse 50% lethal dose [LD50]) was used to inoculate mice intranasally. Mice with body weight loss of ≥25% after inoculation were euthanized. To determine the virus titer, tissues collected at day 4 postchallenge (n = 3 out of 9 challenged mice) were homogenized, and the virus titer was quantified by plaque assay on MDCK cells using methods described previously (19).
Serum antibody responses and HI titers.
Influenza virus-specific antibodies of different isotypes and hemagglutination inhibition (HI) titers were determined using standard methods, as described previously (19). Briefly, 4 μg of inactivated influenza H5N1 virus was used as a coating antigen on 96-well microtiter plates (Nunc, Rochester, NY). Serially diluted serum samples were added and incubated for 1 h at 37°C, and then horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, IgG1, and IgG2a were used as secondary antibodies with O-phenylenediamine as a substrate. Standard IgG molecules were used to determine the antibody concentrations in immune sera from readings at 450 nm. For determination of HI titers, receptor-destroying enzyme-treated serum samples (25 μl) were incubated with equal volumes of inactivated H5N1 virus (4HAU) at room temperature (RT) for 30 min. After incubation, an equal volume of 1% horse erythrocytes was added and incubated at RT for 1 h. The HI titer was expressed as the reciprocal of the highest dilution of the samples preventing hemagglutination.
Virus-specific recall immune responses.
Mice vaccinated with H5 VLPs i.m. and through microneedle procedures (n = 6 out of 15 mice per group) were exposed to the inactivated rgΔH5N1 virus (1 μg) via the intranasal route 6 days prior to sacrifice. Antibody-secreting cell responses in the bone marrow and spleens were determined after 2 or 6 days of in vitro culture as described previously (19).
ELISPOT assay for determination of T-cell responses.
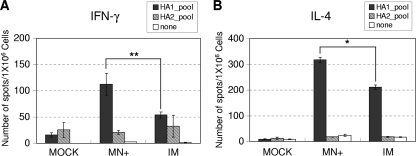
Splenocytes were isolated from immunized mice and cultured in Multiscreen 96-well filtration plates coated with capture antibodies against mouse gamma interferon (IFN-γ) or interleukin 4 (IL-4) cytokine (1.0 × 106 cells/well). The spleen cell cultures were stimulated for 36 h at 37°C with an HA1 or HA2 subunit peptide pool (10 μg/ml) derived from A/Thailand/16/04 (H5N1), which has 99% homology with VN/04 HA. Cytokine IFN-γ- or IL-4-producing cell spots were developed with stable 3,3-diaminobenzidine and counted using an ImmunoSpot enzyme-linked immunospot (ELISPOT) reader as previously described (19).
Langerhans cell distribution in excised human skin.
LC distribution studies were performed in surgically removed but still viable human skin, obtained under informed consent and with full ethical approval at Cardiff University. Microneedle arrays were coated as described previously (21) to deliver H5 VLPs (10 μg) to the human skin following a 5-min insertion period. The treated regions of skin were excised and placed in an optimized air-liquid interface organ culture system, detailed elsewhere (4, 28, 32). To determine LC numbers in the epidermis following VLP microneedle treatment, immunohistochemical staining of human epidermal sheets was achieved using established protocols (28). Briefly, human epidermal sheets were incubated in 0.03% (vol/vol) H2O2 for 5 min and then incubated for 1 h with CD207 mouse monoclonal antibody (Abcam). The epidermal sheets were visualized by light microscopy to count the LCs.
Histological skin sections (4 μm) were generated (Leica 2125 RT microtome; Milton Keynes, United Kingdom) and captured on Superfrost Plus slides. The slides were rehydrated through an ethanol gradient and subjected to heat-mediated antigen retrieval in Tris-EDTA buffer (10 mM Tris base, 1 mM EDTA, 0.05% [vol/vol] Tween 20 in 1 liter double-diluted H2O [ddH2O] adjusted to pH 9.0) at 95°C for 40 min, following which the slides were maintained in antigen retrieval buffer but allowed to cool for 20 min at room temperature. The slides were washed in two changes of PBS-Tween prior to incubation in 0.03% H2O2 for 5 min at room temperature. Detection and visualization of CD207 were the same as for epidermal sheets. The LC distance from the basement membrane was determined in immunohistochemical (IHC)-stained sections using ImageJ software.
Statistical analysis.
Every assay was measured using three to six samples, from which the arithmetic mean and standard error were calculated. A two-tailed paired Student's t test was performed when two groups of animals were compared. A P value of <0.05 was considered statistically significant.
RESULTS
Coating microneedles with influenza H5 VLPs.
The microneedles used in this study were approximately 700 μm in length and 50 μm in cross section at the base (Fig. 1 A) to allow penetration into the epidermal and dermal layers of skin (13, 21, 23, 53). The microneedles were coated with influenza H5 VLPs containing HA from H5N1 (A/VN/1203/04) virus and air dried. A bright-field micrograph of the microneedles after they were coated with influenza H5 VLPs is shown in Fig. 1A. The amount of influenza H5 VLPs coated onto an array with 5 microneedles was 0.4 μg of VLPs, as determined after elution in PBS (Fig. 1B). The addition of trehalose to the coating solution was previously shown to stabilize inactivated influenza virus vaccines (36). We also found that H5 VLPs were stabilized by the presence of trehalose during microneedle coating, as indicated by the retention of over 65% of the hemagglutinating activity after elution of VLPs from microneedles into PBS (Fig. 1C). Trehalose itself did not show any hemagglutination activity (data not shown). In contrast, microneedle coating without trehalose resulted in almost complete loss of the hemagglutinating activity of H5 VLP vaccines (Fig. 1C).
