Abstract
The cellular mechanisms that control arterial diameter in vivo, particularly in hypertension, are uncertain. Here, we report a method that permits arterial intracellular Ca2+ concentration ([Ca2+]i), myosin light-chain kinase (MLCK) activation, and artery external diameter to be recorded simultaneously with arterial blood pressure (BP) in living mice under 1.5% isofluorane anesthesia. The method also enables an assessment of local receptor activity on [Ca2+]i, MLCK activity, and diameter in arteries, uncomplicated by systemic effects. Transgenic mice that express, in smooth muscle, a Ca2+/calmodulin-activated, Förster resonance energy transfer (FRET)-based “ratiometric”, exogenous MLCK biosensor were used. Vasoactive substances were administered either intravenously or locally to segments of exposed femoral or cremaster arteries. In the basal state, mean BP was ∼90 mmHg, femoral arteries were constricted to 65% of their passive diameter, MLCK fractional activation was 0.14, and [Ca2+]i was 131 nM. Phenylephrine (300 ng/g wt iv) elevated mean BP transiently to ∼110 mmHg, decreased heart rate, increased femoral artery [Ca2+]i to 244 nM and fractional MLCK activation to 0.24, and decreased artery diameter by 23%. In comparison, local application of 1.0 μM phenylephrine raised [Ca2+]i to 279 nM and fractional MLCK activation to 0.26, and reduced diameter by 25%, but did not affect BP or heart rate. Intravital FRET imaging of exogenous MLCK biosensor mice permits quantification of changes in [Ca2+]i and MLCK activation that accompany small changes in BP. Based on the observed variance of the FRET data, this method should enable the detection of a difference in basal [Ca2+]i of 29 nM between two groups of 12 mice with a significance of P < 0.05.
Keywords: intravital imaging, blood pressure, femoral artery, cremaster artery, spectral imaging
in the living animal, artery diameter is dynamic, varying over seconds, days, and years. In general, changes in the internal diameter of an artery, which affect its resistance to flow, are thought to be accomplished ultimately by two major mechanisms: 1) changes in phosphorylation of the myosin regulatory light chain (RLC) in smooth muscle (which controls acto-myosin cross-bridge cycling), and 2) structural changes in the walls of arteries (remodeling). Within the smooth muscle cells, Ca2+/calmodulin (Ca2+/CaM)-dependent myosin light-chain kinase (MLCK), and myosin light chain phosphatase (MLCP), are the primary controllers of RLC phosphorylation (17, 31), and both are, therefore, expected to play major roles in rapid changes in arterial diameter in vivo. Nevertheless, the relative importance of each in any particular physiological situation is still not well understood. One reason is that the activities of both are regulated by a large number of different G protein-coupled receptors (21). Both are also regulated by intracellular Ca2+ ([Ca2+]i), but differently: MLCK is regulated directly by [Ca2+]i and calmodulin, whereas MLCP is, in part, regulated indirectly by Ca2+ through Ca2+-dependent protein kinase C and its effectors (CPI-17) (36).
The relative roles of Ca2+, MLCK, MLCP, and arterial remodeling are particularly obscure in the development and maintenance of decreased artery diameter in hypertension. For example, recent data implicate smooth muscle G12/13-coupled receptors, and hence changes in MLCP activity, in salt-induced hypertension (39). Other theories for the decreased artery diameter in salt-induced hypertension attribute a dominant role to [Ca2+]i (2, 15) and hence MLCK activity. In human essential hypertension, however, “eutrophic” remodeling also occurs (24), potentially allowing arteries to maintain a decreased diameter, without increased contractile activation (i.e., increased MLCK activity or decreased MLCP activity). While structural changes can be evaluated ex vivo (22), evaluation of functional changes ex vivo is difficult, as certain factors, e.g., neuronal and humoral vasoactive substances that circulate in living animals and participate in the regulation of diameter, are always lost when the arteries are removed from the animal for ex vivo study.
Here, we report a method that enables the estimation of spatial average [Ca2+]i, fractional activation of exogenous MLCK (exMLCK), and outer diameter of a segment of an individual artery in living, anesthetized mice. The method utilizes transgenic mice that express, specifically in smooth muscle, a Ca2+/CaM-dependent FRET (Förster resonance energy transfer)-type optical biosensor molecule, based on MLCK (exMLCK) (14). “Fractional activation” of exMLCK refers to the fraction of exMLCK molecules that have Ca2+/CaM bound to the site that changes FRET and activates kinase activity. In a cultured cell system, the rate of myosin RLC phosphorylation was directly proportional to this fraction (10). These measurements can be made simultaneously with any of the other conventional measurements of cardiovascular function, such as arterial blood pressure (BP).
The genetically encoded Ca2+ indicator, GCaMP2, has already been used successfully in vivo to explore Ca2+ dynamics in endothelium of cremaster arterioles (32). In contrast to GCaMP2, however, the exMLCK biosensor used here is based on FRET and thus provides a normalized optical signal (the FRET “ratio”) that can be advantageous in several ways: 1) it can be “calibrated” to estimate [Ca2+]i and fractional MLCK activation; 2) it provides usable signals even during artery contraction or relaxation; and 3) it is expected to provide statistical significance for comparison of different groups of animals, as opposed to simply observing changes in the same animal. Fura PE-3/AM has also been used recently to study the local factors controlling blood flow in hamster cheek pouch arterioles (3). Since fura PE-3 is also “ratiometric”, this important study provided the first in vivo quantitative estimates of changes in [Ca2+]i in arterioles during agonist-induced vasomotion and other conditions, using an in vitro calibration. Nevertheless, the use of membrane-permeant Ca2+ indicator dyes presents several well-known methodological difficulties (28). These include the inevitable presence of dye in intracellular organelles, and the technical difficulty of loading the dyes into arteries in vivo. By comparison, the use of biosensor animals that express genetically encoded indicator molecules (20) naturally and specifically in the cytoplasm of a target tissue circumvents some of these problems.
An important feature of the method we report here is that, for the first time, estimates of [Ca2+]i and MLCK activity can be made in conduit or resistance arteries during relatively small changes in BP (20 mmHg), typical of hypertension. We also show that this method can be useful in defining the local roles of α1-adrenergic receptor (α1-AR) in artery function without involving systemic effects that often complicate in vivo experiments. In this case, [Ca2+]i, MLCK activation, and diameter are measured after local application of α1-AR agonists or antagonists to a locally exposed, directly visualized artery, without causing systemic hemodynamic changes.
METHODS
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. The transgenic mouse line, exMLCK (inbred Charles River), was the same as used previously (5, 14, 27, 38). These mice express exMLCK, which monitors the binding of Ca2+/CaM through changes in FRET between cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). A second line of transgenic mice was also created (exMLCK-C57BL/6) that contained the exMLCK biosensor transgene in the genetic background of C57BL/6. These mice were obtained by backcrossing the original exMLCK biosensor mice with wild-type C57BL/6 mice for five generations. There were no detectable functional differences between the two lines. All mice were maintained on a 12:12-h light-dark schedule at 22–25°C and 45–65% humidity and fed ad libitum on a standard rodent diet and tap water. Male mice were used (age 12–26 wk and weight 30–45 g).
Preparation of Mice
Recording of arterial BP and set up for intravenous infusion.
