Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a frequent complication in cystic fibrosis patients. The diagnosis remains difficult and requires a combination of clinical, radiological, biological, and mycological criteria. The aim of this study was to analyze the added value of two recombinant antigens, rAspf4 and rAspf6, associated with the detection of specific IgG; precipitins; total IgE; and Aspergillus fumigatus in sputum for the diagnosis of ABPA. In a retrospective study, we determined the specific IgE responses to these recombinants in 133 sera of 65 cystic fibrosis patients. We selected an average of five serum samples from each of the 17 patients with ABPA (13 proven and 4 probable ABPA) and from 3 patients with Aspergillus bronchitis and rhinosinusitis. One serum sample for the 45 patients without ABPA was tested. The sensitivity of specific IgE detection against rAspf4 calculated per patient (92.3%) was significantly higher (P < 0.05) than that of rAspf6 (53.8%). When rAspf4 IgE detection was associated with anti-Aspergillus IgG enzyme-linked immunosorbent assay (ELISA) and precipitin detection, the sensitivity rose to 100%. The specificities of rAspf4 and rAspf6 IgE detection were 93.7% and 91.6%, respectively. Other diagnostic criteria had slightly lower specificities (87.5% for anti-Aspergillus IgG ELISA, 89.6% for precipitins, 84.4% for total IgE, and 85.0% for positive A. fumigatus culture in sputum). In conclusion, this retrospective study showed the relevance of rAspf4 IgE detection, in combination with other biological markers (Aspergillus IgG ELISA, precipitins, and total IgE), for improving the biological diagnosis of ABPA.
Allergic bronchopulmonary aspergillosis (ABPA) is a frequent complication in patients with cystic fibrosis that causes significant respiratory morbidity (1, 13, 18). The exact prevalence of ABPA is not clearly known; however, it ranges from 2 to 25% in patients with cystic fibrosis (22). ABPA may lead to acute worsening of the respiratory condition and ongoing decline in lung function. Without adequate treatment, ABPA ultimately progresses to a chronic state with lung fibrosis. ABPA is a hypersensitivity pulmonary disease associated with inflammatory destruction of airways in response to Aspergillus allergens (23). Thus, chronic airway colonization with Aspergillus induces strong inflammatory responses with high IgE levels (20). The factors leading to ABPA are not clearly understood, but it is believed that Aspergillus-specific, IgE-mediated type I hypersensitivity reactions and specific IgG-mediated type III hypersensitivity reactions play central roles in the pathogenesis of ABPA (18). Furthermore, host factors, including individual susceptibility, may contribute to the immunopathogenesis of ABPA. Early diagnosis and treatment of ABPA are important to prevent serious and potentially irreversible lung damage (9).
ABPA is defined by five major diagnostic criteria, according to the Cystic Fibrosis Foundation Consensus Conference (19, 23): (i) acute or subacute clinical deterioration and decline in pulmonary function not attributable to another etiology, (ii) elevated serum IgE concentrations, (iii) immediate prick test reactivity to Aspergillus antigen or presence of specific IgE antibodies to Aspergillus fumigatus, (iv) precipitating antibodies to A. fumigatus or elevated serum IgG antibodies to A. fumigatus, and (v) history of pulmonary infiltrates (transient or fixed) and central bronchiectasis. Minor diagnostic criteria include repeated detection of Aspergillus species in sputum samples, a history of expectoration of brown plugs or flecks, and late skin reactivity to Aspergillus antigens. Despite these criteria, diagnosis of ABPA in cystic fibrosis patients remains particularly difficult (3, 16, 21, 22). Therefore, as stated in the most recent consensus document on the diagnosis and therapy of ABPA in cystic fibrosis patients (19), serological findings should strongly contribute to the confirmation or exclusion of clinically suspected ABPA.
The demonstration that some recombinant antigens are specifically expressed only in hyphae explains why an IgE response to these antigens may occur in ABPA patients but not in A. fumigatus-sensitized patients. Thus, cloning, characterization, production, and clinical evaluation of A. fumigatus allergens have been major advances in the diagnosis of ABPA and help in the understanding of the pathophysiological mechanisms underlying the disease (9). The rAspf1, rAspf2, rAspf3, rAspf4, and rAspf6 recombinant allergens have been evaluated for their diagnostic performance in several serological studies concerning patients with both asthma and cystic fibrosis, with or without ABPA, and showed high specificity for the detection of A. fumigatus sensitization, as well as ABPA (14). Serological investigations involving rAspf4 and rAspf6 showed that allergen-specific IgE levels against these proteins increased almost exclusively in samples from patients with ABPA (9). However, de Oliveira et al. (10) showed that the determination of the IgE response against A. fumigatus recombinant antigens in asthmatic patients with immediate cutaneous reactivity to A. fumigatus was not helpful in diagnosing ABPA or in detecting sensitization to fungi.
The aim of the present study was to evaluate the efficiencies of two recombinant allergens (rAspf4 and rAspf6) in IgE detection, combined with anti-Aspergillus IgG, precipitins, total serum IgE concentration, and fungal culture of sputum.
MATERIALS AND METHODS
Patients.