FIG. 1.
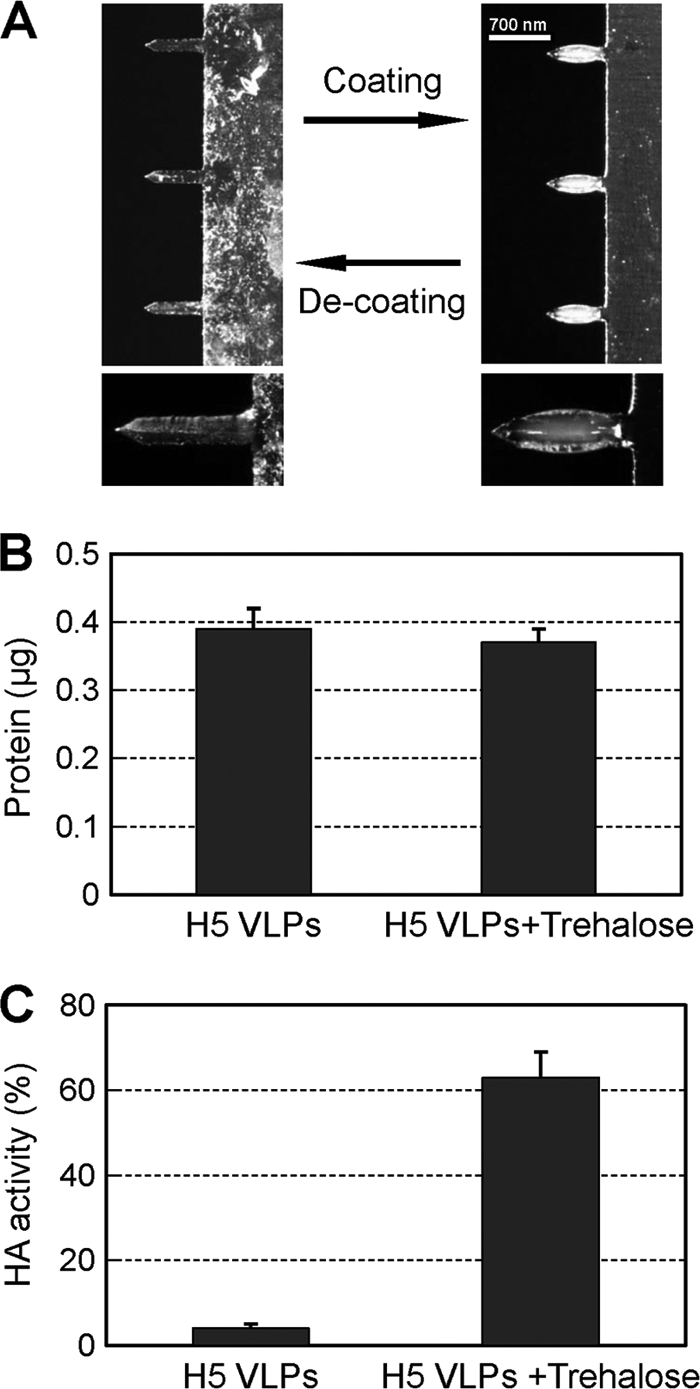
Coating microneedles with H5 VLPs. (A) A microneedle array coated with influenza H5 VLPs is shown as a bright and bulky shape under the micrograph. Influenza H5 VLP-coated microneedles with or without trehalose were used for microneedle vaccination in the skin or redissolved for characterization and intramuscular injection of H5 VLPs in PBS from coated microneedles. (B) H5 VLP vaccine doses on microneedles. The amounts of total protein are represented for an array with 5 microneedles coated with vaccine alone or with VLPs in a trehalose-containing formulation. (C) HA activities (percent of unprocessed control) were determined after dissolving H5 VLP vaccines from microneedles coated or not with trehalose formulation. The data are presented as means ± standard errors.
Microneedle vaccination with H5 VLP induces higher IgG levels.