Anesthesia was induced with 3–4% isoflurane (IsoFlo, Abbott Animal Health, Abbott Park, IL) in O2. Isoflurane was maintained at 2–3% in O2 during surgery and then reduced to 1.5% for the subsequent experiment. To reduce environmental contamination by isoflurane, the exhaust gas from animals was passed through an activated charcoal absorption filter (VaporGuard, VetEquip, Pleasanton, CA). After induction of anesthesia, mice were placed in a supine position on a temperature-controlled platform set to maintain the mouse core temperature at 37–38°C, which was monitored with a rectal thermoprobe.
A 1-cm incision was made on the front of the neck, and the left carotid artery and jugular vein were isolated. The common trunk of the carotid artery was ligated with a suture. A 1.1-Fr Mikro-tip catheter transducer (Millar Instruments, Houston, TX) was inserted, with the help of a 24-gauge needle, into the artery proximal to the ligature and threaded forward into the ascending aorta (43). The jugular vein was ligated, and a PE-10 catheter (Braintree Scientific, Braintree, MA) was inserted caudal to the ligature and threaded down to the superior vena cava. The incision was closed with 5-mm EZ clips (Braintree). Arterial BP was recorded at the sampling rate of 1 kHz using a PowerLab data-acquisition system and LabChart Pro software (ADInstruments, Colorado Springs, CO). Heart rate (HR) was obtained from the arterial BP recording. A programmable syringe pump (Braintree) was used for intravenous (iv) infusion of vasoactive substances at a controlled dose, solution volume, and infusion rate. Injection volumes did not exceed 100 μl/h. At the end of the experiment, the mouse was euthanized with an overdose of isoflurane, followed by cervical dislocation.
Preparation of peripheral arteries for in vivo recording.
Under microscopic observation, a femoral artery was exposed via a cutaneous incision in the midthigh. Over a region ∼2 mm in length of the lower section of the femoral artery, the connective tissue above the artery was lightly dissected, taking care to avoid severing nerves. Methods for exteriorizing the cremaster artery were the same as used by others (9, 11). In brief, the right scrotal skin was opened longitudinally via a vertical midline cutaneous incision to expose the testis and the surrounding cremaster muscle. The cremaster sac was cut open, and the muscle was then spread and anchored with insect pins on a silicon-bottomed, custom-made tissue bath. The testis was retracted into the mouse body. After light dissection of the tissue over ∼2 mm of the main “feed” artery, the animal was moved to the stage of the fluorescence microscope, and superfusion of the artery and muscle was begun. The arrangement of the inlet and outlet for superfusion was made to achieve a laminar flow over the artery at ∼9 ml/min.
Preparation of arteries for ex vivo recording.
For some experiments, femoral or cremaster feed arteries were removed from the animal. These arteries were cleaned and mounted in a pressure myograph placed on the stage of the fluorescence microscope (described below), by methods described in detail (41, 43).
Fluorescence Recording
Microscope.
An upright microscope, with long working distance, high numerical aperture, adequate magnification, and fluorescence illumination was required. We used an Olympus MVX10 MacroView fluorescence microscope (Olympus America, Center Valley, PA), equipped with an objective lens of 0.5 numerical aperture, ×2 magnification, 20-mm working distance, and further variable “zoom” magnification of ×0.63 to ×6.3. Fluorescence illumination was provided by a xenon arc lamp (Lambda LS, Sutter Instrument, Novato, CA). Illumination at 436 ± 10 nm was gated and adjusted in intensity through the use of a programmable shutter (Smart Shutter, Sutter Instrument). In some experiments, this microscope was fitted with an image splitting device (DualView, Photometrics, Tucson, AZ) equipped with a dichroic beam splitter, centered at 505 nm, and two emission filters, 470/30 and 535/30 nm, providing detection of CFP and YFP fluorescence emission, respectively.
Cameras.
For the bulk of the experiments reported here, a sensitive charge-coupled device camera (ORCA ER, Hamamatsu, Bridgewater, NJ), in conjunction with the image splitting device, was used. With a zoom factor of 2.0 and 2 × 2 on-chip binning, this provided “CFP” and “YFP” images at a resolution of 2.0 μm/image pixel, and individual images of 0.672 mm (x) by 1.024 mm (y). The camera was controlled, and images were acquired, using HCImage (Hamamatsu). Image processing was performed with custom software, written using IDL 6.4 (ITT Visual Information Solutions, Boulder, CO). Images, consisting of the CFP channel on their left half, and the YFP channel on their right half, were acquired at 1 frame/s. This system provided the bulk of the experimental data reported here. It is the faster system of the two we used with respect to image acquisition and thus more suitable for dynamic events.
In initial experiments, we used a multispectral imaging camera (Nuance, CRI, or Cambridge Research & Instrumentation, Woburn, MA), that is capable of linear unmixing of images containing multiple kinds of fluorescent molecules. Through the use of an internal array of tunable liquid crystal filters, this camera can provide an “image cube”, consisting of up to 26 image planes, obtained over a selectable spectrum of wavelengths (470–720 nm), of 20 nm in bandwidth, and requiring at least 1.3 s/image cube. When used with a “library” of reference spectra for different fluorescent molecules, including CFP and YFP, the provided software performed linear spectral unmixing to produce separate, spectrally “pure” CFP and YFP images. To produce exMLCK FRET ratio images, these images were then processed with custom software written in IDL.
Reagents and Solutions
Artery superfusing solution is as follows (in mM): 112 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.5 CaCl2, 1.2 MgSO4·7H2O, 1.2 KH2PO4, 11.5 glucose, and 10 HEPES (pH 7.4, equilibrated with gas of 5% O2, 5% CO2, and 90% N2 at 37°C). High (116.9 mM) external K+ ([K+]o) solution was made by replacing NaCl with equimolar KCl of normal superfusion solution. Ca2+-free solution was made by omitting Ca2+ and adding 0.5 mM EGTA. Artery dissection solution for ex vivo experiments is as follows (in mM): 145 NaCl, 4.7 KCl, 1.2 MgSO4·7H2O, 2.0 MOPS, 0.02 EDTA, 1.2 NaH2PO4, 2.0 CaCl2·2H2O, 5.0 glucose, and 2.0 pyruvate, with 1% albumin (pH was adjusted to 7.4 with NaOH).
Reagents and sources were as follows: phenylephrine (PE), prazosin, and α,β-methylene ATP (Sigma-Aldrich, St. Louis, MO); other reagents were reagent grade or the highest grade available. PE was dissolved in deionized water, and prazosin was dissolved in DMSO as stock solutions. Appropriate dilution in experimental superfusing solution was made for local application, whereas sterile saline was used to dissolve PE for systemic iv infusion.
