Abstract
The novel peptide, angiotensin (ANG)-(1–12), elicits a systemic pressor response and vasoconstriction. These effects are blocked by ANG converting enzyme (ACE) inhibitors or AT1 receptor antagonists, suggesting a role as an ANG II precursor. However, ANG-(1–12) can serve as a substrate for either ANG II or ANG-(1–7) formation, depending on the local tissue enzymes. Although levels of ANG-(1–12) are higher than ANG I or ANG II in brain, the role and processing of this peptide for autonomic control of heart rate (HR) has yet to be considered. Thus we examined the effects of nucleus tractus solitarii (NTS) microinjection of ANG-(1–12) on baroreflex sensitivity for control of HR, resting arterial pressure (AP) and HR, and indexes of sympathovagal balance in urethane/chloralose anesthetized Sprague-Dawley rats. NTS injection of ANG-(1–12) (144 fmol/120 nl) significantly impaired the evoked baroreflex sensitivity to increases in AP [n = 7; 1.06 ± 0.06 baseline vs. 0.44 ± 0.07 ms/mmHg after ANG-(1–12)], reduced the vagal component of spontaneous baroreflex sensitivity and HR variability, and elicited a transient depressor response (P < 0.05). NTS pretreatment with an AT1 receptor antagonist or ACE inhibitor prevented ANG-(1–12)-mediated autonomic and depressor responses. ANG-(1–12) immunostaining was observed in cells within the NTS of Sprague-Dawley rats, providing a potential intracellular source for the peptide. However, acute NTS injection of an ANG-(1–12) antibody did not alter resting baroreflex sensitivity, AP, or HR in these animals. Collectively, these findings suggest that exogenous ANG-(1–12) is processed to ANG II for cardiovascular actions at AT1 receptors within the NTS. The lack of acute endogenous ANG-(1–12) tone for cardiovascular regulation in Sprague-Dawley rats contrasts with chronic immunoneutralization in hypertensive rats, suggesting that ANG-(1–12) may be activated only under hypertensive conditions.
Keywords: baroreflex, autonomic
the renin-angiotensin (ANG) system is a series of enzyme-substrate interactions that generates biologically active peptides involved in regulation of the cardiovascular system (27). In the classical renin-ANG system, the precursor angiotensinogen is cleaved by renin into the decapeptide ANG I. ANG I is subsequently cleaved by ANG converting enzyme (ACE) to form ANG II, the primary effector peptide of the renin-angiotensin system, which acts at AT1 receptors to induce sympathetic activation, vasoconstriction, and suppression of baroreflex function (27). New pathways for the production of biologically active peptides have been revealed in the past few decades, including the formation of ANG-(1–7) through cleavage of ANG I or ANG II by neprilysin or ACE2, respectively (39). The vasodilation and facilitation of baroreflex function produced by ANG-(1–7) oppose actions of ANG II (39), suggesting that a balance of these two peptides is important in cardiovascular physiology and pathophysiology.
The complexity of the renin-ANG system is further increased by the recent discovery of ANG-(1–12), a C-terminally extended peptide longer than ANG I (30). ANG-(1–12) is present in plasma and peripheral tissues and elicits a systemic dose-dependent pressor response and vasoconstriction of isolated aortas in Wistar rats. These effects are abrogated by ACE inhibition or ANG II AT1 receptor blockade, suggesting that ANG-(1–12) is processed to ANG II for peripheral cardiovascular actions (30). Similar to findings in the circulation, cardiac perfusion of ANG-(1–12) increases ANG II levels and produces AT1-mediated coronary artery vasoconstriction, providing evidence for primary processing of ANG-(1–12) to ANG II in cardiac tissue (35). However, exogenous ANG-(1–12) is metabolized to both ANG II and ANG-(1–7) independent of renin in isolated perfused hearts from normotensive and hypertensive rats (40). Furthermore, a preliminary report suggests that ANG-(1–12) is processed to ANG-(1–7) by neprilysin in rat kidneys (8), suggesting potential tissue-specific processing of ANG-(1–12) into either ANG II or ANG-(1–7) depending on the local enzyme milieu.
Levels of ANG-(1–12) are reported to be higher than ANG I or ANG II in brain tissue, where a local renin-ANG system exists (30). Central immunoneutralization of ANG-(1–12) lowers arterial pressure (AP) and improves baroreflex sensitivity and heart rate (HR) variability in hypertensive (mRen2)27 rats, suggesting a role for endogenous ANG-(1–12) as an ANG II precursor in brain pathways regulating blood pressure and autonomic function in hypertension (21, 22). However, the sites for ANG-(1–12) actions or processing pathways in the brain have not been elucidated. The nucleus tractus solitarii (NTS), a key brainstem region that mediates baroreflex function (2), is a known site of action for modulation of autonomic balance by ANG peptides (14). Given that enzymatic activities for ACE, ACE2, and neprilysin are all detected in dorsal medulla of Sprague-Dawley rats (12), ANG-(1–12) could be processed to either ANG II or ANG-(1–7) within the NTS for cardiovascular actions. Thus we delineated the local processing and effects of exogenous ANG-(1–12) within this nucleus on baroreflex sensitivity for control of HR, resting AP and HR, and other markers of sympathovagal balance in normotensive Sprague-Dawley rats. We further assessed the expression of ANG-(1–12) within the NTS and determined the role of endogenous ANG-(1–12) to resting cardiovascular and baroreflex function in these animals.
METHODS
The Institutional Animal Care and Use Committee approved the following procedures. All methods have been recently published by our laboratory in detail (3, 4, 37).
