Abstract
Highly pathogenic avian influenza (HPAI) virus causes one of the most economically devastating poultry diseases. An HPAI vaccine to prevent the disease in commercial and backyard birds must be effective, safe, and inexpensive. Recently, we demonstrated the efficacy of an adenovirus-based H5N1 HPAI vaccine (Ad5.HA) in chickens. To further evaluate the potential of the Ad5.HA vaccine and its cost-effectiveness, studies to determine the minimal effective dose and optimal route of administration in chickens were performed. A dose as low as 107 viral particles (vp) of adenovirus-based H5N1 vaccine per chicken was sufficient to generate a robust humoral immune response, which correlated with the previously reported level of protection. Several routes of administration, including intratracheal, conjunctival, subcutaneous, and in ovo routes, were evaluated for optimal vaccine administration. However, only the subcutaneous route of immunization induced a satisfactory level of influenza virus-specific antibodies. Importantly, these studies established that the vaccine-induced immunity was cross-reactive against an H5N1 strain from a different clade, emphasizing the potential of cross-protection. Our results suggest that the Ad5.HA HPAI vaccine is safe and effective, with the potential of cross-clade protection. The ease of manufacturing and cost-effectiveness make Ad5.HA an excellent avian influenza vaccine candidate with the ability to protect poultry from HPAI virus infection. Considering the limitations of the influenza vaccine technology currently used for poultry applications, any effort aimed at overcoming those limitations is highly significant.
Influenza A virus is a segmented, negative-strand RNA virus that belongs to the family Orthomyxoviridae, which is divided into subtypes based on serological reactions of the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Thus far, 16 different HA subtypes (H1 to H16) and 9 different NA subtypes (N1 to N9) (11, 34) have been identified. Each of the subtypes has been isolated from waterfowl species, the natural hosts of all known influenza A viruses. These birds are the reservoir for the spread of influenza virus worldwide in wild birds and poultry (19, 34). Avian influenza (AI) virus strains are further classified into low and highly pathogenic avian influenza (LPAI and HPAI, respectively) viruses based on their pathogenicity.
Continued outbreaks of HPAI viruses of the H5 and H7 subtypes in poultry in Asia, Europe, Africa, and Canada represent a serious risk for animal and public health worldwide. Avian influenza is one of the greatest concerns for public health that has emerged from an animal reservoir in recent times. Since the late 1990s, the number of outbreaks of avian influenza in poultry has dramatically increased. For example, in 2008-2009, 2,770 outbreaks occurred in Vietnam, 1,143 in Thailand, 1,084 in Egypt, and 219 in Turkey; outbreaks have also occurred in many other countries worldwide (http://www.oie.int/eng/en_index.htm).
In 2008-2009, 20 million poultry died or were depopulated because of HPAI outbreaks. This had devastating consequences for the international poultry industry and raised concerns about the potential for transmission to humans and the possible pandemic spread of lethal disease. Culling represents the first line of defense against avian influenza virus; however, continuing outbreaks over the last 6 years revealed that implementation of culling at the farm level was insufficient to halt the spread of disease.
Vaccines, in conjunction with other measures of prevention and management, may represent an alternative to preemptive culling to achieve a reduction in the rate of transmission by reducing the susceptibility of healthy flocks at risk. Although vaccination has been recommended by the World Organization for Animal Health (OIE) and the Food and Agriculture Organization (FAO) to control AI, few effective AI vaccines are available (http://www.oie.int/eng/avian_influenza/OIE_FAO_Recom_05.pdf). Conventional inactivated vaccines containing the same viral subtype as field virus (with differing degrees of antigenic similarity) (4, 5, 22), inactivated vaccines generated through reverse genetic techniques (18, 33), and recombinant vaccines (3, 15, 21, 23) have been tested. However, production constraints associated with conventional inactivated influenza virus vaccines that are manufactured in eggs could severely hinder control of an emerging AI virus with pandemic potential (7).
