Abstract
We hypothesized that the reciprocal association between adiponectin and lectin-like oxidized LDL (ox-LDL) receptor (LOX)-1 contributes to the regulation of aortic endothelial dysfunction in atherosclerosis. To test this hypothesis, endothelium-dependent (ACh) and endothelium-independent (sodium nitroprusside) vasorelaxation of isolated aortic rings from control mice, apolipoprotein E (ApoE) knockout (KO) mice, and ApoE KO mice treated with either adiponectin (15 μg·day−1·mouse−1 sc for 8 days) or neutralizing antibody to LOX-1 (anti-LOX-1, 16 μg/ml, 0.1 ml/mouse ip for 7 days) were examined. Although vasorelaxation to sodium nitroprusside was not different between control and ApoE KO mice, relaxation to ACh was impaired in ApoE KO mice. Adiponectin and anti-LOX-1 restored nitric oxide-mediated endothelium-dependent vasorelaxation in ApoE KO mice. Aortic ROS formation and ox-LDL uptake were increased in ApoE KO mice. Both adiponectin and anti-LOX-1 treatment reduced ROS production and aortic ox-LDL uptake. In mouse coronary artery endothelial cells, TNF-α incubation increased endothelial LOX-1 expression. Adiponectin reduced TNF-α-induced LOX-1 expression. Consistently, in ApoE KO mice, adiponectin treatment reversed elevated LOX-1 expression in aortas. Immunofluorescence staining showed that adiponectin was mainly colocalized with endothelial cells. Although adiponectin expression was lower in ApoE KO versus control mice, anti-LOX-1 increased aortic adiponectin expression, suggesting a reciprocal regulation between adiponectin and LOX-1. Moreover, both adiponectin and anti-LOX-1 reduced NF-κB expression in ApoE KO mice. Thus, adiponectin and LOX-1 may converge on NF-κB signaling to regulate their function. In conclusion, our results indicate that the reciprocal regulation between adiponectin and LOX-1 amplifies oxidative stress and ox-LDL uptake, leading to endothelial dysfunction in atherosclerosis.
Keywords: aorta, oxidative stress, cytokine, vasorelaxation, lectin-like oxidized low-density lipoprotein receptor, apolipoprotein E
endothelial dysfunction plays a crucial role in the development and progression of atherosclerosis (9, 38). A mechanistic understanding of endothelial dysfunction in atherosclerosis is emerging to suggest that atherosclerotic endothelial dysfunction, particularly at early disease stages, is primarily caused by the dysregulation of endothelial nitric oxide (NO) synthase (eNOS) enzymatic activity and inactivation of NO through oxidative stress (38). Thus, recent research to improve endothelial function in atherosclerosis has focused on the promotion of NO production and prevention of NO inactivation by oxidative stress. The integral role of the endothelium in vascular health and of endothelial dysfunction in atherosclerosis has generated considerable interest in the potential for reversal of endothelial dysfunction with various therapies (9). As an adipose-derived hormone, adiponectin has been reported to have vascular protective effects (10). In adiponectin knockout (KO) mice (adiponectin−/− mice), ACh-induced vasorelaxation in aortas was impaired, accompanied by increased superoxide (O2·−) and peroxynitrite (ONOO−) production. eNOS expression was conserved in adiponectin−/− mice, but NO and eNOS phosphorylation were significantly reduced (6). Moreover, we (35) previously found that neutralizing antibody to lectin-like oxidized LDL (ox-LDL) receptor (LOX)-1 (anti-LOX-1 antibody) increased eNOS expression and reduced NAD(P)H oxidase-induced O2·− production in apolipoprotein E (ApoE) KO mice coronary arterioles (35). Thus, both adiponectin and anti-LOX-1 may participate in the regulation of vascular dysfunction by the upregulation of NO production and reduction of oxidative stress. However, the interaction and association between adiponectin and LOX-1 in atherosclerosis-associated endothelial dysfunction have not been shown. In this study, we examine the effects of adiponectin and anti-LOX-1 on aortic endothelial dysfunction in ApoE KO mice. More importantly, we elucidated the reciprocal association between adiponectin and LOX-1, which may contribute to the synergistic vascular protective effects of adiponectin and anti-LOX-1 in atherosclerosis-induced vascular dysfunction.
METHODS
Animal Models
The procedures followed were approved by and in accordance with approved guidelines set by the Animal Care Committee of the University of Missouri. Six- to eight-week-old wild-type (C57BL/6) control mice and ApoE KO mice were obtained from the Jackson Laboratory (Bar Harbor, ME). To accelerate lesion formation in ApoE KO mice, all animals were treated with a Western-type diet (adjusted calories diet, Harlan Teklad TD88137, 42% from milk fat and 0.15% cholesterol) for 12 wk. Animals had free access to water and were kept at a 12:12-h light-dark cycle (35).