Animals.
Experiments were performed in 3- to 5-mo-old male Hannover Sprague-Dawley rats from the Hypertension and Vascular Research Center Colony, Wake Forest University School of Medicine (Winston-Salem, NC). Animals were housed in humidity- and temperature-controlled rooms in group cages (12-h:12-h light-dark cycle) with free access to standard rat chow and water. A total of 35 animals were used, with the numbers for each individual experiment listed below.
Surgical procedures.
Rats were anesthetized with combination urethane-chloralose (750 and 35 mg/kg, respectively) via intraperitoneal injections with supplemental intravenous doses as needed. Rats were instrumented with femoral artery and vein catheters [polyethylene (PE)-50 tubing] and placed in a stereotaxic frame with the head tilted downward (45°) for surgical exposure of the dorsal medulla oblongata. A 30-min stabilization period was allowed before baseline measurements were recorded.
Measurement of resting AP and HR.
Pulsatile AP and electronically derived mean AP were monitored by a strain gauge transducer connected to the femoral artery catheter, recorded, and digitized using a Data Acquisition System (Acknowledge software Version 3.8.1; Biopac System, Santa Barbara, CA), and HR was determined from the AP wave.
Reflex testing.
The baroreflex sensitivity for AP-induced slowing of HR was established by bolus intravenous administration of randomized, graded doses of phenylephrine (2, 5, and 10 μg/kg in 0.9% NaCl) with 5-min periods allowed between doses. Maximum mean AP responses (ΔMAP; mmHg) and associated reflex changes in HR (ΔHR; beats/min) were recorded for each dose of phenylephrine, and ΔHR was converted to pulse interval changes (ΔPI; ms) by the formula: 60,000/HR. The slope of the line fit through the ΔMAP and corresponding ΔPI was used as an index of baroreflex sensitivity for control of HR as previously reported (3, 4, 37). Since ANG peptides do not alter the baroreflex sensitivity for tachycardia to decreases in AP induced by sodium nitroprusside, we performed bolus testing, which is more sensitive to parasympathetic alterations relative to infusion testing (25). Depressor and bradycardic responses to cardiac vagal chemosensitive fiber activation were established by bolus intravenous administration of phenylbiguanide (10 μg/kg in 0.9% NaCl). All reflex testing was completed within 20 min.
NTS microinjections.
At least 30 min was allowed after baseline measurements. Multibarreled glass micropipettes were used to perform bilateral microinjection of drugs into the NTS [0.4 mm rostral, 0.4 mm lateral to the calamus scriptorius (caudal tip of the area postrema), and 0.4 mm below the dorsal surface]. Peak changes in AP and HR were measured, and baroreflex sensitivity and responses to cardiac vagal chemosensitive fiber activation were retested at 10 min after the injection with each animal used as its own control. After experiments, the medulla was removed, frozen, and cryostat sectioned (30 μm) to assess microinjection sites. As recently illustrated (4), data were only included in this study if the pipette tip was localized within the medial NTS at rostrocaudal level −13.3 to −14.0 mm caudal to bregma; the success rate was >90%.
Treatments.
Drugs were dissolved in artificial cerebrospinal fluid (pH 7.4) at doses similar to those found functionally effective in previous microinjection studies (3, 4, 6, 13, 37). We have previously shown that injection of artificial cerebrospinal fluid has no effect on resting AP, HR, baroreflex sensitivity, cardiac vagal chemosensitive fiber activation responses, or indexes of sympathovagal balance in young Sprague-Dawley rats (4, 37). ANG-(1–12) (Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Leu11-Tyr12; Peptide Institute, Osaka, Japan) was administered at three doses (36, 72, or 144 fmol/120 nl; n = 4, 4, and 7); each dose was tested in separate animals. In a subset of animals receiving the 144 fmol dose of ANG-(1–12) (n = 4), the baroreflex sensitivity was assessed at 10, 60, and 120 min after the initial injection to establish a time course for the actions of ANG-(1–12) on baroreflex function. In separate experiments, the AT1 receptor antagonist candesartan (n = 4; CV-11974; 24 pmol/120 nl) or ACE inhibitor bradykinin-potentiating nonapeptide 9-α (n = 4; BPP9α; Bachem; 0.9 nmol/60 nl) was injected into the NTS before subsequent injection of 144 fmol ANG-(1–12). The baroreflex sensitivity was assessed at 10 min after candesartan injection and at 10 and 60 min after BPP9α injection followed immediately by ANG-(1–12) administration and subsequent reflex testing. These protocols were based on previously established time courses of action for candesartan and BPP9α on baroreflex sensitivity (3, 20, 37). An antibody to ANG-(1–12) [n = 5; anti-ANG-(1–12) IgG; AnaSpec; 0.4 μg/120 nl] or control preimmune IgG (n = 4) was injected into the NTS of separate Sprague-Dawley rats to assess the endogenous ANG-(1–12) tone for resting baroreflex regulation. The method for production of the polyclonal ANG-(1–12) antibody has been previously reported (22). In addition, this antibody has been characterized in heart and kidney tissue and shown to be specific to ANG-(1–12) with minimal cross reactivity (<0.01%) for ANG I, ANG II, or ANG-(1–7) (24).
Indexes of sympathovagal balance.