We investigated recombinant replication-defective adenoviruses as possible influenza vaccine vehicles for poultry. Recombinant adenovirus-based vaccines are highly effective inducers of both humoral immunity and cellular immunity in mammals and have shown promise as vaccine vehicle candidates against numerous infectious pathogens (2, 12, 14, 24, 32). Previously, we generated Ad5.HA, an E1/E3-deleted human adenovirus serotype 5 (Ad5)-based vector that expresses the codon-optimized hemagglutinin (HA) gene from the influenza A/Vietnam/1203/04 (H5N1) virus (13). The Ad5.HA vaccine induced humoral and cellular immune responses against HA and protected against influenza virus challenges in both mice and chickens. We have now extended our studies to determine the efficacy of Ad5.HA immunization in chickens when administered at different dosages via different routes.
MATERIALS AND METHODS
Viruses.
Reassortant influenza viruses with the HA lacking the polybasic cleavage site, with the NA from A/VN/1203/04 (H5N1) or A/Indonesia/05/2005 (H5N1), and with the internal genes from A/Puerto Rico/8/34 (PR8) were described previously (35). The designated viruses were A/VN/1203/04xPR8 and A/Indo/05/2005xPR8, respectively, and were propagated in our biosafety level 2+ (BSL-2+) facility in 10-day-old embryonated, specific-pathogen-free chicken eggs at 37°C. Virus-containing allantoic fluid was harvested and inactivated by a 2% β-propiolactone treatment for 2 h at 37°C. Inactivated viruses were titrated by a standard HA assay.
An E1/E3-deleted adenoviral vector expressing the codon-optimized HA gene from A/VN/1203/04 was constructed by using Cre-lox recombination into the adenovirus-packaging cell line Cre8. Recombinant adenovirus was propagated in Cre8 cells, purified by cesium chloride density gradient centrifugation and dialysis, and stored at −70°C as previously described (31).
Immunization of chicken and embryonated chicken eggs.
Five-week-old White Leghorn chickens were immunized with different dosages of Ad5.HA vaccine (1010, 109, 108, 107, 106, 105, and 104 viral particles [vp]/chicken) or Ad5 (as a control). The adenovirus-based vaccine was diluted in 100 μl of phosphate-buffered saline (PBS) and administered through the subcutaneous, intratracheal (with the help of a standard mist applicator), or conjunctival route.
In ovo vaccination of 18-day-old embryonated, specific-pathogen-free chicken eggs was completed by inoculation of 100 μl of Ad5.HA or Ad5 vaccine dilution. Eggs were candled, and based on the visibility of the embryo, a small hole was made through the air cell with a drill. The eggs were injected using a 21-gauge needle at a depth of 1 in. Thereby, seven eggs were inoculated with 1010 vp, five eggs with 109 vp, five eggs with 108 vp, and 4 eggs with 107 vp of Ad5.HA vaccine. Four eggs received 1010 vp of the Ad5 vaccine. Eggs were incubated at 37°C in an egg incubator until they hatched. Serum samples were collected from the jugular vein on a biweekly basis for the determination of hemagglutination inhibition (HI) antibody titers.
All animals were housed and handled according to the University of Pittsburgh's Institutional Animal Care and Use Committee (IACUC) guidelines, and all animal work was approved by the appropriate committee (IACUC 0706924).
Hemagglutination inhibition assay.
The assay was performed as described previously (13). Briefly, receptor-destroying-enzyme-treated chicken sera were diluted in PBS and incubated with 8 HA units of the inactivated A/VN/1203/04xPR8 or A/Indo/2005xPR8 virus for 45 min at room temperature. Equal amounts of 0.5% turkey red blood cells were added, and inhibition of hemagglutination was evaluated after 30 min. Results are given as the log2 of the geometric mean (GMT) of each group.
RESULTS
Route of administration of the Ad5.HA vaccine in chickens.