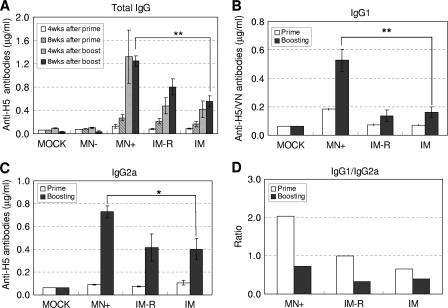
To assess the importance of HA stabilization, mice were vaccinated using microneedles coated with H5 VLP (0.4 μg total protein) formulation containing trehalose (MN+) or lacking trehalose (MN−) into the skin of mice. As comparative controls, groups of mice were intramuscularly immunized with untreated H5 VLPs (0.4 μg; designated i.m.) or H5 VLPs (0.4 μg) previously redissolved from coated microneedles (designated i.m.-R) containing trehalose at weeks 0 and 8 (Fig. 2 A). Thus, the i.m.-R group was immunized with an amount of H5 VLP formulation equivalent to that of the microneedle group (MN+), allowing direct comparison between the routes of delivery. Immunization with microneedles coated with H5 VLPs without trehalose (MN−) did not induce significant levels of antibodies specific for H5N1 virus, and no immune response was observed in this group after boost (Fig. 2A). Microneedles coated with H5 VLPs in trehalose as a stabilizer (MN+) elicited the highest levels of IgG antibodies specific for H5N1 virus after prime-boost vaccination (Fig. 2A). Both i.m. groups (i.m.-R with trehalose and i.m. with untreated H5 VLP vaccine without trehalose) vaccinated with H5 VLPs intramuscularly induced similar IgG antibody responses at the 8-week time point, which were significantly lower than those observed in the trehalose-stabilized microneedle group (MN+) (P < 0.01). The presence of the trehalose stabilizer did not significantly affect host immune responses, as shown in the i.m.-R group. Therefore, these results indicate that microneedle vaccination in the skin was more immunogenic than i.m. immunization. In addition, trehalose was found to stabilize the H5 VLP vaccines, which was an important factor for maintaining their immunogenic property.
FIG. 2.
Total IgG antibody responses and isotypes specific to H5N1 influenza virus. Groups of mice were immunized using microneedles or intramuscular injection at weeks 0 and 8. Virus-specific IgG antibody titers (A), IgG1 (B) and IgG2 (C) isotypes, and the ratios of IgG1 to IgG2a isotypes (D) were determined at 8 weeks after prime or boost vaccination. The data represent mean protein concentrations ± standard errors of 1:100 diluted serum samples from individual mice (n = 9). Mock, microneedle vaccination without vaccine; MN−, vaccination using microneedles coated with 0.4 μg H5 VLPs in the absence of trehalose; MN+, vaccination using microneedles coated with 0.4 μg H5 VLPs in the presence of trehalose; IM-R, intramuscular immunization with 0.4 μg H5 VLPs redissolved from coated microneedles with trehalose formulation; IM, intramuscular immunization with 0.4 μg unprocessed (intact) H5 VLPs. Each group contained 9 BALB/c mice. The asterisks indicate significant differences (*, P < 0.05; **, P < 0.01).
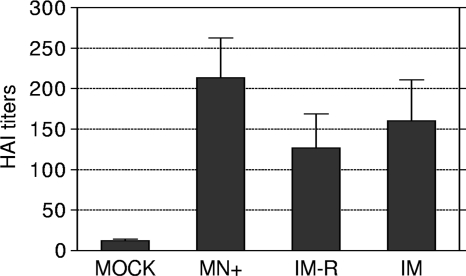
The pattern of antibody isotypes provides insight into the Th1/Th2 polarization of the immune response induced by microneedle vaccination with H5 VLPs. As shown in Fig. 2B and C, microneedle vaccination (MN+) induced significantly higher levels of both IgG1 (Fig. 2B) (P < 0.01) and IgG2a (Fig. 2C) (P < 0.05) antibody responses than in the i.m. groups. Moreover, the ratios of IgG1 to IgG2a elicited by microneedle vaccination were more balanced than those elicited by intramuscular vaccination after boost immunization (Fig. 2D). Microneedle vaccination induced levels of HI titers 40 and 80% higher than those induced by intramuscular vaccination with intact H5 VLPs (i.m.) and redissolved vaccines from microneedles (i.m.-R), respectively, although there was no statistical significance (Fig. 3). These results indicate that there is no good correlation between binding antibodies and HI titers. Previous studies demonstrated that cross-protective immunity against influenza viruses was observed in the absence of detectable HI titers, indicating that nonneutralizing antibodies also contribute to protection (35, 43, 49). Overall, delivery of H5 VLP vaccines in a dry state to the skin using coated microneedles induced higher levels of IgG1 and IgG2a antibody responses and HI titers similar to intramuscular vaccination with liquid formulations.
FIG. 3.
HI titers. Serum samples from individual mice (n = 9) were collected at 8 weeks after boost immunization, and HI titers were determined by standard methods using 4 HA units of inactivated rgΔH5N1 virus and 1% horse erythrocyte suspension. The groups were as described in the legend to Fig. 2. The data are presented as means ± standard errors.
Microneedle vaccination with H5 VLPs provides protection against lethal H5N1 challenge.
To further determine the efficacy of microneedle vaccination with H5 VLPs, groups of vaccinated mice, including a mock control, were challenged with 50 times the mouse 50% lethal dose (LD50) of A/VN/1203/04 (H5N1) virus at 16 weeks after vaccination (Fig. 4). All mice in the mock control group lost body weight rapidly and had to be euthanized by day 7 or 8 postchallenge. The group of mice that received microneedle vaccination with trehalose-stabilized H5 VLPs (MN+) was completely protected against lethal challenge infection (Fig. 4B) and showed a slight increase in body weight. The corresponding intramuscular group (i.m.-R) that received the same H5 VLP trehalose vaccine as in microneedle applications showed a transient but significant body weight loss, on average up to 7.8% compared to the microneedle group (P < 0.05), at day 6 postchallenge (Fig. 4A).