A minimum of 5 min of AP and HR recordings was obtained during baseline and after NTS injections for post-hoc spectral analysis of markers of sympathovagal balance (Nevrokard SA-BRS) (4, 38). Power spectral densities of systolic AP and beat-to-beat interval (RRI) oscillations were computed, transformed, and integrated over specified frequency ranges [low frequency (LF) = 0.25–0.75 Hz; high frequency (HF) = 0.75–3.0 Hz]. The square root of the ratio of RRI and systolic AP powers were used to calculate the HFα and LFα markers of the spontaneous baroreflex sensitivity. In rats, HFα is abolished by atropine and is considered to be a marker of vagal activity of the spontaneous baroreflex sensitivity. Although LFα is a marker of primarily sympathetic activity of the spontaneous baroreflex sensitivity, it is partially controlled by vagal tone (1, 23). The power of RRI spectra in LF and HF ranges was normalized, and the ratio of LFRRI to HFRRI was used as an index of cardiac sympathovagal balance, following a precedent of previously published reports (1, 32). HR variability was measured by time domain analysis as the root mean square of successive differences. The LF component of systolic AP variability (LFSAP) expressed in normalized units was used as a marker of sympathetic tone. LFSAP is abolished after sympathetic blockade in humans and rodents and tracks closely with changes in directly measured peripheral nerve activity in humans (7, 23, 32).
Immunolocalization of ANG-(1–12) in the NTS.
Sprague-Dawley rats (n = 3) were anesthetized with isoflurane and sequentially perfused via the left ventricle with sodium phosphate buffer (pH 7.4) containing 4% paraformaldehyde as previously described (18). Serial cryostat sections (30 μm) were obtained from frozen brains and stored as floating sections. Floating sections were rinsed, blocked in 5% donkey serum, and incubated with the rabbit anti-ANG-(1–12) primary antibody (1:100) overnight at 4°C, followed by 1 h incubation with affinity purified donkey anti-rabbit IgG secondary antibody (1:150; Jackson Immunoresearch Laboratories) conjugated to horseradish peroxidase. Color was developed with 3,3′-diaminobenzidine HCl/H2O2 (Thermo Scientific). Sections were examined using a Leica DM microscope (Leica Microsystems) and photographed with a QImaging Retiga 1300R Camera and the Simple PCI version 6.0 computer-assisted imaging software (Hamamatsu). For immunofluorescent localization, floating brain sections (30 μm) were incubated overnight with anti-ANG-(1–12) (1:100), rinsed and subsequently incubated with a FITC-coupled goat anti-rabbit antibody (1:200), and visualized by fluorescence using the Leica DM microscope. Control sections were treated in the absence of the primary antibody or with preincubation of the primary antibody with the ANG-(1–12) peptide (40 μM) to which the antibodies were directed.
Analysis of data.
Values are presented as means ± SE. ANG-(1–12) dose-response experiments were analyzed by one-way ANOVA with post-hoc Student Newman-Keuls multiple comparisons for the different groups of animals. Changes in the evoked baroreflex sensitivity over time, resting AP and HR over time, and NTS pretreatment experiments were analyzed using repeated-measures ANOVA with post-hoc comparisons by Student-Newman-Keuls. Changes in indexes of sympathovagal balance in response to ANG-(1–12) were compared with baseline using an unpaired t-test. The criterion for statistical significance was P < 0.05, and tests were performed using Prism 4.0 and InStat3 (GraphPad, San Diego, CA).
RESULTS
Resting mean AP and HR in response to NTS microinjection of ANG-(1–12).
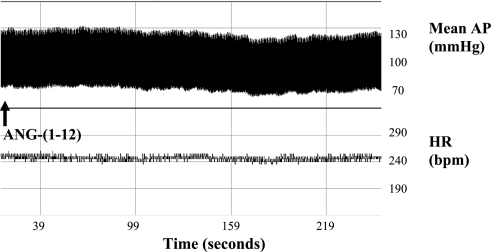
There were no significant differences in resting baseline hemodynamics among animals receiving various doses of ANG-(1–12) (Table 1). NTS injection of 36 fmol ANG-(1–12) had no significant effect on resting mean AP or HR. The 72- and 144-fmol doses of ANG-(1–12) elicited significant depressor responses (Table 1; P < 0.05), which were similar in magnitude and peaked at 3 ± 1 min, with no effect on resting HR (Fig. 1). Mean AP and HR returned to baseline levels and were stable at 10 min after the initial injections, immediately before baroreflex testing.
Table 1.
Resting mean AP and HR in response to NTS microinjection of ANG-(1–12)
| Group | Mean AP, mmHg | HR, Beats/min |
|---|---|---|
| 36 fmol | ||
| Baseline | 89 ± 7 | 283 ± 15 |
| Peak | 91 ± 7 | 274 ± 25 |
| 10 min | 89 ± 7 | 268 ± 19 |
| 72 fmol | ||
| Baseline | 83 ± 3 | 277 ± 12 |
| Peak | 72 ± 5* | 260 ± 24 |
| 10 min | 80 ± 4 | 263 ± 18 |
| 144 fmol | ||
| Baseline | 95 ± 5 | 290 ± 12 |
| Peak | 78 ± 5* | 280 ± 16 |
| 10 min | 92 ± 6 | 282 ± 17 |
Values are means ± SE and represent mean arterial pressure (AP) and heart rate (HR) at baseline, peak changes in response to nucleus tractus solitarii (NTS) injection of ANG-(1–12), and at 10 min after injection (immediately before reflex testing); n = 4 animals for the 36 and 72 fmol groups and n = 7 for the 144 fmol group.
P < 0.05 vs. respective baseline.
Fig. 1.