Measurement of Blood Parameters
Blood was obtained from the vena cava after anesthesia with pentobarbital sodium (50 mg/kg ip) and exposure of the vein. Heparin (1%) was used as anticoagulant. Whole blood samples were centrifuged at 3,000 rpm for 10 min at 4°C, and plasma was collected and stored at −80°C until analysis.
Blood glucose levels.
Blood glucose levels were measured using a OneTouch Ultramini glucometer (LifeScan).
Plasma cholesterol levels.
Total cholesterol, LDL, and HDL levels in plasma were measured with a Cholesterol/Cholesteryl Ester Quantitation kit (Biovision) using spectrophotometry (Multiskan MCC, Fisher Scientific) (37).
Plasma levels of resistin, adiponectin, and LOX-1.
Plasma levels of resistin, adiponectin, and LOX-1 were measured with a commercial ELISA kit from R&D.
LOX-1 Neutralization and Adiponectin Treatment
The neutralizing antibody to LOX-1 used in this study is rat anti-mouse neutralizing antibody to LOX-1 (AF1564, R&D), which blocks the binding of ox-LDL to LOX-1. After 12 wk of the Western diet, ApoE KO mice were treated with either recombinant murine globular adiponectin (gAcrp30; 15 μg·day−1·mouse−1 sc for 8 days, PeproTech) or anti-LOX-1 at a dose of 16 μg/ml (0.1 ml/mouse ip) for 7 days (35).
Functional Assessment of Murine Aortas
After anesthesia, aortas were rapidly excised and rinsed in cold physiological saline solution (PSS), and loose fat and connective tissue were removed. PSS contained (in mM) 118.99 NaCl, 4.69 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 2.50 CaCl2·2H2O, 14.9 NaHCO3, 5.5 d-glucose, and 0.03 EDTA. Aortas were maintained in PSS in 95% O2-5% CO2 at 37°C for the remainder of the experiment. Aortic rings (2 mm) were isometrically mounted in a myograph (model 610M, DMT). After an equilibration period of 45 min, during which an optimal passive tension (15 mN) was applied, aortic rings were precontracted with 1 μmol/l phenylephrine. Dose-response curves were obtained by the cumulative addition of ACh (1 nmol/l–10 μmol/l) and sodium nitroprusside (SNP; 1 nmol/l–10 μmol/l). Relaxation at each concentration was measured and expressed as the percentage of force generated in response to phenylephrine (39). The contribution of NO in vasorelaxation was assessed by incubating the vessels with the NOS (eNOS and neuronal NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 μmol/l for 20 min) (12, 27).
Analysis of the Aortic Uptake of ox-LDL
The aortic uptake of ox-LDL was determined as previously described with minor modifications (20). Briefly, thoracic aortas excised from mice were incubated for 4 h at 37°C in DMEM containing 10 μg/ml DiI-labeled ox-LDL, washed with ice-cold PBS three times, and then snap frozen. Cryosections were prepared, and pictures were taken with fluorescence microscope (IX81, Olympus).
Determination of Aortic ROS Formation
Total ROS release from the aortic pieces was measured by the widely used fluorescence probe 2′,7′-dichlorodihydrofluorescein (DCFH2). Thoracic aortic rings were incubated for 1 h in DMEM culture medium containing 5 μmol/l DCFH2 at 37°C in the dark in a sealed tube. The fluorescence of dichlorofluorescein production was monitored at 485/525 nm. DCFH2 autooxidation was also detected as the blank. The protein content of the aortic rings was determined by a BCA Protein Assay kit (Pierce). ROS formation was expressed in arbitrary units per milligram of protein, and comparison between groups was expressed as folds of the control group.
Mouse Coronary Artery Endothelial Cell Culture
Primary mouse coronary arterial endothelial cells (MCECs; Celprogen, San Pedro, CA) were maintained in culture in the presence or absence of recombinant TNF-α (10 ng/ml for 24 h, R&D). After treatment with 5 μg/ml recombinant murine gArp30 for another 8 h, cells were collected, and LOX-1 protein expression was determined by Western blot analysis.
Western Blot Analyses
Aortas were homogenized in lysis buffer (Celllytic MT Mammalian Tissue Lysis/Extraction Reagent, Sigma). Protein concentrations were assessed with a BCA Protein Assay kit (Pierce). Equal amounts of protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Pierce). Adiponectin, LOX-1, and NF-κB p65 protein expression were detected by Western blot analysis with the use of adiponectin primary antibody (1:500, R&D), LOX-1 primary antibody (1:1,000, R&D), and NF-κB p65 primary antibody (1:1,000, Abcam). Horseradish peroxidase-conjugated secondary antibodies were used. Signals were visualized by ECL (Santa Cruz Biotechnolgy), scanned with a Fuji LAS3000 densitometer, and quantified by Multigauge software (Fujifilm). The relative amounts of protein expression were quantified to those of the corresponding wild-type control, which were set to a value of 1.0.