FIG. 4.
Protection against lethal challenge with the wild-type H5N1 virus. Groups of vaccinated mice and a mock control (PBS) were intranasally challenged with 50 LD50 of A/VN/1203/04 virus (n = 9 per group). (A and B) Body weight changes (A) and survival rates (B) were recorded for 14 days. (C) Challenge virus titers in the lungs, spleens, and brains of mice were determined by plaque assay on MDCK cells. The dotted line in panel C indicates the detection limit of virus titers (50 PFU per ml). The groups were as described in the legend to Fig. 2. The data are presented as means ± standard errors.
Effective control of viral replication in challenge-inoculated animals is an important indicator for assessing the protective efficacy of vaccination. Thus, we also determined the virus titers in lungs, spleens, and brains of inoculated mice on day 4 postinfection. High viral titers in both lungs and spleens were detected in the mock-vaccinated control group, indicating systemic spread of the challenge virus (Fig. 4C). All mice vaccinated with H5 VLPs either intramuscularly (i.m. and i.m.-R) or via microneedles (MN+) showed 2 to 3 log units lower lung viral titers than mock-vaccinated counterparts, and virus was never detected in other organs. Although no statistical difference between the microneedle and intramuscular groups was apparent, these results do indicate that microneedle vaccination with H5 VLP vaccines in the skin provides effective protection in mice.
Microneedle vaccination with H5 VLPs induces long-lived antibody-producing B cells.
The longevity of antibody-producing B cells had not been previously investigated after microneedle vaccination. To determine the H5N1 virus-specific memory B and plasma cell responses, we analyzed spleen (Fig. 5 A) and bone marrow (Fig. 5B) cell populations at 8 months after microneedle vaccination with H5 VLPs. Vaccinated or mock control mice were restimulated by intranasal exposure to inactivated rgΔH5N1 virus 6 days prior to harvesting of spleen and bone marrow cells for ex vivo culture. The levels of virus-specific IgG antibodies were low at day 2 of in vitro culture. Interestingly, after 6 days of in vitro culture, significantly higher levels of virus-specific antibodies were secreted into the culture supernatants of splenocytes from mice that received microneedle vaccination than from those vaccinated intramuscularly (Fig. 5A). Similarly, more than 2.5-fold-higher levels of virus-specific antibodies were secreted in the bone marrow cells from the microneedle-vaccinated group after the 6 days of in vitro culture than from the intramuscularly immunized group (Fig. 5B). These results indicate that virus-specific antibody-producing precursor cells with memory B-cell characteristics were strongly induced in both spleens and bone marrow after microneedle H5 VLP vaccination and that these cells can respond rapidly upon viral antigenic exposure.
FIG. 5.
Antibody-secreting cell responses. Antibody-secreting cell responses from spleen cells (A) and bone marrow cells (B) were determined by ELISA 8 months after immunization with microneedles or intramuscular injection. Splenocytes and bone marrow cells were obtained from mice (n = 6) exposed to inactivated rgΔH5N1 virus 6 days before sacrifice and then cultured in vitro. Culture supernatants harvested on day 2 and day 6 were used to determine the levels of IgG antibodies specific for H5N1 virus. The data are presented as means ± standard errors. The asterisks indicate significant differences (**, P < 0.01).
Microneedle vaccination with H5 VLPs induces cellular immune responses.
Cellular immune responses 8 months following immunizations were determined by analyzing IL-4- and IFN-γ-secreting splenocytes upon stimulation by a pool of H5 HA peptides. Splenocytes isolated from vaccinated mice were stimulated in vitro with a pool of HA1 or HA2 subunit peptide derived from A/Thailand/16/04 (H5N1) influenza virus (Fig. 6). Splenic cell spots secreting IFN-γ were observed in response to the stimulation with the HA1, but not the HA2, peptide pool at 2-fold-higher levels in the group of mice that received microneedle vaccination with H5 VLPs in the skin than in the intramuscular-immunization group (P < 0.01) (Fig. 6A). Similarly, approximately 1.5-fold-higher levels of IL-4-secreting spleen cell spots were detected after microneedle vaccination with H5 VLPs than after intramuscular immunization (P < 0.05) (Fig. 6B). Overall, these results indicate that microneedle vaccination with H5 VLPs effectively induces both Th1 and Th2 types of immune responses, as demonstrated by the patterns of cytokines.
FIG. 6.
Cytokine-secreting cell responses. IFN-γ-secreting (A) and IL-4-secreting (B) cell spots were determined by ELISPOT assay. Groups of mice (n = 6) that were immunized with H5 VLPs by microneedles or intramuscularly 8 months earlier were antigenically challenged with inactivated rgΔH5N1 virus 6 days prior to sacrifice. The cytokine-secreting cellular immune responses were measured by ELISPOT assay using splenocytes isolated from immunized mice. The data are presented as means ± standard errors. The asterisks indicate significant differences (*, P < 0.05; **, P < 0.01).