Representative pressure and heart rate (HR) response to nucleus tractus solitarii (NTS) injection of ANG-(1–12). Representative tracing of mean arterial pressure (AP) and HR in response to NTS injection of the 144 fmol ANG-(1–12) dose is shown. ANG-(1–12) elicited a transient depressor response that peaked at 3 min, with no significant effect on HR. bpm, beats/min.
ANG-(1–12) and baroreflex function.
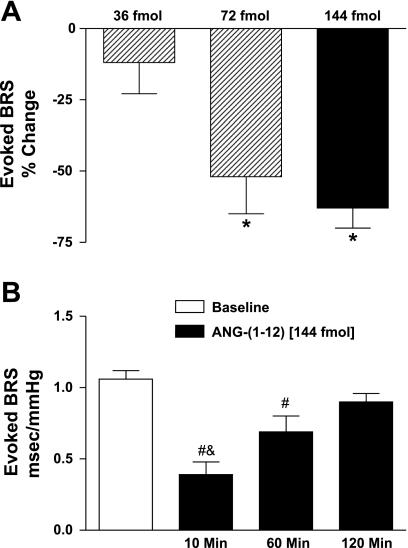
Resting values of baroreflex sensitivity to increases in AP were similar among groups of Sprague-Dawley rats used for the 36-, 72-, or 144-fmol injections (1.19 ± 0.11 vs. 1.29 ± 0.15 vs. 1.07 ± 0.05 ms/mmHg, respectively). NTS injection of 36-fmol ANG-(1–12) did not significantly alter the baroreflex sensitivity at 10 min (Fig. 2A). In contrast, the 72- and 144-fmol doses of ANG-(1–12) significantly impaired the baroreflex sensitivity by 52% and 63%, respectively (Fig. 2A; P < 0.05), at this time point. The 144-fmol dose was used for the remainder of experiments. The time course for the effects of ANG-(1–12) on baroreflex function showed suppression of the baroreflex sensitivity at 10 and 60 min with recovery to baseline levels at 120 min after the initial injection (Fig. 2B; P < 0.05). The specificity of baroreflex modulation is supported by the fact that the ANG-(1–12) injection did not alter depressor [−73 ± 4% baseline vs. −70 ± 3% after ANG-(1–12); n = 7] or bradycardic [−74 ± 6% baseline vs. −73 ± 2% after ANG-(1–12)] responses to activation of cardiac vagal chemosensitive fibers, which converge with baroreceptor inputs in the NTS.
Fig. 2.
ANG-(1–12)-mediated impairments in baroreflex sensitivity (BRS). A: bilateral NTS injection of ANG-(1–12) (36, 72, or 144 fmol; n = 4, 4, or 7) produced dose-dependent impairments in BRS for control of heart rate in response to increases in arterial pressure. B: ANG-(1–12) injection (144 fmol) significantly impaired the BRS at 10 and 60 min with recovery to baseline levels at 120 min (n = 4). *P < 0.05 vs. 36 fmol; #P < 0.05 vs. baseline; &P < 0.05 vs. 60 and 120 min.
Effect of ANG-(1–12) on indexes of sympathovagal balance.
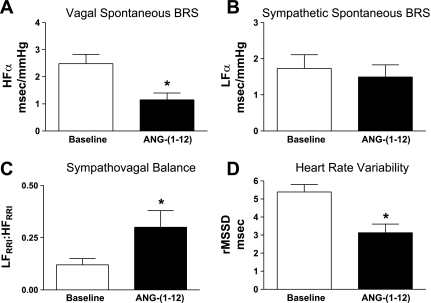
ANG-(1–12) injection significantly reduced the HFα index (P < 0.05; Fig. 3A) with no effect on the LFα component of the spontaneous baroreflex sensitivity (Fig. 3B), resulting in an overall increase in the ratio of LFRRI to HFRRI (P < 0.05; Fig. 3C). In addition, ANG-(1–12) significantly reduced HR variability (P < 0.05; Fig. 3D) with no significant effect on the LFSAP marker of AP variability [15.3 ± 2.2 baseline vs. 9.8 ± 3.0 normalized units after ANG-(1–12)].
Fig. 3.
Spontaneous BRS and indexes of sympathovagal balance. NTS injection of ANG-(1–12) (144 fmol; n = 7) impaired the vagal component (A) with no effect on the sympathetic component (B) of the spontaneous BRS, resulting in an overall increase in sympathovagal balance (C). ANG-(1–12) injection also significantly reduced heart rate variability (D). HF, high frequency; LF, low frequency; rMSSD, root mean square of successive differences; RRI, beat-to-beat interval. *P < 0.05 vs. baseline.
Effect of AT1 receptor antagonist or ACE inhibitor pretreatment on ANG-(1–12) responses.