Data Analysis
Data are presented as means ± SD except where specifically stated (e.g., as means ± SE for the functional experiment), and the significance of intergroup differences was evaluated by one-way ANOVA using SPSS11.5 software. Maximal relaxation (Emax) and half-maximal relaxing effect (EC50) values for ACh were determined from experimental data. EC50 values correspond to the concentration required to produce a half-maximal vasoactive effect. Negative logarithm of EC50 (−log EC50) values were calculated using Prism 5. Statistical differences were considered significant at P < 0.05.
RESULTS
Body Weight, Abdominal Girth, and Blood Glucose, Plasma Resistin, and Plasma Cholesterol Levels
Body weight and plasma parameters were measured at 18–20 wk for control mice, ApoE KO mice, and ApoE KO mice treated with either adiponectin or anti-LOX-1 (Table 1). Body weight, abdominal girth, blood glucose levels, and plasma resistin levels were similar among all groups. Total cholesterol and LDL levels were significantly increased in ApoE KO mice, but HDL levels were reduced. Anti-LOX-1 treatment reduced plasma total cholesterol levels without affecting plasma LDL and HDL levels. Adiponectin did not affect total cholesterol and LDL levels but slightly increased HDL levels in ApoE KO mice.
Table 1.
Basic plasma parameters
| Control Mice | ApoE KO Mice | ApoE KO with anti-LOX-1 Treatment | ApoE KO Mice with Adiponectin Treatment | |
|---|---|---|---|---|
| Body weight, g | 28.9 ± 2.4 | 27.7 ± 2.6 | 34.1 ± 3.2 | 30.9 ± 4.0 |
| Abdominal girth, cm | 8.8 ± 0.2 | 8.5 ± 0.1 | 9.2 ± 0.4 | 9.1 ± 0.6 |
| Total cholesterol, mg/dl | 297.3 ± 128.7 | 646.1 ± 49.1* | 486.8 ± 33.4*† | 514.8 ± 100.1 |
| LDL, mg/dl | 9.3 ± 9.3 | 438.6 ± 170.0* | 539.1 ± 14.6* | 542.1 ± 4.7* |
| HDL, mg/dl | 132.6 ± 39.9 | 35.5 ± 32.4* | 35.1 ± 30.0* | 46.4 ± 28.2*† |
| Resistin, ng/ml | 7.9 ± 2.5 | 7.0 ± 1.7 | 7.7 ± 1.6 | 8.8 ± 2.7 |
| Glucose, mg/dl | 174.9 ± 24.5 | 173.0 ± 46.4 | 182.2 ± 53.6 | 178.1 ± 62.4 |
| Adiponectin, μg/ml | 4.4 ± 0.8 | 2.4 ± 0.9* | 4.6 ± 0.9† | 3.9 ± 0.7† |
| LOX-1, ng/ml | 34.9 ± 15.8 | 96.7 ± 23.3* | 52.9 ± 28.6*† | 57.1 ± 39.2*† |
Data are means ± SD; n = 4–6 animals/group. ApoE, apolipoprotein E; KO, knockout; LOX-1: Lectin-like oxidized LDL receptor-1.
P < 0.05 vs. control mice;
P < 0.05 vs. ApoE KO mice.
Adiponectin and anti-LOX-1 Improved Endothelial Function in Aortas of ApoE KO Mice
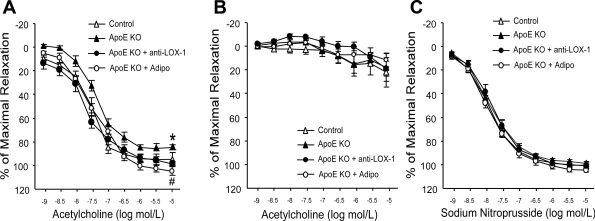
ACh-induced endothelial-dependent vasorelaxation was impaired in ApoE KO mice, whereas SNP-induced endothelial-independent vasorelaxation was comparable (Fig. 1C). The Emax of ACh-induced vasorelaxation was significantly lower in ApoE KO mice compared with control mice (Fig. 1A and Table 2), but EC50 was not statistically different between control and ApoE KO mice (Table 2). Adiponectin and anti-LOX-1 restored aortic endothelial dysfunction in ApoE KO mice. Both adiponectin and anti-LOX-1 treatment increased Emax of ApoE KO mice (Table 2). Incubation with the NOS inhibitor l-NAME completely abolished ACh-induced vasorelaxation (Fig. 1B).
Fig. 1.
Reactivity of the thoracic aorta to ACh in control mice, apolipoprotein E (ApoE) knockout (KO) mice, and ApoE KO mice with anti-lectin-like oxidized LDL (ox-LDL) receptor (LOX)-1 and adiponectin (Adipo) treatment was evaluated. Statistics were conducted on maximal relaxation. A: aortic endothelium-dependent vasorelaxation was impaired in ApoE KO mice versus control mice. Adipo and anti-LOX-1 treatment restored the impaired vasorelaxation in ApoE KO mice. B: vasorelaxation to ACh was abolished in ApoE KO mice treated with either Adipo or anti-LOX-1 after incubation of the aortic rings with the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (100 μM for 20 min), which indicated that vasorelaxation to ACh was nitric oxide mediated in aortas. C: endothelium-independent vasorelaxation was comparable among groups. Data are means ± SE; n = 6–7 mice. *P < 0.05 vs. control mice; #P < 0.05 vs. ApoE KO mice.