Calibration of exMLCK FRET Ratio
Our goal was to determine, as accurately as possible, [Ca2+]i and fractional MLCK activation in arteries in vivo. Parameters needed are as follows: 1) the minimum (Rmin) and maximum exMLCK FRET ratios (Rmax) that correspond to Ca2+/CaM-free and Ca2+/CaM-saturated exMLCK, respectively; and 2) a “calibration curve”, giving the relationships between free [Ca2+]i and both the exMLCK FRET ratio and the exMLCK kinase activity (i.e., rate of myosin RLC phosphorylation). In fact, the relationships between [Ca2+]i and exMLCK FRET ratio, and between [Ca2+]i and myosin RLC phosphorylation (an indication of exMLCK activity) ratio have both been determined previously in permeabilized human embryonic kidney-293 cells that were stably expressing exMLCK (10). A half-maximum FRET ratio change was achieved at [Ca2+]i of 631 nM (pCa 6.2), similar to that of MLCK kinase activity (pCa 6.4) measured concomitantly. These data were well fitted with a Hill coefficient of 1.2. Similarly, the kinase activity of chicken gizzard MLCK was also half-maximally activated at pCa 6.1, with a Hill coefficient of 0.9 (13). Based on this information, we assume that exMLCK in arteries of living mice is half-maximally activated at pCa 6.1, with a Hill coefficient of 1.0. Accepting this assumption, then Rmax and Rmin must be determined in the arteries we study. We obtained information on Rmax by exposing arteries to depolarizing, Ca2+ entry solutions (i.e., high [K+]o, low [Na+]o), and on Rmin by exposing to Ca2+-free/EGTA solutions, as described in results.
Data Analysis and Statistics
The data are expressed as means ± SE; n denotes the number of animals. Comparisons of data were made using Student's paired or unpaired t-test, as appropriate. Differences were considered significant at P < 0.05.
RESULTS
Ex Vivo Arteries
The goal for the methodology developed here was to estimate [Ca2+]i and fractional MLCK activation in vivo. Nevertheless, it was helpful to begin with isolated arteries. In particular, we used isolated arteries to investigate possible effects of motion (i.e., “motion artifacts”) on exMLCK FRET ratios, and possible effects of blood, which absorbs and scatters light (3). This is important because the amount of blood in an artery in vivo will change with the diameter of the artery. For these experiments, isolated, pressurized arteries were superfused externally with a Ca2+-free solution (except during exposure to high [K+]o) to prevent any Ca2+-activated changes in exMLCK FRET ratio. They were filled internally either with albumin-free dissection solution or heparinized blood. Changes in intralumenal pressure were used to change artery diameter.
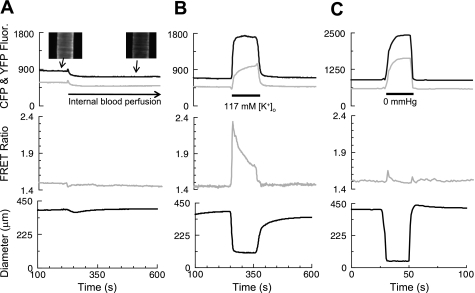
Femoral artery segments were mounted in a pressure myograph at 70 mmHg and subjected to one or more of the protocols illustrated in Fig. 1. Figure 1A shows the effects of blood perfusion on CFP and YFP fluorescence (top), the FRET ratio (middle), and artery diameter (bottom). The insets in Fig. 1A, top, show the CFP fluorescence from an artery when the lumen contained the standard internal perfusion solution (left) and when it contained blood (right). Blood perfusion greatly reduced both CFP and YFP fluorescence, but had no effect on either the FRET ratio or the steady-state artery diameter. The reduced fluorescence, when blood was present in the lumen, can be explained by the increased absorbance and light scattering. Background fluorescence in these images was virtually zero, because there was no fluorescence from surrounding tissues, and the exMLCK FRET ratio was, therefore calculated from the summed fluorescence in an area of interest that contained the full width of the artery at all times. Outer diameter was measured as the average difference between the outer edges of the two walls, which were defined as the positions at which the fluorescence first rose to 67% of the peak value detected in each wall, in each horizontal video line.
Fig. 1.
Artery constriction and internal blood perfusion do not directly affect the biosensor (exogenous myosin light chain kinase, exMLCK) FRET (Forster resonance energy transfer) ratio. All arteries were superfused with a Ca2+-free solution and pressurized internally. Top: solid traces are the cyan fluorescent protein (CFP) fluorescence; shaded traces are the yellow fluorescent protein (YFP) fluorescence corrected for spectral overlap. Middle: exMLCK FRET ratio. Bottom: outer diameter. A: as indicated, the internal perfusion solution was changed from albumin-free dissection solution to heparinzed blood. Insets show that the CFP fluorescence in the artery is visibly decreased by the presence of blood inside, compared with saline. Both CFP and YFP fluorescence decreased substantially. The exMLCK FRET ratio and artery diameter were unaffected. B: same artery, again initially in Ca2+-free solution, then exposed, at the time indicated, to 117 mM external K+ concentration ([K+]o) with 2.5 mM Ca2+ solution. This caused a large contraction and large changes in CFP and YFP fluorescence and the exMLCK FRET ratio. C: effect of changing internal pressure from 120 to 0 mmHg in a different artery. The artery collapsed when internal pressure was dropped to 0 mmHg. This resulted in large changes in CFP and YFP fluorescence and diameter, but no substantial change in the exMLCK FRET ratio.
Superfusion of pressurized, blood-perfused artery segments with the 117 mM [K]o (+Ca2+) depolarizing, Ca2+ entry solution (Fig. 1B) greatly increased the FRET ratio, as well as both the CFP and YFP signals. The high [K+]o solution also induced a large constriction that nearly obliterated the lumen. When intralumenal pressure was reduced from 120 to 0 mmHg (Fig. 1C), a (different) femoral artery collapsed to a similar diameter as with high [K+]o (see Fig. 1B), but, in this case, the FRET ratio did not change. Thus motion and extreme changes in artery diameter do not, per se, affect the FRET ratio. Moreover, these isolated arteries have been “cleaned” and exhibit virtually no “background” fluorescence other than that associated with the artery itself. Thus the changes in the CFP and YFP signals and the FRET ratio in, for example, the high [K+]o depolarizing solution cannot be explained by changes in detection of background tissue fluorescence. The control experiments in Fig. 1 indicate that observed changes in the FRET ratio, even in vivo, are likely to correspond to changes in [Ca2+]i and activation of MLCK. The changes in FRET ratio cannot be explained by changes in light absorbance as a result of changes in blood flow or arterial constriction/dilation.
In Vivo FRET Ratio Methodology
Spectral unmixing.
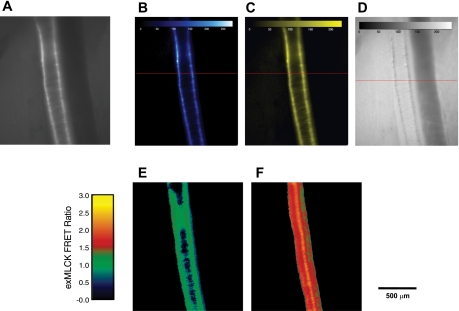
The exMLCK biosensor animals provide an opportunity to measure [Ca2+]i and fractional MLCK activation through the use of a FRET ratio after selective excitation of the CFP moiety of the biosensor (10, 14). Essential, however, is the accurate and precise measurement of the CFP and YFP fluorescence emission, separated from each other and from fluorescence of other molecules in the tissue (namely, the tissue intrinsic fluorescence, or TIF).1 Separation of CFP, YFP, and TIF can be accomplished by linear spectral “unmixing” (7), provided that spectra of the fluorescent molecules are known. Thus we first used the microscope and camera to obtain three reference spectra (see Supplemental Data, Supplemental Fig. S1; The online version of this article contains supplemental data): 1) TIF, from the walls of a wild-type artery in vivo (did not contain exMLCK); 2) CFP, from agarose beads that bound recombinant CFP; and 3) YFP, also from beads. We assume that the reference spectra for CFP and YFP, as obtained from the beads, accurately represents that of the CFP and YFP on the exMLCK biosensor molecule. The spectral imaging camera was then used to obtain 26 separate narrowband (20 nm) images of a biosensor artery, in 10-nm steps, over the wavelength range 470–720 nm. Each narrow band image was acquired in 50 ms, resulting in an “image cube” that was acquired in ∼1.3 s. The image cube was used to create a broad spectrum, “composite” image of the artery (Fig. 2A). The femoral vein lies to the right of, and posterior to, the artery in this image; it is out of the focal plane and is, therefore, barely visible, even though it, too, expresses exMLCK.