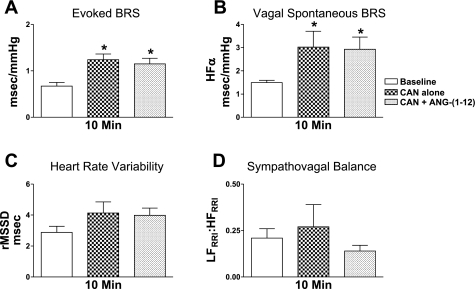
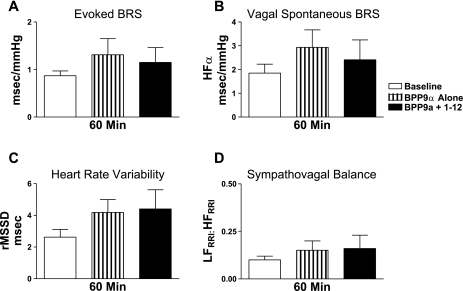
Resting AP and HR were similar among Sprague-Dawley rats receiving pretreatment of the AT1 receptor antagonist candesartan or ACE inhibitor BPP9α (Table 2). Injection of candesartan alone decreased resting mean AP with no effect on HR. In contrast, injection of BPP9α alone decreased resting HR with no effect on mean AP. Importantly, mean AP and HR recovered to baseline levels at 10 min after these injections and were stable immediately before ANG-(1–12) administration. NTS pretreatment with candesartan or BPP9α prevented depressor responses induced by ANG-(1–12) (Table 2). Candesartan alone increased the evoked baroreflex sensitivity for bradycardia and HFα (Fig. 4, A and B; P < 0.05), with a trend for an increase in HR variability (Fig. 4C; P = 0.053) at 10 min after injection. There was no significant effect of candesartan on the ratio of LFRRI to HFRRI (Fig. 4D) at this time point. Injection of BPP9α alone did not significantly alter baroreflex sensitivity, HR variability, or sympathovagal balance at 60 min after the injection (Fig. 5). NTS pretreatment with either candesartan or BPP9α prevented ANG-(1–12)-mediated impairments in baroreflex sensitivity and HR variability as well as the shift in the ratio of LFRRI to HFRRI toward sympathetic dominance (Figs. 4 and 5).
Table 2.
Effect of NTS pretreatment on ANG-(1–12)-mediated mean AP and HR responses
| Group | Mean AP, mmHg | HR, Beats/min |
|---|---|---|
| Candesartan alone | ||
| Baseline | 89 ± 7 | 319 ± 28 |
| Peak | 73 ± 10* | 275 ± 33 |
| 10 min | 81 ± 6 | 277 ± 33 |
| Candesartan + ANG-(1–12) | ||
| Baseline | 89 ± 7 | 302 ± 26 |
| Peak | 87 ± 8 | 305 ± 21 |
| 10 min | 91 ± 7 | 307 ± 23 |
| BPP9 alone | ||
| Baseline | 78 ± 3 | 284 ± 11 |
| Peak | 74 ± 7 | 267 ± 7* |
| 10 min | 73 ± 5 | 269 ± 8 |
| 60 min | 75 ± 7 | 275 ± 10 |
| BPP9α + ANG-(1–12) | ||
| Baseline | 74 ± 6 | 287 ± 15 |
| Peak | 77 ± 7 | 280 ± 19 |
| 10 min | 81 ± 3 | 282 ± 18 |
Values are means ± SE and represent mean AP and HR at baseline (immediately before injections), peak changes in response to NTS injections of inhibitors alone or inhibitors followed by subsequent administration of ANG-(1–12), and values after injection (immediately before reflex testing); n = 4 animals for each group.
P < 0.05 vs. respective baseline.
Fig. 4.
Effect of AT1 receptor antagonist pretreatment on ANG-(1–12) responses. A: candesartan (CAN; n = 4) significantly improved the evoked BRS (A) and vagal component of the spontaneous BRS (B) with no effect on heart rate variability (C) or sympathovagal balance (D) at 10 min after NTS injection. Candesartan pretreatment prevented ANG-(1–12)-mediated impairments in BRS and the shift in sympathovagal balance toward increased sympathetic activity. *P < 0.05 vs. baseline.
Fig. 5.
Effect of ANG converting enzyme (ACE) inhibitor pretreatment on ANG-(1–12) responses. A: NTS injection of bradykinin potentiating peptide 9-α (BPP9α; n = 4) had no significant effect on the evoked BRS (A), parasympathetic component of spontaneous BRS (B), heart rate variability (C), or sympathovagal balance (D) at 60 min. BPP9a pretreatment prevented ANG-(1–12)-mediated impairments in BRS and heart rate variability as well as the shift in sympathovagal balance.
Immunolocalization of ANG-(1–12) within the NTS.
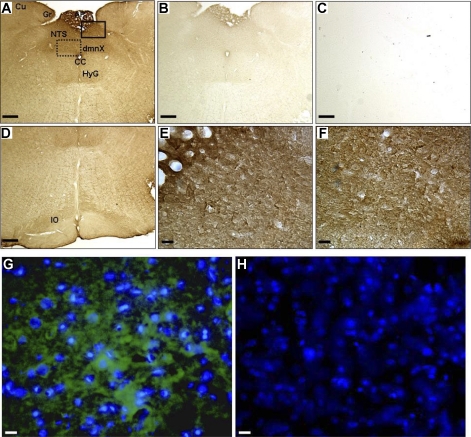
Analysis of the immunostaining pattern in serial sections from rostrocaudal level −14.3 mm to −13.0 mm caudal to bregma revealed the presence of ANG-(1–12) throughout the NTS, encompassing the area of expected microinjection spread. As visualized by immunostaining techniques, ANG-(1–12) was widely expressed in medulla of Sprague-Dawley rats (Fig. 6). Dense ANG-(1–12) staining was observed within the vicinity of the NTS, area postrema, dorsal motor nucleus of the vagus, hypoglossal, cuneate, gracile, and inferior olive nuclei (Fig. 6, A and D). ANG-(1–12) staining was abolished by preincubation of the antibody with the ANG-(1–12) peptide (Fig. 6B), suggesting specific staining for ANG-(1–12). In the absence of primary antibody no endogenous peroxidase activity was detected in brain tissue (Fig. 6C). A higher magnification using either light (Fig. 6, E and F) or fluorescence (Fig. 6, G and H) microscopy revealed ANG-(1–12) localization to cell bodies and fibers but not vascular components within the NTS, area postrema, and dorsal motor nucleus.