Table 2.
−Log EC50 and Emax values for control mice, ApoE KO mice, and ApoE KO mice with anti-LOX-1 and adiponectin treatment
| −Log EC50 | Emax, % | |
|---|---|---|
| Control mice | 7.49 ± 0.15 | 97.30 ± 5.30 |
| ApoE KO mice | 7.37 ± 0.08 | 85.90 ± 2.03* |
| ApoE KO mice with anti-LOX-1 treatment | 7.74 ± 0.08† | 100.37 ± 1.87† |
| ApoE KO mice with adiponectin treatment | 7.43 ± 0.08 | 106.66 ± 3.76† |
Data are means ± SE; n = 6–7 animals/group. Emax, maximal relaxation.
P < 0.05 vs. control mice;
P < 0.05 vs. ApoE KO mice.
Effects of Adiponectin and anti-LOX-1 on Aortic Oxidative Stress
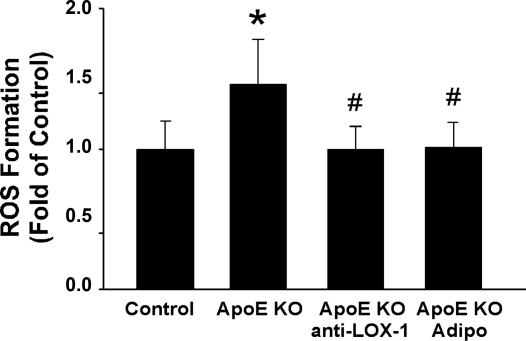
Compared with control mice, total aortic ROS formation was significantly increased in ApoE KO mice. Both adiponectin and anti-LOX-1 treatment reduced aortic ROS production back to the level of control mice (Fig. 2).
Fig. 2.
Effects of Adipo and anti-LOX-1 on ROS formation in aortas of ApoE KO mice. Adipo and anti-LOX-1 significantly decreased ROS formation in the ApoE KO mice aorta. Data are means ± SD; n = 6 mice. *P < 0.05 vs. control mice; #P < 0.05 vs. ApoE KO mice.
Role of Adiponectin and anti-LOX-1 in Aortic ox-LDL Uptake
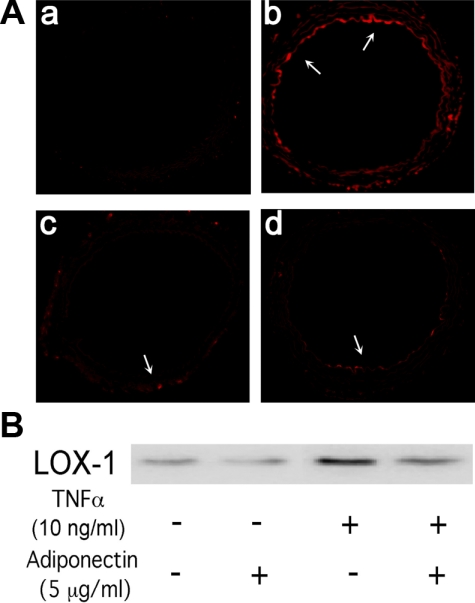
Aortic ox-LDL uptake was significantly elevated in ApoE KO mice. Adiponectin and anti-LOX-1 administration significantly decreased ox-LDL uptake in the ApoE KO mouse aorta (Fig. 3A).
Fig. 3.
A: effects of Adipo and anti-LOX-1 on DiI-labeled ox-LDL (DiI-ox-LDL) uptake in aortas of ApoE KO mice. The control aorta (a) showed very low DiI-ox-LDL uptake, which was significantly higher in the ApoE KO mouse aorta (b). Both anti-LOX-1 (c) and Adipo (d) treatment decreased DiI-ox-LDL uptake. B: murine recombinant TNF-α incubation significantly increased the expression of LOX-1 in mouse coronary artery endothelial cells. Coincubation with Adipo reduced LOX-1 protein expression. The representative blot is from 3 separate experiments.
The Reciprocal Regulation Between Adiponectin and LOX-1
Plasma adiponectin levels were lower in ApoE KO mice versus control mice, whereas plasma LOX-1 levels were higher in ApoE KO mice. Adiponectin treatment increased plasma adiponectin levels but reduced plasma LOX-1 levels. In contrast, anti-LOX-1 reduced plasma LOX-1 levels but increased plasma adiponectin levels (Table 1).