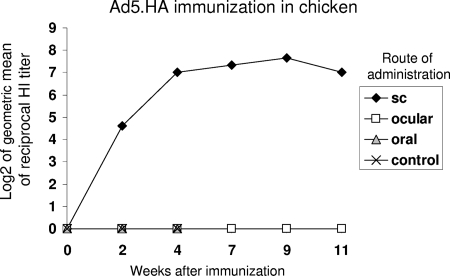
A recombinant, replication-defective adenovirus carrying a codon-optimized hemagglutinin (HA) gene of the influenza A/VN/1203/04 (H5N1) virus was previously generated and tested in mice and chickens. This virus showed promising results by protecting subcutaneously immunized animals against an infection with H5N1 virus (13). To establish the most favorable route for Ad5.HA vaccine delivery in poultry, we investigated subcutaneous, intratracheal, conjunctival, and in ovo routes of administration. Groups of 5-week-old White Leghorn chickens were immunized with 1010 vp of Ad5.HA or Ad5 (control) by subcutaneous injection, intratracheal application using a mist applicator, or conjunctival administration using eye drops. Sera were collected after immunization and tested for the presence of H5N1-specific antibodies measured in a hemagglutination inhibition (HI) assay. Geometric mean HI titers in subcutaneously immunized chickens exceeded 4 log2 (Fig. 1). In contrast, the intratracheal and conjunctival routes of administration failed to induce humoral immune responses measurable by HI (Fig. 1).
FIG. 1.
Evaluation of different routes of administration by measuring induced antibody titers. Four groups of 5-week-old chickens were immunized with 1010 vp of recombinant replication-defective adenovirus encoding the hemagglutinin protein (Ad5.HA) of the influenza A/VN/1203/04 (H5N1) virus or control Ad5 virus. Ad5.HA was administered through the subcutaneous (sc), conjunctival (ocular), or intratracheal (oral) route. Sera from chickens were isolated at the indicated time points and tested in an HI assay using 8 HA units and turkey red blood cells. Results are shown as the log2 value of the geometric mean (GMT) of each group.
Recent reports indicated that the in ovo route of administration can be effective, with the potential to provide an easy and rapid way to immunize a large number of eggs with an adenovirus-based vaccine via robotic in ovo injectors (1, 29). Different doses of Ad5.HA (107 to 1010) or Ad5 (control) vaccine were injected into the allantoic cavity of 18-day-old embryonated chicken eggs. Eggs were further incubated at 37°C until hatching. At 6 weeks of age, sera were collected and tested in the HI assay. As shown in Table 1, only the highest dose (1010 vp) of the Ad5.HA vaccine induced measurable, although low, HI titers in 50% of the chickens. These results revealed that the subcutaneous injection was the most favorable route of administration for the Ad5.HA vaccine, and this route of administration was selected for all the subsequent studies presented here.
TABLE 1.
In ovo immunization with Ad5.HAa
| Vaccine and dosage (vp/chicken) | No. of chickens with positive HI titer/total | Geometric mean HI titerb |
|---|---|---|
| Ad5.HA | ||
| 1010 | 2/4 | 4.32 |
| 109 | 0/4 | 0 |
| 108 | 0/4 | 0 |
| 107 | 0/4 | 0 |
| Ad5 control (1010) | 0/7 | 0 |
Induction of antibodies against H5N1 after in ovo inoculation with dose escalations of Ad5.HA. Eighteen-day-old embryonated chicken eggs were immunized by injection of indicated amounts of Ad5.HA (1010, 109, 108, and 107 vp/chicken) or 1010 vp of Ad5/chicken into the allantoic cavity. Eggs were incubated at 37°C until they hatched. Sera from hatched chickens were isolated at week 6 and tested via the HI test by using 8 HA units and turkey red blood cells.
Results are shown as the log2 of the geometric mean (GMT) of each group.
Dose escalation of the Ad5.HA vaccine in chickens.