Microneedle vaccination with H5 VLPs induces movement of Langerhans cells in human epidermis.
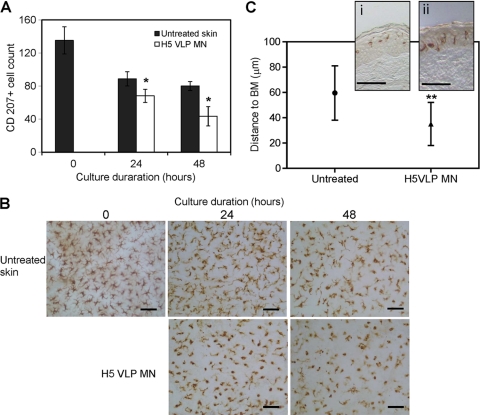
To complement animal studies, excised human breast skin explants were used to determine whether microneedle vaccination with H5 VLPs affected the LC population in human epidermis. The numbers of LCs in human skin were monitored and counted during organ cultures at different time points. Figure 7 A and B show that while the number of LCs in human epidermal sheets was affected by changes in the environment following skin excision and organ culture over 24 and 48 h (untreated skin), the reduction in LC numbers at both of these time points was significantly more pronounced in skin that had been treated with microneedles coated with H5 VLPs. LC numbers in control samples treated with microneedles alone and microneedles coated with placebo coating formulation did not significantly deviate from untreated skin (data not shown). Analysis of skin sections further confirmed that the distribution of LCs was altered following treatment with microneedles coated with H5 VLPs. Figure 7C shows a general trend of LC relocalization from their central epidermal location toward the basement membrane following exposure to the VLPs. These results provide support for the notion that interaction of VLP antigens with LCs may result in their stimulation for subsequent movement in human skin.
FIG. 7.
Change in Langerhans cell distribution in response to H5 VLP microneedle treatment in human epidermal sheets. (A) LC numbers in human skin. The numbers of LCs were determined from randomly chosen, digitally captured images at ×40 magnification corresponding to an area of 0.04 mm2. H5 VLPs were delivered by coated microneedles. Significance was determined relative to untreated skin (*, P < 0.05) at the corresponding time point. The data are presented as means ± standard errors (n = 4). (B) Representative LC images from each time point (bars, 20 μm). Human LCs were visualized by staining the surface marker CD207 with mouse monoclonal antibody as described in Materials and Methods. (C) LC localization to epidermal basement membrane (BM). Shown are the mean distances (±standard deviations) of LCs from the basement membrane in untreated and H5 microneedle-treated (H5 VLP MN) skin samples, both following 24 h of incubation in culture. **, significant difference (P < 0.01). The inset shows magnified sections of untreated (i) and H5 VLP MN-treated (ii) skin (bars, 50 μm).
DISCUSSION
In an attempt to improve H5N1 vaccine delivery approaches, we have determined the immunogenicity and protective efficacy elicited by microneedle administration of an H5 VLP antigen to mouse skin. Our results indicate that prime-boost microneedle vaccination in the skin with low doses of H5 VLPs can induce long-lasting humoral and cellular immune responses, as well as protective immunity. Higher levels of antibody-secreting cell responses in both spleen and bone marrow were found to be induced by the microneedle vaccination than by traditional intramuscular immunization when determined at 8 months postvaccination. More importantly, we found that microneedle delivery of H5 VLP vaccines to viable human skin can mobilize CD207+ human LCs. In addition, microneedle vaccination has logistic advantages, such as potential self-administration and avoiding the use of hypodermic needles and syringes. Therefore, the results in this study have significant implications in demonstrating the feasibility of microneedle vaccination for humans.
We showed that an H5 VLP vaccine delivered via a surface coating on microneedles induces protective immunity at least comparable to that induced by intramuscular immunization. VLPs alone would not cross the skin barrier at a meaningful level, as we have shown that the immune responses without microneedles were minimal even with over 200-fold-higher doses (100 μg) of inactivated whole virus applied to the skin (39). The VLP doses used for microneedle vaccination were 0.4 μg total protein containing approximately 40 ng of HA (19). The immunogenicity of egg-derived inactivated subunit H5N1 vaccines administered without adjuvants in preclinical and clinical studies was far less satisfactory than that of seasonal influenza vaccines (14, 26, 42). We found that a single dose of microneedle vaccination with H5 VLPs induced low levels of antibody responses, which is consistent with results showing low immunogenicity of H5 vaccines in previous studies. High doses and/or multiple injections of H5 vaccines were needed to obtain satisfactory seroconversion rates. In humans, two 90-μg HA doses of baculovirus-expressed or inactivated subunit vaccines produced in eggs were needed to induce antibody responses that were expected to be protective in 54 to 58% of individuals vaccinated (5, 24, 29, 47, 48). A prime-boost immunization regimen of adjuvanted inactivated H5N1 whole virus or a split vaccine containing 3 μg HA has been applied to induce protective immunity or to improve protective efficacy in mice (9, 25, 40). Our results demonstrated that significant levels of H5-specific antibodies were induced after prime-boost vaccination with an even lower dose of H5 VLPs via either microneedle skin delivery or the intramuscular route. Therefore, our study suggests that VLPs are an effective immunogen for inducing protective immunity against H5N1 influenza virus via microneedle vaccination in the skin.