Fig. 2.
In vivo images of a segment of femoral artery in an anesthetized exMLCK FRET biosensor mouse, in the basal state. A: broad spectrum (470–720 nm) image obtained with a spectral imaging camera. Excitation light wavelength: 436 ± 10 nm. Prominent fluorescence within the smooth muscle cells of the artery arises from the exogenous CFP/YFP containing MLCK biosensor molecule (exMLCK). Spectrally “pure” images of CFP (B), YFP (C), and tissue intrinsic (D) fluorescence were created by linear spectral unmixing of image (A), using reference spectra for CFP, YFP, and tissue intrinsic fluorescence. D: image has been scaled up by a factor of 10.0 to facilitate visualizing the low levels of tissue intrinsic fluorescence. E: exMLCK FRET ratio image, CFP/YFP (B/C), in basal state. F: exMLCK FRET ratio image of the same artery segment during exposure of the segment to high [K+]o superfusion solution.
Application of the unmixing algorithm and the reference spectra then provided three “unmixed” images: a spectrally pure CFP image (Fig. 2B), a spectrally pure YFP image (Fig. 2C), and a TIF image (Fig. 2D). The intensity of the TIF image is shown multiplied by a factor of 10, compared with the CFP and YFP images, to facilitate visualizing the low levels of TIF. Thus the average TIF within the regions identifiable as walls of the artery is ∼10% of the corresponding average pure CFP fluorescence. The backgrounds of the CFP and YFP images are virtually zero, as a result of the complete removal of TIF from these images. The ratio of these images was then obtained (Fig. 2E), using a spatial mask to set to zero all pixels in the background of the artery. The resulting exMLCK FRET ratio image appeared quite uniform, with an average value of 1.2. This artery was then subjected to local application of a high [K+]o superfusing solution to elevate [Ca2+]i and activate MLCK (this calibration procedure is described in more detail below), and a second image cube was acquired, during a time when the artery was fully constricted, and not changing diameter. Spectral unmixing and “ratioing” produced an exMLCK FRET ratio image with an average value of 1.8 in the artery in response to high [K+]o solution (Fig. 2F).
Image splitting.
A method offering better temporal resolution and relative simplicity is the use of an image splitter and bandpass emission filters to isolate CFP and YFP fluorescence before ratioing, similar to that described in detail for ex vivo biosensor arteries mounted on a wire myograph (27, 38) or urinary bladder strips (5, 14). An important modification of those methods was needed here to study contracting arteries in vivo. We used images to define the artery walls to restrict analysis to regions with the highest signal-to-noise ratio, even during contraction, and to measure artery diameter during contraction. It was also important with this method to use the microscope's field stop at its minimum aperture, to limit interference by scattered light and TIF.
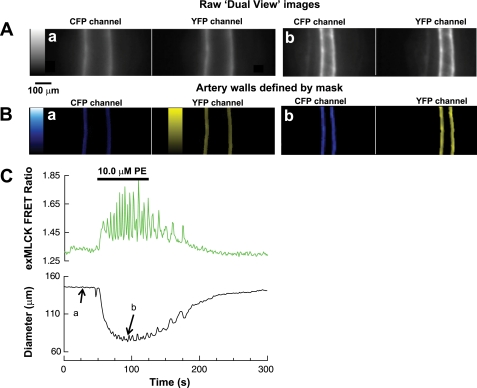
Unprocessed images of a cremaster artery, as obtained directly from the image splitter, are shown in Fig. 3A. The first step in processing was to define the artery walls. Peak fluorescence in the (obvious) region of each wall was found at each video line of each image. Artery walls were then defined arbitrarily, as a region, 10–20 μm wide (depending on the experiment), with the peak at its center (Fig. 3B; see Supplemental Data, Supplemental Fig. S2, which shows the image intensity graph). Areas outside the walls were set to zero. This provided a sharp edge from which artery external diameter was readily obtained and eliminated from consideration any area of the image not containing artery wall, even during motion. Changes in wall thickness during vasoconstriction were neglected. In the example of Fig. 3, when the cremaster muscle feed artery was superfused for 60 s with 10 μM PE, it constricted strongly (Fig. 3, Ab, Bb, and C; see video clips in Supplemental Data, Supplemental Fig. S3). We then summed the fluorescence from both walls to obtain the spatially averaged FRET ratio. The summed fluorescence was corrected for TIF and spectral “bleed through” or overlap. It is necessary with this system to account for TIF (details in Supplemental Data, Supplemental Figs. S4 and S5), since it is not otherwise removed in this method. Spectral bleed through of the CFP fluorescence into the YFP channel was measured at 35% using CFP- and YFP-labeled agarose beads. Bleed through was readily corrected by subtracting 35% of the CFP fluorescence from the YFP fluorescence, before taking the ratio. There was no detectable direct excitation of YFP. Photobleaching of CFP was ∼5% over the 5 min of recording of this experiment, while photobleaching of YFP was not detectable (for details, see Supplemental Data, Supplemental Fig. S6). The exMLCK FRET ratio calculated in this way, and the diameter change of the artery, are shown in Fig. 3C. In association with vasoconstriction, the exMLCK FRET ratio increased and oscillated. This likely corresponds to the agonist-induced Ca2+ oscillations that were observed in isolated arteries ex vivo (42). Analysis of the time derivative of diameter revealed that the peak of each oscillations in FRET ratio was approximately coincident with a peak in the rate of decrease in diameter (Supplemental Data, Supplemental Fig. S7).
Fig. 3.
In vivo imaging of a segment of small cremaster artery in an anesthetized exMLCK FRET biosensor mouse during local application (superfusion) of phenylephrine (PE; 10.0 μM) to the artery. A: unprocessed images from the “dual view” image splitter. a: CFP and YFP channel images at 25 s after beginning of recording. b: Corresponding images at 90 s, at the peak of the contraction elicited by PE. B: colored, processed images illustrating the definition of artery walls from the images shown in A. Gray-scale and color bars indicate fluorescence intensity levels from 0 to 4,095 in all cases. 250 frame movies of the images in A and B are available in the Supplemental Data. C: spatially averaged exMLCK FRET ratio was computed from the summed fluorescence of the artery walls, after correction for spectral overlap and tissue intrinsic fluorescence (see methods for details). PE elicited a large decrease in diameter with some small oscillations. Oscillations in the exMLCK FRET ratio, indicative of Ca2+ oscillations, are evident.