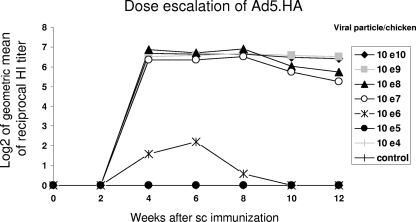
Dose escalation studies were performed to determine the lowest dose necessary to achieve an immune response that correlated with protection. Groups of nine chickens were immunized subcutaneously with escalating doses of the Ad5.HA vaccine. Sera were collected at different time points and tested in the HI assay for the presence of HA-specific antibodies. Immunizations with 107 vp of the Ad5.HA vaccine were able to induce high HI antibody titers (Fig. 2), whereas 106, 105, and 104 vp of Ad5.HA did not induce any measurable HI titers. Immunizing doses above 107 vp (108 to 1010) did not result in an increase in antibody titer. Thus, we concluded that 107 vp of Ad5.HA is the minimal dosage in chickens that correlates with protection from an H5N1 challenge. HI titers were analyzed at 2-week intervals. The earliest time point at which robust titers of H5N1 virus-specific antibodies could be detected was serum collection at 4 weeks postimmunization. In addition, these results demonstrate the duration of immunity. A high HI titer was observed in sera collected 12 weeks postimmunization, indicating a potential long-term protection against H5N1.
FIG. 2.
Dose escalation of Ad5.HA and induction of antibodies. Five groups of nine 5-week-old chickens were subcutaneously immunized with indicated doses of Ad5.HA (1010, 109, 108, 107, 106, 105, or 104 vp/chicken) or Ad5 (1010 vp). Sera from chickens were isolated at indicated time points and tested in an HI assay using 8 HA units and turkey red blood cells. Results are shown as the log2 value of the geometric mean (GMT) of each group.
Induction of cross-reactive HA-specific antibody by the Ad5.HA vaccine.
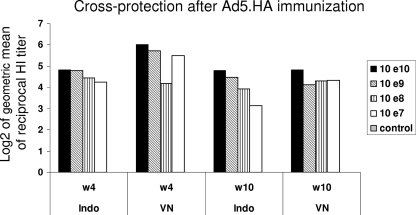
The rapid evolution of the H5N1 HPAI virus in Asia has resulted in considerable antigenic diversity of the HA protein among viruses from different regions. The HA of the H5N1 lineage viruses has diverged into distinct clades and subclades. Currently, clade 1 viruses antigenically related to the A/Vietnam/1203/04 strain circulate in Vietnam and clade 2 viruses related to A/Indonesia/05/2005 circulate in Indonesia. To characterize the capability of the Ad5.HA vaccine to induce cross-reactive immune responses against these strains, sera from immunized chickens were tested against the heterotypic A/Indonesia/05/2005 (H5N1) clade 2 virus. HI titer results at two different time points (weeks 4 and 10) are shown in Fig. 3. All doses of the Ad5.HA vaccine induced antibody responses against the homologous influenza virus strain A/VN/1203/04 with cross-reactivity against the heterotypic A/Indo/05/2005 clade 2 strain and demonstrated similar levels of HI antibody titers. These results indicate that cross-protection against other influenza virus strains within the same serotype may be achieved in Ad5.HA-immunized chickens.
FIG. 3.
Induction of cross-reactive antibodies by Ad5.HA. Cross-reactivity of sera against other H5N1 strains was tested by using the influenza A/Indo/05/2005 (H5N1) × PR8 virus strain in an HI test. Sera collected at weeks 4 (w4) and 10 (w10) from Ad5.HA (1010, 109, 108, or 107 vp) or control Ad5 (1010 vp)-immunized chickens were tested in parallel against 8 HA units of the influenza A/VN/1203/04 (H5N1) × PR8 or influenza A/Indo/05/2005 (H5N1) × PR8 virus. Results are shown as the log2 value of the geometric mean (GMT) of each group.