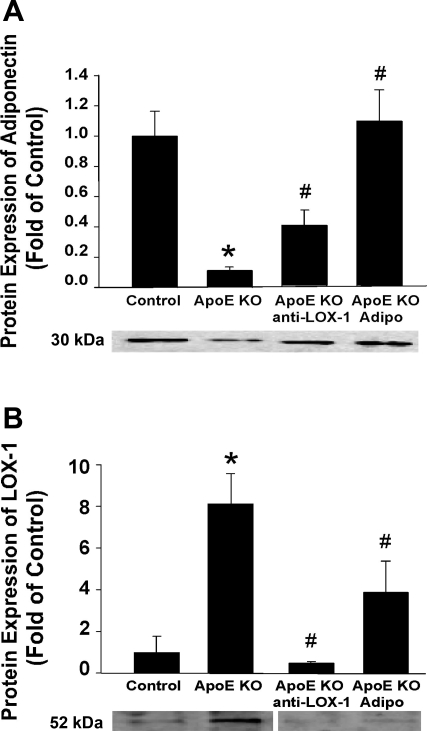
Furthermore, the protein expression of adiponectin was lower in the ApoE KO mouse aorta, whereas LOX-1 expression was higher. Both adiponectin and anti-LOX-1 treatment increased aortic adiponectin expression but inhibited LOX-1 expression in ApoE KO mice (Fig. 4). Additionally, in MCECs, incubation with TNF-α markedly increased endothelial cell LOX-1 expression, which was reversed by adiponectin treatment (Fig. 3B).
Fig. 4.
A: Adipo protein expression was lower in ApoE KO mice compared with control mice. Both anti-LOX-1 and Adipo treatment increased Adipo expression in the aortas of ApoE KO mice. B: protein expression of LOX-1 was higher in ApoE KO mice versus control mice. Both anti-LOX-1 and Adipo treatment reduced aortic LOX-1 expression in ApoE KO mice. The representative image shown in B was rearranged from the original capture by removing the gel segment between ApoE KO and ApoE KO + anti-LOX-1. Space was inserted to disclose this manipulation. Data are means ± SD; n = 6 mice. *P < 0.05 vs. control mice; #P < 0.05 vs. ApoE KO mice.
Cellular Source of Adiponectin
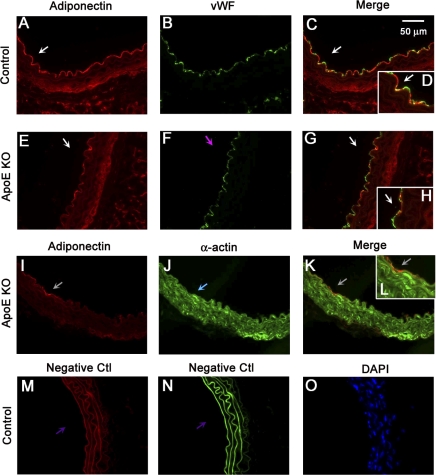
Immunofluorescence staining results suggested that adiponectin is colocalized with aortic endothelial cells but not with smooth muscle cells (Fig. 5).
Fig. 5.
Dual fluorescence combining Adipo with markers for endothelial cells [von Willebrand factor (vWF)] and vascular smooth muscle cells (α-actin) with the use of specific primary antibodies followed by fluorescent-labeled secondary antibodies. A–C: dual labeling of Adipo (red) and vWF (green) in a control mouse aorta. E–G: dual labeling of Adipo (red) and vWF (green) in an ApoE KO mouse aorta. The white arrows in C and G show the colocalization of Adipo and endothelial cells (yellow). I–K: dual labeling of Adipo (red) and α-actin (green) in an ApoE KO mouse aorta. The gray arrow in K shows specific α-actin staining with the absence of Adipo staining. M and N: negative controls (Ctl). The purple arrows show an absence of staining in vessels with the secondary antibodies. O: nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI; blue) in a control mouse aorta. Data are representative of 4 separate experiments.
Adiponectin and LOX-1 Reduced NF-κB p65 Expression in the ApoE KO Mouse Aorta
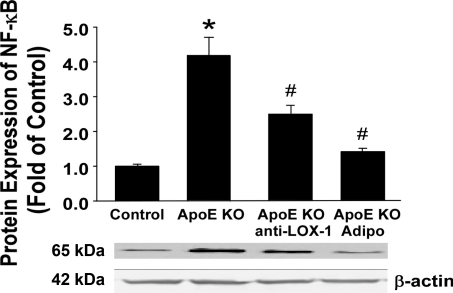
Aortic NF-κB p65 protein expression was elevated in ApoE KO mice compared with control mice. Both adiponectin and anti-LOX-1 reduced NF-κB levels in ApoE KO mice (Fig. 6).
Fig. 6.
Aortic NF-κB expression was higher in ApoE KO mice than in control mice. Anti-LOX-1 and Adipo significantly reduced NF-κB expression in the ApoE KO mouse aorta. Data are means ± SD; n = 6 mice. *P < 0.05 vs. control mice; #P < 0.05 vs. ApoE KO mice.