Fig. 6.
Immunolocalization of ANG-(1–12). Paraformaldehyde-fixed, frozen brain sections (n = 3 and 30 μm) were incubated with the ANG-(1–12) antibody to determine expression of ANG-(1–12) in dorsal medulla of Sprague-Dawley rats. 3,3′-Diaminobenzidine (A–F) and immunofluorescence (G and H) staining show ANG-(1–12) expression in various brainstem regions, including the NTS, in representative adjacent sections at ∼−13.8 mm caudal to bregma. ANG-(1–12) immunostaining was widely expressed in the medulla (A and D; 5×). Control sections (5×) were incubated with 40 μM ANG-(1–12) peptide together with the ANG-(1–12) antibody (B) or no ANG-(1–12) antibody (C). A higher magnification (40×) shows ANG-(1–12)-like immunoreactivity in cell bodies and fibers in the area postrema (E), NTS (E), and dorsal motor nucleus of the vagus (F). Adjacent sections were incubated with the ANG-(1–12) antibody (G) or no primary antibody (H) for immunofluorescence localization. ANG-(1–12) fluorescence is shown in green and nuclei in blue (40×). dmnX, Dorsal motor nucleus of the vagus; Cu, cuneate nucleus; Gr, nucleus gracilis; CC, central canal; HyG, hypoglossal nucleus; IO, inferior olivary nucleus. Scale bars: A–D, 100 μm, and E–H, 10 μm.
Endogenous ANG-(1–12) for baroreflex modulation.
NTS administration of the ANG-(1–12) antibody did not alter resting mean AP [n = 5; 91 ± 3 baseline vs. 86 ± 5 at 10 min vs. 81 ± 6 mmHg at 60 min after ANG-(1–12) IgG], HR [289 ± 8 baseline vs. 312 ± 11 at 10 min vs. 279 ± 12 beats/min at 60 min after ANG-(1–12) IgG], or baroreflex sensitivity [0.96 ± 0.14 baseline vs. 0.89 ± 0.16 at 10 min vs. 0.95 ± 0.33 ms/mmHg at 60 min after ANG-(1–12) IgG] in Sprague-Dawley rats. There was also no effect of control preimmune IgG on resting mean AP (n = 4; 91 ± 8 baseline vs. 86 ± 9 at 10 min vs. 74 ± 12 mmHg at 60 min after preimmune IgG), HR (262 ± 12 baseline vs. 276 ± 7 at 10 min vs. 280 ± 26 beats/min at 60 min after preimmune IgG), or baroreflex sensitivity (1.00 ± 0.31 baseline vs. 1.05 ± 0.39 at 10 min vs. 1.35 ± 0.71 ms/mmHg at 60 min after preimmune IgG). A higher dose of either ANG-(1–12) IgG or preimmune IgG caused nonspecific reductions in the baroreflex (data not shown).
DISCUSSION
The goal of the present study was to determine the processing of exogenous ANG-(1–12) and role of endogenous ANG-(1–12) for autonomic control of HR within the NTS of Sprague-Dawley rats. The novel findings of this study are that within the NTS of normotensive rats exogenous ANG-(1–12) elicits 1) a transient decrease in resting AP with no change in HR; 2) impairments in the vagal component of the evoked and spontaneous baroreflex sensitivity, with no effect on the sympathetic component of the spontaneous baroreflex sensitivity; 3) reductions in HR variability; and 4) a shift in overall sympathovagal balance toward sympathetic dominance. These effects mimic those of ANG II and are opposite to those of ANG-(1–7) injected into this brain region, suggesting that although these peptides have sequences in common, the C-terminal residues dictate functional specificity. Pretreatment with either an AT1 receptor antagonist or ACE inhibitor prevented ANG-(1–12)-mediated depressor and autonomic effects, providing further support that exogenous ANG-(1–12) is processed to ANG II for cardiovascular actions at AT1 receptors within the NTS. In Sprague-Dawley rats, immunohistochemistry and immunofluorescence techniques showed localization of ANG-(1–12)-like staining in cell bodies and fibers within the NTS, suggesting an intracellular source for the peptide. However, NTS injection of an antibody to ANG-(1–12) had no short-term effect on resting AP or baroreflex sensitivity, suggesting a lack of acute ANG-(1–12) tone for central control of baroreflex function in normotensive animals at sites accessible to the antibody.
Low dose injection of either ANG II or ANG-(1–7) within the NTS results in transient depressor responses, in part mediated by glutamate and substance P release, that peak between 40–45 s in normotensive rats (6, 10, 17). The decrease in AP elicited by ANG-(1–12) was similar in degree and dose range as previously reported for both ANG II and ANG-(1–7), but with a peak response at 3 min. The slower time course for reaching a maximum effect on AP is consistent with the interpretation that ANG-(1–12) requires conversion before manifesting its actions either extraneuronally or following its uptake intraneuronally. In the face of the transient decrease in AP, there was no alteration in resting HR, which would be consistent with a blunting of baroreflex sensitivity by ANG-(1–12).