DISCUSSION
Previously, we generated a recombinant adenovirus encoding the HA protein derived from the A/VN/1203/04 (H5N1) HPAI virus strain (13). Evaluation of the efficacy of the adenovirus-based influenza vaccine in challenge studies confirmed that a single subcutaneous inoculation in chickens provided complete protection against a lethal challenge with the influenza A/VN/1203/04 (H5N1) virus in combination with a significant reduction of virus isolates in cloacal and oral swabs. Furthermore, high HI antibody responses to influenza virus were generated in chickens and were correlated with protection, with chickens with low HI titers showing incomplete protection when HI titers were lower than 4 (log2 values) (13). Our goal for the present study was to expand upon these findings and investigate different routes of administration and the minimal dosage required by correlating the induced HI titer in order to manufacture a cost-effective poultry vaccine. Considering the use of chickens as broilers, breeders, or layers, the site of injection of the adenovirus-based vaccine is an important question to address. Although a human adenovirus-based vaccine is replication incompetent, the direct injection of such a vaccine into the “meat” would not be favorable and would require further safety investigations. In breeders and layers, it would be more acceptable, since vaccine traces are not expected to be detected in eggs and offspring. Toro et al. described an interesting vaccination technology that proposed the use of robotic injectors for in ovo immunization of an adenovirus-based vaccine encoding the H5N9-derived hemagglutinin (28, 29). Therefore, we also tested the in ovo immunization route for comparison. Among the different routes of administration studied here (intratracheal, conjunctival, subcutaneous, and in ovo), only the subcutaneous route for Ad5.HA was able to induce an HI titer correlating with protection. Despite the previously reported successful in ovo immunization using an adenovirus-based vaccine, we failed to consistently detect an HI titer in the hatched chickens after egg injection of high doses of Ad5.HA. One possible explanation for this unfavorable outcome could be the technique used to administer in ovo vaccines. The success of in ovo vaccination depends not only on the manner in which the vaccine is applied but also on the timing of the injection in relation to the stage of embryonic development and the exact site of injection (route) in the developing egg. An in ovo injection can access five different areas of the egg during late stage incubation. These areas include the air cell, the allantoic sac, the amniotic fluid, the body of the embryo, and the yolk sac. Each area represents a distinct route of vaccine administration to the embryo, and these in turn represent distinct types of vaccine and antigen presentation to the avian immune system. Additionally, these in ovo compartments change quickly during the window of injection timing. Thus, we do not exclude the possibility that a different route and/or timing could lead to a successful in ovo application of adenovirus-based vaccines.
Using the subcutaneous route of administration, subsequent dose escalation studies were performed with Ad5.HA; in these studies, 107 vp were sufficient to induce a high HI titer similar to those induced by higher doses (1010, 109, and 108 vp) of Ad5.HA. Remarkably, the antibodies elicited were cross-reactive with a heterotypic H5N1 influenza virus strain from a different clade. These data indicate potential resistance to other circulating H5N1 influenza virus strains in the immunized chicken, as observed with other poultry vaccines (4, 18, 21, 30). We hypothesize that Ad5.HA at the dosage of 107 vp will provide protection against an influenza A/VN/1203/04 (H5N1) virus infection because HI titers correlate with the previously reported level of protection in chickens (13). However, challenge studies that measure viral titers in cloacal and oral swabs in chickens are warranted to determine the impact of subcutaneous vaccination with 107 vp of Ad5.HA on protection and viral shedding (sterilizing immunity).
Current licensed avian influenza (AI) virus vaccines used in poultry around the world include the inactivated oil adjuvanted whole-virus vaccine and a recombinant fowlpox virus-vectored vaccine with an H5 AI virus antigen insert. Historically, AI virus strains selected for manufacturing inactivated vaccine have been based on low-pathogenicity viruses obtained from field outbreaks that have homologous HA proteins. HPAI virus strains have rarely been used to manufacture inactivated vaccines because they require specialized, high-biocontainment manufacturing facilities. More importantly, HPAI virus strains are difficult to grow in eggs due to their toxicity, which results in poor yields. The fowlpox-vectored H5 vaccine is safe and effective in chickens and has the advantage of early application on the first day of life (3). However, this vaccine cannot be used in older animals, as their immunity to fowlpox virus prevents the development of effective immunity (27).