In our retrospective study, a total of 133 sera from 65 patients (30 males and 35 females; mean age, 13.2 ± 9.9 years; range, 1 to 53 years of age) with cystic fibrosis, regularly followed up at the Grenoble cystic fibrosis center between 1997 and 2008, were analyzed. Among the 65 patients, 13 were considered to present clinical, radiological, and biological criteria of proven ABPA and 4 of probable ABPA; 2 had Aspergillus bronchitis and 1 had Aspergillus rhinosinusitis; and 45 presented no criteria for ABPA. One serum sample was collected from these 45 patients. An average of five sequential samples were obtained from each of the 20 patients with A. fumigatus-associated diseases; one or two samples were collected before aspergillosis diagnosis to study how early the serological tests were positive, and three or four sera were sampled after the ABPA diagnosis to study the evolution of biological criteria. The ABPA patients were treated with steroids, and the patients with bronchitis or rhinosinusitis were treated with voriconazole. In the serum samples, the following parameters were measured: (i) specific IgE against the A. fumigatus recombinant allergens (rAspf4 and rAspf6), (ii) specific anti-A. fumigatus IgG by ELISA, (iii) precipitins by immunoelectrophoresis (IE), and (iv) total IgE level. Sputum samples for performing mycological cultures and serum samples were collected from all patients at the same time.
Biological tests. (i) IgE detection.
Total IgE and specific IgE against two A. fumigatus recombinants (rAspf4 and rAspf6) were measured by the Pharmacia UniCAP method (Phadia SAS, Saint Quentin en Yvelines, France). The results of the total IgE measurements were expressed in kIU/liter, and the cutoff used was 500 kIU/liter. This cutoff corresponds to the lowest limit for the IgE level in favor of ABPA in the guidelines. The results of specific IgE measurements against rAspf 4 and rAspf6 were given in kAU/liter, where A represents allergen-specific antibodies (1 kAU/liter = 2.4 ng/ml). The cutoff IgE against rAspf4 and rAspf6 was 0.1 kAU/liter, according to the manufacturer's instructions.
(ii) Anti-A. fumigatus IgG detection.
Specific anti-A. fumigatus IgG was measured by a homemade enzyme-linked immunosorbent assay (ELISA) method. Metabolic antigens (concentrated culture medium) and somatic antigens (mechanically extracted from fungal mycelium) were prepared. Two A. fumigatus strains, IHEM 9073 (isolated from an aspergilloma) and IHEM 22145 (isolated from a human cerebral biopsy specimen) were cultured for antigenic preparation. The strains were grown at 30°C for 21 days on a liquid modified Czapek medium (saccharose, 15 g/liter; yeast nitrogen base, 1 g/liter; brain heart, 1 g/liter; NaNO3, 3 g/liter; K2HPO4, 1 g/liter; KCl, 0.5 g/liter; MgSO4, 0.5 g/liter; and FeSO4·7H2O, 0.01g/liter). The culture flasks were shaken in a rotary shaker (60 rpm). All the cultures were left at 4°C overnight before antigenic preparation. Then, they were mechanically extracted with a homogenizer (Ultraturrax) in the presence of 0.01 g sodium azide/liter and filtered at 4°C through 0.7-μm, 0.45-μm, and 0.22-μm filters, respectively. The filtrates were dialyzed at 4°C for 48 h, concentrated, and lyophilized. The two preparations from the two strains were mixed, and the total protein level was determined using QuickStart Bradford Dye Reagent (Bio-Rad protein assay 500-0205) with bovine serum albumin as a standard (Bio-Rad 500-026). Catalases and chymotrypsin were detected and had to be present to validate the antigenic preparation. In ELISA, the best results were obtained with a fungal-protein concentration of 0.15 mg/ml. Briefly, the microplates were coated with soluble antigens (0.15 mg/ml) in carbonate buffer (carbonate-bicarbonate buffer; Sigma) and left at 4°C overnight. The plates were stored at −80°C until they were used. Before use, the plates were washed with washing buffer at pH 7.2 (phosphate buffer; Na2HPO4, 5.325 g; NaH2PO4·2H2O, 0.975 g; NaCl, 21.25 g; Tween 20, 0.125% [vol/vol]; distilled water, total volume of 1 liter). They were then left for 15 min with a blocker (5% nonfat milk in washing buffer) before being washed three times with washing buffer. Both the patients' sera and positive and negative controls were diluted at 1/400 in washing buffer and nonfat milk. Then, 100 μl each was placed in duplicate in each well and incubated at 37°C for 30 min. The plates were first washed three times with the washing buffer, and then 100 μl of anti-human IgG conjugated to peroxidase (Interchim, Montluçon, France) was added and incubated at 37°C for 30 min. After four washings, 100 μl of orthophenylenediamine dihydrochloride was added. After 10 min at room temperature in the dark, the enzymatic reaction was stopped by adding 200 μl of 2 N sulfuric acid; the optical density (OD) was then read at 492 nm on a spectrophotometer (Power Wave XS; Bio Tek, Colmar, France). The final value was the sample OD times the correction factor (positive-control OD/positive reference value). The positive reference value was the mean of 10 OD results for the positive control obtained in 10 different experiments. The positive control was a pool of sera from a patient with proven aspergilloma. The negative control was a pool of sera from patients without aspergillosis. The IgG ELISA cutoff was 0.75.
(iii) A. fumigatus precipitin detection.
IE for precipitin detection was performed with the metabolic and somatic antigens obtained from the IHEM 22145 strain that regularly gave a high level of catalase and chymotrypsin antigens (5). The cutoff was two precipitin lines.
(iv) Fungal cultures of sputum.
The samples were incubated for 6 days on CAID 2 (bioMérieux, Marcy-l'Etoile, France) at 35°C to detect yeasts and on Sabouraud medium (bioMérieux, Marcy-l'Etoile, France) at 27°C to detect molds. If mold colonies were detected, morphological identification was performed.
Statistical analysis.
The χ2 test was used to compare sensitivities, specificities, and negative and positive predictive values of the test with Yates correction (see Table 3). The Mann-Whitney U test was used to compare biological values between the proven-ABPA group and the non-ABPA group, excluding the small group of three patients with Aspergillus-related diseases (Fig. 1). A P value of <0.05 was considered significant.