DISCUSSION
The endothelium and its product, NO, are key regulators of vascular health. Reduced bioavailability of NO is involved in the initiation, progression, and complications of atherosclerosis. Basic mechanisms involved in endothelial dysfunction have been clarified and suggest many new therapeutic targets (13). In particular, studies investigating the antioxidative effects of therapeutics on vascular function constitute a promising avenue of research. Our results suggest that although adiponectin and anti-LOX-1 did not demonstrate significant lipid-lowering effects in ApoE KO mice, both adiponectin and anti-LOX-1 improved aortic endothelial function and reduced aortic ox-LDL uptake. Furthermore, our molecular evidence indicates a reciprocal association between adiponectin and LOX-1. This interaction may contribute to the development of oxidative stress, which initiates and perpetuates endothelial dysfunction as well as atherosclerosis in aortas of ApoE KO mice.
Role of Endothelial Dysfunction and Vascular Protection by Adiponectin and anti-LOX-1
Endothelial dysfunction is considered to be an early marker for atherosclerosis, preceding angiographic or ultrasonic evidence of atherosclerotic plaques (9). An essential factor contributing to endothelial dysfunction is the reduced bioavailability of NO, with the consequent loss of its beneficial vascular actions (30). Oxidative stress, which can be defined as an imbalance between the production of endogenous ROS and the presence of antioxidant enzymes, is one of the most important mechanisms contributing to endothelial dysfunction (30). Indeed, NO is rapidly inactivated by the oxidative action of O2·−. Therefore, antioxidative interventions may pave the way to better targeted pharmaceutical agents that prevent or improve endothelial dysfunction associated with atherosclerosis.
Both adiponectin and anti-LOX-1 have been reported to participate in the regulation of vascular function by mediating the imbalance between oxidative stress and NO bioavailability, although the role of adiponectin and anti-LOX-1 in aortic endothelial dysfunction in ApoE KO mice have not yet been determined. Our previous studies (30, 35) suggested that antibody neutralization of LOX-1 prevented coronary endothelial dysfunction in ApoE KO mice by reducing NAD(P)H oxidase-induced O2·− generation. Anti-LOX-1 also increased eNOS protein expression in the coronary arterioles of ApoE KO mice (35). In the aortas of ApoE KO mice, ACh-induced endothelial-dependent vasorelaxation is impaired, accompanied by increased LOX-1 protein expression and aortic ROS formation. Anti-LOX-1 significantly improves aortic endothelial function and decreases ROS formation (Figs. 1 and 2).
Adiponectin induced eNOS activation and NO production in endothelial cells (7). Although a negative correlation between adiponectin expression and oxidative stress in nondiabetic humans with chronic kidney disease has been reported (4), the effects of adiponectin treatment on vascular oxidative stress in atherosclerosis remain to be completely understood. Our results suggest that in aortas of ApoE KO mice, adiponectin reduces ROS production as well as improves endothelial function. Thus, the antioxidative effects of adiponectin and anti-LOX-1 may contribute to their vascular protective effects in atherosclerotic mice.
Role of Adiponectin and LOX-1 in Atherosclerosis
Most cardiovascular disease results from complications of atherosclerosis (16). ox-LDL is now thought to be a more potent mediator of atherogenesis than native LDL (19). ox-LDL is internalized by a group of scavenger receptors (SRs), leading to macrophage activation, foam cell formation, and secretion of growth factors and proinflammatory cytokines (18). An important initiating event for atherosclerosis may be the transport of ox-LDL across the endothelium into the artery wall (21). This is likely to occur at sites of endothelial damage that are caused by ox-LDL itself as well as by physical or chemical forces and infection (28). LOX-1 was first identified and cloned as a mammalian endothelial receptor for ox-LDL. The induced expression of LOX-1 in endothelial cells may provide a molecular link for the incorporation of ox-LDL into the cells as well as the resultant cellular activation, dysfunction, and injury. Early human carotid atherosclerotic plaques displayed increased levels of LOX-1 mRNA with greater endothelial LOX-1 expression compared with advanced lesions. Our previous study (35) showed that the mRNA expression of LOX-1 in left ventricular coronary arterioles is elevated in ApoE KO mice. In murine models, deletion of LOX-1 (20) decreased the formation of atherosclerotic lesions in an ApoE KO or LDL receptor-null background, suggesting a crucial role for LOX-1 in the pathogenesis of atherosclerosis.
Adiponectin exerts atheroprotective effects. A previous study (22) has suggested that an adenovirus-mediated increase in plasma adiponectin significantly suppressed the progression of atherosclerotic lesions in the aortic sinus by 30% in ApoE KO mice. The mechanism may be that adiponectin significantly suppressed the expression of VCAM-1 and class A SRs (SR-A) and reduced the lipid accumulation in macrophages in atherosclerotic lesions (22). Globular adiponectin also showed effects of amelioration of atherosclerosis, which was associated with decreased expression of SR-A and TNF-α (36). Furthermore, adiponectin reduced lipid accumulation in macrophage foam cells (31) and prevented atherosclerosis by increasing cholesterol efflux from macrophages (32). In in vitro studies, adiponectin inhibited the TNF-α-induced increase in the endothelial expression of ICAM-1, VCAM-1, and E-selection (23) and suppressed vascular smooth muscle cell proliferation (2) as well as macrophage to foam cell transformation (24). Thus, adiponectin modulates multiple pathways to impact the development of atherosclerosis.