In addition to the vaccines mentioned above, other novel recombinant vaccine technologies for use in poultry, such as the reverse-genetics-produced live attenuated influenza A virus vaccine (18, 25, 33) and a Newcastle disease virus (NDV)-based live attenuated vaccine (15, 21), have recently been developed and show promising results. Reverse-genetics-produced vaccines, which sometimes take longer to generate, have the advantage of being able to overcome egg toxicity. Moreover, biocontainment for the production process could be lowered and would result in easier and less costly manufacturing. Using an NDV virus as a vector encoding the H5N1 HA protein, Steel et al. developed a bivalent vaccine strategy that induced protective immune responses against both H5N1 and NDV infections (26). In addition to resulting in protection in H5N1 challenges and reduction of viral shedding, they both are effective when delivered in ovo, which would reduce the cost of administration dramatically.
Among the strategies for vaccinating poultry against influenza, the use of adenovirus-based influenza vaccines may complement current technologies and offer some advantages. First, adenovirus is known to produce strong humoral and cell-mediated immune responses that are not confounded by natural preexisting immunity to the viral vector. However, the efficacy of sequential immunizations with Ad5-based vaccines in poultry could potentially be reduced by the induction of Ad5-specific neutralizing antibodies after the first immunization. Studies performed both with mice and with humans have shown that the level of neutralizing antibodies, route of administration, and serotype all played important roles in the effective delivery of an adenovirus-based vaccine (10, 17, 20, 31). This suggests that vector-specific immunity may be overcome by enhancing the vaccine dosage or by using alternative human and animal adenovirus serotypes. However, given the short lifespan of poultry (particularly for the broiler), we believe this might constitute a relatively minor limitation in this vaccine target population.
In comparison to the use of live attenuated influenza vaccines, adenoviral technology based on a recombinant incompetent human pathogen does not replicate in avian species and therefore poses no risk of reactivation in the host.
The second advantage is that the culture of recombinant adenovirus is both rapid and efficient. The ability to scale up to large quantities makes this technology one of the most promising DNA-based platforms for vaccination. Adenovirus-based vaccines, either in solution or lyophilized, can be stored at room temperature or at 4°C for up to 2 years without substantial loss of viral titer (8, 9). The ability to store the vaccine at room temperature in a lyophilized form, the low-dose application (107 vp/chicken), and the high production yield (with one cell producing 1,000 to 10,000 vp) all lower the burden of cost for vaccination at all stages of vaccine production and administration.
From an initial cost evaluation analysis that we performed for large-scale manufacturing of an adenovirus-based vaccine, we estimated the cost of 1 to 5 cents per dose (data not shown), which is within the acceptable cost range for poultry vaccines (6, 16).
In conclusion, our findings support the development of replication-defective adenovirus-based vaccines for the prevention of HPAI. An adenovirus-based vaccine could be additional ammunition in the current vaccine armamentarium to control the disease and to reduce the economic damage.
Acknowledgments
We thank S. Schoonover, T. Kenniston, and the staff of the Central Animal Facility of the University of Pittsburgh for their assistance in collecting serum samples from the chickens and K. Okada for the adenovirus preparation. We also thank S. Rea for the administrative support and the help with the preparation of the manuscript. We thank the Ministries of Health of Indonesia and Vietnam, as well as the WHO Global Influenza Surveillance Network, for providing specimens containing HPAI virus. We thank C. O'Connell from the University of Pittsburgh Medical Center for helpful suggestions.
The findings and conclusions in this report are ours and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
This work was supported by NIH grant 5R01AI069282-02 (A.G.).