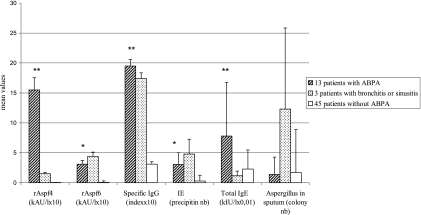
FIG. 1.
Mean results obtained for three groups of cystic fibrosis patients, 13 with ABPA, 3 with other fungal diseases, and 45 without ABPA. The mean values of IgE detection using rAspf4 and rAspf6, as well as specific IgG ELISA detection, were multiplied by 10; total IgE detection was multiplied by 10−2 in order to obtain the same range values. The results of the samples collected before diagnosis of proven ABPA were excluded. Significant differences between the ABPA patient group and the control group (patients without ABPA) were observed. **, P < 0.001 for rAspf4, specific IgG, and total IgE; *, P < 0.05 for rAspf6. No significant difference was observed for the detection of Aspergillus in the sputum. Statistical analysis was not performed for the group of three patients with bronchitis or sinusitis, as it was too small.
RESULTS
The biological results for 20 patients with proven and probable ABPA are detailed in Table 1 and those for the 3 patients with aspergillosis diseases other than ABPA in Table 2. The sensitivity, specificity, and negative and positive predictive values were calculated for each patient (Table 3). If at least one serum was positive during the survey, the biological test was considered positive for the patient and was used for the calculation of biological criteria. The three patients with A. fumigatus-associated diseases and the four probable ABPA patients were excluded from the calculation. In Table 4, the performance of the techniques per serum was calculated for 49 sera sampled after ABPA diagnosis, for 15 sera of patients with A. fumigatus-associated diseases, and for 45 sera of patients without ABPA. In the group with proven ABPA, nine sera were collected before the ABPA diagnosis (Table 1); they allowed us to compare the positivity of the biological tests in the suspected ABPA cases. In Fig. 1, the mean values and standard deviations of the biological test results after ABPA diagnosis were calculated for the three groups of patients: proven ABPA (13 patients), fungal bronchitis or rhinosinusitis (3 patients), and without ABPA (45 patients).
TABLE 1.
Biological results for 17 cystic fibrosis patients with proven or probable ABPAh
| Patient no. | Age (yr) | Gendera | Aspergillosis diagnosis | Serum sample no.b | Antibodies to A. fumigatus |
Total IgEf (kIU/liter) | No. of Aspergillus colonies in sputum | |||
|---|---|---|---|---|---|---|---|---|---|---|
| rAspf4d (kAU/liter) | rAspf6d (kAU/liter) | IgGc index | IE (precipitin no., enzymatic activitiese) | |||||||
| 1 | 10 | M | ABPA | 1 (m − 17) | <0.10 | <0.10 | 0.37 | 0 | 15.5 | ND |
| 2 (m − 2) | <0.10 | <0.10 | 0.58 | 3, cat+ | 553 | ND | ||||
| 3 (m + 7) | 0.11 | 0.10 | 1.58 | 7, cat+, chym+ | 3,090 | 0 | ||||
| 4 (m + 20) | 0.60 | 0.42 | 1.53 | 2, cat+ | 422 | 0 | ||||
| 5 (m + 32) | 6.52 | 2.75 | 2.92 | 3, cat+ | ND | 0 | ||||
| 2 | 6 | F | ABPA | 6 (m − 17) | NDg | ND | 0.4 | 0 | ND | ND |
| 7 (m0) | <0.10 | <0.10 | 1.83 | 4, cat+ | 289 | 3 | ||||
| 8 (m + 13) | 0.13 | 0.13 | 2.12 | 2, cat+ | 292 | 0 | ||||
| 9 (m + 30) | 3.25 | 1.66 | 3.37 | 2, cat+ | ND | 0 | ||||
| 10 (m + 40) | 7.28 | 1.7 | 0.7 | 2, cat+ | 2,140 | 1 | ||||
| 3 | 14 | M | ABPA | 11 (m0) | <0.10 | <0.10 | 3.34 | 4, cat+ | 945 | 0 |
| 12 (m + 18) | 0.98 | 0.20 | 1.48 | 3, cat+ | 362 | 0 | ||||
| 13 (m + 30) | 3.09 | 1.00 | 2.6 | 2 | 889 | 0 | ||||
| 14 (m + 38) | 2.81 | 0.34 | 1.16 | 1 | 711 | 0 | ||||
| 15 (m + 47) | 0.52 | 2.27 | 5.34 | 3, cat+ | 839 | 0 | ||||
| 4 | 13 | F | ABPA | 16 (m0) | 1.62 | 0.34 | 1.38 | 3, cat+ | 184 | 0 |
| 17 (m + 6) | 0.47 | 0.11 | 0.64 | 2, cat+ | 70 | 0 | ||||