Systemic Application of Vasoactive Substances
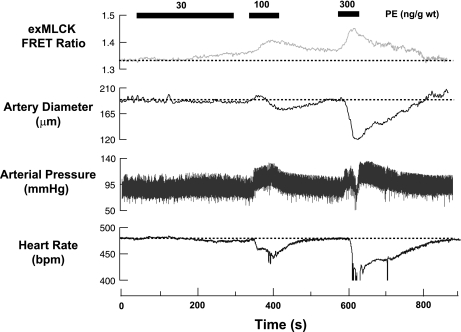
One of our major goals was to determine whether or not changes in [Ca2+]i, MLCK activation, and artery diameter could be measured reliably during small, physiologically relevant changes in BP. To produce such changes, we administered PE intravenously at doses of 30, 100, and 300 ng/g mouse weight over periods of 300, 60, and 45 s, respectively (Fig. 4). At the lowest dose, very small increases in exMLCK FRET ratio began to be evident midway through the infusion. This occurred simultaneously with a small decrease in HR, a very small increase in BP, and a very small decrease in artery diameter. At the two higher doses, simultaneous increases in exMLCK FRET ratio and BP were readily apparent, as were simultaneous decreases in HR and arterial diameter. For example, PE (300 ng/g) elevated mean BP transiently to ∼110 from ∼90 mmHg, decreased HR to 410 from 480 beats/min at the basal level, increased the exMLCK FRET ratio from 1.35 to 1.42, and decreased artery diameter from 189 to 121 μm (∼23% reduction of passive external diameter, 295 μm).
Fig. 4.
In vivo recording of exMLCK FRET ratio, femoral artery external diameter, arterial pressure, and heart rate during systemic administration (intravenous) of PE in an exMLCK biosensor mouse. PE was administered at a constant rate via a syringe pump connected to a catheter in the jugular vein, as indicated. At total doses of 100 and 300 ng/g body wt, PE increased the exMLCK FRET ratio, constricted the femoral artery, increased mean arterial pressure, and decreased the heart rate. bpm, Beats/min.
The bradycardia presumably reflects operation of the baroreceptor reflex in response to the elevated BP, as expected after systemic application of a vasoconstrictor to a normal animal. This reflex involves increased parasympathetic nerve activity to the heart and decreased sympathetic nerve activity to the blood vessels, which tend to decrease HR and increase artery diameter. The observed vasoconstriction likely arose as a net result of direct activation of arterial α1-AR by circulating PE. This would have overwhelmed an expected vasorelaxant effect caused by reflexly decreased activation of α1-AR by neurally released norepinephrine. The results show that changes in exMLCK FRET ratio and artery diameter are readily observable in arteries, even during small changes in BP that result from systemwide changes in artery diameter. In the case of systemic application of vasoactive substances, these changes are clearly complex in origin. They reflect the effects of the exogenous substances, possibly acting at many sites in the animal, as well as changes in other systems that control BP.
Local Application of Vasoactive Substances
The complex effects of systemic drug application make it difficult to sort out the contributions of individual components. We, therefore, sought to determine whether meaningful results could be obtained by application of vasoactive substances directly to the exposed segment of an artery. Such local application has the potential advantage of eliciting effects solely from receptors and cellular mechanisms in the artery, without involving nonvascular receptors or cardiovascular reflexes. For this method to be valid, vasoactive substances in the solution superfusing the artery must 1) reach the artery quickly, 2) achieve a concentration around the artery similar to that in the solution, and 3) not diffuse into the blood in sufficient quantities to have significant systemic effects.
PE.
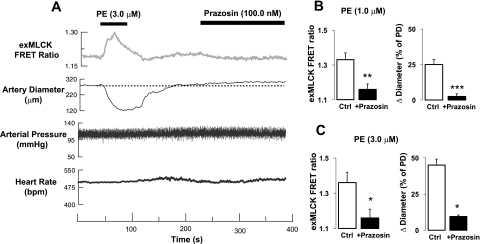
PE, at concentrations of 1.0, 3.0, and 10.0 μM, in the solution superfusing a femoral artery segment, induced vasoconstriction and increases in exMLCK FRET ratio without causing any change in BP or HR (Fig. 5A). Presumably, the superfusion of only a relatively small segment of the artery with PE, for a relatively short period of time, avoided any significant diffusion of PE into the blood. Although full dose-response curves were not obtained, the arteries appeared highly sensitive to PE. For example, 1.0 μM PE increased exMLCK FRET ratio and reduced diameter by 25 ± 3% (n = 8), but did not affect BP or HR. PE (3.0 μM) elicited a larger change in FRET ratio and vasoconstriction, similar to that observed ex vivo in pressurized small arteries (41). Corresponding values for [Ca2+]i and fractional activation of exMLCK are presented below, after methods of calibration have been described.
Fig. 5.
In vivo recording of exMLCK FRET ratio, femoral artery external diameter, arterial pressure, and heart rate during local administration (superfusion) of PE and prazosin in an exMLCK biosensor mouse. A: PE and prazosin were administered locally to an exposed segment of femoral artery. PE increased the exMLCK FRET ratio and decreased diameter, but had no effect on mean arterial pressure or heart rate. Prazosin elicited a small decrease in exMLCK FRET ratio and vasodilation. B: summary of exMLCK FRET ratio (left) and diameter changes (Δ) as a percentage of passive diameter (PD; right) in response to local application of PE (1.0 μM) in the absence (open bars, n = 8) and presence (solid bars, n = 4) of 100 nM prazosin. Ctrl, control PE response. **P < 0.01; ***P < 0.001. C: summary of local application of PE (3.0 μM) on exMLCK FRET ratio (left) and diameter (right) in the absence (n = 6) and presence (n = 3) of 100 nM prazosin. *P < 0.05. Thus locally applied prazosin blocks local α1-adrenoceptors, but does not produce any systemic hemodynamic effects.
Prazosin.
Local application of receptor antagonists should be very useful for delineating the in vivo roles of various vascular receptors (as opposed to nonvascular receptors). Systemic administration of the α1-AR antagonist, prazosin, for example, caused a decrease in mean BP (37), undoubtedly because it acts, not only on vascular α1-ARs, but also on the α1-ARs in the central nervous system, kidney, and adrenals. When applied locally, however, prazosin does not block these nonvascular α1-ARs. This permits observation, exclusively, of local vascular α1-AR effects in the living animal in the context of the basal state function of the cardiovascular system. Indeed, local application of a high concentration of prazosin (100 nM, Fig. 5A) to a femoral artery segment caused a small vasodilatation and a corresponding decrease in exMLCK FRET ratio, without any change in arterial BP or HR. Local prazosin essentially abolished responses to locally applied PE (Fig. 5, B and C).
Calibration of exMLCK
Rationale and procedure.
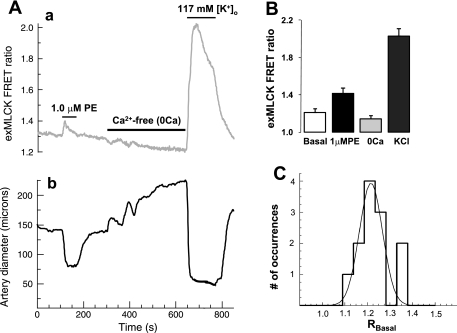
As explained in methods, the information needed to calculate [Ca2+]i and fractional MLCK activity are Rmin and Rmax, which correspond to Ca2+/CaM-free exMLCK and Ca2+/CaM-saturated exMLCK, respectively, and a calibration curve. Ideally, these values would be determined in permeabilized arteries, in which [Ca2+]i is controlled through the use of Ca2+-buffers (+EGTA), but control of [Ca2+]i is not likely to be achieved in arteries of the living animal. Nevertheless, simply exposing arteries in vivo to Ca2+-free solution caused the exMLCK FRET ratio to decrease significantly and the arteries to dilate (Fig. 6A). The response to Ca2+-free solution appeared similar to that of isolated arteries, which reach their “passive diameter”, as [Ca2+]i falls to levels below those at which MLCK is activated (as required for measurement of Rmin). Furthermore, exMLCK FRET ratios and diameter in Ca2+-free solution were not affected further by exposure to caffeine and ryanodine. This procedure, which unloads the sarcoplasmic reticulum, has been reported to permeabilize arteries by locking open Ca2+-permeant, store-operated channels (1).