After recovery of ANG-(1–12)-induced hypotensive effects, the evoked baroreflex testing and spectral analysis methods were conducted. Although eliciting similar initial cardiovascular responses, ANG II and ANG-(1–7) have opposing effects on baroreflex function, suggesting specificity of responses despite only one amino acid difference in these peptides (5, 9, 26). ANG-(1–12) injection produced dose-dependent impairments in the baroreflex sensitivity to evoked increases in AP, consistent with known actions of ANG II. The highest dose of ANG-(1–12) suppressed baroreflex function to levels observed in hypertensive rodents (11, 31) with an extended time course of action. The time course for the effects of ANG peptides on baroreflex sensitivity and other indexes of sympathovagal balance has not been previously documented. The baroreflex sensitivity is typically assessed within 15 min after exposure to NTS microinjection of ANG peptides and is altered at these longer time points (5, 9, 37) well after the cessation of the depressor responses. Since previous reports show that different neural mechanisms are involved in the regulation of blood pressure and baroreflex function (3, 4, 37), the timing of these hemodynamic and autonomic effects may differ. At the present time, we do not know the mechanisms underlying the prolonged baroreflex suppression in response to ANG-(1–12) administration within the NTS. However, alterations in nitric oxide and GABA as well as monoamine and other neuropeptide transmitter systems could be involved (15, 34).
To complement the evoked baroreflex testing, spectral analysis methods were employed to assess the effects of ANG-(1–12) on markers of spontaneous autonomic balance. Spectral analysis, although controversial, is widely used to indirectly assess sympathetic and parasympathetic cardiovascular control (1, 7, 23, 32). A highly significant correlation exists between baroreflex sensitivity values obtained by evoked and spectral analysis methods in humans and rodents (33, 38). Consistent with known actions of ANG II to specifically modulate the evoked baroreflex sensitivity for bradycardia, injection of ANG-(1–12) reduced the parasympathetic component with no effect on the primarily sympathetic component of the spontaneous baroreflex sensitivity in the HR spectrum. As a result, the ratio of LFRRI to HFRRI was higher, suggesting a shift in cardiac sympathovagal balance toward sympathetic dominance. Either an elevation in sympathetic or reduction in parasympathetic activity could contribute to this shift in autonomic balance. ANG-(1–12) reduced indexes of parasympathetic tone (evoked BRS for bradycardia, HFα, HR variability) with no significant effect on indexes of sympathetic (LFSAP, LFα) tone. Thus the ANG-(1–12)-mediated shift in sympathovagal balance appears to result primarily from parasympathetic inhibition, similar to known actions of ANG II.
In conscious and anesthetized rodents, administration of AT1 receptor antagonists improves baroreflex sensitivity and HR variability, suggesting that endogenous ANG II tone suppresses parasympathetic function (28, 37). Consistent with these findings, NTS injection of the AT1 blocker candesartan alone significantly facilitated the evoked and spontaneous baroreflex sensitivity, with a trend for an increase in HR variability. Administration of the ACE inhibitor BPP9α alone did not significantly alter autonomic function. The finding that candesartan, but not BPP9α or the ANG-(1–12) antibody, acutely improves baroreflex function within the NTS suggests that ANG-(1–12) or ANG I has to be processed intracellularly with release of ANG II from cell bodies and terminals or is coming from an extracellular source. ANG-(1–12)-mediated depressor and autonomic effects were abolished by NTS pretreatment with either an AT1 receptor antagonist or ACE inhibitor providing evidence for specific ANG II-mediated actions. Consistent with the ability to block exogenous ANG-(1–12) actions, BPP9α is a competitive extracellular peptide inhibitor of ACE (13). In addition to preventing formation of ANG II, BPP9α also potentiates bradykinin and ANG-(1–7) levels, all of which may contribute to abrogating ANG-(1–12)-mediated impairments in baroreflex sensitivity and HR variability.
Collectively, the present studies suggest that exogenous ANG-(1–12) is processed to ANG II for modulation of cardiovascular and baroreflex function at AT1 receptors within the NTS of normotensive rats. The present study supports enzymatic cleavage of ANG-(1–12) in the brain by ACE, which is similar to observations in the circulation (30) but may be distinct from cardiac processing (35) where chymase is implicated in the conversion of ANG-(1–12) into ANG II in isolated perfused hearts of Sprague-Dawley rats. It remains possible that ANG-(1–12) exerts direct effects on the cardiovascular system at AT1 receptors; however, this seems unlikely based on the combined observations that peripheral (30) and central actions of ANG-(1–12) are abolished by ACE inhibitors.
Consistent with previous observations of brain content of ANG-(1–12) by radioimmunoassay (30), immunohistochemistry and immunofluorescence techniques revealed discrete ANG-(1–12) staining in cell bodies and fibers within the NTS and other brainstem nuclei involved in cardiovascular regulation in Sprague-Dawley rats. In the present study, we could not differentiate whether ANG-(1–12) was localized to glia versus neurons, elements which have been shown to differentially contribute to modulation of resting baroreflex sensitivity and AP (29, 36). ANG-(1–12) endogenous to the NTS does not appear to contribute to resting baroreflex regulation in Sprague-Dawley rats since NTS injection of an antibody to ANG-(1–12) did not acutely alter resting AP, HR, or baroreflex sensitivity in these animals. However, the antibody is not likely to penetrate the cell resulting only in the immunoneutralization of the extracellular peptide. Since chronic central immunoneutralization of ANG-(1–12) lowers AP and improves baroreflex function and HR variability in (mRen2)27, but not Sprague-Dawley, rats (21, 22), endogenous ANG-(1–12) existing at global brain sites accessible to the antibody may only alter cardiovascular regulation in hypertensive conditions associated with elevations in ANG II. Further suggesting pathological activation of ANG-(1–12) in cardiovascular disease, ANG-(1–12) levels are elevated in cardiac and renal tissue from hypertensive relative to normotensive rats (24), and preconditioning of the heart with ANG-(1–12) worsens ischemia-reperfusion injury (35).