| 18 (m + 14) | 0.27 | 0.1 | 0.35 | 0 | 33 | 0 | ||||
| 19 (m + 21) | 0.11 | <0.10 | 0.58 | 0 | <18 | 0 | ||||
| 20 (m + 32) | 0.77 | 0.14 | 2.15 | 3, cat+ | 138 | 0 | ||||
| 5 | 5 | M | ABPA | 21 (m − 2) | <0.10 | 0.10 | 0.51 | 1 | 805 | ND |
| 22 (m0) | 3.49 | 0.28 | 1.77 | 5, cat+ | 3,190 | 3 | ||||
| 23 (m + 30) | 3.74 | 0.28 | 0.63 | 0 | 1,650 | 0 | ||||
| 24 (m + 48) | 8.15 | 0.55 | 3.9 | 3 | 4,250 | ND | ||||
| 25 (m + 54) | 7.21 | 1.74 | 0.88 | 2 | 3,580 | 0 | ||||
| 6 | 8 | M | ABPA | 26 (m − 12) | 1.17 | <0.10 | 1.61 | 2 | 403 | 0 |
| 27 (m − 6) | 2.79 | <0.10 | 2.29 | 2 | 417 | ND | ||||
| 28 (m0) | 4.37 | <0.10 | 1.87 | 2, cat+ | 777 | 1 | ||||
| 29 (m + 6) | 5.45 | <0.10 | 2.82 | 3, cat+ | 726 | 0 | ||||
| 30 (m + 12) | 4.24 | <0.10 | 1.77 | 2, cat+ | 918 | 0 | ||||
| 7 | 10 | F | ABPA | 31 (m − 4) | <0.10 | <0.10 | 0.36 | 0 | QI | 0 |
| 32 (m + 2) | 0.64 | <0.10 | 3.43 | 4, cat+ | 227 | 3 | ||||
| 33 (m + 11) | 0.59 | <0.10 | 4.61 | 7, cat+ | 212 | 0 | ||||
| 34 (m + 12) | 0.35 | <0.10 | 3.88 | 5, cat+ | 142 | ND | ||||
| 35 (m + 23) | <0.10 | <0.10 | 0.66 | 0 | 24 | 0 | ||||
| 8 | 18 | F | ABPA | 36 (m − 15) | 0.80 | <0.10 | 1.59 | 1 | 798 | 0 |
| 37 (m0) | 2.56 | <0.10 | 1.92 | 3, cat+ | 2,380 | 0 | ||||
| 38 (m + 9) | 0.86 | <0.10 | 1.06 | 1, cat+ | 924 | 0 | ||||
| 39 (m + 15) | 1.07 | <0.10 | 1.13 | 2 | 866 | 0 | ||||
| 40 (m + 26) | 0.37 | <0.10 | 0.88 | 0 | 338 | 0 | ||||
| 41 (m + 34) | 0.58 | <0.10 | 1.12 | 2 | 918 | 0 | ||||
| 9 | 16 | F | ABPA | 42 (m0) | <0.10 | <0.10 | 0.82 | 6, cat+ | 198 | ND |
| 43 (m + 7) | <0.10 | <0.10 | 0.92 | 4 | 137 | 3 | ||||
| 44 (m + 15) | 0.20 | <0.10 | 1.04 | 7, cat+ | 169 | 3 | ||||
| 45 (m + 31) | 0.63 | <0.10 | 1.70 | 4, cat+, chym+ | 351 | 5 | ||||
| 46 (m + 38) | 1.64 | <0.10 | 1.95 | 4, cat+ | 622 | 15 | ||||
| 10 | 16 | F | ABPA | 47 (m − 25) | <0.10 | <0.10 | 0.03 | 0 | 426 | ND |
| 48 (m + 20) | 2.03 | <0.10 | 1.80 | 4, cat+ | 1,080 | ND | ||||
| 49 (m + 23) | 1.03 | <0.10 | 1.81 | 3, cat+ | 716 | ND | ||||
| 50 (m + 34) | 0.51 | <0.10 | 1.22 | 2, cat+ | 612 | ND | ||||
| 51 (m + 60) | 0.16 | <0.10 | 3.03 | 2, cat+ | 531 | ND | ||||
| 11 | 18 | M | ABPA | 52 (m0) | <0.10 | <0.10 | 3.06 | 7, cat+, chym+ | 268 | ND |
| 53 (m + 12) | 0.10 | 0.27 | 2.78 | 3 | 407 | 1 | ||||
| 54 (m + 19) | <0.10 | 0.14 | 1.56 | 5, cat+ | 272 | 5 | ||||
| 55 (m + 24) | <0.10 | 0.18 | 3.12 | 5, cat+ | 304 | ND | ||||
| 56 (m + 27) | 0.11 | 0.19 | 2.57 | 5, cat+ | 449 | 6 | ||||
| 12 | 8 | M | ABPA | 57 (m0) | <0.10 | <0.10 | 1.21 | 6, cat+ | 18 | 6 |
| 13 | 21 | F | ABPA | 58 (m + 84) | 1.58 | 2.04 | 0.48 | 0 | 618 | 0 |
| 14 | 18 | F | Probable ABPA | 59 (m − 28) | <0.10 | <0.10 | 1.13 | 2 | ND | 1 |
| 60 (m − 16) | <0.10 | <0.10 | 1.30 | 6, cat+ | ND | 5 | ||||
| 61 (m − 8) | <0.10 | <0.10 | 2.04 | 4, cat+ | 11 | 1 | ||||
| 62 (m + 13) | <0.10 | <0.10 | 2.35 | 7, cat+ | 10 | 9 | ||||
| 63 (m + 26) | <0.10 | <0.10 | 2.70 | 6, cat+ | ND | 15 | ||||
| 15 | 19 | M | Probable ABPA | 64 (m − 53) | <0.10 | <0.10 | 0 | 0 | 24 | 3 |
| 65 (m − 12) | <0.10 | <0.10 | 0.48 | 0 | 792 | 2 | ||||
| 66 (m0) | <0.10 | <0.10 | 1.14 | 3 | 1,090 | ND | ||||
| 67 (m + 13) | 0.76 | <0.10 | 2.35 | 1 | 1,540 | ND | ||||
| 16 | 9 | F | Probable ABPA | 68 (m − 5) | <0.10 | <0.10 | 0.51 | 0 | 76 | 0 |
| 69 (m0) | <0.10 | <0.10 | 0.75 | 0 | 526 | 1 | ||||
| 70 (m + 1) | <0.10 | 0.24 | 0.41 | 1, cat+ | 224 | ND | ||||
| 71 (m + 8) | <0.10 | 0.10 | 0.27 | 0 | 137 | 1 | ||||
| 72 (m + 23) | <0.10 | 0.63 | 0.32 | 2 | 180 | 0 | ||||
| 17 | 13 | M | Probable ABPA | 73 | <0.10 | <0.10 | 1.16 | 3 | 17 | 20 |
M, male; F, female.