Our results show that LOX-1 protein expression was increased in the ApoE KO mouse aorta, whereas both adiponectin and anti-LOX-1 treatment reduced aortic LOX-1 expression (Fig. 4). ox-LDL uptake was increased in the ApoE KO mouse aorta; anti-LOX-1, as well as adiponectin, significantly suppressed the cellular uptake of ox-LDL in aortas of ApoE KO mice (Fig. 3). This suggests that the atheroprotective effects of adiponectin may be partially due to its inhibition of LOX-1 expression in our experiments.
Reciprocal Regulation Between Adiponectin and LOX-1
The role of adiponectin in the regulation of LOX-1 expression in macrophage has been reported in a recent publication (26). Macrophage LOX-1 expression was decreased when macrophages were cocultured with adipocytes or when exposed to adipocyte-conditioned medium. The addition of adiponectin-neutralizing antibody resulted in a twofold increase in LOX-1 gene expression, demonstrating that adiponectin regulated LOX-1 by reducing its expression (26). TNF-α plays an important role in both atherogenesis and vascular dysfunction since inhibition of TNF-α reduces atherosclerosis (5) and improves endothelial function in ApoE KO mice (unpublished observations). TNF-α expression was increased in aortas of ApoE KO mice versus control mice (unpublished observations), and a TNF-α-induced increase in LOX-1 expression has been demonstrated in various cell types, including endothelial cells, macrophages, vascular smooth muscle cells, etc. (8, 15, 29). Our results demonstrate that in MCECs, TNF-α upregulates endothelial LOX-1 expression, which was significantly inhibited by adiponectin treatment (Fig. 3). Thus, this adiponectin-mediated LOX-1 expression exists in both endothelial cells and macrophages in vitro. Furthermore, we demonstrated, for the first time, that adiponectin administration to ApoE KO mice decreases aortic LOX-1 expression and circulating levels of LOX-1 in vivo (Table 1 and Fig. 4).
In contrast, the role of LOX-1 in the regulation of adiponectin expression had not been determined in the past. We found that aortic adiponectin expression and plasma adiponectin levels are lower in ApoE KO mice compared with control mice. Anti-LOX-1 treatment increased adiponectin expression in aortas as well as circulating levels of adiponectin (Table 1 and Fig. 4). These data suggest a reciprocal regulation between adiponectin and LOX-1 as well as a critical role for adiponectin in LOX-1-mediated endothelial dysfunction relating to atherosclerosis.
Signaling of Interactions Between Adiponectin and LOX-1
A previous study (3) has revealed that ox-LDL activated NF-κB signaling through LOX-1. ox-LDL treatment caused a significant increase in the expression of ICAM-1 and ROS formation in human umbilical vein endothelial cells, which was associated with a dramatic augmentation in the phosphorylation of inhibitor of NF-κB (IκB) and translocation of NF-κB into the nuclei (14). In the endothelial cell line EA.hy926, ox-LDL led to the upregulation of LOX-1, phosphorylation of p38 MAPK, translocation of NF-κB, and downregulation of eNOS expression (3). Results from an in vitro study (17) suggested that selected fatty acids, and especially linoleic acid, could elicit endothelial dysfunction and LOX-1 upregulation. Pretreatment of human aortic endothelial cells with antioxidants and inhibitors of NF-κB inhibited the stimulatory effect of linoleic acid on LOX-1 protein expression (17). Those results suggested that NF-κB may play a significant role in ox-LDL-induced LOX-1 activation and downstream signaling.
Adiponectin has been shown to disrupt the activation of NF-κB in human aortic endothelial cells (23). Ouchi el al. (23) found that adiponectin suppressed TNF-α-induced IκB-α phosphorylation and subsequent NF-κB activation in human aortic endothelial cells without affecting other TNF-α-mediated phosphorylation signals, including JNK, p38 kinase, and Akt kinase. Our results also showed that adiponectin inhibits NF-κB expression (Fig. 6) without affecting JNK phosphorylation in the aorta of ApoE KO mice (data not shown), which further supports the in vitro data of Ouchi et al. Although Ouchi el al. (25) suggested that adiponectin inhibits NF-κB activation through the cAMP/PKA-dependent pathway, Wu et al. (33) reported that both cAMP/PKA signaling and the activation of the AMP-activated protein kinase (AMPK) pathway played a role in the suppression by adiponectin in TNF-α-mediated IκB kinase-β activation (33). Furthermore, high-molecular-weight adiponectin has been observed to activate AMPK, thereby increasing the phosphorylation of eNOS and NO production in endothelial cells, which leads to a potential reduction in the activation of NF-κB against inflammatory stimuli (11). Thus, cAMP/PKA and AMPK pathways activated by adiponectin may contribute to the inhibition of NF-κB activation, which suppresses aortic ROS formation and improves endothelial function. Therefore, NF-κB might act as a pivot in the reciprocal regulation between adiponectin and LOX-1. Adiponectin and anti-LOX-1 may converge on NF-κB signaling to interactively regulate their expression and function in the atherosclerosis-associated vascular dysfunction.