Although there is a wide distribution of angiotensinogen in brain regions involved in cardiovascular regulation, central levels of renin are low (19). This mismatch has been an ongoing topic of controversy for the biosynthesis of ANG peptides within the brain. The enzymatic pathways responsible for the generation of ANG-(1–12) remain unknown, but do not appear to involve renin (8, 16, 40). Thus the discovery of ANG-(1–12) may provide an alternate, renin-independent pathway for the production of bioactive ANG peptides within the central nervous system. Indeed, exogenous administration of the peptide appears to be a functional precursor for ANG II in brain pathways regulating baroreflex function. Why ANG-(1–12) is not processed into ANG-(1–7) for cardiovascular actions in the brainstem, especially given the local expression of ACE2 and neprilysin (12), is of interest for further studies. Furthermore, understanding the regulation of processing of endogenous ANG-(1–12) for central cardiovascular regulation may improve our understanding of the RAS and allow for improvements in current therapies.
GRANTS
We gratefully acknowledge support from National Heart, Lung, and Blood Institute Grants HL-51952 and HL-56973, Farley-Hudson Foundation, and Unifi (Greensboro, NC). H. A. Shaltout is a faculty member in the Department of Pharmacology and Toxicology, School of Pharmacy, University of Alexandria, Egypt. K. Isa is an assistant professor in the Department of Neurology, University of the Ryukyus, and was supported by American Heart Association fellowship 0825482E.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol Heart Circ Physiol 249: H867–H875, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Arnold AC, Sakima A, Ganten D, Ferrario CM, Diz DI. Modulation of reflex function by endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension 51: 1326–1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension 54: 1001–1008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnole-Santos MJ, Diz DI, Ferrario CM. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension 11: I167–I171, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Campagnole-Santos MJ, Diz DI, Santos RA, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1–7) injected into the dorsal medulla of rats. Am J Physiol Heart Circ Physiol 257: H324–H329, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: assessment by spectral analysis. Am J Physiol Heart Circ Physiol 266: H1993–H2000, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Chappell MC, Westwood BM, Pendergrass KD, Jessup JA, Ferrario CM. Distinct processing pathways for the novel peptide angiotensin-(1–12) in the serum and kidney of the hypertensive mRen2.Lewis rat (Abstract). Hypertension 50: E139, 2007 [Google Scholar]
- 9.Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens 18: 1841–1848, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Diz DI, Fantz DL, Benter IF, Bosch SM. Acute depressor actions of angiotensin II in the nucleus of the solitary tract are mediated by substance P. Am J Physiol Regul Integr Comp Physiol 273: R28–R34, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol 51: 542–548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann TE, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol 93: 694–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diz DI, Jacobowitz DM. Cardiovascular effects of discrete intrahypothalamic and preoptic injections of bradykinin. Brain Res Bull 12: 409–417, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exp Pharmacol Physiol 29: 473–482, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Diz DI, Pirro NT. Differential actions of angiotensin II and angiotensin-(1–7) on transmitter release. Hypertension 19: II41–II48, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell AT, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol 296: H1184–H1192, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fow JE, Averill DB, Barnes KL. Mechanisms of angiotensin-induced hypotension and bradycardia in the medial solitary tract nucleus. Am J Physiol Heart Circ Physiol 267: H259–H266, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Espinosa MA, Wallin R, Hutson SM, Sweatt AJ. Widespread neuronal expression of branched-chain aminotransferase in the CNS: implications for leucine/glutamate metabolism and for signaling by amino acids. J Neurochem 100: 1458–1468, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 23: 187–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isa K, Arnold AC, Chappell MC, Diz DI. Angiotensin-converting enzyme inhibition, but not AT1 receptor blockade, in the solitary tract nucleus improves baroreflex sensitivity in anesthetized transgenic hypertensive (mRen2)27 rats (Abstract). Hypertension 54: e98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isa K, Garcia-Espinosa MA, Shaltout HA, Arnold AC, Chappell MC, Ferrario CM, Diz DI. Intracerebroventricular anti-Ang-(1–12)-IgG improves baroreflex sensitivity and heart rate variability in transgenic (mRen2)27 hypertensive rats (Abstract). FASEB J 23: 612.–13., 2009 [Google Scholar]
- 22.Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol 297: R111–R115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Japundzic N, Grichois ML, Zitoun P, Laude D, Elghozi JL. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst 30: 91–100, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 294: H2614–H2618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korner PI. Cardiac baroreflex in hypertension: role of the heart and angiotensin II. Clin Exp Hypertens 17: 425–439, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 275: R1611–R1619, 1998 [DOI] [PubMed] [Google Scholar]
- 27.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Michelini LC, Bonagamba LG. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension 15: I45–I50, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 277: 33235–33241, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Oliveira DR, Santos RA, Santos GF, Khosla M, Campagnole-Santos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension 27: 1284–1290, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95: 1441–1448, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Parati G, Di RM, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12: 214–222, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med 86: 705–710, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res 82: 40–50, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Sakai K, Chapleau MW, Morimoto S, Cassell MD, Sigmund CD. Differential modulation of baroreflex control of heart rate by neuron- vs. glia-derived angiotensin II. Physiol Genomics 20: 66–72, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension 46: 333–340, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Shaltout HA, Abdel-Rahman AA. Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther 314: 1328–1337, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Trask AJ, Ferrario CM. Angiotensin-(1–7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev 25: 162–174, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 294: H2242–H2247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]