The microneedles used in this study were designed to deliver coated vaccines in a dry form to the epidermis and dermis of the skin, as previously described (21, 23, 34, 53). H5 VLPs delivered to the skin via microneedles induced effective humoral and cellular immune responses after boost immunization, including IgG1 and IgG2a isotype antibodies and cytokine (IFN-γ and IL-4)-secreting splenocytes, which were observed to be significantly higher in the microneedle group than in the intramuscular group. Microneedle immunization also induced more balanced immune responses than the intramuscular route, including both T helper type 1 (Th1) and Th2, as shown by increases in both IgG1 and IgG2a antibody levels after boost vaccination. In contrast, intramuscular immunization with influenza VLPs induced IgG2a antibody as a dominant isotype, probably due to preferential activation and differentiation of conventional B2 cells to secrete IgG2a isotype antibody by VLP immunization (52). It was previously reported that epidermal gene gun delivery of DNA vaccines induced IgG1 as a dominant antibody, as well as IFN-γ- and IL-4-secreting T-cell responses (10, 33), whereas the same DNA vaccines delivered by intramuscular injection induced a Th1 pattern of immune responses, including IgG2a (10, 33). Therefore, the route of vaccination can influence the pattern of immune responses, as well as protective efficacy.
It was demonstrated that aggregates of inactivated split influenza vaccines can influence the Th1/Th2 immune responses by increasing the Th2-type cytokine-producing cells (IL-4 and IL-5), but not by changes in the ratios of antibody isotypes IgG1 and IgG2a (2). Microneedle vaccination with inactivated influenza H1N1 viral vaccines (A/PR/8/34) without trehalose stabilization induced high IgG1 and low IgG2a antibody levels compared to trehalose-stabilized microneedle vaccination, which was partially attributed to the formation of particle aggregates (21). A Th2-type immune response was implicated as being undesirable in humans (2). Thus, it is important to improve the Th1-type immune responses after microneedle vaccination with H5 VLPs. Although trehalose stabilization was shown to prevent the formation of influenza vaccine aggregates and to induce higher levels of IgG2a antibodies (21), the low immunogenicity of H5 vaccines (A/VN/1203/04) might also have contributed to the types of immune responses. Microneedle coating with H5 VLPs needs further investigation to improve Th1-type immune responses.
A recent study demonstrated that simian-human immunodeficiency VLPs trafficked to and were associated with local lymph nodes for longer times after intradermal immunization on the abdominal site than after intramuscular injection of mice and resulted in enhanced immune responses (8). Also, we demonstrated that microneedle delivery on the dorsal skin can load a model compound into dendritic cells, more effectively draining to the local lymph nodes than intramuscular injection, using a fluorescent dye as a model compound (22). These studies indicate that different anatomical sites of the skin did not significantly affect immune responses in a mouse model, but it remains to be determined in humans. Our complementary experiments investigated the effects on LCs after delivery of VLPs from coated microneedles using a previously validated excised-human-skin model (28). Importantly, this study demonstrated that the numbers of human CD207+ LCs in a given epidermal plane were significantly lower after administration of influenza H5 VLP vaccine to the skin using microneedles. Tissue sections further confirmed changes in the LC distribution pattern following VLP exposure, suggesting their mobilization in the human skin environment. VLP vaccines were shown to effectively activate dendritic cells, enhancing the cell surface activation markers CD80 and CD86 (38). Therefore, effective uptake and stimulation of antigen-presenting cells, such as LCs, by delivery of particulate VLP vaccines to the skin is likely to be a mechanism for inducing long-lasting immune responses, as evidenced by high levels of antibody-secreting B-cell responses and IFN-γ cytokine-producing T cells after skin vaccination.
Vaccine delivery using coated microneedles is a new platform for vaccination. The vaccines are delivered in a dry formulation, and delivery does not involve hypodermic needles and syringes. The patch-based format of microneedles should simplify vaccination and may enable self-administration. Production of VLP vaccines does not require the handling of live influenza viruses during vaccine manufacture and does not rely on problematic egg supplies. Therefore, microneedle vaccination with VLPs provides an attractive approach to improve both vaccination efficacy and coverage for seasonal and pandemic viruses that warrants further studies.
Acknowledgments
A/Thailand/16/05 (H5N1) HA peptide pools were provided by the Biodefense and Emerging Infections Research Resources Repository. We thank Vladimir Zarnitsyn for microneedle fabrication and Mark Allen for use of his laser microfabrication facilities.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
This work was supported in part by NIH/NIBIB grant EB006369 (M.R.P.), NIH/NIAID grants AI0680003 and AI074579 (R.W.C.), the Georgia Research Alliance (S.-M.K.), and the Korea Research Foundation grant KRF-2007-357-C00088 (J.-M.S.).