Fig. 6.
In vivo exMLCK calibration procedure. Segments of femoral artery, in vivo, are exposed locally to superfusing solution containing 1.0 μM PE, then to “Ca2+-free (+0.5 mM EGTA)” (“0Ca”) solution, and, finally, to high [K+]o solution with 2.5 mM Ca2+. A: representative experiments show changes in exMLCK FRET ratio (a) and arterial diameter (b) during standard calibration procedure. Record of (outer) diameter has been retouched slightly to remove an artifact that occurred during exposure to high [K+]o (+Ca2+). PE elicited a transient increase in exMLCK FRET ratio and constricted the artery. Ca2+-free solution reduced the exMLCK FRET ratio and induced a slow vasodilatation, abolishing the basal vascular tone. High [K+]o (+Ca2+) caused a large increase in the exMLCK FRET ratio and a maximal vasoconstriction. B: summary of exMLCK FRET ratios (n = 5 arteries), as measured from records such as in A. C: histogram of RBasal (basal state exMLCK FRET ratio) values measured from 12 arteries. The smooth curve is a three-parameter Gaussian distribution fit to the data (correlation coefficient, R2 = 0.8223).
To estimate Rmax in vivo, we exposed arteries locally to a solution high in [K+]o (117 mM), to optimize Ca2+ entry through L-type voltage-gated Ca2+ channels, and low in [Na+]o, to minimize Ca2+ extrusion via Na+/Ca2+ exchange. Figure 6A shows that this caused maximal vasoconstriction (i.e., near closure of the artery lumen) and a very large increase in exMLCK FRET ratio (compared with that induced by agonists). In a few cases, a P2X receptor agonist (α,β-methylene ATP) (25) was added to increase the Ca2+ permeability further, but this had no further effect on the exMLCK FRET ratios. As was the case with local application of receptor agonists and antagonists (Fig. 5), local application of the calibrating solutions had no effect on BP or HR (not shown). Average values of FRET ratios in five femoral arteries in the basal state, in 1.0 μM PE, in the “Ca2+-free” solution, and in the high [K+]o solution are shown in Fig. 6B. These arteries were constricted to ∼65% of their passive diameter in the basal state in vivo, indicating a significant vascular “tone” of unknown origin. In contrast, femoral arteries pressurized ex vivo had no pressure-induced (myogenic) tone (data not shown), as expected for a large artery. The distribution of basal state FRET ratio (RBasal) was approximately “Gaussian” (Fig. 6C) with a mean value of 1.21 and SE of 0.04 (n = 12).
Determination of [Ca2+]i and fractional activation of MLCK.
The Ca2+-free and high [K+]o (+Ca2+) calibrating solutions appeared to change [Ca2+]i to very low levels and to very high levels, respectively, as judged by artery diameter, but the [Ca2+]i values that were actually achieved are not known. In particular, the true exMLCK Rmin is likely to be somewhat lower than the value measured in Ca2+-free solution, “R0Ca”, because cytosolic Ca2+ is bound to proteins and buffered and is, therefore, difficult to remove completely.
The absolute FRET ratios in both calibrating solutions varied slightly from animal to animal (for unknown reasons), while the difference between Rmax and Rmin varied very little (0.954 ± 0.091). Therefore, we used a normalization procedure to calculate RBasal (RBasal,Norm) and [Ca2+]i. Accordingly, RBasal,Norm = (RBasal − Rmin)/(Rmax − Rmin), where RBasal is the observed basal FRET ratio and RBasal,Norm is the normalized ratio.
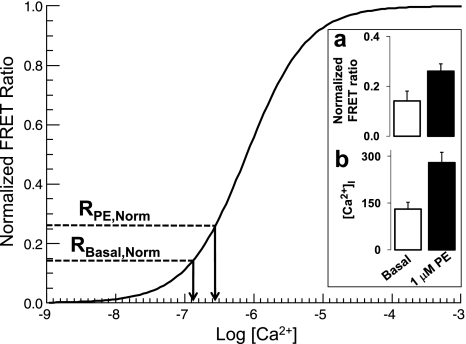
The calibration curve in Fig. 7 is based on the assumption that [Ca2+]i was 63 nM (i.e., pCa = 7.2) in the Ca2+-free solution and 32 μM (i.e., pCa = 4.5) in the high [K+]o solution. If we assumed that arterial [Ca2+]i in the “Ca2+-free” solution actually fell below 10 nM, then Rmin would have been 1.14 (i.e., equal to R0Ca) and the basal arterial [Ca2+]i would have been 68 nM; this seems improbably low for an artery with substantial tone. If we assumed, however, that the arterial [Ca2+]i reached in the Ca2+-free solution was actually 63 nM (pCa = 7.2), then Rmin would have been 1.07 and the basal arterial [Ca2+]i would have been 131 nM. For these two cases, fractional activation of exMLCK was 0.08 and 0.14, respectively. In contrast, uncertainty in the Rmax value had negligible effect on the calculated [Ca2+]i.
Fig. 7.
Estimation of intracellular Ca2+ concentration ([Ca2+]i) and fractional activation of MLCK in arteries in vivo. Smooth curve is a graph of the Hill equation with EC50 at pCa 6.1 and Hill coefficient (n) of 1.0. Curve was calculated on the assumption that arterial [Ca2+]i achieved during exposure to Ca2+-free/0.5 mM EGTA solution was 63 nM (pCa 7.2), as described in the text. Inset: normalized exMLCK FRET ratio (a) and calculated [Ca2+]i (b) under “basal” physiological conditions (open bars) and in response to locally applied 1.0 μM PE (solid bars). Normalized basal exMLCK FRET ratio, RBasal,Norm = (RBasal − Rmin)/(Rmax − Rmin). For RBasal, n = 12. Rmin and Rmax, minimum and maximum exMLCK FRET ratios that correspond to Ca2+/calmodulin-free (Ca2+-free/EGTA solution) and Ca2+/calmodulin-saturated (high [K+]o solution) exMLCK, respectively. RPE,Norm (normalized PE exMLCK FRET ratio) was calculated similarly (n = 8).
Based on this method, local application of 1.0 μM PE to eight femoral artery segments elevated estimated [Ca2+]i from 131 to 279 nM, and fractional MLCK activation from 0.14 to 0.26 (see Fig. 7, inset). During iv administration of PE (30 ng/g wt) (Fig. 4), which marginally increased BP and HR, estimated [Ca2+]i rose from 106 to 133 nM, and fractional MLCK activation increased from 0.12 to 0.14 (calculated as described above). At 100 ng PE/g wt, [Ca2+]i and fractional MLCK activation increased to 179 nM and 0.18, respectively; 300 ng PE/g wt, which raised mean BP by 20 mmHg, increased peak [Ca2+]i to 244 nM and increased fractional activation of exMLCK to 0.24.