The month of serum sampling (m +/−) in respect to the month of aspergillosis diagnosis (m0) for each patient is given in parentheses.
Anti-Aspergillus ELISA IgG. An index value of >0.75 was considered positive.
Specific IgE against two A. fumigatus recombinants measured by the Pharmacia UniCAP method. A result of >0.1 kAU/liter was considered positive.
IE for precipitin detection. More than 2 precipitin lines was considered positive. Positive enzymatic activities (catalase and chymotrypsin) are indicated (+).
Total IgE measured by the Pharmacia UniCAP method. A result of >500 kIU/liter was considered positive.
ND, not determined.
Positive results are shown in boldface type.
TABLE 2.
Biological results for 3 cystic fibrosis patients with Aspergillus rhinosinusitis and bronchitisg
| Patient no. | Age (yr) | Gendera | Aspergillosis diagnosis | Serum sample no. | Antibodies to A. fumigatus |
Total IgEe (kIU/liter) | No. of Aspergillus colonies in sputum | |||
|---|---|---|---|---|---|---|---|---|---|---|
| rAspf4c (kAU/liter) | rAspf6c (kAU/liter) | IgGb index | IE (precipitin no., enzymatic activitiesd) | |||||||
| 18 | 6 | M | Bronchitis | 74 | <0.10 | <0.10 | 2.19 | 5, cat+ | 48 | ND |
| 75 | <0.10 | <0.10 | 1.78 | 6, cat+ | 44 | 3 | ||||
| 76 | <0.10 | <0.10 | 0.38 | 0 | 26 | ND | ||||
| 77 | <0.10 | <0.10 | 2.33 | 4, cat+ | 94 | 2 | ||||
| 78 | <0.10 | <0.10 | 2.2 | 6 | NDf | 1 | ||||
| 19 | 23 | F | Bronchitis | 79 | 0.55 | 1.33 | 0.25 | 1 | 96 | 12 |
| 80 | 0.86 | 2.33 | 2.58 | 3 | 200 | 5 | ||||
| 81 | 0.35 | 1.03 | 3.41 | 3 | 124 | 12 | ||||
| 82 | 0.32 | 1.06 | 2.29 | 4, cat+ | 115 | 8 | ||||
| 83 | 0.19 | 0.87 | 0.99 | 10, cat+ | 101 | 41 | ||||
| 20 | 18 | F | Colonization | 84 | <0.10 | <0.10 | 0.91 | 6 | 162 | 8 |
| 85 | <0.10 | <0.10 | 0.86 | 7, cat+ | 298 | 5 | ||||
| Sinusitis | 86 | <0.10 | <0.10 | 1.36 | 7, cat+, chym+ | ND | 40 | |||
| 87 | <0.10 | <0.10 | 3.24 | 5, cat+ | ND | 20 | ||||
| 88 | <0.10 | <0.10 | 1.35 | 5 | ND | 3 | ||||
M, male; F, female.
Anti-Aspergillus ELISA IgG. An index value of >0.75 was considered positive.
Specific IgE against two A. fumigatus recombinants measured by the Pharmacia UniCAP method. A result of >0.1 kAU/liter was considered positive.
IE for precipitin detection. More than 2 precipitin lines was considered positive. Positive enzymatic activities (catalase and chymotrypsin) are indicated (+).
Total IgE measured by the Pharmacia UniCAP method. A result of >500 kIU/liter was considered positive.
ND, not determined.
Positive results are shown in boldface type.
TABLE 3.
Sensitivity, specificity, and positive and negative predictive values of biological tests calculated per patient, excluding the four patients with probable ABPA
| Test type | Sensitivity (%)a | Specificity (%)b | Negative predictive value (%)c | Positive predictive value (%)d |
|---|---|---|---|---|
| rAspf4 IgE detection | 92.3 (12/13) | 93.7 (45/48) | 97.8 (45/46) | 80.0 (12/15) |
| rAspf6 IgE detection | 53.8 (7/13) | 91.6 (44/48) | 88.0 (44/50) | 63.6 (7/11) |
| Anti-Aspergillus IgG ELISA | 92.3 (12/13) | 87.5 (42/48) | 97.7 (42/43) | 66.6 (12/18) |
| IE | 92.3 (12/13) | 89.6 (43/48) | 97.7 (43/44) | 70.6 (12/17) |
| Total IgE detection | 69.2 (9/13) | 84.4 (27/32) | 87.1 (27/31) | 64.3 (9/14) |
| Culture of Aspergillus in sputum | 53.8 (7/13) | 85.0 (34/40) | 85.0 (34/40) | 58.3 (7/12) |
Sensitivity = true positive/(true positive + false negative).
Specificity = true negative/(true negative + false positive).
Negative predictive value = true negative/(true negative + false negative).
Positive predictive value = true positive/(true positive + false positive).
TABLE 4.