M.R.P. is a consultant and inventor of patents licensed to companies with an interest in microneedles. R.W.C. and S.-M.K. have equity in Zetra Biologicals, which is developing VLP technology under license from Emory University. The associated conflict of interest is being managed by Georgia Tech and Emory University.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Auewarakul, P., U. Kositanont, P. Sornsathapornkul, P. Tothong, R. Kanyok, and P. Thongcharoen. 2007. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 25:659-663. [DOI] [PubMed] [Google Scholar]
- 2.Babiuk, S., D. M. Skowronski, G. De Serres, K. HayGlass, R. C. Brunham, and L. Babiuk. 2004. Aggregate content influences the Th1/Th2 immune response to influenza vaccine: evidence from a mouse model. J. Med. Virol. 72:138-142. [DOI] [PubMed] [Google Scholar]
- 3.Belshe, R. B., F. K. Newman, K. Wilkins, I. L. Graham, E. Babusis, M. Ewell, and S. E. Frey. 2007. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine 25:6755-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birchall, J., S. Coulman, M. Pearton, C. Allender, K. Brain, A. Anstey, C. Gateley, N. Wilke, and A. Morrissey. 2005. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch micro-fabricated micro-needles. J. Drug Target. 13:415-421. [DOI] [PubMed] [Google Scholar]
- 5.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657-1664. [DOI] [PubMed] [Google Scholar]
- 6.Chang, L. Y., S. R. Shih, P. L. Shao, D. T. Huang, and L. M. Huang. 2009. Novel swine-origin influenza virus A (H1N1): the first pandemic of the 21st century. J. Formos. Med. Assoc. 108:526-532. [DOI] [PubMed] [Google Scholar]
- 7.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 8.Cubas, R., S. Zhang, S. Kwon, E. M. Sevick-Muraca, M. Li, C. Chen, and Q. Yao. 2009. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J. Immunother. 32:118-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desheva, J. A., X. H. Lu, A. R. Rekstin, L. G. Rudenko, D. E. Swayne, N. J. Cox, J. M. Katz, and A. I. Klimov. 2006. Characterization of an influenza A H5N2 reassortant as a candidate for live-attenuated and inactivated vaccines against highly pathogenic H5N1 viruses with pandemic potential Vaccine 24:6859-6866. [DOI] [PubMed] [Google Scholar]
- 10.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 11.Flacher, V., M. Bouschbacher, E. Verronese, C. Massacrier, V. Sisirak, O. Berthier-Vergnes, B. de Saint-Vis, C. Caux, C. Dezutter-Dambuyant, S. Lebecque, and J. Valladeau. 2006. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J. Immunol. 177:7959-7967. [DOI] [PubMed] [Google Scholar]
- 12.Galarza, J. M., T. Latham, and A. Cupo. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18:244-251. [DOI] [PubMed] [Google Scholar]
- 13.Gill, H. S., and M. R. Prausnitz. 2007. Coated microneedles for transdermal delivery. J. Control Release 117:227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillim-Ross, L., and K. Subbarao. 2006. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19:614-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, J. R., L. Dokken, J. A. Wiley, A. G. Cawthon, J. Bigger, A. G. Harmsen, and C. Richardson. 2009. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27:530-541. [DOI] [PubMed] [Google Scholar]
- 16.Holland, D., R. Booy, F. De Looze, P. Eizenberg, J. McDonald, J. Karrasch, M. McKeirnan, H. Salem, G. Mills, J. Reid, F. Weber, and M. Saville. 2008. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J. Infect. Dis. 198:650-658. [DOI] [PubMed] [Google Scholar]
- 17.Hon, H., and J. Jacob. 2004. Tracking dendritic cells in vivo: insights into DC biology and function. Immunol. Res. 29:69-80. [DOI] [PubMed] [Google Scholar]
- 18.Hung, C. F., B. Ma, A. Monie, S. W. Tsen, and T. C. Wu. 2008. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin. Biol. Ther. 8:421-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, S. M., D. G. Yoo, A. S. Lipatov, J. M. Song, C. T. Davis, F. S. Quan, L. M. Chen, R. O. Donis, and R. W. Compans. 2009. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One 4:e4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295-2301. [DOI] [PubMed] [Google Scholar]
- 21.Kim, Y. C., F. S. Quan, R. W. Compans, S. M. Kang, and M. R. Prausnitz. 2010. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control Release 142:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, Y. C., F. S. Quan, D. G. Yoo, R. W. Compans, S. M. Kang, and M. R. Prausnitz. 2010. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J. Infect. Dis. 201:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsonanos, D. G., M. del Pilar Martin, V. G. Zarnitsyn, S. P. Sullivan, R. W. Compans, M. R. Prausnitz, and I. Skountzou. 2009. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One 4:e4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, J., J. Zhang, X. Dong, H. Fang, J. Chen, N. Su, Q. Gao, Z. Zhang, Y. Liu, Z. Wang, M. Yang, R. Sun, C. Li, S. Lin, M. Ji, Y. Liu, X. Wang, J. Wood, Z. Feng, Y. Wang, and W. Yin. 2006. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 368:991-997. [DOI] [PubMed] [Google Scholar]
- 25.Lu, X., L. E. Edwards, J. A. Desheva, D. C. Nguyen, A. Rekstin, I. Stephenson, K. Szretter, N. J. Cox, L. G. Rudenko, A. Klimov, and J. M. Katz. 2006. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine 24:6588-6593. [DOI] [PubMed] [Google Scholar]