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Avakian, A. P., R. M. Poston, F. K. Kong, K. R. Van Kampen, and D. C. Tang. 2007. Automated mass immunization of poultry: the prospect for nonreplicating human adenovirus-vectored in ovo vaccines. Expert Rev. Vaccines 6:457-465. [DOI] [PubMed] [Google Scholar]
- 2.Barratt-Boyes, S. M., A. C. Soloff, W. Gao, E. Nwanegbo, X. Liu, P. A. Rajakumar, K. N. Brown, P. D. Robbins, M. Murphey-Corb, R. D. Day, and A. Gambotto. 2006. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J. Gen. Virol. 87:139-149. [DOI] [PubMed] [Google Scholar]
- 3.Bublot, M., N. Pritchard, J. S. Cruz, T. R. Mickle, P. Selleck, and D. E. Swayne. 2007. Efficacy of a fowlpox-vectored avian influenza H5 vaccine against Asian H5N1 highly pathogenic avian influenza virus challenge. Avian Dis. 51:498-500. [DOI] [PubMed] [Google Scholar]
- 4.Capua, I., G. Cattoli, and S. Marangon. 2004. DIVA—a vaccination strategy enabling the detection of field exposure to avian influenza. Dev. Biol. (Basel) 119:229-233. [PubMed] [Google Scholar]
- 5.Capua, I., and S. Marangon. 2003. The use of vaccination as an option for the control of avian influenza. Avian Pathol. 32:335-343. [DOI] [PubMed] [Google Scholar]
- 6.Capua, I., and S. Marangon. 2007. The challenge of controlling notifiable avian influenza by means of vaccination. Avian Dis. 51:317-322. [DOI] [PubMed] [Google Scholar]
- 7.Check, E. 2005. Avian flu special: is this our best shot? Nature 435:404-406. [DOI] [PubMed] [Google Scholar]
- 8.Croyle, M. A., X. Cheng, and J. M. Wilson. 2001. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther. 8:1281-1290. [DOI] [PubMed] [Google Scholar]
- 9.Evans, R. K., D. K. Nawrocki, L. A. Isopi, D. M. Williams, D. R. Casimiro, S. Chin, M. Chen, D. M. Zhu, J. W. Shiver, and D. B. Volkin. 2004. Development of stable liquid formulations for adenovirus-based vaccines. J. Pharm. Sci. 93:2458-2475. [DOI] [PubMed] [Google Scholar]
- 10.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouchier, R. A., V. Munster, A. Wallensten, T. M. Bestebroer, S. Herfst, D. Smith, G. F. Rimmelzwaan, B. Olsen, and A. D. Osterhaus. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79:2814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallichan, W. S., and K. L. Rosenthal. 1995. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine 13:1589-1595. [DOI] [PubMed] [Google Scholar]
- 13.Gao, W., A. C. Soloff, X. Lu, A. Montecalvo, D. C. Nguyen, Y. Matsuoka, P. D. Robbins, D. E. Swayne, R. O. Donis, J. M. Katz, S. M. Barratt-Boyes, and A. Gambotto. 2006. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, W., A. Tamin, A. Soloff, L. D'Aiuto, E. Nwanegbo, P. D. Robbins, W. J. Bellini, S. Barratt-Boyes, and A. Gambotto. 2003. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet 362:1895-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge, J., G. Deng, Z. Wen, G. Tian, Y. Wang, J. Shi, X. Wang, Y. Li, S. Hu, Y. Jiang, C. Yang, K. Yu, Z. Bu, and H. Chen. 2007. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 81:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halvorson, D. A. 2002. The control of H5 or H7 mildly pathogenic avian influenza: a role for inactivated vaccine. Avian Pathol. 31:5-12. [DOI] [PubMed] [Google Scholar]
- 17.Harro, C., X. Sun, J. E. Stek, R. Y. Leavitt, D. V. Mehrotra, F. Wang, A. J. Bett, D. R. Casimiro, J. W. Shiver, M. J. DiNubile, and E. Quirk. 2009. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin. Vaccine Immunol. 16:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadhao, S. J., C. W. Lee, M. Sylte, and D. L. Suarez. 2009. Comparative efficacy of North American and antigenically matched reverse genetics derived H5N9 DIVA marker vaccines against highly pathogenic Asian H5N1 avian influenza viruses in chickens. Vaccine 27:6247-6260. [DOI] [PubMed] [Google Scholar]