Sensitivity, specificity, and positive and negative predictive values of biological tests calculated per sample of the patient groups examineda
| Test type | Sensitivity (%) | Specificity (%) | Negative predictive value (%) | Positive predictive value (%) |
|---|---|---|---|---|
| rAspf4 IgE detection | 81.6 (40/49) | 88.3 (53/60) | 85.5 (53/62) | 85.1 (40/47) |
| rAspf6 IgE detection | 46.9 (23/49) | 86.6 (52/60) | 66.6 (52/78) | 74.2 (23/31) |
| Anti-Aspergillus IgG ELISA | 87.7 (43/49) | 73.3 (44/60) | 88.0 (44/50) | 87.7 (43/49) |
| IE | 85.7 (42/49) | 73.3 (44/60) | 86.3 (44/51) | 87.5 (42/48) |
| Total IgE detection | 47.9 (23/48) | 87.5 (35/40) | 58.3 (35/60) | 82.1 (23/28) |
| Culture of Aspergillus in sputum | 32.5 (13/40) | 69.2 (36/52) | 57.1 (36/63) | 44.8 (13/29) |
Samples of probable ABPA and samples collected before diagnosis of proven ABPA have been excluded.
Sensitivity of biological markers.
The sensitivity of rAspf4 IgE detection per patient (92.3%) was significantly higher (P < 0.05) than the sensitivity of rAspf6 detection (53.8%) (Table 3). Among the 13 proven ABPA cases, 12 had at least one positive result with rAspf4 and 7 with rAspf6 (Table 1). When the rAspf6 IgE detection yielded a positive result, the rAspf4 IgE detection was also positive. Anti-Aspergillus ELISA IgG and IE showed high sensitivity (92.3%). Only one patient (patient no. 13; serum no. 58 in Table 1) had positive results with rAspf4 and rAspf6 but negative results with anti-A. fumigatus IgG ELISA detection and IE. A sensitivity of 100% was reached for ABPA patients using the combination of rAspf4 IgE, anti-A. fumigatus IgG ELISA detection, and precipitins. The sensitivity calculated per serum was lower than that calculated per patient (Tables 3 and 4). The results in Table 1 and Fig. 1 show that the levels of total IgE in serum samples fluctuated greatly during the course of the disease. The mean value was elevated in the ABPA group (Fig. 1). Moreover, the four patients (no. 4, 7, 11, and 12 in Table 1) with low IgE levels (<500 kIU/liter) were treated with corticosteroids and should have had higher IgE levels in serum samples collected before treatment. Among the four patients, one (no. 7) was not tested on the day of ABPA diagnosis and one (no. 12) was tested only once. The presence of A. fumigatus in sputum, listed as a minor diagnostic criterion, was observed in only 7/13 patients (53.8%) (Table 3). The four patients with probable ABPA (patients no. 14 to 17 in Table 1) had elevated values with anti-A. fumigatus IgG ELISA and IE. Two patients in this group showed positive IgE levels against recombinant allergens: one patient (no. 15) for rAspf4 and one patient (no. 16) for rAspf6.
Specificity of biological markers.
Table 2 shows that three patients with Aspergillus bronchitis or rhinosinusitis had positive results for anti-A. fumigatus IgG detection, IE, and sputum culture. The specific IgE detections against rAspf4 and rAspf6 were positive for one patient (no. 19 in Table 2). The total IgE level was under 500 kIU/liter for these three patients. The specificity calculated per patient was generally higher than those per serum (Tables 3 and 4). Among the 45 patients without ABPA, although only two false-positive results with rAspf4 IgE detection were observed, the levels obtained were very low (0.12 and 0.11 kAU/liter). Figure 1 clearly shows the high specificity of IgE detection using rAspf4. Among the 45 patients without ABPA, three false-positive results with rAspf6 IgE detection were observed. Anti-A. fumigatus IgG detection was positive for 3 sera and total IgE for 5 sera. The specificity per patient was slightly higher for rAspf4 and rAspf6 IgE detection (93.7% and 91.6%, respectively) than the specificities of the other tests (anti-Aspergillus IgG ELISA, 87.5%; IE, 89.6%; total IgE detection 84.4%; and Aspergillus detection in sputum, 85.0%), but the differences were not significant (P < 0.05).
Negative and positive predictive values.
The negative predictive values of rAspf4 IgE, anti-Aspergillus IgG ELISA, and IE were significantly higher than those of rAspf6 IgE, total IgE, and Aspergillus culture in sputum (Tables 3 and 4) (P < 0.05). The positive predictive value of the rAspf4 test (80.0% calculated per patient) was slightly higher than those of the other five biological tests (from 58.3% to 70.6% calculated per patient) (Table 3), but the differences were not significant (P > 0.05).
Importance of biological criteria before ABPA diagnosis.
A total of 15 sera from 10 patients were sampled before the ABPA diagnosis (Table 1). Anti-Aspergillus IgG ELISA and IE were positive for 6/15 serum samples, total IgE for 4/11 samples, and fungal culture for 5/7 sputum samples. The rAspf4 IgE tests were positive for 3/14 sera sampled before ABPA was diagnosed, and the rAspf6 IgE tests were negative. Thus, biological tests, such as anti-Aspergillus IgG ELISA, IE, total IgE, and fungal culture, were often positive before ABPA diagnosis.
DISCUSSION
Difficulties in diagnosing ABPA in cystic fibrosis patients are frequently reported in the literature. The wide variation in diagnostic practices among clinics, different estimates of prevalence, and diagnosis time delays lead to undertreatment (25). Consequently, combined biological and clinical criteria were described and graded in the consensus conference (19).