- 26.Luke, C. J., and K. Subbarao. 2006. Vaccines for pandemic influenza. Emerg. Infect. Dis. 12:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitragotri, S. 2005. Immunization without needles. Nat. Rev. Immunol. 5:905-916. [DOI] [PubMed] [Google Scholar]
- 28.Ng, K. W., M. Pearton, S. Coulman, A. Anstey, C. Gateley, A. Morrissey, C. Allender, and J. Birchall. 2009. Development of an ex vivo human skin model for intradermal vaccination: tissue viability and Langerhans cell behaviour. Vaccine 27:5948-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937-1943. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas, J. F., and B. Guy. 2008. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev. Vaccines 7:1201-1214. [DOI] [PubMed] [Google Scholar]
- 31.Normile, D. 2006. Avian influenza. Human transmission but no pandemic in Indonesia. Science 312:1855. [DOI] [PubMed] [Google Scholar]
- 32.Pearton, M., C. Allender, K. Brain, A. Anstey, C. Gateley, N. Wilke, A. Morrissey, and J. Birchall. 2008. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm. Res. 25:407-416. [DOI] [PubMed] [Google Scholar]
- 33.Pertmer, T. M., T. R. Roberts, and J. R. Haynes. 1996. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J. Virol. 70:6119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prausnitz, M. R., and R. Langer. 2008. Transdermal drug delivery. Nat. Biotechnol. 26:1261-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan, F. S., R. W. Compans, H. H. Nguyen, and S. M. Kang. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 82:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan, F. S., Y. C. Kim, D. G. Yoo, R. W. Compans, M. R. Prausnitz, and S. M. Kang. 2009. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One 4:e7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross, T. M., K. Mahmood, C. J. Crevar, K. Schneider-Ohrum, P. M. Heaton, and R. A. Bright. 2009. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One 4:e6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sailaja, G., I. Skountzou, F. S. Quan, R. W. Compans, and S. M. Kang. 2007. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology 362:331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skountzou, I., F. S. Quan, J. Jacob, R. W. Compans, and S. M. Kang. 2006. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine 24:6110-6119. [DOI] [PubMed] [Google Scholar]
- 40.Subbarao, K., H. Chen, D. Swayne, L. Mingay, E. Fodor, G. Brownlee, X. Xu, X. Lu, J. Katz, N. Cox, and Y. Matsuoka. 2003. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology 305:192-200. [DOI] [PubMed] [Google Scholar]
- 41.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 42.Subbarao, K., B. R. Murphy, and A. S. Fauci. 2006. Development of effective vaccines against pandemic influenza. Immunity 24:5-9. [DOI] [PubMed] [Google Scholar]
- 43.Takada, A., S. Matsushita, A. Ninomiya, Y. Kawaoka, and H. Kida. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212-3218. [DOI] [PubMed] [Google Scholar]
- 44.Tao, P., M. Luo, D. Zhu, S. Qu, Z. Yang, M. Gao, D. Guo, and Z. Pan. 2009. Virus-like particle vaccine comprised of the HA, NA, and M1 proteins of an avian isolated H5N1 influenza virus induces protective immunity against homologous and heterologous strains in mice. Viral Immunol. 22:273-281. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, C. B. Bridges, N. J. Cox, and K. Fukuda. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333-1340. [DOI] [PubMed] [Google Scholar]
- 46.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, J. de Menno, C. Schultsz, P. Cheng, W. Lim, P. Horby, and J. Farrar. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 47.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1343-1351. [DOI] [PubMed] [Google Scholar]
- 48.Treanor, J. J., B. E. Wilkinson, F. Masseoud, J. Hu-Primmer, R. Battaglia, D. O'Brien, M. Wolff, G. Rabinovich, W. Blackwelder, and J. M. Katz. 2001. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 19:1732-1737. [DOI] [PubMed] [Google Scholar]
- 49.Tumpey, T. M., M. Renshaw, J. D. Clements, and J. M. Katz. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ungchusak, K., P. Auewarakul, S. F. Dowell, R. Kitphati, W. Auwanit, P. Puthavathana, M. Uiprasertkul, K. Boonnak, C. Pittayawonganon, N. J. Cox, S. R. Zaki, P. Thawatsupha, M. Chittaganpitch, R. Khontong, J. M. Simmerman, and S. Chunsutthiwat. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 352:333-340. [DOI] [PubMed] [Google Scholar]
- 51.Van Damme, P., F. Oosterhuis-Kafeja, M. Van der Wielen, Y. Almagor, O. Sharon, and Y. Levin. 2009. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27:454-459. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, S., R. Cubas, M. Li, C. Chen, and Q. Yao. 2009. Virus-like particle vaccine activates conventional B2 cells and promotes B cell differentiation to IgG2a producing plasma cells. Mol. Immunol. 46:1988-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, Q., V. G. Zarnitsyn, L. Ye, Z. Wen, Y. Gao, L. Pan, I. Skountzou, H. S. Gill, M. R. Prausnitz, C. Yang, and R. W. Compans. 2009. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 106:7968-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]