- 19.Kida, H., R. Yanagawa, and Y. Matsuoka. 1980. Duck influenza lacking evidence of disease signs and immune response. Infect. Immun. 30:547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasaro, M. O., and H. C. Ertl. 2009. New insights on adenovirus as vaccine vectors. Mol. Ther. 17:1333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayak, B., S. N. Rout, S. Kumar, M. S. Khalil, M. M. Fouda, L. E. Ahmed, K. C. Earhart, D. R. Perez, P. L. Collins, and S. K. Samal. 2009. Immunization of chickens with Newcastle disease virus expressing H5 hemagglutinin protects against highly pathogenic H5N1 avian influenza viruses. PLoS One 4:e6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poetri, O. N., A. Bouma, S. Murtini, I. Claassen, G. Koch, R. D. Soejoedono, J. A. Stegeman, and M. van Boven. 2009. An inactivated H5N2 vaccine reduces transmission of highly pathogenic H5N1 avian influenza virus among native chickens. Vaccine 27:2864-2869. [DOI] [PubMed] [Google Scholar]
- 23.Qiao, C., Y. Jiang, G. Tian, X. Wang, C. Li, X. Xin, H. Chen, and K. Yu. 2009. Recombinant fowlpox virus vector-based vaccine completely protects chickens from H5N1 avian influenza virus. Antiviral Res. 81:234-238. [DOI] [PubMed] [Google Scholar]
- 24.Santosuosso, M., S. McCormick, and Z. Xing. 2005. Adenoviral vectors for mucosal vaccination against infectious diseases. Viral Immunol. 18:283-291. [DOI] [PubMed] [Google Scholar]
- 25.Song, H., G. R. Nieto, and D. R. Perez. 2007. A new generation of modified live-attenuated avian influenza viruses using a two-strategy combination as potential vaccine candidates. J. Virol. 81:9238-9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steel, J., S. V. Burmakina, C. Thomas, E. Spackman, A. Garcia-Sastre, D. E. Swayne, and P. Palese. 2008. A combination in-ovo vaccine for avian influenza virus and Newcastle disease virus. Vaccine 26:522-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swayne, D. E., J. R. Beck, and N. Kinney. 2000. Failure of a recombinant fowl poxvirus vaccine containing an avian influenza hemagglutinin gene to provide consistent protection against influenza in chickens preimmunized with a fowl pox vaccine. Avian Dis. 44:132-137. [PubMed] [Google Scholar]
- 28.Toro, H., and D. C. Tang. 2009. Protection of chickens against avian influenza with nonreplicating adenovirus-vectored vaccine. Poult. Sci. 88:867-871. [DOI] [PubMed] [Google Scholar]
- 29.Toro, H., D. C. Tang, D. L. Suarez, M. J. Sylte, J. Pfeiffer, and K. R. Van Kampen. 2007. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine 25:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Goot, J. A., G. Koch, M. C. de Jong, and M. van Boven. 2005. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc. Natl. Acad. Sci. U. S. A. 102:18141-18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Kampen, K. R., Z. Shi, P. Gao, J. Zhang, K. W. Foster, D. T. Chen, D. Marks, C. A. Elmets, and D. C. Tang. 2005. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 23:1029-1036. [DOI] [PubMed] [Google Scholar]
- 32.Wang, J., L. Thorson, R. W. Stokes, M. Santosuosso, K. Huygen, A. Zganiacz, M. Hitt, and Z. Xing. 2004. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173:6357-6365. [DOI] [PubMed] [Google Scholar]
- 33.Webby, R. J., D. R. Perez, J. S. Coleman, Y. Guan, J. H. Knight, E. A. Govorkova, L. R. Clain-Moss, J. S. Peiris, J. E. Rehg, E. I. Tuomanen, and R. G. Webster. 2004. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 363:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. 2005. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 11:1515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]