The total IgE level is considered a major and useful test to support the diagnosis and follow-up of ABPA (2). A normal serum IgE level should exclude ABPA as the cause of the patient's symptoms. The situations in which IgE levels can be normal in active ABPA have been described by Knutsen et al. (12). Glucocorticoid therapy can explain a normal serum IgE level (2). Different cutoffs for total IgE levels exist: some authors used a cutoff of 1,000 IU/ml, while others used a cutoff of 500 IU/ml or 1,000 ng/ml (equivalent to 417 IU/ml). In the present study, the cutoff used was 500 IU/ml, and the 3 patients with rhinosinusitis and bronchitis due to A. fumigatus had values under 300 IU/ml. However, 4/13 patients with ABPA had IgE levels under the cutoff. For them, corticotherapy could explain the absence of elevated levels. High IgE levels (>500 IU/ml) were revealed in 5 patients without ABPA: four of them were atopic, with allergic reactions to pollens and acaridan allergens, while two presented intestinal helminthiasis.
Specific anti-Aspergillus IgG and precipitins indicate Aspergillus antigen sensitization, as well as ABPA. These tests have low discriminative power for ABPA diagnosis (4). Increased ELISA values were nevertheless observed in patients with an Aspergillus-associated disease, as shown in Fig. 1. In addition, the specific IgG antibodies measured in ABPA sera were elevated, as observed in our in-house ELISA for 12/13 patients with proven ABPA. However, in the three other clinical forms of aspergillosis, the IgG levels were also high. The precipitins were highly suggestive of aspergillosis. The presence of anti-catalase antibody was able to discriminate between sensitized cystic fibrosis patients and ABPA cystic fibrosis patients (17). Chymotrypsin detection has very low sensitivity in this population (Table 1). Although the quality of Aspergillus antigens is of great importance for reliable diagnosis, inconsistencies in commercial Aspergillus extracts have been noted (7). The quality of our own antigens used in homemade ELISA and IE was regularly checked, and both the repeatability and reproducibility of the results have been highly satisfactory. The presence of A. fumigatus in sputum is not specific to ABPA (22), but Aspergillus colonization is the first step leading to ABPA, depending on the individual susceptibilities of cystic fibrosis patients (2). Thus, A. fumigatus was more often isolated from the 17 ABPA patients (64.7%) and from the 3 patients with other forms of aspergillosis than from the ABPA-free population (6.6%). However, the colonization intensity did not vary among the three groups (Fig. 1).
The major difficulty in the biological diagnosis of ABPA remains the lack of standardization of the methods, as well as of the antigens used. Recombinant technology is known to enhance the reproducibility of antigen production (7, 8, 17, 24). For this reason, six A. fumigatus recombinants are now commercially available for specific antibody detection. The new standardized allergens are supposed to increase the accuracy of ABPA diagnosis. rAspf1, rAspf3, rAspf4, and rAspf6 recombinant allergens represent a useful measure to diagnose ABPA but have not been successful for monitoring therapy (7). Nevertheless, the data in the literature showed discordant results. de Oliveira et al. (10) have shown the lack of advantage of these recombinants (rAspf1, rAspf2, rAspf3, rAspf4, and rAspf6) in diagnosing ABPA. Other authors (11) have reported that rAspf3 and rAspf4 allow discrimination between ABPA and non-ABPA patients with atopic A. fumigatus-positive skin tests. According to the data in the literature (2, 11, 21), rAspf4 (a cytoplasmic protein with unknown function) and rAspf6 recombinants have provided powerful tools for the accurate diagnosis of ABPA. Kraemer et al. (13) have shown that allergen-specific IgE against these two recombinants could be detected almost exclusively in the sera of patients with ABPA. rAspf4 sensitivity is usually higher than that of rAspf6 (6, 11), but not for Latzin et al. (15). In comparison, the sensitivities obtained in our work were 92.3% for rAspf4 and 53.8% for rAspf6. These results are quite similar to those reported by Knutsen et al. (11) (82% with rAspf4 and 25% with rAspf6) and Bowyer et al. (6) (70% with rAspf4 and 30% with rAspf6). The cutoff used (0.1 kAU/liter) was very low but gave good sensitivity for specific IgE detection, especially with the two recombinants (specificity, up to 90%). These two recombinants had slightly higher reactivities than other biological markers. In our study, early suspected diagnosis was essentially linked to the increase in specific IgG and the presence of precipitins (Fig. 1). The IgE responses to the two recombinants were rarely detectable before the acute ABPA stage.
In conclusion, our study showed how complementary different biological markers are in diagnosing ABPA in cystic fibrosis patients. While the most sensitive techniques per patient were rAspf4 IgE detection (92.3%), anti-Aspergillus IgG ELISA (92.3%), and IE (92.3%), the most specific were rAspf4 and rAspf6 IgE detection (93.7% and 91.6%). The sensitivity of rAspf4 IgE detection was 92.3%, whereas rAspf6 had lower sensitivity (53.8%). Thus, our work confirmed the importance of rAspf4 in the panel of biological markers of ABPA and the limited efficiency of rAspf6.
Acknowledgments
We thank Evelyne Bouge, Marie-Paule Bernard, and Sonia Montlhuc for their technical assistance and Sabine Durville for reading the manuscript. We are indebted to Roland Carbonnel for kindly providing Pharmacia UniCAP tests (Phadia SAS, Saint Quentin en Yvelines, France).