DISCUSSION
Numerous factors contribute to the control of vascular tone and BP in vivo, and many of these are lost when arteries are isolated and studied ex vivo. Moreover, study of arterial function by systemic application of drugs does not permit separation of the local regulation from reflex and endocrine control. Many investigators have explored the effects of local application of vasoactive agents to arteries in vivo, but most such studies have been limited to measurements of artery diameter (6, 19, 30). Just a few laboratories have reported on [Ca2+]i measurements in arteries. Nonratiometric dyes were used to monitor uncalibrated [Ca2+]i changes in endothelial cells in vivo (8, 32); the latter study employed the genetically encoded, but nonratiometric, fluorochrome, GCaMP-2. On the other hand, the ratiometric dye, fura PE-3 was used to estimate [Ca2+]i in myocytes and endothelial cells (4). Especially in intact arteries, however, calibration of Ca2+-sensitive fluorochromes, such as fura 2 (18) and fura PE-3 (4), is severely limited by problems such as dye sequestration in organelles and dye leakage (28).
To circumvent some of the aforementioned problems, we have presented methods for quantitative determination of [Ca2+]i and fractional MLCK activation in segments of exMLCK biosensor arteries in vivo. We used transgenic mice that express a genetically encoded, “ratiometric” sensor molecule (10), specifically in smooth muscle cytosol (14, 38).
The spectral unmixing method has the potential to produce the most accurate measurements of FRET ratio, but suffers from relatively poor temporal resolution, due to the requirement of obtaining a large image cube at each point in time. The image-splitting method offers better temporal resolution, but the separation of CFP and YFP fluorescence from TIF is less certain. Nevertheless, the two methods produced very similar estimates of the exMLCK FRET ratio in arteries in the basal state and in a highly activated state (high [K+]o): ∼1.2 in the basal state, and 1.8–2.0 when depolarized with high [K+]o. This probably reflects the fact that the major source of uncertainty in the image-splitting method, the TIF, is relatively small compared with the CFP and YFP fluorescence.
Quantification of [Ca2+]i
Several other considerations also apply to our estimates of [Ca2+]i and fractional activation of exMLCK using in vivo calibration (Fig. 6). If the [Ca2+]i achieved during exposure to high [K+]o is not sufficient to saturate exMLCK, then we have overestimated basal [Ca2+]i. Unfortunately, reliable estimates of [Ca2+]i during exposure to high [K+]o in artery smooth muscle are not yet available, since the affinity of the most commonly used Ca2+ indicator, fura 2, is too high. In skeletal muscle, however, the use of low-affinity Ca2+ indicators, such as furaptra, has resulted in a recent estimate of spatial average [Ca2+]i of ∼18 μM (12) during a single action potential; although not yet measured, the [Ca2+]i is certainly much higher during KCl contracture. In smooth muscle, given an adequate amount of calmodulin, such a [Ca2+]i would saturate exMLCK with Ca2+/CaM, as we have assumed. If the concentration of calmodulin is “limiting” (33), however, then our estimate of Rmax is too low, and we will have overestimated both the [Ca2+]i and fractional activation of exMLCK. In either case, the effects on basal [Ca2+]i are likely to be small because these values lie near the foot of the calibration curve (Fig. 7).
Remarkably, the range of basal [Ca2+]i we estimate in vivo (68–131 nM) is nearly identical to that measured previously in ex vivo rat mesenteric arteries (wire myograph) with Ca2+-selective microelectrodes (115 nM) and fura 2 (129 nM) (16). [Ca2+]i in pressurized rat cerebral arteries increased from 119 to 245 nM, as pressure was increased from 10 to 100 mmHg (18). [Ca2+]i was reported to be ∼55 nM (23) in rat cremaster arterioles, ∼185 nM (at 70 mmHg) in mouse mesenteric small arteries (43), and ∼170 nM in hamster cremaster arterioles (40). The single previous in vivo measurement (3) produced a higher value (406 nM) than all of these, a difference that may be attributable to the high level of vasomotor tone in the hamster cheek pouch arterioles (3) and/or to problems of calibration (28).
The image-splitter method provides spatial average values of the measured quantities at a temporal resolution of 1 s−1. In this form, the method does not reveal inhomogeneous Ca2+ signaling in arteries, either within individual cells or among different cells. Nevertheless, the method should be particularly well suited for determining the involvement of [Ca2+]i and MLCK activation in (mouse) models of cardiovascular disease. Because the method utilizes a “ratio”, the variability of the measurements is relatively small (Fig. 6). It is likely, therefore, that statistical significance can be achieved in measurements made in different groups of animals so that arterial mechanisms involved in experimentally acquired or genetic hypertension may be studied. Thus we ask: What is the minimum increase in [Ca2+]i that could be detected between two groups of 12 animals each (as in Fig. 6), if the variance (σ2) in each group was the same as that obtained here? The basal FRET ratios are normally distributed (Fig. 6C), and, therefore, standard “parametric” statistical tests, such as Student's t-test, may be used. For the present experiments, RBasal,Norm is 0.14, with a standard deviation (σ) of 0.04, requiring P < 0.05, then the minimum larger mean value of RBasal,Norm that could be detected would be 0.168. Calculating arterial [Ca2+]i with this method indicates that an increase of just 29 nM Ca2+ could be detected; i.e., a rise from 131 to 160 nM (P < 0.05).2
The exMLCK biosensor gene can be put into any desired genetic background through backcrossing, as we have done here. The method appears capable of measuring, reliably, the changes in [Ca2+]i that underlie even small changes in arterial pressure, such as occur with many models of hypertension. Finally, it has often been postulated that drug or hormone effectiveness may be increased or decreased under pathological conditions in vivo as that ex vivo. The method offers the possibility to test this in vivo by combining both systemic and local applications (Figs. 4 and 5).
In Vivo Measurement
Although exMLCK biosensor arteries have been studied previously on a wire myograph (27, 38), there have been no studies previously of pressurized biosensor arteries, either ex vivo or in vivo. The clear advantage of making these measurements in vivo is that the arteries are in a state closer to physiological than can be reproduced in experiments outside the animal (ex vivo), even in the pressurized state. The measurements can be made either on conduit arteries (femoral) or resistance arteries (cremaster). Thus this method presents new opportunities for examining the mechanisms and effects on arteries of tonic nervous system activity, blood flow (as it affects endothelium), pulsatile BP, and circulating and locally produced endogenous factors in the blood. When combined with the measurement of BP and other cardiovascular parameters, such as cardiac output and regional blood flow, the method should provide new information on mechanisms controlling arterial BP. Nevertheless, the method requires anesthetization, which can have several consequences for cardiovascular function, including reduction of sympathetic nerve activity (29) and increased activity of the renin-angiotensin system (34). We believe that the use of an appropriate concentration (1.5%) of isofluorane for anesthesia (29) is important in this regard. It is easier to produce a constant effect, compared with injectable anesthetics, which must be adjusted periodically throughout the experiment and may result in more substantial depression of cardiovascular function.
Summary
The success of the exMLCK biosensor molecule is especially notable with respect to providing an adequate signal-to-noise ratio and high dynamic range without detectable perturbation of function (27). This indicates, clearly, the potential for creating other FRET-type ratiometric biosensor animals (35), particularly those of the intramolecular type (as is exMLCK). FRET-based biosensors of RhoA activation (26) are particularly appealing, as these would provide a view into regulation of MLCP. Together with exMLCK, this would enable optical measurement of the activation of the two major enzymes that control cross-bridge cycling by regulating phosphorylation and dephosphorylation of myosin RLCs. Finally, the methods devised here can certainly be extended to spatially resolved imaging techniques, such as two-photon and confocal microscopy. Such applications will, however, have to deal with the motion of arteries that occurs during many physiological situations of interest.