We conclude that anti-LOX-1 and adiponectin reduced the enhanced aortic uptake of ox-LDL and aortic ROS formation, which might contribute to their vascular protective effects in atherosclerosis. Adiponectin and anti-LOX-1 converged on NF-κB signaling to reciprocally regulate their expression and function. This reciprocal regulation may account for the attenuation of atherogenesis and endothelial dysfunction in atherosclerosis induced by adiponectin and anti-LOX-1 treatment. Therefore, replenishment of adiponectin and anti-LOX-1 may provide a novel treatment modality for atherosclerosis.
GRANTS
This work was supported by Pfizer Atorvastatin Research Award 2004-37, American Heart Association Scientist Development Grant 110350047A, and National Heart, Lung, and Blood Institute Grant RO1-HL-077566 (to Dr. C. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors appreciate Dr. Tzyh-Chang Hwang and Dr. Haoyang Liu for providing training and access to the confocal microscope.
REFERENCES
- 1. Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 288: R1220–R1225, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105: 2893–2898, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bao M, Lou Y. Isorhamnetin prevent endothelial cell injuries from oxidized LDL via activation of p38MAPK. Eur J Pharmacol 547: 22–30, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Barazzoni R, Bernardi A, Biasia F, Semolic A, Bosutti A, Mucci M, Dore F, Zanetti M, Guarnieri G. Low fat adiponectin expression is associated with oxidative stress in nondiabetic humans with chronic kidney disease–impact on plasma adiponectin concentration. Am J Physiol Regul Integr Comp Physiol 293: R47–R54, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 24: 2137–2142, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B, Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol 46: 413–419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chiba Y, Ogita T, Ando K, Fujita T. PPARγ ligands inhibit TNF-α-induced LOX-1 expression in cultured endothelial cells. Biochem Biophys Res Commun 286: 541–546, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–III32, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med 6: 27–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-κB activation in vascular endothelial cells. FEBS Lett 582: 1719–1724, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension 44: 956–962, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Lahera V, Goicoechea M, de Vinuesa SG, Miana M, de las Heras N, Cachofeiro V, Luno J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem 14: 243–248, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Li R, Chen B, Wu W, Bao L, Li J, Qi R. Ginkgolide B suppresses intercellular adhesion molecule-1 expression via blocking nuclear factor-κB activation in human vascular endothelial cells stimulated by oxidized low-density lipoprotein. J Pharmacol Sci 110: 362–369, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-α promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett 258: 31–37, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Maingrette F, Renier G. Linoleic acid increases lectin-like oxidized LDL receptor-1 (LOX-1) expression in human aortic endothelial cells. Diabetes 54: 1506–1513, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Martin-Fuentes P, Civeira F, Recalde D, Garcia-Otin AL, Jarauta E, Marzo I, Cenarro A. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J Immunol 179: 3242–3248, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Mehta JL. Oxidized or native low-density lipoprotein cholesterol: which is more important in atherogenesis? J Am Coll Cardiol 48: 980–982, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100: 1634–1642, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM. The yin and yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol 16: 831–842, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106: 2767–2770, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103: 1057–1063, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102: 1296–1301, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Rasouli N, Yao-Borengasser A, Varma V, Spencer HJ, McGehee RE, Jr, Peterson CA, Mehta JL, Kern PA. Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Arterioscler Thromb Vasc Biol 29: 1328–1335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richter A, Loschmann PA, Loscher W. Antidystonic efficacy of nitric oxide synthase inhibitors in a rodent model of primary paroxysmal dystonia. Br J Pharmacol 131: 921–926, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M. Mulberry leaf aqueous fractions inhibit TNF-α-induced nuclear factor κB (NF-κB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis 193: 20–27, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis 202: 152–161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsubakio-Yamamoto K, Matsuura F, Koseki M, Oku H, Sandoval JC, Inagaki M, Nakatani K, Nakaoka H, Kawase R, Yuasa-Kawase M, Masuda D, Ohama T, Maeda N, Nakagawa-Toyama Y, Ishigami M, Nishida M, Kihara S, Shimomura I, Yamashita S. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun 375: 390–394, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ. Adiponectin suppresses IκB kinase activation induced by tumor necrosis factor-α or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab 293: E1836–E1844, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316: 924–929, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol 27: 871–877, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278: 2461–2468, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Yang J, Park Y, Zhang H, Gao X, Wilson E, Zimmer WE, Abbott L, Zhang C. Role of MCP-1 in tumor necrosis factor-α-induced endothelial dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 297: H1208–H1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res 4: 53–65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]