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Accurso, F. J. 2007. Update in cystic fibrosis. 2006. Am. J. Respir. Crit. Care. Med. 175:754-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal, R. 2009. Allergic bronchopulmonary aspergillosis. Chest 135:805-826. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal, R., D. Gupta, A. N. Aggarwal, D. Behera, and S. K. Jindal. 2006. Allergic bronchopulmonary aspergillosis. Lessons from 126 patients attending a chest clinic in North India. Chest 130:442-448. [DOI] [PubMed] [Google Scholar]
- 4.Barton, R. C., R. P. Hobson, M. Denton, D. Peckham, K. Brownlee, S. Conway, and M. A. Kerr. 2008. Serologic diagnosis of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis through the detection of immunoglobulin G to Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 62:287-291. [DOI] [PubMed] [Google Scholar]
- 5.Biguet, J., P. Trav Van Ky, J. Fruit, and S. Andrieu. 1967. Identification d'une activité chymotrypsique au niveau de fractions remarquables de l'extrait antigénique d'Aspergillus fumigatus. Répercussion sur le diagnostic immunologique de l'aspergillose. Rev. Immunol. Paris 31:317-328. [PubMed] [Google Scholar]
- 6.Bowyer, P., O. Blightman, and D. W. Denning. 2006. Relative reactivity of Aspergillus allergens used in serological tests. Med. Mycol. 44:S23-S28. [DOI] [PubMed] [Google Scholar]
- 7.Casaulta, C., S. Flückiger, R. Crameri, K. Blaser, and M. H. Schoeni. 2005. Time course of antibody response to recombinant Aspergillus fumigatus antigens in cystic fibrosis with and without ABPA. Pediatr. Allergy Immunol. 16:217-225. [DOI] [PubMed] [Google Scholar]
- 8.Chan, C., P. C. Y. Woo, A. S. P. Leung, S. K. P. Lau, X. Y. Che, L. Coa, and K. Yuen. 2002. Detection of antibodies specific to an antigenic cell wall galactomannoprotein for serodiagnosis of Aspergillus aspergillosis. J. Clin. Microbiol. 40:2041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crameri, R., M. Weichel, S. Flückiger, A. G. Glaser, and C. Rhyner. 2006. Fungal allergies: a yet unsolved problem. Chem. Immunol. Allergy 91:121-133. [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira, E., P. Giavina-Bianchi, L. A. M. Fonseca, A. T. França, and J. Kalil. 2007. Allergic bronchopulmonary aspergillosis diagnosis remains a challenge. Respir. Med. 101:2352-2357. [DOI] [PubMed] [Google Scholar]
- 11.Knutsen, A. P., P. S. Hutcheson, R. G. Slavin, and V. P. Kurup. 2004. IgE antibody to Aspergillus fumigatus recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy 59:198-203. [DOI] [PubMed] [Google Scholar]
- 12.Knutsen, A. P., B. Noyes, M. R. Warrier, and J. Consolino. 2005. Allergic bronchopulmonary aspergillosis in a patient with cystic fibrosis: diagnostic criteria when the IgE level is less than 500 IU/mL. Ann. Allergy Asthma Immunol. 95:488-493. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer, R., N. Delosea, P. Ballinari, S. Gallati, and R. Crameri. 2006. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care. Med. 174:1211-1220. [DOI] [PubMed] [Google Scholar]
- 14.Kurup, V. P., A. P. Knutsen, R. B. Moss, and N. K. Bansal. 2006. Specific antibodies to recombinant allergens of Aspergillus fumigatus in cystic fibrosis patients with ABPA. Clin. Mol. Allergy 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latzin, P., D. Harti, N. Regamey, U. Frey, M. H. Schoeni, and C. Casaulta. 2008. Comparison of serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur. Respir. J. 31:36-42. [DOI] [PubMed] [Google Scholar]
- 16.Máiz, L., M. Cuevas, S. Quirce, J. F. Canon, A. Pacheco, A. Sousa, and H. Escobar. 2002. Serologic IgE immune responses against Aspergillus fumigatus and Candida albicans in patients with cystic fibrosis. Chest 121:782-788. [DOI] [PubMed] [Google Scholar]
- 17.Sarfati, J., M. Monod, P. Recco, A. Sulahian, C. Pinel, E. Candolfi, T. Fontaine, J.-P. Debeaupuis, M. Tabouret, and J.-P. Latgé. 2006. Recombinant antigens as diagnostic markers for aspergillosis. Diagn. Microbiol. Infect. Dis. 55:279-291. [DOI] [PubMed] [Google Scholar]
- 18.Soubani, A. O., and P. H. Chandrasekar. 2002. The clinical spectrum of pulmonary aspergillosis. Chest 121:1988-1999. [DOI] [PubMed] [Google Scholar]
- 19.Stevens, D. A., R. B. Moss, V. P. Kurup, A. P. Knutsen, P. Geenberger, M. A. Judson, D. W. Denning, R. Crameri, A. S. Brody, M. Light, M. Skov, W. Malsh, G. Mastella, and participants in the Cystic Fibrosis Foundation Consensus Conference. 2003. Allergic bronchopulmonary aspergillosis in cystic fibrosis-state of the art. Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 37:225-264. [DOI] [PubMed] [Google Scholar]
- 20.Takhar, P., T. P. Corrigan, L. Smurthwaite, B. J. O'Connor, S. R. Durham, T. H. Lee, and H. J. Gould. 2007. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 119:213-218. [DOI] [PubMed] [Google Scholar]
- 21.Tillie-Leblond, I., and A. B. Tonnel. 2005. Allergic bronchopulmonary aspergillosis. Allergy 60:1004-1013. [DOI] [PubMed] [Google Scholar]
- 22.Virnig, C., and R. K. Bush. 2007. Allergic bronchopulmonary aspergillosis: a US perspective. Curr. Opin. Pulm. Med. 13:67-71. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. Van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 24.Weig, M., M. Frosch, K. Tintelnot, A. Haas, U. Gross, B. Linsmeier, and J. Heesemann. 2001. Use of recombinant mitogillin for improved serodiagnosis of Aspergillus fumigatus-associated diseases. J. Clin. Microbiol. 39:1721-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zirbes, J. M., and C. E. Milla. 2008. Steroid-sparing effect of Omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis. Pediatr. Pulm. 43:607-610. [DOI] [PubMed] [Google Scholar]