GRANTS
This work was supported by a National Scientist Development Grant from the American Heart Association (to J. Zhang) and an International Society of Hypertension-Pfizer Award (to J. Zhang). This work was also supported by National Heart, Lung, and Blood Institute Grant P01-HL-78870, Projects 1 (to M. P. Blaustein) and 3 (to W. G. Wier), and Core C (to W. G. Wier). The work was also supported by R01-HL-091969 (to W. G. Wier) and R01-HL-045215 (to M. P. Blaustein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. James Stull (University of Texas Southwestern Medical Center, Dallas, TX) for generously providing exMLCK mice to start our colony, and Meng Li for transgenic mouse genotyping and breeding.
Footnotes
We prefer the term “tissue intrinsic fluorescence” to the more commonly used term “autofluorescence.” That term is typically used to refer to fluorescence arising from all molecules other than the one(s) of interest. The fluorescence emission from these molecules does not arise automatically, however, and, therefore, the term is a misnomer.
For comparison, if the data are not normalized, the minimum increase in RBasal that could be detected with a P < 0.05 (n = 12) is from 1.07 to 1.26, which corresponds to a rise in basal [Ca2+]i from 131 to 199 nM, an increase of 68 nM.
REFERENCES
- 1.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 291: L208–L221, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53: 291–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brekke JF, Jackson WF, Segal SS. Arteriolar smooth muscle Ca2+ dynamics during blood flow control in hamster cheek pouch. J Appl Physiol 101: 307–315, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Rivers RJ. Measurement of membrane potential and intracellular Ca(2+) of arteriolar endothelium and smooth muscle in vivo. Microvasc Res 62: 55–62, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Ding HL, Ryder JW, Stull JT, Kamm KE. Signaling processes for initiating smooth muscle contraction upon neural stimulation. J Biol Chem 284: 15541–15548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dora KA, Damon DN, Duling BR. Microvascular dilation in response to occlusion: a coordinating role for conducted vasomotor responses. Am J Physiol Heart Circ Physiol 279: H279–H284, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ducros M, Moreaux L, Bradley J, Tiret P, Griesbeck O, Charpak S. Spectral unmixing: analysis of performance in the olfactory bulb in vivo. PLoS One 4: e4418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duza T, Sarelius IH. Localized transient increases in endothelial cell Ca2+ in arterioles in situ: implications for coordination of vascular function. Am J Physiol Heart Circ Physiol 286: H2322–H2331, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Frame MD, Sarelius IH. Arteriolar bifurcation angles vary with position and when flow is changed. Microvasc Res 46: 190–205, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Geguchadze R, Zhi G, Lau KS, Isotani E, Persechini A, Kamm KE, Stull JT. Quantitative measurements of Ca(2+)/calmodulin binding and activation of myosin light chain kinase in cells. FEBS Lett 557: 121–124, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Hill MA, Meininger GA. Calcium entry and myogenic phenomena in skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 267: H1085–H1092, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol 586: 5063–5075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong F, Haldeman BD, John OA, Brewer PD, Wu YY, Ni S, Wilson DP, Walsh MP, Baker JE, Cremo CR. Characterization of tightly associated smooth muscle myosin-myosin light-chain kinase-calmodulin complexes. J Mol Biol 390: 879–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci U S A 101: 6279–6284, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jensen PE, Mulvany MJ, Aalkjaer C, Nilsson H, Yamaguchi H. Free cytosolic Ca2+ measured with Ca2+-selective electrodes and fura 2 in rat mesenteric resistance arteries. Am J Physiol Heart Circ Physiol 265: H741–H746, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koeppen M, Feil R, Siegl D, Feil S, Hofmann F, Pohl U, de Wit C. cGMP-dependent protein kinase mediates NO- but not acetylcholine-induced dilations in resistance vessels in vivo. Hypertension 44: 952–955, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kotlikoff MI. Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology. J Physiol 578: 55–67, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maguire JJ, Davenport AP. Regulation of vascular reactivity by established and emerging GPCRs. Trends Pharmacol Sci 26: 448–454, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24: 45–57, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol Heart Circ Physiol 261: H950–H959, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Mulvany MJ. Small artery remodelling in hypertension: causes, consequences and therapeutic implications. Med Biol Eng Comput 46: 461–467, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Pakdeechote P, Rummery NM, Ralevic V, Dunn WR. Raised tone reveals purinergic-mediated responses to sympathetic nerve stimulation in the rat perfused mesenteric vascular bed. Eur J Pharmacol 563: 180–186, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Pertz O, Hahn KM. Designing biosensors for Rho family proteins–deciphering the dynamics of Rho family GTPase activation in living cells. J Cell Sci 117: 1313–1318, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Raina H, Zacharia J, Li M, Wier WG. Activation by Ca2+/calmodulin of an exogenous myosin light chain kinase in mouse arteries. J Physiol 587: 2599–2612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rembold CM, Chen XL. The buffer barrier hypothesis, [Ca2+]i homogeneity, and sarcoplasmic reticulum function in swine carotid artery. J Physiol 513: 477–492, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seagard JL, Hopp FA, Bosnjak ZJ, Osborn JL, Kampine J. Sympathetic efferent nerve activity in conscious and isoflurane-anesthetized dogs. Anesthesiology 61: 266–270, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol Heart Circ Physiol 256: H832–H837, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Tran QK, Black DJ, Persechini A. Intracellular coupling via limiting calmodulin. J Biol Chem 278: 24247–24250, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Ullman J, Härgestam R, Lindahl S, Chan SH, Eriksson S, Rundgren M. Circulatory effects of angiotensin II during anaesthesia, evaluated by real-time spectral analysis. Acta Anaesthesiol Scand 47: 532–540, 2003 [DOI] [PubMed] [Google Scholar]
- 35.VanEngelenburg SB, Palmer AE. Fluorescent biosensors of protein function. Curr Opin Chem Biol 12: 60–65, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Walsh MP, Susnjar M, Deng J, Sutherland C, Kiss E, Wilson DP. Phosphorylation of the protein phosphatase type 1 inhibitor protein CPI-17 by protein kinase C. Methods Mol Biol 365: 209–223, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Wang EQ, Fung HL. Prazosin potentiates the acute hypotensive effects of nitroglycerin but does not attenuate nitrate tolerance in normal conscious rats. J Cardiovasc Pharmacol 43: 341–346, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Wier WG, Rizzo MA, Raina H, Zacharia J. A technique for simultaneous measurement of Ca2+, FRET fluorescence and force in intact mouse small arteries. J Physiol 586: 2437–2443, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind JS, Offermanns S. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Yashiro Y, Duling BR. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ Res 87: 1048–1054, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Zacharia J, Zhang J, Wier WG. Ca2+ signaling in mouse mesenteric small arteries: myogenic tone and adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol 292: H1523–H1532, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Zang W, Balke CW, Wier WG. Graded alpha1-adrenoceptor activation of arteries involves recruitment of smooth muscle cells to produce “all or none” Ca(2+) signals. Cell Calcium 29: 327–334, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Lee M, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.